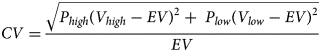Research has demonstrated the importance of the role that peers play in the development of psychopathology (e.g., Dishion & Tipsord, Reference Dishion and Tipsord2011). Specifically, prior work on peer contagion—a mutual influence process that occurs between an individual and a peer that potentially undermines one's own development—points to peer socialization effects on the development of risky behaviors, including substance use (Hawkins, Catalano, & Miller, Reference Hawkins, Catalano and Miller1992 for review). Adolescence is a developmental period characterized by heightened susceptibility to social influences, particularly by peers (Blakemore, Reference Blakemore2008). That is, adolescents are more sociable and more sensitive to acceptance and rejection by peers compared with children. It follows that development of the social brain during adolescence may make some adolescents more vulnerable to the effects of adverse social environments. To date, little is known about the role of neural processes in developmental pathways connecting peer contexts and adolescent health risk behaviors. Informed by Thomas Dishion's research on deviant peer affiliation and substance use in adolescence (e.g., Dishion, Véronneau, & Myers, Reference Dishion, Véronneau and Myers2010), we tested developmental cascade models to evaluate joint contributions of adolescent risk-taking behaviors, peer substance use, and neurobehavioral variables of risk processing and cognitive control to adolescent substance use behaviors. Within a developmental psychopathology framework, we used multiple levels of analysis, including neurobiological, behavioral, and socioenvironmental levels.
Explaining peer influences on the development of adolescent substance use requires consideration of two symbiotic processes: selection, through which adolescents affiliate with peers whose attitudes and behaviors are similar to theirs, and socialization, through which affiliations with peers with similar attitudes and behaviors augment adolescents’ own substance use (Dishion, Capaldi, Spracklen, & Li, Reference Dishion, Capaldi, Spracklen and Li1995; see Deater-Deckard, Reference Deater-Deckard2001 and Dishion & Patterson, Reference Dishion, Patterson and Cicchetti2016 for reviews). In congruence with the selection processes, research suggests that adolescents who use substances are more likely to select peers who also use substances (i.e., homophily; Kandel, Reference Kandel1978). Furthermore, there is evidence that adolescents who smoke are more susceptible to peer influences on risk-taking behaviors compared with those who are non-smokers (Cavalca et al., Reference Cavalca, Kong, Liss, Reynolds, Schepis, Lejuez and Krishnan-Sarin2013). For peer socialization, it has been theorized that affiliation with peers who engage in risk-taking behaviors increases adolescents’ own engagement in risk-taking behaviors, partly through deviancy training (Dishion, Spracklen, Andrews, & Patterson, Reference Dishion, Spracklen, Andrews and Patterson1996). Indeed, empirical research has demonstrated that affiliation with delinquent or substance-using peers predicts early onset of substance use and amplifies increases in substance use in adolescence and young adulthood (Buchmann et al., Reference Buchmann, Schmid, Blomeyer, Becker, Treutlein, Zimmermann and Laucht2009; Otten, Mun, & Dishion, Reference Otten, Mun and Dishion2017; Van Ryzin & Dishion, Reference Van Ryzin and Dishion2013; Van Ryzin, Fosco, & Dishion, Reference Van Ryzin, Fosco and Dishion2012).
Available empirical studies examining both selection and socialization have yielded inconsistent findings. Some longitudinal studies investigating bidirectional associations between peer substance use and adolescent substance use supported both selection and socialization effects (Bray, Adams, Getz, & McQueen, Reference Bray, Adams, Getz and McQueen2003; Dishion & Owen, Reference Dishion and Owen2002; Simons-Morton & Chen, Reference Simons-Morton and Chen2006). For example, in a longitudinal study, the positive effect of adolescent substance use on changes in friend substance use and the positive effect of friend substance use on changes in adolescent substance use were both significant and comparable in magnitude (Farley & Kim-Spoon, Reference Farley and Kim-Spoon2015). Still, other longitudinal studies yielded findings that supported either selection or socialization only. A longitudinal study of middle school students revealed evidence for selection effects but not socialization effects: Adolescent substance use was related to changes in peer substance use, whereas peer substance use was not related to changes in adolescent substance use (Farrell & Danish, Reference Farrell and Danish1993). In contrast, another longitudinal study reported evidence for socialization effects, but not selection effects. In a sample involving adolescents with and without alcoholic parents, affiliation with substance-use-promoting peers predicted increased adolescent substance use, but not vice versa (Haller, Handley, Chassin, & Bountress, Reference Haller, Handley, Chassin and Bountress2010).
These previous studies focused on contrasting the effect of adolescent substance use on peer substance use against the effect of peer substance use on adolescent substance use. From a developmental psychopathology perspective, we believe that both selection and socialization processes play important roles in the development of substance use behaviors at different points in adolescence, such that minor deviations of normative development progressively lead to associations with deviant peers, which in turn lead to more serious forms of problem behavior (Dishion & Owen, Reference Dishion and Owen2002; Dishion et al., Reference Dishion, Véronneau and Myers2010). Clearly, understanding how these processes work would inform effective prevention and intervention efforts for adolescent substance use and its transition into more serious forms of substance use disorders into adulthood.
Although it has been proposed that peer relationships are an important source of individual differences that should be addressed when investigating neurocognitive development in adolescence (Foulkes & Blakemore, Reference Foulkes and Blakemore2018; Schriber & Guyer, Reference Schriber and Guyer2016), few studies have addressed the implications that peer groups may have for development on a neurobiological level. Peer influences on the brain are particularly germane to adolescence, given the dramatic development in socioemotional regions (e.g., medial prefrontal cortex, amygdala, insula) during this period (Blakemore, Reference Blakemore2008). This effect has been demonstrated empirically, demonstrating that the simple physical presence of a peer has been shown to promote greater risky decisions, accompanied by greater activation in reward systems in the brain (Albert, Chein, & Steinberg, Reference Albert, Chein and Steinberg2013). To date, neuroimaging studies on peer influences have focused on linking neural and behavioral indices of risk taking to social exclusion. For example, adolescents who were more susceptible to peer influences demonstrated greater sensitivity in regions associated with social cognition (e.g., temporoparietal junction) when making decisions following peer exclusion (Peake, Dishion, Stormshak, Moore, & Pfeifer, Reference Peake, Dishion, Stormshak, Moore and Pfeifer2013). Furthermore, these adolescents were more likely to take more risks during a driving simulation following exclusion.
While there is a dearth of studies investigating the link between deviant peer group selection and brain function, initial evidence lends support for this association. Saxbe and colleagues (Reference Saxbe, Del Piero, Immordino-Yang, Kaplan and Margolin2015) reported that adolescents showed greater activation in mentalizing structures of the brain (including the posterior cingulate cortex, precuneus, and temporoparietal junction) when viewing images of peers relative to images of their parents, and a greater degree of contrast in activation between parent and peer conditions was related to greater affiliation with risk-taking peers. Taken together, these experimental studies demonstrated the effects that peer influence may have on both a behavioral and neurobiological level. However, these studies were cross-sectional and based on small samples (n = 20–22), and further comprehensive longitudinal research is warranted to clarify the association between peer influences and brain development, particularly in regions associated with risky decision making.
