Published online by Cambridge University Press: 04 March 2004
Echinococcus granulosus exhibits substantial genetic diversity that has important implications for the design and development of vaccines, diagnostic reagents and drugs effective against this parasite. DNA approaches that have been used for accurate identification of these genetic variants are presented here as is a description of their application in molecular epidemiological surveys of cystic echinococcosis in different geographical settings and host assemblages. The recent publication of the complete sequences of the mitochondrial (mt) genomes of the horse and sheep strains of E. granulosus and of E. multilocularis, and the availability of mt DNA sequences for a number of other E. granulosus genotypes, has provided additional genetic information that can be used for more in depth strain characterization and taxonomic studies of these parasites. This very rich sequence information has provided a solid molecular basis, along with a range of different biological, epidemiological, biochemical and other molecular-genetic criteria, for revising the taxonomy of the genus Echinococcus. This has been a controversial issue for some time. Furthermore, the accumulating genetic data may allow insight to several other unresolved questions such as confirming the occurrence and precise nature of the E. granulosus G9 genotype and its reservoir in Poland, whether it is present elsewhere, why the camel strain (G6 genotype) appears to affect humans in certain geographical areas but not others, more precise delineation of the host and geographic ranges of the genotypes characterised to date, and whether additional genotypes of E. granulosus remain to be identified.
An important feature of the biology of Echinococcus granulosus is the fact that it comprises a number of intraspecific variants or strains that exhibit considerable variation at the genetic level (Thompson & McManus, 2001). By contrast, there appears to be very limited genetic variation within E. multilocularis (McManus & Bryant, 1995; Haag et al. 1997; Rinder et al. 1997; Kedra et al. 2000a), and there are no available data to indicate that either E. vogeli or E. oligarthrus is variable. The term strain is used to describe variants that differ from other groups of the same species in gene frequencies or DNA sequences, and in one or more characters of actual or potential significance to the epidemiology and control of echinococcosis (Thompson & Lymbery, 1988; Bowles, Blair & McManus, 1995).
The extensive intra-specific variation in nominal E. granulosus may influence life cycle patterns, host specificity, development rate, antigenicity, transmission dynamics, sensitivity to chemotherapeutic agents and pathology (Thompson & Lymbery, 1988; Thompson, 1995; Thompson & McManus, 2001, 2002). This may have important implications for the design and development of vaccines, diagnostic reagents and drugs impacting on the epidemiology and control of echinococcosis (McManus & Bowles, 1996). For example, the adult parasite of the cattle strain of E. granulosus exhibits a precocious development in the definitive host with a short pre-patent period of only 33–35 days, nearly a week earlier than that of the common sheep strain (Thompson, 1995). This complicates control efforts where drug treatment of definitive hosts is utilised as a means of breaking the cycle of transmission, as it necessitates an increase in frequency of adult cestocidal treatment.
A number of well-characterized strains are now recognized that all appear to be adapted to particular life cycle patterns and host assemblages (Thompson & McManus, 2001; McManus, 2002). To date, molecular studies, using mainly mitochondrial DNA (mtDNA) sequences, have identified 9 distinct genetic types (genotypes G1-9) within E. granulosus (McManus, 2002). This categorization follows very closely the pattern of strain variation emerging based on biological characteristics. Here, the various DNA-based approaches that have been used in accurate identification of these genetic variants are briefly reviewed as are their use in molecular epidemiological surveys of echinococcosis in different host assemblages and geographical settings.
Genetic variation in Echinococcus has been investigated in both the nuclear and mitochondrial genomes. The nuclear ribosomal RNA gene (rDNA) repeat unit has different regions evolving at varying rates and so has been used extensively to study variation and phylogeny in Echinococcus (Bowles, Blair & McManus, 1995; Kedra et al. 1999) at a number of different taxonomic levels. Mitochondrial DNA (mtDNA) is useful for the discrimination of closely related organisms because of its relatively rapid rate of evolution. Furthermore, as mtDNA is haploid, allele haplotypes can be determined unambiguously. Mitochondrial DNA has the additional advantage that, as far as is known, it is maternally inherited and does not recombine, thus simplifying analysis. The advent of the polymerase chain reaction (PCR) has provided a highly sensitive approach that is now widely used for Echinococcus identification purposes, including discrimination of eggs.
Earlier studies of molecular genetic variation in Echinococcus involved restriction fragment length polymorphism (RFLP) analysis using the conventional Southern blotting approach (McManus & Simpson, 1985; McManus, Simpson & Rishi, 1987; Rishi & McManus, 1987; McManus & Rishi, 1989). The technique was able to distinguish several distinct strains of E. granulosus and extensive study showed that the RFLP patterns were stable within a particular strain. The conventional RFLP procedure was simplified, without loss of resolution or accuracy, by linking RFLP analysis with PCR targeting the nuclear rDNA ITS1 region (Bowles & McManus, 1993a). Characteristic PCR-amplified ITS1 and PCR-ITS1 RFLP banding patterns were produced when samples within Echinococcus species and strain group were analysed. The approach proved rapid and, although its usefulness has been questioned (Kedra et al. 1999), it has proved applicable and reliable in the hands of a number of researchers for the identification of newly collected isolates and for the investigation of E. granulosus transmission patterns where strains occur sympatrically (Bowles & McManus, 1993a; Wachira et al. 1993; Bowles, Blair & McManus, 1994; Scott et al. 1997; Rosenzvit et al. 1999; Snabel et al. 2000; Gonzalez et al. 2002).
The random amplified polymorphic DNA-polymerase chain reaction (RAPD-PCR) is another method that has been used under carefully controlled conditions for distinguishing the four recognized Echinococcus species and genetically distinct forms of E. granulosus (Eckert et al. 1993; Scott & McManus, 1994; Siles-Lucas et al. 1994; Turcekova, Snabel & Dubinsky, 1998; Turcekova et al. 2003).
