The hypotheses of this study are that the liver derived metabolites of vitamin D: 25-hydroxyvitamin D2 (25OHD2) and 25-hydroxyvitamin D3 (25OHD3) are transported in different plasma fractions, causing the difference in physiological efficiency between D2 and D3, and that 25OHD3 produced from D3 of endogenous or synthetic origin, respectively, are transported in different plasma fractions, contributing to their difference in toxicity.
Plasma from six calves was fractionated by ultracentrifugation into five fractions: chylomicron, lipoprotein fraction (LPF) 1, LPF2, LPF3, and protein. Samples were subsequently analysed for contents of 25OHD2 and 25OHD3. Results showed that 25OHD3 was transported in the protein fraction regardless of its origin, whereas 25OHD2 was transported in chylomicron and LPF2/LPF3 fractions. Associating with designated binding proteins is vital to the function and stability of 25OHD2 and 25OHD3, hence transport in other fractions than protein may explain the reduced circulating levels of 25OHD2 compared to 25OHD3, when D2 or D3, respectively, is supplied in similar amounts.
Vitamin D has become one of the most discussed and studied nutrients in human nutrition. This has led to an increased interest in milk and dairy products as sources of vitamin D; and milk is routinely fortified with synthetic vitamin D in many countries. Fortification of milk with synthetic vitamin D is littered with difficulties related to poor stability of naked synthetic vitamin D in watery solutions (Diarrassouba et al. Reference Diarrassouba, Garrait, Remondetto, Alvarez, Beyssac and Subirade2015), which can be overcome by securing a naturally high content of vitamin D in the produced milk via the dairy cow (Kurmann & Indyk, Reference Kurmann and Indyk1994).
A sufficient vitamin D status of cattle can be secured by two different vitamin D compounds: vitamin D2 (D2) and vitamin D3 (D3). Vitamin D2 originates from roughage, e.g. hay and silage, used for cattle feeding, and it is synthesised during sun-curing at harvest by fungi growing on the plant material (Richardson & Logendra, Reference Richardson and Logendra1997). Vitamin D3 is either synthesised endogenously in the skin of the animals during exposure to sunlight (Hymøller & Jensen, Reference Hymøller and Jensen2010b), thus never entering the digestive tract, or supplied in the feed as a synthetic additive. Studies in cattle have shown that D2 is much less physiologically effective than D3, and it is not particularly good for securing a sufficient plasma status of the liver derived metabolites 25-hydroxyvitamin D2 (25OHD2) and 25-hydroxyvitamin D3 (25OHD3) (25OHDx), respectively (Hymøller & Jensen, Reference Hymøller and Jensen2010a, Reference Hymøller and Jensen2011a). This physiological difference appears illogical from a biochemical point of view since only minor differences in the side-chain of the general steroid ring-structure of the vitamin D molecule exist between D2 and D3 (Nigwekar et al. Reference Nigwekar, Bhan and Thadhani2012).
In vitamin research there is an on-going discussion about whether synthetic vitamins are as efficient as natural vitamins (Thiel, Reference Thiel2000) and it is often claimed that synthetic vitamins work similar to their natural counterparts in the organism. However, an increasing number of researchers in physiology, nutrition, and medicine are expressing their doubt, and results in direct opposition to these claims have been published (Thiel, Reference Thiel2000). Many synthetic forms of vitamins are not supplemented in the same physiochemical form as their natural forms but as derivatives stabilised as salts, esters etc. without being chaperoned into circulation by their naturally adjacent cofactors (Thiel, Reference Thiel2000). This is not the case for D3 because the synthetic dietary form is chemically similar to the natural endogenous form produced in the skin of cattle during exposure to sunlight but the two forms act very different within the living organism, since synthetic dietary D3 is toxic and can be easily overdosed, in contrast to endogenous natural D3.