Theoretical work in the current neuroscience literature suggests that risk taking in adolescence is, in part, derived from the neurological maturation gap between a faster-maturing system sensitive to rewards and a slower-maturing system involved in behavioral control (Casey, Getz, & Galvan, Reference Casey, Getz and Galvan2008; Ernst, Pine, & Hardin, Reference Ernst, Pine and Hardin2006; Steinberg, Reference Steinberg2008). Following the imbalance model, we focus on the valuation system that is involved in estimating the incentive value of different options and the control system that is involved in action selection, maintaining future goals, and inhibiting prepotent responses (van den Bos et al., Reference van den Bos, Vahl, Güroğlu, van Nunspeet, Colins, Markus and Crone2014). In adolescents, the pursuit of high-risk yet rewarding options may be, in part, driven by anomalies in neural processes that evaluate the risk associated with the options. That is, choosing high-risk behavioral options in adolescence may be driven by biases in risk preference. For example, an adolescent may have a normal understanding of the positive and negative consequences of underage drinking but engage in drinking anyway because of a preference for risky options. Indeed, value-based decision-making research has shown that risky choices are driven by neural computations associated with the likelihood of receiving rewards as well as the value of rewards (d'Acremont & Bossaerts, Reference d'Acremont and Bossaerts2008; Mohr, Biele, & Heekeren, Reference Mohr, Biele and Heekeren2010). A key region consistently implicated in the processing of risk information is the anterior insular cortex (Mohr et al., Reference Mohr, Biele and Heekeren2010; Richards, Plate, & Ernst, Reference Richards, Plate and Ernst2013). The insular cortex acts as a signal, guiding adolescents toward or away from risky choices consistent with individual preferences for risk. Research has shown that adolescents recruit the insular cortex during risky decision-making more than children or adults, and adolescents’ hypersensitivity of the insular cortex to increasing variance of potential outcomes is related to making safer choices (van Duijvenvoorde et al., Reference van Duijvenvoorde, Huizenga, Somerville, Delgado, Powers, Weeda and Figner2015).
From a developmental psychopathology perspective, we are interested in how risk factors may be modulated by protective factors when explaining the emergence of maladaptation. We focus on cognitive control as a protective factor given its buffering role against detrimental effects of deviant peer affiliation and neurobiological vulnerability to risk taking. First, research has reported significant moderating effects of self-regulation, a psychological construct defined similarly to cognitive control (Nigg, Reference Nigg2017) in the link between peer influences and adolescent risk-taking behaviors. Specifically, one previous study reported that substance use lifestyle during adolescence (i.e., adolescent alcohol use, peer alcohol use, and peer drug-related talk) was significantly predictive of problematic alcohol use in early adulthood, but only for individuals with low effortful control (Piehler, Véronneau, & Dishion, Reference Piehler, Véronneau and Dishion2012). The main effects of effortful control, but not the moderation, were significant for cigarette and marijuana use. Studies on adolescent antisocial behaviors have also reported significant moderating effects of effortful control (Gardner, Dishion, & Connell, Reference Gardner, Dishion and Connell2008) or inhibitory control (Hinnant & Forman-Alberti, Reference Hinnant and Forman-Alberti2018) against the negative effects of deviant peer affiliation.
Similarly, evidence from neuroimaging research points toward more consistent and stronger regulating effects of cognitive control over reactivity related to adolescent substance use, potentially accounting for the weak main effects of cognitive control found in previous behavioral studies (see Kim-Spoon, Maciejewski, Lee, Deater-Deckard, & King-Casas, Reference Kim-Spoon, Maciejewski, Lee, Deater-Deckard and King-Casas2017 for a review). For example, lower levels of activity of the behavioral activation system significantly predicted earlier onset of substance use, but only among adolescents with poor inhibition who showed high activation in the regulatory neural network (e.g., medial and dorsolateral frontal cortices) during a cognitive interference task (Kim-Spoon, Deater-Deckard, Holmes, et al., Reference Kim-Spoon, Deater-Deckard, Holmes, Lee, Chiu and King-Casas2016). Further, blunted hemodynamic activity in the anterior insula during anticipation of uncertain outcomes predicted health risk behaviors only among adolescents with greater dorsal anterior cingulate cortex activity during a cognitive control task (i.e., poor cognitive control), but not among those with lower dorsal anterior cingulate cortex activity (i.e., good cognitive control; Kim-Spoon, Deater-Deckard, Lauharatanahirun, et al., Reference Kim-Spoon, Deater-Deckard, Lauharatanahirun, Farley, Chiu, Bickel and King-Casas2016).
Taken together, these findings suggest significant moderating effects of the neural substrates involved in cognitive control on the link between valuation systems and health risk behaviors among adolescents. Within the neuroscience literature, previous studies have identified brain regions involved in inhibition, including the basal ganglia, that are thought to be involved in the inhibition of inappropriate responses and prefrontal regions (such as the inferior, medial, and dorsolateral prefrontal cortices) that receive inputs from the limbic basal ganglia thalamocortical circuit and represent and maintain relevant information for goal-directed behaviors (Aron, Robbins, & Poldrack, Reference Aron, Robbins and Poldrack2014; Casey, Durston, & Fossella, Reference Casey, Durston and Fossella2001). Here, we focus on brain regions that are closely related to cognitive control over interference, as measured by brain activation during an inhibitory control task primarily involving medial prefrontal cortices.
In the current longitudinal study, we investigated developmental pathways through which adolescent risk-taking behaviors and peer substance use contribute to later adolescent substance use behaviors. We evaluated how neural risk processing (i.e., functional brain activation to high-risk options) may be affected by deviant peer affiliation or promote deviant peer affiliation to ultimately contribute to the development of substance use. We also tested modulating effects of cognitive control, measured by behavioral performance and prefrontal functioning. Given the importance of deviant friendship processes (Dishion et al., Reference Dishion, Capaldi, Spracklen and Li1995; Dishion & Owen, Reference Dishion and Owen2002) as well as deviant peer association, we considered substance use by both peers and best friends. We examined the average frequency of the use of three most commonly used substances—cigarettes, alcohol, and marijuana—given the evidence that involvement in a substance-using peer group is a common factor leading to all forms of substance use (Hawkins et al., Reference Hawkins, Catalano and Miller1992).
Specifically, we examined whether risky decision making may play an important role in the socialization process or the selection process. We were particularly interested in the role of neural risk processing in these pathways and tested two alternative models. In the first model, we hypothesized that risk processing is involved in the socialization process such that adolescent risk-taking behaviors would be related to adolescents’ associations with substance-using peers, which in turn would be associated with brain activation during decision making for high-risk options, and this neural risk processing would then contribute to substance use behaviors. In the second model, we hypothesized that neural risk processing is involved in the selection process such that neurobiological vulnerability to risk taking (indicated by the neural processing of risk) would be associated with adolescent risk-taking behaviors, which in turn would be related to affiliation with substance-using peers, and such affiliation would then be related to adolescents’ own substance use behaviors.