Comparison of the nucleotide sequence of defined DNA segments between organisms provides the most direct and sensitive means of detecting genetic variation. PCR has made sequence comparison a feasible approach for the study of genetic variation. Mitochondrial sequences, particularly fragments of the mitochondrial protein-coding genes, cox1 (cytochrome c oxidase subunit 1 (CO1) (366 bp)) (Bowles, Blair & McManus, 1992), and nad1 (NADH dehydrogenase I (ND1) (471 bp)) (Bowles & McManus, 1993a), have proved invaluable for E. granulosus strain identification. The consensus view of the strain pattern within E. granulosus, based on a variety of other criteria (Thompson, 1995), was broadly upheld when the DNA sequences of the different genotypes were compared. Furthermore, remarkable intra-genotypic strain homogeneity was found at the DNA sequence level. A recent comparison of the complete mtDNA sequences for the horse-dog and sheep-dog strains of E. granulosus relative to the mtDNA sequence of E. multilocularis (Nakao et al. 2002) has shown them to be almost as distinct from each other as either is from E. multilocularis (Le et al. 2002). This will be discussed further below.
Mutation scanning methods (Gasser, 1997; Gasser & Zhu, 1999) provide alternatives to DNA sequencing for the high resolution analysis of PCR-amplified fragments and they can be used to rapidly screen large numbers of Echinococcus isolates. One such method is single strand conformation polymorphism (SSCP) which has the capacity to distinguish PCR-amplified fragments of 530 bp differing by a single nucleotide. Once the utility of SSCP for the categorization of Echinococcus genotypes was established (Gasser, Zhu & McManus 1998a) the method was applied successfully for the genetic analysis of a number of isolates of E. granulosus collected from China and Argentina (Zhang et al. 1999). The analysis identified representative SSCP profiles, which corresponded to 4 variant sequence types of nad1 and cox1 defined subsequently by DNA sequencing. The approach has also been used to assess the genetic variability of coding and noncoding regions of the genome of E. granulosus and to test whether or not E. granulosus populations are mainly self-fertilizing (Haag et al. 1999).
Another useful mutation scanning method is dideoxy fingerprinting (ddF) which is a hybrid between SSCP and conventional dideoxysequencing. The technique has been used for the direct display of sequence variation in the cox1 gene to genetically type and differentiate all of the Echinococcus genotypes examined by their characteristic and reproducible ddF fingerprinting profiles (Gasser et al. 1998b).
A recently developed base excision sequence scanning thymine-base method incorporating cox1 and cob (cytochrome b) genes as targets has been for differential DNA diagnosis of human Taenia cestodes (Yamasaki et al. 2002). Characteristic thymine-base peak profiles indicated four distinct types, unique for T. saginata, T. saginata asiatica and two genotypes of T. solium. This approach, which provides a useful tool for the identification and diagnosis of human taeniid cestodes without DNA sequencing, should be readily applicable to similar studies on Echinococcus isolates.
A virtually untapped area for studying diversity in Echinococcus is the use of microsatellite DNA. Microsatellites have become one of the most useful genetic markers used in a large number of organisms due to their abundance and high level of polymorphism. Microsatellites have been used for individual identification, paternity tests, forensic studies and population genetics. Microsatellites are short stretches of repeated DNA (the repeats usually being of 2 to 6 nucleotides each in length) that show exceptional variability in humans and most other species. This variability has made microsatellites the genetic marker of choice for most applications, including genetic mapping and studies of the evolutionary connections between species and populations (Schlotterer, 2000; Barker, 2002). The repetitive nature of microsatellite sequences results in variability in the number of repeats that can be found at specific loci. By contrast, the sequences surrounding the microsatellites are generally well conserved within a species and, on occasion, even among higher taxa. Accordingly, PCR primers complementary to sites flanking the microsatellite loci can be used to amplify the intervening repeat region. Diploid organisms including E. granulosus will exhibit two alleles at each of the amplified loci, which might be the same or different lengths, depending upon the number of repeated motifs that constitute each microsatellite locus. An individual's alleles, which may differ by as little as a single dinucleotide repeat, can then be determined using gel electrophoresis techniques. Microsatellites are thus valuable diagnostic markers for studies on genetic variation within populations and of population structure of sexually reproducing organisms such as tapeworms.
Data on microsatellite abundance comes preferentially from microsatellite-enriched libraries and DNA sequence databases. DNA microsatellites have been used as molecular markers to analyse the population structure of schistosomes (Curtis & Minchella, 2000; Curtis, Sorensen & Minchella 2002; Rodrigues et al. 2002). Microsatellite loci have also been isolated and characterized from the pseudophyllidean cestode Schistocephalus solidus (Binz et al. 2000). Some microsatellite markers are available for E. multilocularis, following the studies of Bretagne et al. (1996) who were able to use microsatellite DNA to divide isolates of E. multilocularis into three groups: European, North American (Montana) and Japanese. The provision of microsatellite markers for E. granulosus and additional microsatellites from E. multilocularis, will provide exquisitely sensitive markers for studying the population genetics and transmission biology of the Echinococcus organisms. The ability to detect genetic variation within strains and species of Echinococcus will allow a better understanding of the transmission dynamics of the causative agents in localised endemic foci. For example, on the Australian mainland, it will enable the interactions between wild and domestic cycles of transmission to be determined, and in other areas where more than one species of domestic intermediate hosts are susceptible to infection, as in the Middle East and China, their role in maintaining cycles of transmission could be evaluated if appropriate microsatellite markers can be identified.
A description follows of the utility of DNA-based approaches in helping to clarify the complex issue of strain variation in E. granulosus and their value for molecular epidemiological studies of cystic echinococcosis. The various genotypes of E. granulosus that have been identified together with their host and geographical ranges are presented in Table 1.