The physiological distinction between natural endogenous and synthetic dietary D3 in cattle may be facilitated by different transport mechanisms in the blood stream. In a study by Haddad et al. (Reference Haddad, Matsuoka, Hollis, Hu and Wortsman1993) in humans, natural endogenous D3 was recovered almost exclusively bound to Vitamin D Binding Protein (VDBP), a protein responsible for storing and transporting vitamin D metabolites in plasma. In contrast, orally administered D2 was recovered from other plasma fractions than VDBP (Haddad et al. Reference Haddad, Matsuoka, Hollis, Hu and Wortsman1993). This has been suggested to be due to dietary D2 entering circulation through the lymph system after being absorbed with the fat-fraction of the diet in the distal part of the small intestine (Nechama et al. Reference Nechama, Hoff, Harell and Edelstein1977; Maislos et al. Reference Maislos, Silver and Fainaru1981; Dueland et al. Reference Dueland, Pedersen, Helgerud and Drevon1983). The same route of uptake would apply to synthetic dietary D3. It has, however, never been studied if differences in plasma transport mechanisms between D2 and D3 or between natural endogenous and synthetic dietary D3 is also present in the 25OHD2 and 25OHD3 metabolites, respectively. Differences in transport mechanisms between vitamin D metabolites in the body of cows could affect the choice of form and route of vitamin D supplementation to secure the highest possible vitamin D content in the produced milk.
The hypotheses of the present study are that differences between routes of plasma transport of 25OHDx exist, which cause the observed physiological inefficiency of D2 compared to D3 in securing a sufficient plasma status of 25OHDx in cattle, and cause the difference in toxicity between chemically similar D3 compounds of endogenous and dietary origin, respectively.
Materials and methods
The study was carried out at Aarhus University, Department of Animal Science in Tjele, Denmark between June 7th and August 16th 2013 at 55°N. The presented study complied with the Danish Ministry of Justice Law No. 1306 (November 23rd 2007) concerning experiments with animals and care of animals used for experimental purposes.
Animals
Six Danish Holstein bull calves were used in the study. The calves were between 9 and 13 d of age at the beginning of the study and weighed between 41 and 56 kg. At the end of the study the calves weighed between 88 and 118 kg. Initial plasma contents of 25OHD3 were between 0·3 and 5·7 ng/ml and contents of 25OHD2 between 0·2 and 1·3 ng/ml. The coat colour of the calves was assessed from silhouettes of animals (Sneddon et al. Reference Sneddon, Walton and Bond2004) as mainly black or mainly white and the calves were blocked according to predominant coat colour and initial plasma content of 25OHD3. Isolated cases of disease among the young calves were treated according to guidelines of the supervising veterinarian.
Treatments, feeding, and housing
The six calves were divided into two treatment groups: Three calves were let out for pasture between 10·00 and 16·00 daily in order to facilitate endogenous D3 synthesis from sunlight (SUN), whereas the other three calves were housed indoor and fed a synthetic supplement of 75 µg (3·000 IU) D3 daily (SUP).
Between day 0 and 31 of the study all calves were fed 6 litres of fresh cow milk per day in two servings at 07·00 and 16·30 and in addition the calves in the SUP group were given 75 µg D3 dissolved in 6 ml ethanol in the milk at the morning feeding. All calves had ad libitum access to pelleted concentrate without added synthetic D3 containing: barley (57%), soybean meal (28%), dried grass pellets (13%), and sugar beet molasses (3%). Between day 32 and day 36 of the study all calves were gradually weaned off milk. Between day 37 and day 71 of the study the calves in the SUN group had ad libitum access to the concentrate mixture void of D3, whereas calves in the SUP group had ad libitum access to concentrate (Grønkalv valset, DLG, Copenhagen, Denmark) containing 25 µg (1·000 IU) D3 per kg. This concentrate consisted of: barley (50%), soybean meal (15%), linseed cake (10%), sugar beet molasses (7%), maize (5%), wheat (4%), and dried grass pellets (3%), palm fat (1%), macro and micro minerals and vitamins (5%). All calves had ad libitum access to fresh water and hay at all times. This sun cured hay was the source of D2 in all calves (Horst et al. Reference Horst, Reinhardt, Russel and Napoli1984).
Before weaning calves were housed in individual pens on straw bedding. After weaning SUN calves were group housed, whereas SUP calves remained individually housed to facilitate registration of individual daily concentrate and, consequently, D3 intakes. From registered feed intakes it was calculated that calves in the SUP group ate on average 75 µg D3/d approximately 10 d after weaning, which was equivalent to the amount supplied in the milk prior to weaning.
