Published online by Cambridge University Press: 02 March 2005
Objectives: The aim of this study is to explain factors influential to the diffusion of computed tomography (CTs) and magnetic resonance imaging (MRIs).
Methods: Variables were identified from a review of the literature on the diffusion of health technologies. A formal process was applied to build a conceptual model of the mechanism that drives technology diffusion. Variables for the analysis were classified as predisposing, enabling, or reinforcing factors, in keeping with a model commonly used to explain the diffusion of health behaviors. Multiple regression analysis was conducted using year 2000 OECD data.
Results: The results of this study showed that total health expenditure per capita (p < .01, both CTs and MRIs) and flexible payment methods to hospitals (p < .05, both CTs and MRIs) were significantly associated with the diffusion of CTs and MRIs (adjusted R2 = 0.477, 0.656, respectively).
Conclusions: This study presents a systematically developed model of the mechanism governing technology diffusion. Important findings from the study show that purchasing power, represented by total health expenditure per capita and economic incentives to hospitals in the form of flexible payment methods, were positively correlated with diffusion. Another important achievement of our model is that it accounts for all thirty OECD member countries without excluding any as outliers. This study shows that variation across countries in the diffusion of medical technology can be explained well by a logical model with multiple variables, the results of which hold profound implications for health policy regarding the adoption of innovations.
The cost of advanced medical technologies is commonly considered to be a major factor in the overall escalation of expenditures on health. Many countries have focused on limiting the use of medical technology, particularly those referred to as “big ticket” items, in an effort to contain costs. Computed tomography (CT) scanners and magnetic resonance imaging (MRI) scanners are often targeted, because they have been rapidly adopted, despite their high cost (2;3).
Although many studies have sought factors to explain patterns of technology diffusion, few have succeeded, particularly in international comparisons. It may not be practical to define optimal numbers of CTs and MRIs, but a comparison of their diffusion in different countries would be helpful. The aim of this study, therefore, is to provide an international comparison of the diffusion of CTs and MRIs and to identify factors that can explain the observed variation.
We conducted a review of the literature to identify variables that have been used in other qualitative and quantitative studies of medical technology diffusion. To ensure that we would be able to provide both general and specific justification for our own diffusion model, our review included a wide range of medical technologies, in addition to CTs and MRIs. After this survey, we constructed a conceptual model and selected relevant variables, subject to availability of data for international comparison. Statistical analysis was then conducted, culminating in the construction of a regression model to describe international variations in the diffusion of CTs and MRIs.
From our review of fifty-one medical technology diffusion studies, we identified a broad range of factors. We then grouped these factors into categories, using an approach similar to that described by Banta (2). The five categories we defined were as follows: (i) Purchasing Power; (ii) Patient Needs; (iii) Physician Demand; (iv) Government Regulation; and (v) Payment Methods. These five categories became the dimensions of our conceptual model and from each we selected one factor for later inclusion in a statistical model.
To illustrate the role that each of these dimensions plays in our model, we have further classified them as being predisposing factors, enabling factors, or reinforcing factors. Predisposing factors are those that provide the basic motivation for diffusion; enabling factors are factors that enable a motivation to be realized; and reinforcing factors are factors that sustain a level of diffusion once it has been reached. In our conceptual model, Patient Needs and Physician Demand are what we would consider predisposing factors—they set the stage and provide the actors' motivations. Purchasing Power is the enabling factor in our model and determines to what extent needs and demands can ultimately be met. Finally, Payment Methods and Government Regulation are reinforcing factors that provide the incentives necessary to sustain diffusion. Figure 1 presents a graphic representation of our diffusion model mapped in this way. Readers familiar with health promotion planning may recognize that we have borrowed this metaphor from the PRECEDE framework (11). It is not surprising that common mechanisms of behavior diffusion can be found between the diffusion of behavior to adopt health promotion practices and the diffusion of behavior to adopt health care technology.
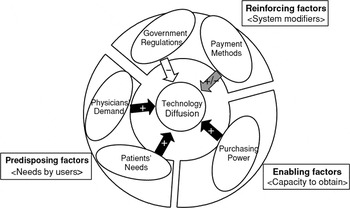
A proposed model of determinants of technology diffusion.
Green et al. (10) explain behavioral determinants of preventive practices by physicians using a model with similar structure to ours, in which physician's values, beliefs, attitudes, and perspectives are described as predisposing factors. The authors include necessary skills and resources, such as space or materials, as enabling factors; and physician rewards as reinforcing factors.
It is widely assumed that factors belonging to the category Purchasing Power are positively related with the diffusion. Simply put, countries that can afford it will tend to adopt expensive new technologies faster (14;23). Gross domestic product (GDP) per capita is an example of the kinds of factors found in this category and can be interpreted as a crude measure of purchasing power. Another more health-care specific measure, total health expenditure (THE) as a fraction of GDP, is also included in this category.