As the result of extensive study, instigated by Professor Des Smyth in the 1970s, discrete horse/dog and sheep/dog forms of E. granulosus have been shown to be present in the United Kingdom that differ in a wide spectrum of biological criteria (Smyth, 1977; Thompson & Lymbery, 1988; Thompson, 1995). Conventional RFLP analysis (McManus & Simpson, 1985; McManus & Rishi, 1989), PCR/RFLP analysis (Bowles & McManus, 1993b) and sequence comparison of the cox1 (Bowles, Blair & McManus, 1992) and nad1 genes (Bowles & McManus, 1993a) confirmed the distinctiveness between, but uniformity within, these two forms of E. granulosus. Further, analysis of isolates collected world-wide using these approaches indicated that the sheep/dog strain is cosmopolitan in its geographical distribution, that it is remarkably uniform genetically and that the horse/dog form is genetically similar to that infecting equines in other countries. This early DNA sequence data indicated that these ‘strains’ were as distinct as the accepted species of Echinococcus (Bowles, Blair & McManus, 1992), suggesting that they may be more appropriately regarded as sibling species, especially in light of the considerable biological and biochemical differences that were shown to exist between them.
No evidence of gene exchange was found in examination of their rDNA sequences (Bowles & McManus, 1993b), implying that the sheep and horse strain parasites do not interbreed despite the fact that they use the same definitive host and occur sympatrically. Careful phylogenetic analysis of the mitochondrial sequence data, in combination with additional nuclear sequence data (Bowles et al. 1995), formally demonstrated the evolutionary distinctiveness of the sheep and horse strains of E. granulosus. Confirmation of species identity was obtained after analysis of the complete mitochondrial genomes that were recently obtained for both strains and another taeniid cestode, Taenia crassiceps (Le, Blair & McManus, 2002). Pair-wise comparisons of concatenated protein-coding genes indicated that the sheep-dog and the horse-dog forms were almost as distant from each other as each was from E. multilocularis. In addition, sequences for the variable genes atp6 and nad3 were obtained from additional genotypes of E. granulosus, from E. vogeli and E. oligarthrus. Again, pair-wise comparisons showed the distinctiveness of the G1 and G4 genotypes. Phylogenetic analyses of concatenated atp6, nad1 (partial) and cox1 (partial) genes from E. multilocularis, E. vogeli, E. oligarthrus, 5 genotypes of E. granulosus, and using T. crassiceps as an outgroup, yielded the same results (Fig. 1).

Fig. 1. Inferred relationships among species and genotypes of Echinococcus, using Taenia crassiceps as an outgroup (After Le et al. 2002). Concatenated sequences of atp6, nad1 (partial) and cox1 (partial) were analysed (Bowles, Blair & McManus, 1992; Bowles & McManus, 1993a; Le et al. 2002). A distance matrix was constructed from inferred amino acid (aa) sequences (alignment was 451 aa long with 168 variable aa sites 67 aa parsimony-informative sites) using a Poisson correction for multiple hits and the tree constructed using the minimum evolution approach. Five hundred bootstrap resamplings were carried out. Branches with bootstrap support values less than 50% are indicated with an asterisk. EgrG1, EgrG4, EgrG6-EgrG8 are the different genotypes of E. granulosus. Units on scale bar: changes per site. The branches indicated by an asterisk were supported by fewer than 50% of the resampled data sets and therefore should be regarded as poorly supported. It is clear that EgrG4, EgrG1, E. vogeli and E. oligarthrus are almost equidistant from each other in terms of mt sequences. Furthermore, the E. granulosus G1 and G4 genotypes are also almost equidistant from the G6-8 genotype cluster, although there is some structure in this latter group. E. multilocularis appears as basal within the genus, but again the branch placing it there is rather poorly supported. Given this, recognition of the sheep-dog (G1 genotype) and the horse-dog (G4 genotype) strains (and possibly also the G6-8 genotypes) as separate species is appropriate. The discrete nature of the two forms is quite clear and the molecular and phylogenetic evidence from this and previous studies suggests the case for reinstatement of their formal taxonomic status as subspecies/species is now proven.
These data and a range of different biological, epidemiological, biochemical and other molecular-genetic criteria provide an overwhelming argument in favour of separate species status for the horse-dog and sheep-dog strains. Of public health significance is the fact that the sheep strain is infective to humans but, probably, non-infective to horses. The horse strain appears to be poorly infective to sheep and may prove to be non-infective to humans. This is borne out by the DNA data as, to date, the horse strain (G4 genotype) has not been reported in sheep or humans, and the sheep strain (G1 genotype) has not been identified by DNA analysis in horses.
The fact that the genetic characteristics of the horse-dog and sheep-dog forms of E. granulosus are maintained in sympatry in endemic areas where the life cycles overlap (e.g. UK, Spain and Jordan; Kamhawi & Hijawi, 1992; Siles Lucas et al. 1994) reinforces the argument that the two forms are separate species. Rausch (1967) quite correctly identified the problem of recognizing the form in horses as a sub-species since Williams & Sweatman (1963) provided no evidence of a segregating mechanism, since subspecies by definition can interbreed. Consequently, if Williams & Sweatman (1963) had proposed species status for the form in horses its taxonomic status is unlikely to have been questioned as rigorously. Considering the additional evidence that has accumulated in the intervening 40 years, we have proposed (Thompson & McManus, 2002) that E. equinus is recognised as a distinct species, following the description given by Williams & Sweatman (1963).
DNA-based techniques have shown that cattle from a number of countries harbour the common sheep strain (G1 genotype) of E. granulosus (McManus & Rishi, 1989; Bowles, Blair & McManus, 1992; Bowles & McManus, 1993b). Bovines are, however, susceptible to other genotypes of E. granulosus. For example, a morphologically and developmentally distinct form of E. granulosus has been reported in India and comparison of cox1 sequences and/or PCR/RFLP patterns (Bowles, Blair & McManus, 1992; Bowles & McManus, 1993b) indicated that Indian buffaloes are infected with three different E. granulosus genotypes. These include the common sheep strain and a genotype that is slightly different, either a variant of the common sheep strain or a genetically distinct but very closely related parasite. The third genotype, which was designated G5, was quite distinct from these two forms but it was genetically indistinguishable from the well characterized ‘Swiss’ cattle strain (Thompson, Kumaratilake & Eckert, 1984).