Method
Participants
Participants included 167 adolescents (53% male) and their primary caregiver (82% biological mothers). Adolescents were ages 13 to 14 years (M = 14.13, SD = 0.54) at Time 1, participating annually in the longitudinal study across four years (M = 15.05, SD = 0.54 at Time 2, M = 16.07, SD = 0.56 at Time 3, and M = 17.01, SD = 0.55 at Time 4). Our sample was representative of the region from where we sampled families. For this Appalachian area, comprised of small cities and rural towns and counties, 2010 US Census data showed median annual household income to be in the $36,000 to $59,000 range. Median family income in the current sample was $35,000 to $49,999 per year at all time points. Based on an income-to-needs (ITN) ratio calculation, about half of the sample was deemed to be “poor” (25% of the sample, with ITN < 1) or “near-poor” (25%, with ITN < 2). Regarding race/ethnicity, the 2010 US Census data show 82% to 91% White and 4% to 13% Black for the region. In the current sample, adolescents identified as Caucasian (82%), African-American (12%), and other (6%). Exclusion criteria were claustrophobia, history of head injury resulting in loss of consciousness for > 10 minutes, orthodontia impairing image acquisition, and contraindications to magnetic resonance imaging.
At Time 1, 157 families participated. At Time 2, 10 families were added for a final sample of 167 parent–adolescent dyads. However, 24 families did not participate at all possible time points for reasons including: ineligibility for tasks (n = 2), declined participation (n = 17), and lost contact (n = 5) during the follow-up assessments. We performed attrition analyses using a general linear model (GLM) univariate procedure to determine whether there were systematic predictors of missing data. Results indicated that rate of participation (indexed by proportion of years participated to years invited to participate) was not significantly predicted by demographic variables (p = .61 for age, p = .67 for income, p = .62 for sex, p = .73 for race, contrasted as White vs. non-White).
Procedure
Participants were recruited by advertisement methods including flyers, recruitment letters, and email. Adolescent participants and their primary caregivers visited the laboratory to complete behavioral measures and MRI scans at four annual time points, and they were compensated for their participation. All adolescent participants provided written assent and their parents provided written permission for a protocol approved by the university's institutional review board.
Measures
Adolescent substance use
Adolescents reported frequency of cigarette, alcohol, and marijuana use at Time 4, using a substance use index adapted from the CDC Youth Risk Behavior Survey (Kann et al., Reference Kann, Kinchen, Shanklin, Flint, Hawkins, Harris and Zaza2014). This index consisted of three items that ask what is most true regarding the individual's substance use. For example, the item for alcohol use is “Which is the most true for you about using alcohol?” Participants responded using a 6-point response scale ranging from 1 (never used) to 6 (usually use every day). A substance use composite score was computed using an average of all three items, with higher scores indicating greater use. The scale reliability was acceptable (α = .75). In our sample, adolescent substance use rates were higher than the national average (Kann et al., Reference Kann, McManus, Harris, Shanklin, Flint, Hawkins and Zaza2016) with 37% for cigarette/tobacco use, 71% for alcohol use, and 40% for marijuana use.
Peer substance use
Adolescents were asked to estimate the number of their friends that they perceived to use cigarettes, alcohol, and marijuana, using items adapted from the CDC Youth Risk Behavior Survey (Kann et al., Reference Kann, Kinchen, Shanklin, Flint, Hawkins, Harris and Zaza2014) at Time 2 and Time 3. This scale consisted of three items (e.g., “How many of your friends would you estimate smoke cigarettes?”) and uses a 5-point response scale ranging from 0 (none) to 4 (all). The scale demonstrated acceptable reliability in the current sample (α = .77 at both time points). Separately, adolescents were also asked to report their closest or best friend's substance use based on six items (Hussong, Reference Hussong2002). The questions ask for the participants’ best approximation of their best friend's use of cigarettes, other tobacco, alcohol, marijuana, and drugs other than marijuana. The scale demonstrated acceptable reliability in the current sample (α = .81–.82). Responses on the friend substance use scale were highly correlated with the best friend substance use scale (r = .51–.62), so we created a composite peer substance use score by standardizing and then averaging the scales together. Higher scores were indicative of greater peer substance use.
Risk-taking behaviors
Risk-taking behaviors were assessed using adolescent report on the Things I Do questionnaire and parent report on the Things Your Child May Do questionnaire (Conger, Elder, Lorenz, Simons, & Whitbeck, Reference Conger, Elder, Lorenz, Simons and Whitbeck1994) at Time 1 and Time 2. In the self-report version, adolescents reported on 19 items about things they may have done in the last year (0 = not at all, 1 = once or twice, and 2 = more than twice). In the parent-report version, parents reported on 30 items about things their child may have done in the past year (1 = not at all, 2 = once or twice, 3 = more than two times, 0 = I don't know). Sample items included “Done something dangerous on a dare,” “Told a lie to get another kid in trouble,” and “Skipped school without permission.” Responses were summed and standardized for each scale and then averaged across the parent and adolescent reports, given the consistency between them shown by significant correlations (r = .31 to .39). Higher scores indicated higher risk-taking behaviors and the scale demonstrated acceptable reliability for both parent (α = .69–.72) and adolescent (α = .74–.77) report.
Risk processing
To assess neural risk processing at Time 1 and Time 3, adolescents completed an adapted economic lottery choice task while blood-oxygen-level-dependent (BOLD) responses were measured using functional magnetic resonance imaging (fMRI). During this task (see Figure 1A and 1B), adolescents made choices between pairs of gambles, in which each gamble included a high and low monetary outcome, each associated with a specific probability (Holt & Laury, Reference Holt and Laury2002). Corresponding colors (orange or blue) were used to indicate associations between potential monetary outcomes and probabilities. Pie charts were used to visualize probabilities associated with potential payoffs to increase comprehension of numerical information for adolescents. Each gamble included 10 slices in each pie that corresponded to a probability of 10%. The proportion of slices relative to the total pie indicated the likelihood of receiving a high or low monetary outcome. Monetary outcomes and probabilities varied across trials. The risk of each gamble was measured using the coefficient of variation (CV)Footnote 1, a scale-free metric calculated by dividing the standard deviation by expected value of each gamble. Compared with other economic metrics of risk (i.e., standard deviation), previous studies indicate that CV is a stronger predictor of choice behavior (Bach, Symmonds, Barnes, & Dolan, Reference Bach, Symmonds, Barnes and Dolan2017; Weber, Shafir, & Blais, Reference Weber, Shafir and Blais2004). Within each pair of gambles, one option was always riskier (higher CV) than the other (lower CV). Adolescents made a total of 72 decisions and were told that they would be paid a bonus based on their actual choices from four randomly selected trials, which was added to their compensation for participating in the study. Prior to beginning the task, adolescents were instructed that each trial was independent from all other trials and was equally likely to be selected for compensation. On average, adolescents took approximately 30 minutes to complete the task.