Sample material
Approximately 30 ml of blood was collected once weekly between 08·00 and 09·00 from the jugular vein in EDTA coated Vacuette ® tubes (Greiner Bio-One GmbH, Kremsmünster, Austria). Blood samples were kept on ice until they were centrifuged for 10 min at 1500 g (SL 40 centrifuge, Thermo Scientific, Hvidovre, Denmark). Plasma was subsequently transferred to micro tubes (Deltalab S.L., Barcelona, Spain) and stored at −18°C until analysis.
For fractioning plasma into five fractions: chylomicron, lipoprotein fraction (LPF) 1, LPF2, LPF3, and protein, 6 ml of plasma was ultra-centrifuged at 100·000 g for 1 h in a Beckman Coulter 70·1 Ti fixed angle rotor using a Beckman Coulter Optima™ L-80 XP ultracentrifuge (Beckman Coulter Inc., Copenhagen, Denmark). The upper chylomicron fraction was subsequently removed (Axis-Shield, 2011). The remaining plasma was fractionated by gradient ultracentrifugation at 300·000 g for 18 h after mixing the remaining plasma with 1·5 ml OptiPrep™ (60% iodixanol/water, density 1·32 g/ml) (Axis-Shield, Oslo, Norway) and layering Hepes buffered saline 0·85% (w/v) pH 7·4 on top (Gardner et al. Reference Gardner, Ogden, Cripps and Billington2003; Axis-Shield, 2011). Fractions of LPF1, LPF2, LPF3, and protein were subsequently removed from the top down by use of Pasteur glass pipettes.
Laboratory analyses
Plasma and plasma fractions were analysed for content of 25OHD2 and 25OHD3 as described by Hymøller & Jensen (Reference Hymøller and Jensen2011b). Proper separation of fractions was confirmed by analysing selected samples for content of triacylglycerides, cholesterol, total protein, and albumin according to standard procedures (Siemens Diagnostics® Clinical Methods for ADVIA 1650) and phosphorlipids according to the Wako Choline Oxidase, DAOS Method. All analyses were performed using an Autoanalyzer ADVIA 1650® Chemistry System (Siemens Medical Solutions, Tarrytown NY, USA). To further confirm the distribution of the obtained fractions, they were also analysed for content of vitamin A and vitamin E as described by Jensen et al. (Reference Jensen, Jensen, Jakobsen, Engberg, Andersen, Lauridsen, Sørensen, Henckel, Skibsted and Bertelsen1998).
Statistics and calculations
Analysis of variance on repeated measures of plasma contents of 25OHD2 and 25OHD3 was performed using the MIXED models procedure of SAS® (SAS Institute Inc., Cary, NC). The statistical model used was: Y ijk = μ + α i + β j + (αβ)ij + c ijk + e ijk where the dependent variable Y ijk is the content of 25OHD2 or 25OHD3, μ is the overall mean, α i is the fixed effect of treatment i (SUN, SUP), β j is the fixed effect of day j (0, 7, 14, 21, 28, 35, 42, 49, 56, 63, 70), (αβ)ij is the effect of the interaction between treatment i and day j, c k is the random effect of calf k, and e ijk is the random residual error. To account for the covariance structure of the repeated measures during consecutive days within calf the covariance was modelled using the repeated statement in the MIXED procedure of SAS® (Littell et al. Reference Littell, Milliken, Stroup, Wolfinger and Schabenberger2006). The best model fit was obtained using the auto regressive first order covariance structure. Random effects were assumed normally distributed with mean value zero and constant variance c ijk ~ N (0, σ c2) and e ijk ~ N (0, σ 2). Distribution of 25OHD2 and 25OHD3 between different plasma fractions in each sample was calculated as the amount (ng) of a given metabolite found in each fraction in per cent of the total amount (ng) found in all fractions. Differences were compared by Student's two-sample, two-tailed heteroscedastic t-test on averages of all samples between calves and sampling times. Differences were considered statistically significant when P ⩽ 0·05 and as tendencies if 0·05 < P ⩽ 0·1.