The Swiss cattle strain of E. granulosus differs from other strains in its unique morphology, biochemistry, precocious development in dogs and in its predilection for the lungs in the intermediate host where the cysts, in contrast to other strains infecting cattle, are usually highly fertile (Thompson et al. 1984). High fertility rates have been reported for E. granulosus in cattle from a number of other countries (Thompson & Lymbery, 1988), suggestive of widespread distribution of this strain. Genetic evidence (McManus & Rishi, 1989; Bowles, Blair & McManus, 1992; Bowles, van Knapen & McManus, 1992; Bowles & McManus, 1993b) indicates that it certainly occurs in Switzerland and India and, as mentioned in this review, in Holland, Nepal and South America.
We have advocated that the form of Echinococcus which is adapted to cattle as its intermediate host also warrants taxonomic recognition (Thompson & McManus, 2002). This form is characterized by the nature of its pulmonary metacestode development with the production of predominantly fertile cysts, its unusual strobilar morphology and rapid rate of development of the adult worm. In addition, although the molecular data are not as rich as those available for comparing the horse and sheep strains, there is no question of its genetic distinctiveness as clearly shown by pairwise distance matrix and phylogenetic analysis using nuclear and mitochondrial genes (Bowles et al. 1995). The cattle-adapted form has a widespread geographical distribution that includes parts of central Europe, South Africa, India, Sri Lanka, Nepal and possibly South America (Thompson & McManus, 2001). Although cattle are commonly found to harbour hydatid cysts throughout the world, the aetiological agent is usually the sheep strain of E. granulosus and infected cattle are an accidental host with resultant cysts rarely fertile. The cysts of the cattle strain are invariably fertile and well developed (Thompson et al. 1984). As with Echinococcus of horse origin, the cattle form of Echinococcus was given taxonomic status by Lopez-Neyra and Soler Planas in 1943 based on their re-evaluation of a description by Ortlepp in 1934 of Echinococcus in South Africa where we now know the cattle strains occurs. The taxonomic status of E. ortleppi was not accepted by Rausch and Nelson in 1963, but subsequent studies by Verster (1965) revealed that previous taxonomic considerations were based on only a limited appraisal of the morphological features which characterize this form of Echinococcus. We have, therefore, proposed that E. ortleppi should be reinstated and recognised as the cattle-adapted form of Echinococcus (Thompson & McManus, 2002), a comprehensive morphological description of which has been provided (Verster, 1965).
It has been recognised for a considerable period that E. granulosus is maintained in 2 cycles of transmission on mainland Australia (Thompson & Kumaratilake, 1982). One cycle principally involves domestic sheep as the major intermediate host, with cattle and pigs as potential accidental intermediate hosts, while the other involves numerous species of macropod marsupials (kangaroos and wallabies). There is interaction between these cycles through a range of carnivores (domestic dogs, feral dogs, dingoes and red foxes) which act as definitive hosts.
Early evidence of morphological, biochemical and developmental differences between isolates of E. granulosus of domestic and sylvatic origin led to their proposed designation as distinct strains (Thompson & Kumaratilake, 1985). However, this hypothesis was questioned following additional morphological studies, isoenzyme analysis, conventional RFLP, PCR/RFLP and mitochondrial DNA sequencing (Hobbs, Lymbery & Thompson, 1990; Lymbery, Thompson & Hobbs 1990; Hope et al. 1992) which indicated that only the common sheep strain was present. Indeed, cox1 and nad1 sequences of an additional 24 E. granulosus samples collected from various Australian hosts including sheep, macropods, humans, pigs, cattle and dingoes (Bowles, Blair & McManus, 1992; Bowles & McManus, 1993c) were identical to the common sheep strain, again arguing strongly against the theory that a distinct Australian sylvatic strain occurs on the mainland.
In biological, epidemiological and molecular features the common sheep strain can be regarded as homogeneous except in Tasmania where morphological distinctiveness and a significantly shortened pre-patency period have been reported (Kumaratilake, Thompson & Dunsmore 1983; Thompson & Lymbery, 1988). Molecular evidence (Bowles, Blair & McManus, 1992) also indicated that a variant of the common E. granulosus genotype occurred in Tasmania. Two out of a total of nine Tasmanian sheep isolates were shown to differ slightly in mitochondrial cox1 sequence from the common sheep strain. Three (out of 366) nucleotides were variant, causing two amino-acid changes in the protein. The same 2 Tasmanian isolates could not be distinguished from UK and Australian mainland sheep isolates by conventional RFLP (Hope, Bowles & McManus, 1991) or PCR/RFLP analysis (Bowles & McManus, 1993a) although, as indicated above, the well-established strain groups can be clearly distinguished by these approaches. A slightly different nad1 sequence was also found when these isolates were compared with other available sheep strain isolates. These results suggested that the biologically atypical Tasmanian form of E. granulosus had diverged only relatively recently from the common sheep strain, possibly under the influence of changed environmental conditions such as intensive drug treatment of the definitive host. Alternatively, the slightly distinct Tasmanian form may have represented a genotype that is relatively rare in the Australian mainland population and has perhaps become established in Tasmania as the result of a founder effect. Subsequent work (see below) has shown that the G2 genotype is also present in Argentina, possibly having been introduced with Merino sheep exported from Australia to Argentina (Rosenzvit et al. 1999).
Kenya has a very high prevalence of human hydatid disease which is hyperendemic among two pastoral communities, the Turkana in the northwest and the Maasai in the southwest. These regions are geographically separated by a non-hydatid zone, the length of which varies between 250 and 800 kilometres. The range of intermediate hosts for E. granulosus includes cattle, sheep, goats and humans in both regions and camels in Turkana, while domestic dogs are the main definitive hosts. E. granulosus isolates from the various Kenyan hosts have a uniform morphology and developmental rates that are similar in vitro and in vivo (Wachira et al. 1993). However, two distinct strains of the parasite were readily identifiable by isoenzyme analysis and RFLP analysis of rDNA and cox1 sequencing (Bowles, Blair & McManus, 1992; Bowles & McManus, 1993b) confirmed the results of the earlier enzyme studies. In Kenya, the sheep strain of E. granulosus occurs in sheep, cattle, goats and man, with the camel strain (G6 genotype) infecting camels and occasionally goats. Comparison of PCR/RFLP patterns (Bowles & McManus, 1993b) and cox1 sequences (Bowles, Blair & McManus, 1992) indicated that the camel strain was closely related to a form of E. granulosus found in pigs from eastern Europe and morphological evidence (Eckert et al. 1993) supported this relatedness.