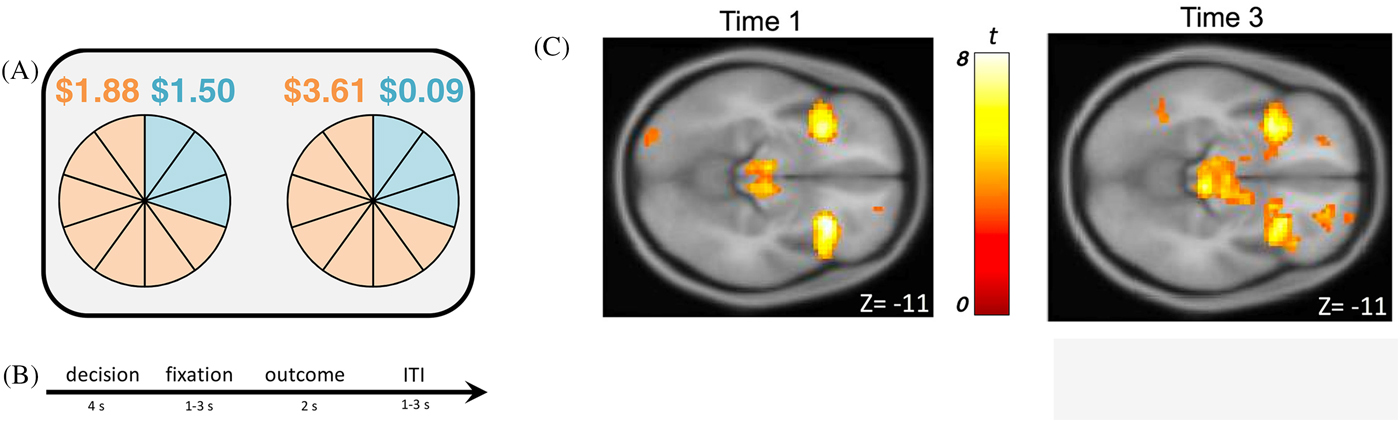
Figure 1. (A) Adolescents were asked to make decisions between pairs of uncertain gambles in an economic lottery choice task. For each gamble, there was a high and a low monetary outcome, each associated with a probability. Outcomes and probabilities were represented with corresponding colors (orange and blue). (B) Each trial consisted of a decision phase, a fixation phase, an outcome phase, and an inter-trial-interval (ITI). (C) During the decision phase of the economic lottery choice task, adolescents exhibited increased BOLD responses in the bilateral anterior insular cortex to chosen gambles that were of higher relative to lower levels of risk (i.e., coefficient of variation; CV) at both Time 1, t (145) = 7.22, p (cluster family-wise error correction) < .05, and Time 3, t (125) = 7.07, p (cluster family-wise error correction) < .05.
For each individual, a general linear model (GLM) was constructed including decision and outcome events of the task modeled with a duration of four and two seconds, respectively. To assess neural risk processing, a parametric regressor of decision phase activation representing the risk level (i.e., CV) for chosen gambles was entered into the model. Additionally, a parametric regressor indicating whether participants received high or low monetary outcomes during the outcome phase was included in the model. At the group level of the GLM, whole brain analysis was conducted to determine how CV for chosen gambles modulated BOLD responses during the decision phase. Individual- and group-level analyses were conducted for Time 1 and Time 3 separately. Given the robust literature implicating the insular cortex as a key region involved in risk processing (Kuhnen & Knutson, Reference Kuhnen and Knutson2005; Mohr et al., Reference Mohr, Biele and Heekeren2010; Paulsen, Carter, Platt, Huettel, & Brannon, Reference Paulsen, Carter, Platt, Huettel and Brannon2012; Paulus, Rogalsky, Simmons, Feinstein, & Stein, Reference Paulus, Rogalsky, Simmons, Feinstein and Stein2003; Platt & Huettel, Reference Platt and Huettel2008; Preuschoff, Quartz, & Bossaerts, Reference Preuschoff, Quartz and Bossaerts2008; van Duijvenvoorde et al., Reference van Duijvenvoorde, Huizenga, Somerville, Delgado, Powers, Weeda and Figner2015), we hypothesized that BOLD responses in the bilateral insular cortex would correspond to the level of CV (i.e., level of risk) such that greater responses would be observed for chosen gambles that were of relatively higher risk.
To test a priori hypotheses, region of interest (ROI) analyses were performed using Statistical Parametric Mapping (SPM8: Wellcome Centre for Human Neuroimaging, London, UK) for Time 1 and Time 3. Eigenvariate values were extracted for the left and right insular cortex using a 6mm sphere around the peak voxel coordinates for each region for Time 1 (leftT1: x = −30, y = 17, z = −14; rightT1: x = 30, y = 20, z = −11) and Time 3 (leftT3: x = −33, y = 14, z = −11; rightT3: x = 36, y = 17, z = −11). Figure 1C illustrates activation in the bilateral insular cortex during the lottery choice task for Time 1 and Time 3 (see Supplemental Information Appendices A and B for all regions associated with increasing CV during the decision phase for Time 1 and Time 3). We created a latent factor score to operationalize insular risk processing using bilateral insular eigenvariate values, with higher scores indicating higher BOLD responses in the insular cortex. We conducted confirmatory factor analyses, in which standardized left and right anterior insula activation scores loaded on an overall insula factor score. Factor loadings were constrained to be equal for model identification purposes. In this fully saturated model (χ2 = 0, df = 0), both factor loadings were significant (.90 at Time 1 and .93 at Time 3, all p’s < .001).
Cognitive control
At each time point, adolescents completed a multiple-source interference task (MSIT; Bush, Shin, Holmes, Rosen, & Vogt, Reference Bush, Shin, Holmes, Rosen and Vogt2003) while BOLD response was monitored. In the MSIT, adolescents were presented with three digits, two of which were identical, and they were asked to indicate the identity of the oddball digit using a button press. In neutral trials, the target's identity matched the digit's presented location, but in interference trials, the target's identity was incongruent with the digit's presented location (see Figure 2A). In line with previous studies (Bush et al., Reference Bush, Shin, Holmes, Rosen and Vogt2003), we found significant MSIT interference effects in reaction time for correct responses, such that reaction time was higher for interference compared with neutral trials, t (153) = 69.58 at Time 1; t (148) = 69.41 at Time 2; t (142) = 63.30 at Time 3; and t (142) = 59.87 at Time 4; all p’s < .001. We calculated reaction time difference scores (interference minus neutral trials), with low scores indicating higher cognitive control.