Results
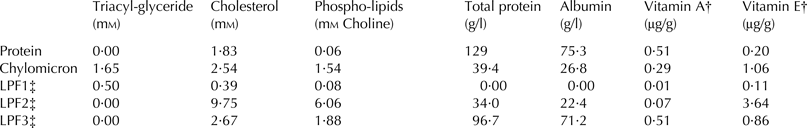
Contents of lipids, proteins, vitamin A, and vitamin E in the different plasma fractions are shown in Table 1. Total protein was highest in the protein fraction whereas no protein or albumin was encountered in LPF1. Cholesterol and phospholipids were mainly found in LPF2 and LPF3 whereas triacylglyceride was mainly found in chylomicrons. The highest concentration of vitamin A was encountered in the protein fraction and the highest concentration of vitamin E in the LPF2 fraction.
Table 1. Content of triacylglyceride, cholesterol, phosphorlipids, total protein, albumin, vitamin A, and vitamin E in fractions of cattle plasma obtained by gradient ultracentrifugation

† The intake of vitamin A was 9 µg/d and vitamin E was 360 µg/d calculated from registered feed intakes
‡ Lipoprotein fractions 1, 2, and 3; LPF1 contains VLDL and LPF2 and LPF3 contain a mixture of LDL and HDL
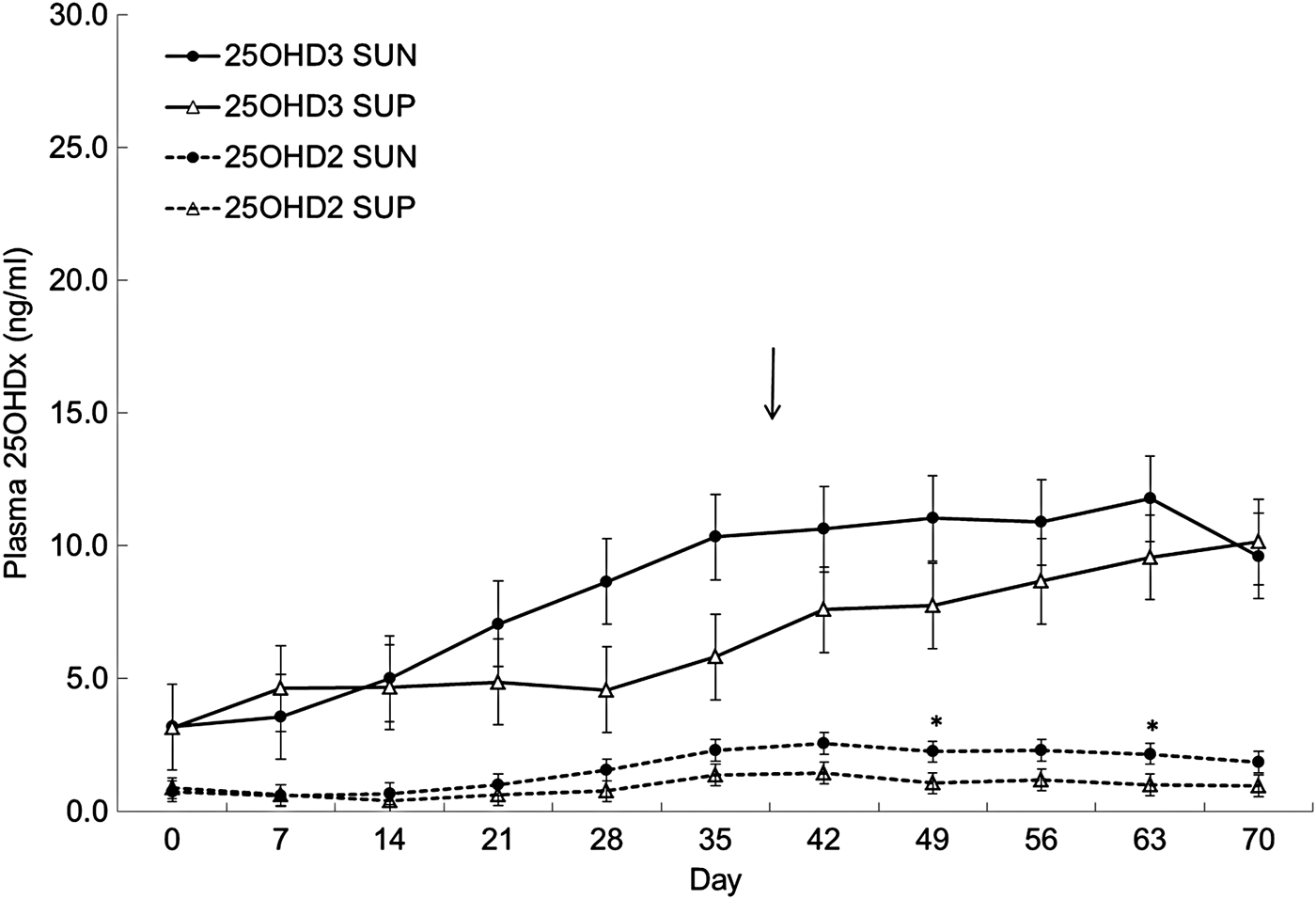