The rDNA PCR/RFLP approach was subsequently used (Wachira et al. 1993) to examine a much larger number of E. granulosus isolates than had previously been possible. Existence of the sheep/dog and camel/dog strains in Kenya was confirmed and it was shown that the camel strain appeared restricted to the Turkana region, where camels are kept as livestock. The intermediate host range for both strains appeared to be similar except that no evidence was found of human infections with the camel strain in Turkana.
There has been one molecular epidemiological survey of E. granulosus isolates in eastern Libya where the incidence of surgically confirmed cystic echinococcosis was estimated to be at least 4·2 cases/100000 with significantly more female cases than male (Tashani et al. 2002). The prevalences of infection with E. granulosus among 1087 sheep, 881 goats, 428 camels and 614 cattle from the same region, determined post mortem in abattoirs, were 20%, 3·4%, 13·6% and 11%, respectively. Infection in the livestock was age-dependent and generally the female animals were more often infected than the male. The measurements of rostellar hooks on protoscoleces collected from sheep and cattle were similar but significantly different from the corresponding measurements of parasites of human or camel origin. However, when a portion of the cox1 gene from each of 30 protoscolex samples (12 from cattle, three from humans, five from camels and 10 from sheep) was sequenced, the sequences were all found to be identical to that published for the common sheep strain of E. granulosus.
Iran is an important endemic focus of cystic echinococcosis where several species of intermediate host are commonly infected with E. granulosus. Two molecular epidemiological studies have been carried out on Iranian isolates. In one, sixteen isolates of E. granulosus, collected from Iranian patients at surgery and from domestic animals including sheep, goats, cattle and camels at slaughterhouses in Tehran and central and southern Iran were analysed for sequence variation within the cox1 and nad1 genes (Zhang et al. 1998b). A PCR-RFLP method, based on the DNA sequence variation in the nad1 gene, was also used to survey the E. granulosus isolates rapidly. The isolates were categorized into two distinct and uniform genotype groupings. The analysis clearly indicated that the camel/dog strain (G6 genotype) of E. granulosus as well as the cosmopolitan, common sheep strain (G1 genotype) occur in Iran. The G1 genotype was found to be present in all four human isolates examined and it was more prevalent in domestic animals than the camel-restricted G6 genotype. In E. granulosus-endemic areas of Iran it is evident, therefore, that the majority of E. granulosus-infected livestock animals can potentially act as reservoirs of human infection, and this has important implications for hydatid control and public health.
In the second study, isolates of E. granulosus were collected from humans and other animals from different geographical areas of Iran and characterized using both DNA (PCR-RFLP of ITS1) and morphological criteria (metacestode rostellar hook dimensions) (Harandi et al. 2002). The sheep and camel strains/genotypes were again shown to occur in Iran. As previously, the sheep strain was shown to be the most common genotype of E. granulosus affecting sheep, cattle, goats and occasionally camels. The majority of camels were infected with the camel (G6) genotype as were 3 of 33 human cases. This was the first time that cases of cystic echinococcosis in humans had been identified in an area where a transmission cycle for the camel strain exists (but see the situation in Mauritania, below). In addition, the camel genotype was found to cause infection in both sheep and cattle. The results of this study also demonstrated that both sheep and camel strains could be readily differentiated on the basis of hook morphology alone.
Mauritania lies between West-Central Africa where human cystic echinococcosis (CE) is considered extremely rare and West Maghreb where CE accounts for a real public health problem. Until 1992, Mauritania was considered as human CE-free even through CE seemed well known in livestock. In 1992, the introduction of ultrasonography led to the diagnosis of the first human CE cases. In 1997, a veterinary study revealed that dogs living around one region in Mauritania, Nouakchott, were commonly infected by E. granulosus. A combined epidemiological and molecular biology survey was undertaken by Bardonnet and colleagues (Bardonnet et al. 2002) to assess E. granulosus transmission and to identify the most relevant animal reservoir responsible for human CE emerging in Mauritania. The field studies included sample collection and investigation of relationship between intermediate hosts, definitive hosts and humans. Typing of E. granulosus strains was performed using comparison of PCR-amplified DNA sequences with one nuclear (BG 1/3) and 2 mitochondrial (cox1, nad1) targets. The results indicated that the camel strain is infectious to humans and circulates between intermediate hosts including camels and cattle. The G1 genotype (sheep strain) was not found in the survey. Although its presence could not be ruled out completely, this study suggests that if the sheep strain is present in Mauritania, it is probably rarely found.
Echinococcosis is a major public health problem in China where it has been recorded in 22 provinces, including autonomous and municipality regions. Examination by a combination of DNA techniques of a large number of E. granulosus isolates collected from different provinces of north-western China showed that all were genetically identical to the common domestic sheep/dog strain (G1 genotype) (McManus, Ding & Bowles, 1994). Subsequently, sequence analysis of the nad1 and cox1 genes of an additional group of isolates collected from this region indicated the presence of the camel/dog strain of E. granulosus as well (Zhang et al. 1998a). Furthermore, as a result of the variation in the nad1 sequences of the G1 and G6 genotypes, a PCR-RFLP assay was developed that allowed rapid discrimination of the two strains.