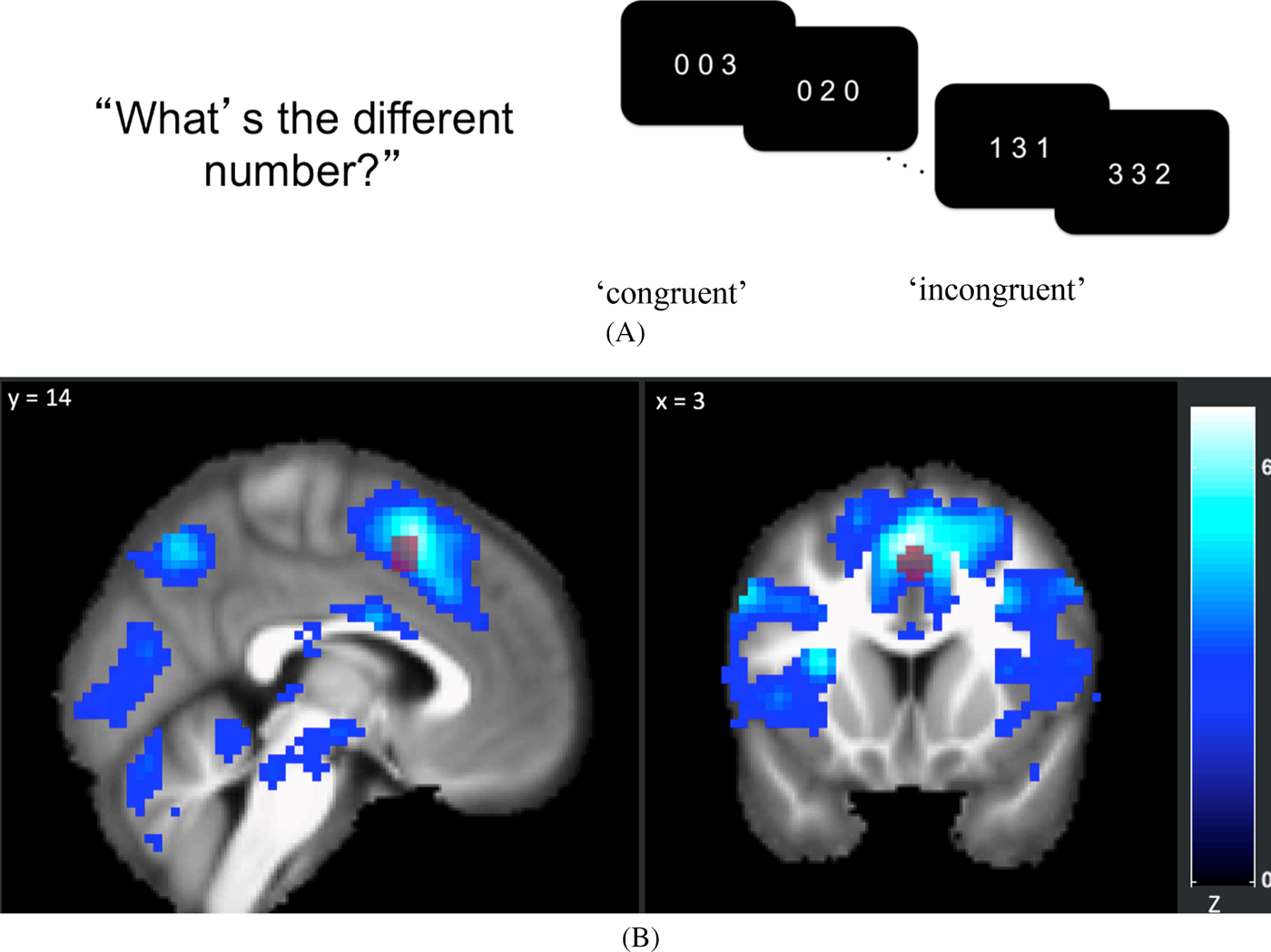
Figure 2. (A) In the multi-source interference task (MSIT), adolescents were asked to identify the digit that differed from two other concurrently presented digits, ignoring its position in the sequence. (B) 6-mm-radius spherical ROI at (x = 4, y = 14, z = 48) overlaid on top of map showing a significant negative linear relationship between the time points and the interference effect on BOLD using the Sandwich Estimator Toolbox. Displayed using voxel-wise false discovery rate corrected threshold of p < .05.
Imaging acquisition and analysis
Functional neuroimaging data were acquired on a 3T Siemens Tim Trio MRI scanner with a standard 12-channel head matrix coil. Structural images were acquired using a high-resolution magnetization prepared rapid acquisition gradient echo sequence with the following parameters: repetition time (TR) = 1200 ms, echo time (TE) = 2.66 ms, field of view (FoV) = 245 x 245 mm, and 192 slices with the spatial resolution of 1 x 1 x 1 mm. Echo-planar images were collected using the following parameters: slice thickness = 4mm, 34 axial slices, FoV = 220 x 220mm, TR = 2 s, TE = 30 ms, flip angle = 90 degrees, voxel size = 3.4 x 3.4 x 4 mm, 64 x 64 grid, and slices were hyperangulated at 30 degrees from the anterior-posterior commissure. Imaging data were preprocessed and analyzed using SPM8 (Wellcome Trust Neuroimaging Center). For each scan, data were corrected for head motion using a six-parameter rigid body transformation and realigned. The mean functional image was co-registered to the anatomical image, and then the anatomical image was segmented and registered to the MNI template and functional volumes were normalized using parameters from the segmented anatomical image and were smoothed using a 6mm full-width-half-maximum Gaussian filter.
Preprocessed scans were entered into a General Linear Model (GLM) for each participant using SPM8. Interference and neutral trials were modeled using a boxcar function convolved with a canonical hemodynamic response function. A low-pass filter with applied cutoff of 168 seconds was used to remove low-frequency noise. To control for the effect of motion, six motion realignment parameters were included as nuisance covariates. Framewise displacement (FD) was calculated using the head realignment parameters (Power, Barnes, Snyder, Schlaggar, & Petersen, Reference Power, Barnes, Snyder, Schlaggar and Petersen2012; Siegel et al., Reference Siegel, Power, Dubis, Vogel, Church, Schlaggar and Petersen2014). Volumes with FD > 0.9mm were scrubbed by adding a TR-specific regressor to the GLM for each censored volume. For each participant and for each time point, beta maps for the neutral condition were subtracted from the beta maps of the interfere condition to create interference minus neutral contrasts. For each time point, interference minus neutral contrast images were entered into a second level one-sample t-test using the root mean square of frame displacement as a regressor of no interest to account for age-related changes to within-scanner motion (Satterthwaite et al., Reference Satterthwaite, Wolf, Loughead, Ruparel, Elliott, Hakonarson and Gur2012). A significant MSIT interference effect on BOLD was observed at each time point consistent with previously reported effects of the MSIT (see Supplemental Information Appendices C, D, E, and F for all regions associated with the interference effect at each time point). Finally, a longitudinal group-level whole-brain analysis was performed using the Sandwich Estimator Toolbox (Guillaume, Hua, Thompson, Waldorp, & Nichols, Reference Guillaume, Hua, Thompson, Waldorp and Nichols2014) to assess the linear effect of the time points on the interference effect on BOLD after controlling for within-scanner motion. A significant decrease in the interference effect was observed across time points in multiple regions implicated in the MSIT (see Figure 2B).
Individual-level ROI values were extracted for each participant using a gray matter mask and an a priori 6mm radius spherical mask centered at the MNI coordinate (x = 4, y = 14, z = 48) of peak activation in the dACC/MPFC/SMA cluster (dorsal anterior cingulate cortex/medial prefrontal cortex/supplementary motor area) identified in the meta-analysis reported by Deng, Wang, Wang, and Zhou (Reference Deng, Wang, Wang and Zhou2018). The first eigenvariate values of the contrast images were extracted after adjusting for an F-contrast of the effects of interest (see Figure 2B).
Statistical Analyses
We conducted longitudinal moderated mediation analyses using structural equation modeling (SEM) in Mplus version 8.1 (Muthén & Muthén, Reference Muthén and Muthén1998–2007) based on syntax developed by Stride, Gardner, Hatley, and Thomas (Reference Stride, Gardner, Catley and Thomas2015). Little's MCAR test indicated that the missing data pattern for all study variables resembled a completely at random pattern (χ2 = 325.44, df = 303, p = .180). Therefore, we used full information maximum likelihood estimation (FIML) to account for missing data. We calculated bias-corrected bootstrapped confidence intervals (CIs) for indirect effects using 1,000 bootstrapping samples.