Plasma contents of 25OHD3 were not affected by treatment (P = 0·36) but there was a significant effect of day (P ⩽ 0·001) and a significant interaction between treatment and day (P ⩽ 0·05), probably due to the plasma content of 25OHD3 increasing more rapidly in SUN than in SUP during the study. However, the only differences between plasma contents of 25OHD3 in SUN and SUP were found at day 28 (P = 0·08) and day 35 (P = 0·05), where SUN had a higher plasma content of 25OHD3 than SUP. Plasma contents of 25OHD2 were generally very low and were, as expected due to its source in the hay fed to all calves, not affected by treatment (P = 0·14) but there was a significant effect of day (P ⩽ 0·001). No interaction between treatment and day was found (P = 0·76) but plasma contents of 25OHD2 were higher in SUN than in SUP between day 42 and 63 (P ⩽ 0·06) (Fig. 1).

Fig. 1. Plasma concentration (mean ± sem) of 25(OH)-vitamin D3 (25OHD3) and 25(OH)-vitamin D2 (25OHD2) in calves supplied with vitamin D3 from the sun (SUN) or from synthetic supplements (SUP) during 70 d (n = 3). Arrow indicates time of weaning. *Significant difference between SUN and SUP in plasma content of 25OHD2 or 25OHD3 *P ⩽ 0·05, **P ⩽ 0·01, ***P ⩽ 0·001.