The second study of Chinese isolates showed that cattle could harbour both the G1 and G6 genotypes, thus confirming the earlier report by Wachira et al. (1993) which identified the camel strain in Kenyan cattle. Three human isolates examined were each categorised as being the G1 genotype which added to the accumulating evidence (Bowles & McManus, 1993c) at the time that humans were refractory or poorly susceptible to infection with the camel strain (G6 genotype) of E. granulosus. Subsequent studies on isolates of E. granulosus collected from other areas have indicated this genotype to be infective to humans (see below). Despite the fact the camel strain was identified it was, nevertheless, evident from the two surveys in north western China (McManus, Ding & Bolwes, 1994; Zhang et al. 1998a) that the common sheep strain was the most predominant in the region and, from the public health perspective, the majority of infected livestock there could act as reservoirs of human infection.
Hydatid disease is recognised as a significant public health and veterinary problem in all urban areas of Nepal; water buffaloes, goats, sheep and pigs are commonly found infected (prevalence 3–8%) in local abattoirs as are domestic dogs (6–15%) (Joshi et al. 1997). During 1994/95, 120 operations were performed for hydatid cyst removal in different Kathmandu hospitals; human serum samples tested by ELISA in several surveys have suggested a very high prevalence (14%) of cystic echinococcosis in humans (Joshi, Joshi & Joshi, 1997).
Twenty-seven isolates were collected from buffaloes (liver and lung cysts), sheep (lung cysts) and goats (lung cysts) from abattoirs in and around Kathmandu; human lung cysts were obtained at surgery at Bir Hospital, the largest hospital in Nepal. Three E. granulosus genotypes (G1, G5 and G6) were identified in the mammalian hosts from Kathmandu based on a comparison of cox1 and nad1 sequences and alignment with the published E. granulosus genotypic sequences (Zhang, Joshi & McManus, 2000). Eighteen samples, including fourteen buffalo isolates, two sheep isolates and two goat isolates, produced identical cox1 and nad1 sequences to the cattle strain (G5 genotype), whereas three buffalo isolates, two sheep isolates and two goat isolates, shared identical sequences with those of the common sheep strain (G1 genotype). Notably, the two human isolates examined produced identical cox1 and nad1 sequences to the G6 (camel strain) genotype; neither were infected with the G1 or G5 genotype, the latter being the predominant strain (18/25 isolates examined) identified in the study.
Studies of Iranian (Zhang et al. 1998b), and as discussed earlier, Kenyan and Chinese isolates suggested that the camel strain (G6 genotype) has a low or no infectivity to humans although on epidemiological grounds, camels appear to be an important reservoir for human infection (Eckert et al. 1989). The molecular genetic studies of E. granulosus from Argentina (Rosenzvit et al. 1999), Mauritania (Bardonnet et al. 2002) and Iran (Harandi et al. 2002), summarised in this review, reported the presence of the G6 genotype in several human subjects. The Nepalese report is the fourth study showing human infection with the G6 genotype. This has potentially important implications for public health and the implementation of hydatid control programmes in Nepal and elsewhere where the camel strain is involved in E. granulosus transmission. The camel strain has a shorter maturation time in dogs compared with the common sheep strain which is the form generally associated with human infection. There are no reports of camels infected with E. granulosus in Nepal so the reservoir of the G6 genotype there remains undetermined although, as is the case in Argentina, goats are a likely source of infection.
Cystic echinococcosis is a major public health problem in Argentina, being endemic in many areas of the country and numerous human cases are reported. Despite the importance of the disease, strain identification and characterization studies were, until recently, limited to one report where a single isolate from sheep was reported to be infected with the common sheep strain (McManus & Rishi, 1989). In light of the extensive geographic and climatic diversity in the country and also because of the importation of different kinds of livestock from other regions of the world, the presence of other strains would be anticipated. Earlier observations indicated a high percentage (over 60%) of fertile E. granulosus cysts in pigs in one area (Santa Fe Province). This observation led to the speculatation that these pigs might be infected by a strain other than the common sheep strain, since it had been reported that this strain produces only sterile cysts in pigs (Eckert et al. 1993).
A combination of rDNA-PCR-RFLP analysis and cox1 sequencing was undertaken on a sample of 33 E. granulosus isolates collected from different regions and hosts in Argentina (Rosenzvit et al. 1999). The study demonstrated the presence of at least four distinct genotypes; the common sheep strain (G1 genotype) in sheep from Chubut Province and in humans from Río Negro Province, the Tasmanian sheep strain (G2 genotype) in sheep and one human subject from Tucumán Province, the pig strain (G7 genotype) in pigs from Santa Fe Province and the camel strain (G6 genotype) in humans from RíoNegro and Buenos Aires Provinces. The finding that pigs harboured the pig strain and the occurrence of the Tasmanian sheep strain again has considerable implications for hydatid control due to the shorter maturation time of both strains in dogs compared with the common sheep strain. Furthermore, this was the first report of the presence of the G2 and G6 genotypes in humans which may also have important consequences for human health. Previous studies had suggested that humans were refractory or poorly susceptible to infection with the camel and pig strains of E. granulosus (Thompson & Lymbery, 1988; Zhang et al. 1998a,b; McManus & Rishi, 1989; Wachira et al. 1993), whereas this survey showed unequivocally that 4 of 9 patients were infected with the G6 genotype. It is possible that some genetic mutation may have arisen in the E. granulosus G6 genotype in Argentina that has made this strain more infective for humans. What was not clear is why the G6 but not the G7 genotype was present in these patients and what might be the reservoir, if not pigs, of the G6 genotype in Argentina. There are no camels in Argentina but other American camelids, including the Guanaco, Llama and Alpaca can be found. Analysis of isolates of E. granulosus from these animals, though they are not easy to obtain, would be rewarding as would genetic studies of hydatid material from Argentinian goats, which have been shown also to harbour the G6 genotype (Bowles, Blair & McManus, 1992; Wachira et al. 1993).
Until the early 90s, all surgically obtained human isolates of E. granulosus examined by isoenzyme and DNA analysis (Wachira et al. 1993) conformed to the common domestic sheep strain. However, a partly calcified hydatid cyst removed from a 11 year old Dutch boy typed by PCR/RFLP analysis and cox1 and nad1 sequence comparisons with known genotypic sequences showed clearly that the patient was infected, not with the sheep strain, but with the genetically distinct cattle strain (G5 genotype) of E. granulosus (Bowles, Blair & McManus, 1992; Bowles, van Knapen & McManus, 1992). Thus, in regions where the bovine strain occurs, cattle may act as reservoirs of human infection. As is evident from scrutiny of Table 1, more recent DNA analysis indicates that other E. granulosus genotypes are also infective to humans.