In Model 1, we tested the association between early adolescent risk taking (Time 1) and later substance use (Time 4) via peer substance use at Time 2 and risk processing at Time 3. In Model 2, we tested the association between risk processing at Time 1 and substance use at Time 4, via risk taking at Time 2 and peer substance use at Time 3. We first tested each model including main effects of cognitive control on all endogenous variables. Next, in the interaction effect model, we tested the moderating effects of neural cognitive control on all possible paths, as well as the indirect effect.
Results
Descriptive Statistics
Prior to analysis, statistical outliers (n = 31 across all variables) were winsorized to the next value that was not an outlier (i.e., within 3 SD) to retain statistical power and attenuate bias resulting from elimination. Table 1 depicts descriptive statistics and correlations for all study variables. Multivariate general linear modeling (GLM) analyses indicated that none of the demographic variables (i.e., parent education, family income, gender, and race) had a significant effect on endogenous study variables (all p’s > .30), with the exception of adolescent age which was significantly associated with peer substance use (p = .003) and adolescent substance use (p = .001). Thus, the effect of age on peer and adolescent substance use was controlled for in all models.
Table 1. Descriptive statistics and bivariate correlations of model variables

*p < .05; **p < .01.
Main Effect Models
We fit the two competing indirect effect models to test whether adolescent risk taking predicted later substance use via peer substance use and risk processing (Model 1) or whether risk processing predicted later substance use via risk taking and peer substance use (Model 2). In the main effect model for Model 1, main effects of cognitive control on peer substance use, risk processing, and adolescent substance use were estimated (i.e., cognitive control at Time 1 → peer substance use at Time 2, cognitive control at Time 2 → risk processing at Time 3, and cognitive control at Time 3 → adolescent substance use at Time 4). The full model demonstrated good fit (χ2 = 4.87, df = 9, p = .84; CFI = 1.00; RMSEA = .00). Within the model, the effect of age on adolescent substance use was nonsignificant (b = 0.19, SE = .12, p = .13); thus, this path was trimmed from the model. The nested model comparison between the full model and the trimmed model indicated that the trimmed model (i.e., χ2 = 7.13, df = 10, p = .71; CFI = 1.00; RMSEA = .00) was the preferred, more parsimonious model over the full model (∆χ2 = 2.26, ∆df = 1, p = .13). As shown in Table 2, results of Model 1 indicated that greater adolescent risk-taking behaviors at Time 1 predicted greater peer substance use at Time 2, which in turn predicted higher activation in the insular cortex during risk processing at Time 3. However, there was not a significant association between risk processing at Time 3 and substance use at Time 4. Finally, there was a significant direct effect of adolescent risk taking at Time 1 on substance use at Time 4. The main effects of cognitive control on risk taking, peer substance use, or risk processing were not significant.
Table 2. Parameter estimates for the path model of longitudinal associations among risk-taking behavior, peer substance use, neural risk processing, and adolescent substance use moderated by neural cognitive contrrol

In the main effect model for Model 2, main effects of cognitive control on risk-taking behavior, peer substance use, and adolescent substance use were estimated (i.e., cognitive control at Time 1 → risk-taking behavior at Time 2, cognitive control at Time 2 → peer substance use at Time 3, and cognitive control at Time 3 → adolescent substance use at Time 4). The full model demonstrated good fit (χ2 = 3.29, df = 9, p = .92; CFI = 1.00; RMSEA = .00). Within the model, the effect of age on peer substance use (b = 0.12, SE = .12, p = .31) and the effect of insular risk processing on substance use at Time 4 (b = -0.03, SE = .07, p = .66) were not significant; thus, these paths were trimmed from the model. The trimmed model also demonstrated good fit (χ2 = 4.48, df = 10, p = .92; CFI = 1.00; RMSEA = .00) and was selected as the final, more parsimonious model based on the nested model comparison between the full model and the trimmed model (∆χ2 = 1.00, ∆df = 1, p = .32). As shown in Table 3, results of Model 2 indicated that greater adolescent risk-taking behaviors at Time 2 were associated with greater peer substance use at Time 3, which in turn predicted adolescents’ own substance use at Time 4. However, activation in the insular cortex during risk processing at Time 1 did not significantly predict risk-taking behavior at Time 2. Furthermore, there were no significant main effects of cognitive control on adolescent risk-taking behaviors, peer substance use, or adolescent substance use.
Table 3. Parameter estimates for the path model of longitudinal associations among neural risk processing, risk-taking behavior, peer substance use, and adolescent substance use moderated by neural cognitive control

Interaction Effect Models
After determining the direction of effects between adolescent risk-taking behaviors, peer substance use, risk processing, and adolescent substance use (using the main effect models), we continued to test for the moderating effect of cognitive control in each of the hypothesized models. In Model 1, when interaction effects were introduced in addition to the main effects, the model demonstrated good fit (χ2 = 9.90, df = 16, p = .87; CFI = 1.00; RMSEA = .00). The results indicated that neural cognitive control moderated only the path between insular risk processing at Time 3 and adolescent substance use at Time 4 (see Table 2 and Figure 3). In Model 2, though the model demonstrated good fit (χ2 = 6.86, df = 16, p = .98; CFI = 1.00; RMSEA = .00), there were no significant moderating effects of cognitive control (see Table 3 and Figure 4).

Figure 3. Summarized model fitting results of the path model of longitudinal associations among risk-taking behavior, peer substance use, neural risk processing, and adolescent substance use moderated by neural cognitive control. Standardized estimates are presented.

Figure 4. Summarized model fitting results of the path model of longitudinal associations among neural risk processing, risk-taking behavior, peer substance use, and adolescent substance use moderated by neural cognitive control. Standardized estimates are presented.
To further evaluate the moderating effect of cognitive control on the association between risk processing and substance use in Model 1, we conducted simple slope analyses to examine the effects of insular risk processing at varying levels of cognitive control: low BOLD responses during the MSIT task (i.e., 1 SD below the mean, higher cognitive control) versus high BOLD responses (i.e., 1 SD above the mean, lower cognitive control). Before probing conditional effects, all nonsignificant paths were trimmed for model parsimony. As shown in Figure 5, results indicated that the association between insular risk processing at Time 3 and substance use at Time 4 was significant for adolescents with higher cognitive control, such that higher insular activation was associated with lower substance use (B = −0.20, SE = 0.10, p = .048). This association was also significant for adolescents with lower cognitive control, such that higher insular activation during risk processing was associated with higher substance use (B = 0.29, SE = 0.11, p = .008). However, this association was not significant at mean levels of cognitive control (B = 0.04, SE = 0.07, p = .558). Moreover, the indirect effect from risk-taking behavior at Time 1 to substance use at Time 4 via peer substance use at Time 2 and neural risk processing at Time 3 was significant for adolescents with higher cognitive control, B = 0.02, SE = 0.01, 95% CI [0.001, 0.055], and adolescents with lower cognitive control, B = −0.02, SE = 0.02, 95% CI [−0.072, −0.003], but not for adolescents at mean levels of cognitive control, B = −0.004, SE = 0.006, 95% CI [−0.027, 0.007].

Figure 5. Simple slope analyses comparing the relation between insular risk processing and substance use for adolescents with high cognitive control (low interference-related BOLD responses) and adolescents with low cognitive control (high interference-related BOLD responses).