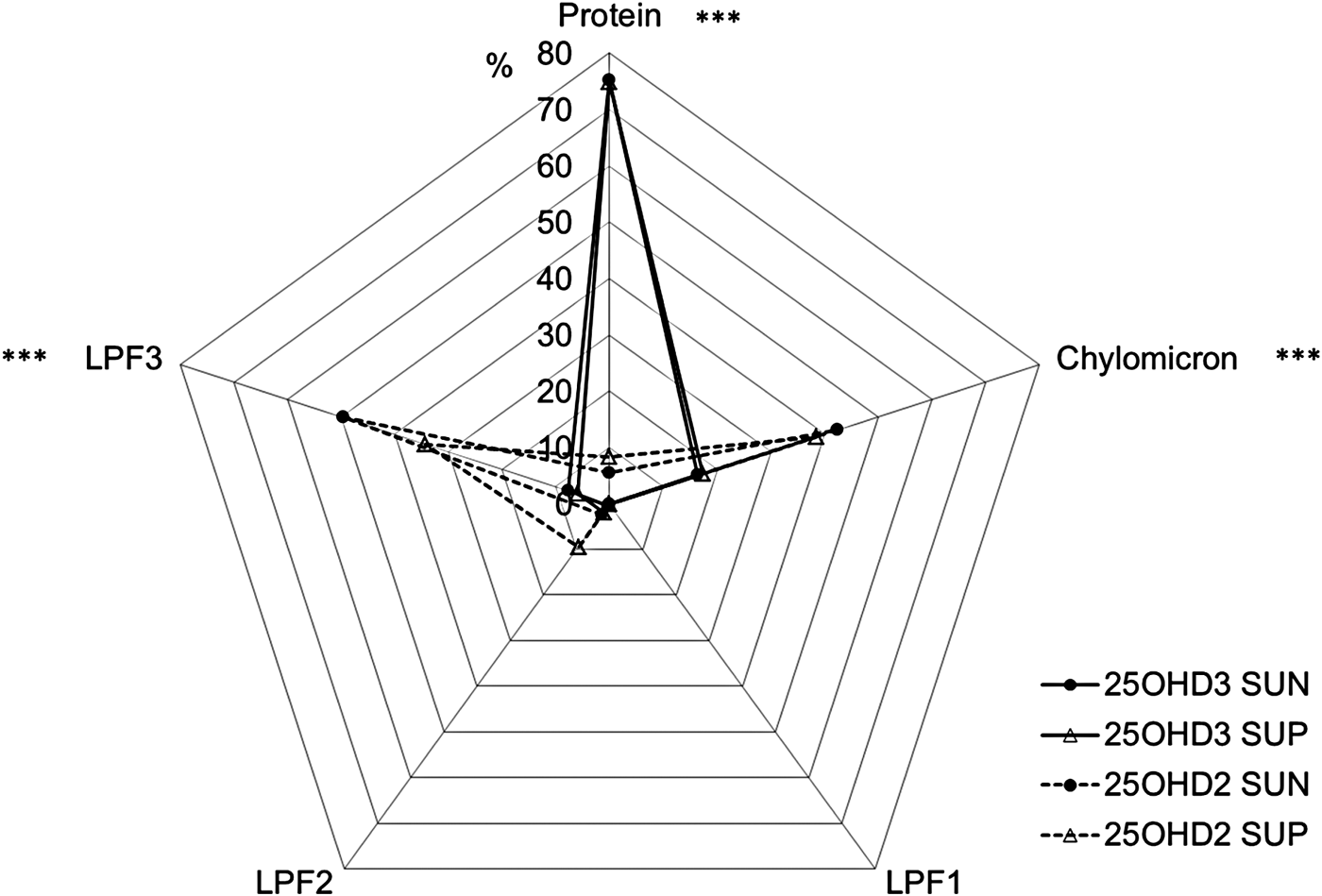
The percentage of 25OHD3 was higher than the percentage of 25OHD2 in the protein fraction (P ⩽ 0·001), whereas the percentage of 25OHD2 was higher than the percentage of 25OHD3 in the chylomicron (P ⩽ 0·001), the LPF2 (P ⩽ 0·05), and the LPF3 (P ⩽ 0·001) fractions. No 25OHD2 or 25OHD3 was found in the LPF1 fraction (Fig. 2).

Fig. 2. Distribution (% of total content in all fractions) of 25(OH)-vitamin D3 (25OHD3) and 25(OH)-vitamin D2 (25OHD2) in plasma fractions of protein, chylomicrons, and lipoprotein fractions (LPF) in calves supplied with vitamin D3 from the sun (SUN) or from synthetic supplements (SUP) during 70 d (mean of all samples, n = 3). LPF1 equals VLDL and LPF2 and LPF3 is a mixture of LDL and HDL. *Significant difference between fraction content of 25OHD2 and 25OHD3 in both SUN and SUP *P ⩽ 0·05, **P ⩽ 0·01, ***P ⩽ 0·001.