It had been suspected, on epidemiological grounds, that E. granulosus from pigs has low infectivity to humans (Pawlowksi, 1985; Pawlowski et al. 1993; Eckert et al. 1993) but this needed to be confirmed by identification of isolates taken from humans residing in an area (Poznan) where sheep were rarely bred, where pig hydatidosis was highly prevalent and where the pig strain of E. granulosus was the most common form found in domesticated animals. Nuclear ribosomal ITS1-PCR-RFLP patterns and nad1 sequences were compared for human isolates of Polish origin (Scott et al. 1997) collected by fine needle aspiration biopsy (FNAB). This is a procedure that, along with ultrasonography, allows differential diagnosis of suspected hepatic cysts in the liver and permits collection of parasite material for analysis from patients residing in areas where hydatid disease is relatively uncommon (Stefaniak & Lemke, 1995). The data indicated clearly that the Polish patients were not infected with the common sheep strain (G1 genotype) of E. granulosus, normally associated with human cystic echinococcosis. Instead, the form of E. granulosus infecting the Polish patients shared very similar nad1 sequence with the previously characterized pig (G7) genotype but it exhibited some clear differences. In particular, a single ITS1 fragment of 1·04 kb in size was amplified by PCR (the G7 genotype produces 2 distinct bands of 1 kb and 1·1 kb) and unique RFLP patterns were obtained after restriction digestion.
Accordingly it was proposed that these human isolates represented a distinct E. granulosus genotype (designated G9). A subsequent study of human and pig isolates from Poland, Slovakia and Ukraine (Kedra et al. 1999) failed to confirm the existence of this genotype. This second study suggested that, based on nad1 sequences, pigs (56 isolates examined) and humans (4 isolates examined) were infected with the G7 genotype or pig strain. Separate isoenzyme and DNA-based investigations of E. granulosus isolates (Snabel et al. 2000; Turcekova et al. 1998, 2003), using RAPDs, nad1 sequence comparisons and PCR-RFLP analysis of the nuclear ITS1 region, have provided additional evidence for the almost exclusive presence of the G7 genotype in Slovakia. DNA from an isolate of E. granulosus taken from a wild boar (Sumy region, Ukraine) had identical nad1 sequence to the G7 genotype earlier found in pigs from the same region (Kedra et al. 2000b).
Major questions that are outstanding concern confirmation of the existence of the G9 genotype and the reservoir(s) of human hydatid disease in Poland and other countries in Central and East Europe. It is unlikely to be sheep in Poznan Province in Poland as ovine infections with E. granulosus are rarely seen there, whereas the prevalence of echinococcosis in pigs is higher than in other parts of the country (Pawlowski et al. 1993). In Poland in 1985, the national figures for cystic echinococcosis in slaughtered animals showed prevalences of 5·35% in pigs, 1·08% in sheep and 0·04% in cattle (see Scott et al. 1997). Furthermore, the G7 genotype had not, until recently, been shown by molecular analysis to definitively infect sheep (McManus, 2002). However, Gonzalez et al. (2002) showed that two of four Spanish pig isolates had identical molecular characteristics to the G1 genotype whereas the other two conformed to the G7 genotype (pig strain). Scott et al. (1997) speculated that in Poland pigs naturally harbour the G9 genotype although, unlike in humans, it may develop poorly, producing small yet viable cysts in this host. Clearly, this is an important epidemiological question that needs to be further addressed. Examination of additional E. granulosus isolates from Poland and surrounding countries from humans, pigs and other potential intermediate hosts is clearly warranted to resolve this controversial issue. Interestingly, an isolate of E. granulosus obtained from a wild European beaver, Castor fiber, from North-Easter Poland was typed, on the basis of identical nad1 sequence, as the G7 genotype (Tkach et al. 2002). This is the first report of E. granulosus from the European beaver but it is unlikely that this host plays any significant role in the transmission of echinococcosis.
The ‘cervid’ strain, ‘sylvatic strain’ or ‘northern form’ of E. granulosus occurs in North America and Eurasia. The wolf is the principal definitive host while moose and reindeer (family Cervidae) serve as intermediate hosts; cycles involving sled dogs and domesticated reindeer also occur (Rausch, 1986). E. granulosus of cervid origin differs in a number of biological and clinical respects from domestic strains of the parasite (Rausch, 1986; Thompson & Lymbery, 1988). Rausch (1986) considered the northern form to be ancestral to the domestic strains of E. granulosus which he contended became adapted to synanthropic hosts with the development of animal husbandry.
A number of molecular genetic approaches were used to characterize 4 isolates of the cervid strain obtained from Alaskan moose (Bowles et al. 1994). PCR-RFLP analysis of the nuclear ITS1 region of the rDNA repeat could readily distinguish the cervid form from other strains of E. granulosus. The complexity of the RFLP patterns obtained suggested, however, that a number of distinct ITS1 types were present in this strain which may represent an inter-strain E. granulosus hybrid. Furthermore, mitochondrial cox1 sequence of the cervid genotype was ambiguous at 18 positions and closely resembled a cluster of previously characterized E. granulosus genotypes, G1 (common, domestic sheep)/G2 (Tasmanian sheep)/G3 (buffalo). In contrast, mitochondrial nad1 sequence, although unique, suggested that the cervid form was most similar to strains represented by the G6 (camel)/G7 (pig) genotypes. Based on its unique nad1 sequence and ITS1 PCR-RFLP pattern, the cervid strain appeared to represent a distinct genotype of E. granulosus which was designated G8 (Bowles et al. 1994).