Supplementary Behavioral Analyses
To examine whether our hypotheses were supported on a behavioral level, we tested the two alternative models using behavioral performance in the economic lottery choice and cognitive control task, instead of neural variables. The behavioral risk processing was represented by adolescents’ risk preference (α)Footnote 2, which represented behavioral risk taking estimated using a standard power utility function (Pratt, Reference Pratt1964; Arrow, Reference Arrow1965) from each participant's set of decisions in the economic lottery choice task. Behavioral cognitive control was reflected by reaction time for correct trials in the MSIT. Both Model 1 (χ2 = 22.66, df = 15, p = .09; CFI = .93; RMSEA = .06) and Model 2 (χ2 = 16.56, df = 16, p = .41; CFI = 1.00; RMSEA = .02) demonstrated good fit. However, in Model 1, peer substance use at Time 2 did not predict the proportion of risky responses at Time 3 (b = −0.07, SE = 0.04, p = .09), and the proportion of risky responses at Time 3 did not predict substance use at Time 4 (b = 0.12, SE = 0.15, p = .42). Furthermore, there were no main or interaction effects for behavioral cognitive control. Similarly, in Model 2, the proportion of risky responses at Time 1 did not predict risk-taking behavior at Time 2 (b = 0.12, SE = 0.14, p = .38) and there were no main or interaction effects for behavioral cognitive control.
Discussion
Middle adolescence is a developmental milestone for substance use, and individuals who use substances before age 15 are significantly more likely to have substance abuse problems as adults (e.g., Grant & Dawson, Reference Grant and Dawson1997). Concomitantly, middle adolescence appears to be when brain sensitivity to social environments, such as peer contexts, peaks (Schriber & Guyer, Reference Schriber and Guyer2016). Yet, little is known about the roles that brain functioning may play in the developmental pathways through which adolescent risk-taking behaviors and associations with substance-using peers may progressively lead to adolescent substance use behaviors. The current study tested a developmental cascade model to demonstrate a developmental sequence of risk processes, whereby an earlier risk factor (i.e., adolescent risk-taking behaviors) may generate subsequent vulnerabilities in development (i.e., deviant peer affiliation), which in turn transact to produce further risk for competent adaptation (i.e., adolescent substance use behaviors).
In a recent review of fMRI and behavioral studies, Foulkes and Blakemore (Reference Foulkes and Blakemore2018) noted that an adolescent's peer environment can affect their development, and that individual differences in neurobiology can determine how sensitive an adolescent is to their social context. Our data supported both the socialization process and the selection process, and thus are consistent with the study by Dishion and Owen (Reference Dishion and Owen2002), which suggested that peer selection and influence go hand in hand. In addition, our findings, in general, are consistent with previous studies that have suggested developmental cascades delineating significant contributions of early problem behaviors to substance use problems in adolescence (Martel et al., Reference Martel, Pierce, Nigg, Jester, Adams, Puttler and Zucker2008; Rogosch, Oshri, & Cicchetti, Reference Rogosch, Oshri and Cicchetti2010). Particularly relevant are findings from Dodge and colleagues (Reference Dodge, Malone, Lansford, Miller, Pettit, Bates and Maslowsky2009) showing that children with behavior problems associated with deviant peers and in turn those associations provided exposure to substances, which ultimately resulted in substance use initiation in adolescence. The unique contribution of our finding lies in testing the role of neural processes that are involved in the progressive pathways linking early problems behaviors and later substance use via deviant peer affiliation. We found evidence indicating that brain functioning is involved in socialization processes rather than selection processes. Specifically, adolescents who associated with substance-using peers showed lower insular activation during risk-related decision making. Adolescents who were affiliating with substance-use-promoting peers were more likely to be afforded opportunities to use substances or to see substance use as more normative, which resulted in decreased sensitivity to risk over time.
As such, our findings provide important insights regarding how brain functioning is involved in the well-documented association between adolescent substance use and that of their peers (Dishion, McCord, & Poulin, Reference Dishion, McCord and Poulin1999; Hawkins et al., Reference Hawkins, Catalano and Miller1992). This association has been attributed to maladaptive peer socialization processes, in which peers provide opportunities for substance use, model substance use behavior, and encourage attitudes that are positively biased toward substance use (Deater-Deckard, Reference Deater-Deckard2001). Our neuroimaging data revealed that adolescents’ insular activation during risky decisions was involved in socialization processes such that it was a significant mediator that linked earlier peer substance use and later adolescent substance use. In the peer socialization processes, research has suggested deviancy training—active peer reinforcement through positive affect and social attention shown for deviant behavior—as a mechanism for peer contagion (Dishion & Tipsord, Reference Dishion and Tipsord2011). Peer contagion has also been demonstrated by the finding that an adolescent's desire to conform to peers’ standards of behavior is associated with adolescent risky behaviors including substance use (Farrell, Kung, White, & Valois, Reference Farrell, Kung, White and Valois2000). Considering peer contagion, our findings regarding the interaction between insular and medial prefrontal cortices are convergent with prior evidence indicating that these brain regions play important roles in determining peer influences on decision making. In an experimental study, Chung and colleagues (Reference Chung, Christopoulos, King-Casas, Ball and Chiu2015) showed that others’ choices of risky options increased the subjective value (utility) of those options, and that this other-conferred utility interacted with individual risk preference shown in the insula and the dorsal anterior cingulate cortex. That is, risky choices made by others worked as a ‘gentle nudge’ to risk-averse individuals or a ‘strong push’ to risk-seeking individuals. This finding clarifies why the pernicious effects of negative peer influence are most evident in vulnerable adolescents who are already engaging in risky behaviors (Dishion & Tipsord, Reference Dishion and Tipsord2011). Our findings further elucidate how neural processes underlying risky decision making are involved in linking associations with substance-using peers and real-world substance use behaviors.
Interestingly, we found that the effects of risk-related insular activation on real-world substance use behaviors were contingent upon the individual's cognitive control abilities. Specifically, for adolescents with high cognitive control abilities (i.e., low neural activation during cognitive control), higher levels of insular activation during risk-processing were predictive of lower substance use. This finding is consistent with previous research on risk processing indicating that heightened insular activation is related to risk avoidance in adolescents (van Duijvenvoorde et al., Reference van Duijvenvoorde, Huizenga, Somerville, Delgado, Powers, Weeda and Figner2015). However, for adolescents with low cognitive control abilities (i.e., high neural activation during cognitive control), higher levels of insular activation during risk-processing were predictive of higher substance use. One interpretation of this finding is that the role of the insula in risk processing may be different between adolescents with high cognitive control abilities versus those with low cognitive control abilities. That is, adolescents with low cognitive control abilities may show heightened activation in broader brain regions (including the insula) because risk information is not evaluated in the same way as in adolescents with high cognitive control abilities. Such difficulties in risk processing within the brain may be related to greater susceptibility to engaging in risky behaviors.
An alternative explanation for our data might be that those with low cognitive control abilities may show heightened activation in the insula in response to potentially negative affect and other somatic sensory input instead of encoding the potential negative outcomes of their actions. The insular cortex, while known to be a key region in the neural representation of risk, also plays a role in integration of cognitive and affective signals and has been shown in other contexts to play a role in the processing of pain and negative emotions. Perhaps, adolescents with low cognitive control abilities may be more susceptible to distracting emotions evoked by high-risk options which may interfere with somatic markers—emotional factors that are relevant to the task (Bechara & Damasio, Reference Bechara and Damasio2005).