Discussion
Separation between fractions of protein, chylomicrons, and LPF1, which contains very low density lipoprotein (VLDL), was obtained and confirmed by their individual contents of proteins, lipids, and fat soluble vitamins. Based on the contents of proteins, lipids, and fat soluble vitamins, the fractions LPF2 and LPF3 appeared to contain a mixture of low density lipoprotein (LDL) and high density lipoprotein (HDL); whereas the chylomicron fraction appeared to contain traces of VLDL. Particularly LDL and HDL can be difficult to separate completely in cattle plasma, when using methods developed for human plasma fractionation (Gardner et al. Reference Gardner, Ogden, Cripps and Billington2003), and overlaps in density between LDL and HDL in cattle, particularly in calves, are previously reported (Bauchart, Reference Bauchart1993; Leplaix-Charlat et al. Reference Leplaix-Charlat, Bauchart, Durand, Laplaud and Chapman1996). Furthermore, in humans LDL is the main cholesterol transporter (Kayden & Traber, Reference Kayden and Traber1993), whereas in cattle a significant proportion of cholesterol is found circulating in HDL (Herdt & Smith, Reference Herdt and Smith1996; Leplaix-Charlat et al. Reference Leplaix-Charlat, Bauchart, Durand, Laplaud and Chapman1996; Pysera & Opalka, Reference Pysera and Opalka2000). This may further contribute to an ineffective separation of cattle LDL and HDL in density gradients developed for human plasma. In the present study it appeared, from the contents of cholesterol and vitamin E in the LPF2 and LPF3 fractions, that they contained a mixture of LDL and HDL. Cholesterol contents were slightly higher in LPF2 than LPF3 and the highest vitamin E concentration was found in LPF2. Vitamin E is reported by others to be circulating in the highest concentration in HDL in cattle or at least in even concentrations between LDL and HDL in ruminants (Herdt & Smith, Reference Herdt and Smith1996; Senaidy, Reference Senaidy1996; Ametaj et al. Reference Ametaj, Nonnecke, Franklin, Horst, Bidlack, Stuart and Beitz2000). Hence it appears that satisfactory separation of plasma fractions was obtained.
Regardless of origin, endogenous D3 or synthetic D3, respectively, 25OHD3 was similarly distributed between plasma fractions, which indicated that differences in toxicity between endogenous and synthetic D3 must be found at other levels of the vitamin D metabolic pathways than in the 25-hydroxylated metabolites. All calves had the same plasma content of 25OHD2 and 25OHD3, respectively, regardless of the treatments and there was no difference between treatments in distribution of 25OHD2 and 25OHD3, respectively, in different plasma fractions. However, when comparing the distribution of 25OHD2 to the distribution of 25OHD3, it became obvious that the two forms of hydroxylated vitamin D circulate bound to completely different plasma fractions. Haddad et al. (Reference Haddad, Matsuoka, Hollis, Hu and Wortsman1993) found in humans that endogenously synthesised D3 is recovered almost exclusively bound to VDBP whereas D2 supplemented orally is recovered from other plasma fractions e.g. chylomicrons. This difference is speculated to be caused by dietary D2 entering systemic circulation through the lymph system after absorption with the fat fraction of the feed (Nechama et al. Reference Nechama, Hoff, Harell and Edelstein1977; Maislos et al. Reference Maislos, Silver and Fainaru1981; Dueland et al. Reference Dueland, Pedersen, Helgerud and Drevon1983). However, results from the present study showed that this difference in distribution between different plasma fractions is also found between 25OHD2 and 25OHD3, which can be caused by a low binding affinity of 25OHD2 to VDBP. The protein fraction, which should cover most of the circulating VDBP designated to binding and transporting vitamin D, contained almost 80% of the circulating 25OHD3 whereas next to none of the circulating 25OHD2 was encountered in this fraction. In contrast 25OHD2 was found in chylomicron and LPF3 fractions, which may not offer the same level of protection to the molecule against degradation or excretion as binding to designated transport proteins. This could be part of the explanation to why D2 is less efficient at securing a sufficient vitamin D status in cattle, measured as 25OHD2, than D3, measured as 25OHD3, as observed by Hymøller & Jensen (Reference Hymøller and Jensen2010a, Reference Hymøller and Jensen2011a). However, the consequences of the difference in the plasma transport of different vitamin D metabolites for their transfer into milk needs investigation.
Conclusions
Transport of 25OHD2 and 25OHD3 in different plasma fractions may explain the relatively low physiological efficiency of D2 compared to dietary D3 in cattle. However, no difference in plasma transport between 25OHD3 of synthetic dietary or natural endogenous origin exist, hence the physiological distinction and discrimination between these two D3 sources must be related to other levels of the vitamin D metabolic pathway than the liver derived 25OHD3.
The study was financed via a grant from the Danish Council for Independent Research – Technology and Production (DFF-FTP) under the Danish Ministry of Higher Education and Science. The authors wish to thank laboratory technicians E.L. Petersen and M.W. Reeh and Research technician T.N. Jakobsen for kind assistance in blood sampling and laboratory analyses, and senior scientist T. Larsen for performing lipid and protein analyses.