Case-based data have suggested that the course of sylvatic disease is less severe than that of domestic disease, which led to the recommendation to treat cystic echinococcosis patients in the Arctic by careful medical management rather than by aggressive surgery. The first two documented E. granulosus human cases in Alaska with accompanying severe sequelae in the liver were recently reported (Castrodale et al. 2002). The results of molecular genetic analysis of the cyst material of one of the subjects supported identification of the parasite as the sylvatic (cervid) (G8 genotype) strain and not the domestic (common sheep strain), which was initially thought to be implicated in these unusually severe Alaskan cases (McManus et al. 2002). The adverse outcomes could have been rare complications that are part of the clinical spectrum of disease caused by sylvatic CE, an indication that the sylvatic form of E. granulosus, especially when affecting the liver, has potential for severe clinical consequences.
The range of DNA techniques now available for the study of genetic variation in Echinococcus granulosus and the molecular epidemiology of cystic echinococcocosis is impressive and much valuable information on the molecular categorisation of the different genotypes is now available. Importantly, in many cases, molecular techniques have validated the genetic basis of important morphological differences that can now be used with confidence as a reliable and simple means of identifying and differentiating between strains and species of Echinococcus (e.g. Tashani et al. 2002; Harandi et al. 2002). The recent publication of the complete sequences of the mt genomes of the horse and sheep strains of E. granulosus (Le et al. 2002) and E. multilocularis (Nakao et al. 2002) and mt DNA sequences for a number of other E. granulosus genotypes (Pearson et al. 2002; Le et al. 2002; Le, Blair & McManus, 2002), has provided additional genetic information that can be used for even more in-depth strain characterization and phylogenetic study of the hydatid organisms. Already, the availability of this very rich sequence information has provided a solid molecular basis for revising the taxonomy of the genus Echinococcus (Thompson & McManus, 2002; Le et al. 2002), a controversial issue for decades. Furthermore, the accumulating genetic data may allow insight to several other unresolved questions such as confirming the presence and precise nature of the G9 genotype and its reservoir in Poland, whether it occurs elsewhere, why the camel strain (G6 genotype) appears to affect humans in certain geographical areas but not others, more precise delineation of the host and geographic ranges of the genotypes characterized to date, and whether additional genotypes of E. granulosus remain to be identified. In this context, the recent studies of Gonzalez et al. (2002) are important and are worthy of particular comment as they highlight the complexity and genomic organisation differences that exist in E. granulosus. Based on two E. granulosus DNA multiplex-PCR amplification fragments they had previously reported, this group developed three PCR protocols (Eg9-PCR, Eg16-PCR and Eg9-PCR-RFLP) for discrimination of E. granulosus genotypes. They used the approach to identify distinct G1 and G7 genotypes within E. granulosus Spanish pig isolates. Sequencing of the nad1 and cox1 genes and ITS1-PCR coupled to RFLP (Bowles & McManus, 1993b) confirmed these observations. The Eg9-PCR-RFLP and Eg16-PCR protocols could thus be used as additional methods to discriminate the recognised E. granulosus genotypes and they might be especially useful for resolving the issue of the G7/G9 genotypes and human infection in Poland.
Finally, it should be emphasised that as well as proving of value for investigating genetic variation in Echinococcus, DNA approaches can be used to identify and discriminate Echinococcus eggs from those of other taeniid eggs in definitive hosts. A polymerase chain reaction (PCR)-based assay has been developed for detecting DNA of E. multilocularis in faecal samples of foxes after isolation of the parasite eggs by a sieving procedure (Mathis & DePlazes, 2002). There is no similar test available yet for E. granulosus although one is being developed (Cabrera et al. 2002). The copro-PCR is a valuable method for confirmation of positive coproantigen results by ELISA and for diagnosis in individual animals.
DPM acknowledges the National Health and Medical Research Council of Australia, the Australian Research Council, The Wellcome Trust and the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) for financial support of his research. He would also like to thank numerous collaborators, especially Josephine Bowles, Robin Gasser, Thanh Hoa Le, David Blair, Mark Pearson, Mara Rosenzvit and Li Hua Zhang, for their contributions to the DNA analysis. RCAT acknowledges the support provided by the Australian Research, Council, World Health Organization and Murdoch University, and the collaboration of Russ Hobbs, Alan Lymbery, John Eckert, Peter Deplazes, Clare Constantine, Majid Fasihi Harandi and Lakshmi Kumaratilake.

Table 1. Genotypes/strains of E. granulosus categorised by DNA analysis with their host and geographical range
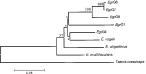
Fig. 1. Inferred relationships among species and genotypes of Echinococcus, using Taenia crassiceps as an outgroup (After Le et al. 2002). Concatenated sequences of atp6, nad1 (partial) and cox1 (partial) were analysed (Bowles, Blair & McManus, 1992; Bowles & McManus, 1993a; Le et al. 2002). A distance matrix was constructed from inferred amino acid (aa) sequences (alignment was 451 aa long with 168 variable aa sites 67 aa parsimony-informative sites) using a Poisson correction for multiple hits and the tree constructed using the minimum evolution approach. Five hundred bootstrap resamplings were carried out. Branches with bootstrap support values less than 50% are indicated with an asterisk. EgrG1, EgrG4, EgrG6-EgrG8 are the different genotypes of E. granulosus. Units on scale bar: changes per site. The branches indicated by an asterisk were supported by fewer than 50% of the resampled data sets and therefore should be regarded as poorly supported. It is clear that EgrG4, EgrG1, E. vogeli and E. oligarthrus are almost equidistant from each other in terms of mt sequences. Furthermore, the E. granulosus G1 and G4 genotypes are also almost equidistant from the G6-8 genotype cluster, although there is some structure in this latter group. E. multilocularis appears as basal within the genus, but again the branch placing it there is rather poorly supported. Given this, recognition of the sheep-dog (G1 genotype) and the horse-dog (G4 genotype) strains (and possibly also the G6-8 genotypes) as separate species is appropriate. The discrete nature of the two forms is quite clear and the molecular and phylogenetic evidence from this and previous studies suggests the case for reinstatement of their formal taxonomic status as subspecies/species is now proven.