Our findings reveal the significance of neural activation related to cognitive control in risk-taking behaviors and complement previous findings demonstrating the important role played by the right temporoparietal junction, which is known to be involved in mentalizing and social cognition, in linking social exclusion and risk taking (Peake et al., Reference Peake, Dishion, Stormshak, Moore and Pfeifer2013). Specifically, adolescents with lower resistance to peer influences exhibited higher activity in the right temporoparietal junction during social exclusion, which in turn was predictive of increases in laboratory-based risk-taking behaviors after social exclusion. Our study using longitudinal data expands this cross-sectional experimental study by showing that neurobiological vulnerability can be manifested in different ways in terms of peer-related risk factors that promote real-world risk-taking behaviors throughout adolescence.
Although our findings converge with extant literature, they differ from a recent report of cross-sectional analysis indicating a significant interaction between risk processing and cognitive control (Kim-Spoon, Deater-Deckard, Lauharatanahirun, et al., Reference Kim-Spoon, Deater-Deckard, Lauharatanahirun, Farley, Chiu, Bickel and King-Casas2016). In that study, lower insular activation during the outcome phase (i.e., during anticipation of uncertain outcomes) was associated with greater health risk behaviors (including substance use) for late adolescents with poor cognitive control indicated by greater dorsal anterior cingulate cortex activation during cognitive control, whereas there was no association between insular activation and health risk behaviors among those with lower dorsal anterior cingulate activation. As we discuss in the following section, the longitudinal findings of the current study provide a more nuanced understanding of the role that insular activation during risk processing (i.e., during the decision phase) plays in predicting adolescent substance use. Most importantly, the significant interactions between neural risk processing and neural cognitive control in linking peer substance use and adolescent substance use underscore the criticality of considering interactions between the two neural systems involved in determining risk taking, as emphasized in a review by Kim-Spoon and colleagues (Reference Kim-Spoon, Maciejewski, Lee, Deater-Deckard and King-Casas2017).
We found that the indirect effects linking earlier risk-taking behaviors and later substance use through peer substance use and neural risk processing were significant for adolescents with lower or higher levels of neural cognitive control but not for those with average levels. Further, the developmental pathways to substance use operated differently between high versus low levels of neural cognitive control. Depending on the levels of neural activation during cognitive control, insular activation during risk processing appeared to dissuade adolescents from substance use or propel them toward substance use. Therefore, high insular activation coupled with low neural cognitive control activation (i.e., good control) may represent a neural protective factor against adolescents’ substance use in the context of peer substance use, whereas high insular activation coupled with high neural cognitive control activation (i.e., poor control) may represent a neural vulnerability factor that puts adolescents at risk for substance use.
Further, we propose that the nature of substance use behaviors committed by adolescents with high versus low cognitive control may represent two distinctive subtypes of adolescent risk taking: reasoned versus reactive risk-taking behaviors (Reyna & Farley, Reference Reyna and Farley2006). That is, adolescents with high neural activation during cognitive control (i.e., low cognitive control abilities) may engage in substance use out of their reactive impulse, and their heightened insular activation during risk processing predicts greater propensity to such unplanned substance use behaviors. In contrast, adolescents with low neural activation during cognitive control (i.e., high cognitive control abilities) may engage in substance use out of their reasoned intention to take those risks, and their heightened insular activation during risk processing suggests greater aversion to such planned substance use behaviors. Indeed, research has shown that reasoned risk taking (that involved behavioral intention), compared with reactive risk taking (that did not involve behavioral intention), was associated with better performance on neurocognitive tasks measuring abilities to gauge relative risks and benefits (Maslowsky, Keating, Monk, & Schulenberg, Reference Maslowsky, Keating, Monk and Schulenberg2011). If the different neural activation patterns shown in our data underlie distinctive subtypes of risk taking (i.e., reasoned versus reactive), they may provide important information for effective prevention by considering the different causes of such behavior.
Our cross-level (brain-behavior) analyses involving neural variables in the prediction of risk-taking behaviors demonstrated significant moderating effects of prefrontal functioning on the longitudinal association between insular risk-processing and substance use. However, we did not find evidence for such moderating effects when using behavioral performance data of risk processing and cognitive control. It may be that neural response can sensitively capture individual differences in neurobiological vulnerability, whereas laboratory-based behavioral performance is relatively limited in representing vulnerability to real-life risk-taking behaviors (Richards et al., Reference Richards, Plate and Ernst2013). Thus, the finding highlights the advantage of brain function measures. Imaging data, in conjunction with behavioral data, can provide a powerful tool for identifying adolescents susceptible to substance use.
There are several limitations of the current study. First, our correlational analyses do not allow us to infer causality in the identified relationships. Second, our sample was representative of the region where we recruited families with respect to socioeconomic status and ethnicity/race. Accordingly, although our sample was economically diverse (about half of the sample qualifying as poor or near-poor), ethnic/racial diversity was relatively limited. Thus, further research is warranted for evaluating generalizability of our findings to more ethnically/racially diverse samples. Third, we primarily focused on examining insular functioning during risk processing. Future research may find it informative to examine multiple affective and cognitive networks as well as frontolimbic connectivity. Finally, although we primarily focused on examining peer selection and socialization processes in the current study, we acknowledge that other social relationship factors (e.g., parenting practices, relationships with teachers) may also contribute to substance use development (e.g., Allen, Chango, Szwedo, Schad, & Marston, Reference Allen, Chango, Szwedo, Schad and Marston2012). Future research should consider the interface of peer systems and other contexts such as family, school, and neighborhood contexts as risk and protective processes for the development of risky decision making and behaviors in adolescence. Considering other social contexts that may interface with peer systems is particularly important because of evidence that peer-based interventions, under certain circumstances, can be harmful (Dishion et al., Reference Dishion, McCord and Poulin1999). These other social agencies may positively structure adolescents’ environments in ways that do not aggregate youth into peer group settings that have dynamics that promote deviance.
In conclusion, our study is the first to investigate brain function in relation to the developmental processes of peer selection and socialization and adolescent substance use. Our findings illustrate how brain functioning related to risk processing and cognitive control contributes to the development of psychopathology, particularly substance use in adolescence. Understanding the developmental pathways of neural and behavioral phenotypes associated with risky decision making and how they interact with social environments can provide a powerful tool for identifying individuals susceptible to health risk behaviors. Our findings elucidate a specific brain-behavior mechanism that may be a biomarker for identifying those who are particularly vulnerable to peer contagions, potentially preventing powerful iatrogenic effects arising from aggregating deviant youth in group interventions (Dishion et al., Reference Dishion, McCord and Poulin1999). Furthermore, the modulating effects of neural cognitive control may suggest a new direction for prevention and intervention efforts to reduce adolescent substance use behaviors that focus on not only risk processing but also cognitive control abilities.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579419001056
Acknowledgments
We thank the former and current JK Lifespan Development Lab members for their help with the data collection for this study. We are also grateful to the adolescents and parents who participated in our study.
Financial Support
This work was supported by grants from the National Institute on Drug Abuse (R01 DA036017 to Jungmeen Kim-Spoon and Brooks King-Casas and F31 DA042594 to Nina Lauharatanahirun).
Conflict of interest
None.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any agency of the U.S. government.