Lázaro and Fitch (14) analyzed the relationships between technology diffusion and GDP per capita and health-care expenditure (HCE) per capita, both separately and together. However, HCE per capita is a function of GDP per capita and the fraction of GDP spent on health care, so HCE per capita (or THE per capita) alone should be enough to represent the influence purchasing power exerts on health care. We, therefore, selected THE per capita to represent purchasing power in our statistical model, with the expectation that it would allow us to measure the effect of the real money expenditure on health.
The category of Patient Needs includes measures that directly or indirectly express the level of demand generated by patients. Technology is more rapidly diffused where there are populations with a high prevalence of disease requiring treatment with advanced medical technologies (18;27). To the extent that this is true, technology diffusion then is disease-specific. A comparison of countries by disease structure would be very complicated; however, the percentage of a population over 65 years of age may be a reasonable proxy. As a population ages, demand for sophisticated medical technologies is likely to increase. We, therefore, selected this simple measure of population age structure to represent Patient Needs in our statistical model.
Physician Demand represents demand generated by technology adopters. Aspects, for example types of ownership, and the level of competition between health-care providers can be included in this category. The number of physicians per capita is a good indication of Physician Demand, because competition is proportional to the number of physicians. If the population remains fixed and the number of physicians increases, each physician on average will see fewer patients. As the number of patients per physician decreases, physicians feel increasing pressure to order more tests, prescribe more drugs, and perform more procedures on a per patient basis to maintain their income. Thus, as the number of physicians per capita increases, supplier-induced demand increases, positively stimulating technology diffusion (24). Based on this assumption, we selected the number of physicians per capita to represent Physician Demand in later statistical analysis.
The category of Government Regulation includes metrics that represent the level and nature of regulation. Most of these metrics will be negatively related with the extent of technology diffusion, given that regulation typically works against market orientation in the health sector (8;13;16;22). Strict regulation to limit the diffusion and utilization of medical technologies is a comparably easy strategy to contain health care costs. International comparisons in this category, however, are complicated by the fact that there is no simple quantitative measure of government controls. Public expenditure on health, however, is an indirect way to gage government involvement. When a large share of total health expenditure is covered by public funds, the government will have more leverage to restrict spending. Conversely, when private funds cover the majority of spending on health, the government will tend to have less control over the diffusion of technology. By this logic, the ratio of public spending on health to total health expenditure will vary inversely to technology diffusion. This ratio, therefore, was selected to represent Government Regulation in our regression model.
The last category in our model is Payment Methods. The method of payment can alternatively encourage or discourage the diffusion of new technologies. For example, fixed or inflexible payment methods, like global budgets, provide neutral or negative incentives for the adoption of new technologies, whereas flexible fee-for-service reimbursement mechanisms positively encourage adoption.
Many studies have acknowledged the important influence payment methods have on technology diffusion (4;5;7;12;15;18;21;26) , but none have addressed payment to hospitals and physicians separately. And yet, especially where capital-intensive technologies such as CTs and MRIs are concerned, hospitals and physicians play very different roles in the adoption and use of medical technology. For this reason, we elected to treat them separately.
Hospital payment methods can be grouped several different ways. Donaldson and Gerard characterized payment methods as being either retrospective or prospective, and indicated that a third category could be added if competition (e.g., capitation and per-capita payments to HMO's) were included (6). Another commonly used scheme includes global budgeting, per diem payments, and diagnosis-related group reimbursement (17). Yet another combination includes fixed global budgets, additional payments for the provision of more costly treatments, and fee-for-service payments (1).
Physician payment methods are more easily differentiated. Fee-for-service payments and salary payments represent two ends of a spectrum. Capitation payments, typically used in the context of primary care, fit between these two polls. In this study, capitation payments are ignored, because we are only concerned with hospital-based specialists. Instead, a hybrid of fee-for-service and salary payment is considered as a third option.
To simplify nomenclature, we elected to classify hospital and physician payment systems into three categories—fixed, moderate, and flexible—based on regulatory intensity (Table 1). “Fixed” payment methods leave little room for providers to influence revenue. Global budget methods to hospitals or salary payment methods in general terms are included in this category. “Flexible” methods are those that allow providers more autonomy. This category is composed of methods that calculate reimbursements on a per case or per diem basis. Payment systems that fall between these two poles, prospective payment methods for example, are deemed “moderate.”

Before moving on, it is important to note that we have made a distinction between payment methods to physicians and demand generated by physicians. The Payment Methods category just described includes supply factors that are directly derived from payment systems, whereas the Physician Demand category described earlier contains factors related to characteristics of physicians or their environment, for example, competition. Although it may seem subtle, this distinction is in keeping with supply-induced theory.
In addition to these five categories, it could be argued that the nature of the technology itself should play a part in determining the extension of its own diffusion. For example, the marginal clinical advantage CTs and MRIs hold over X-rays should encourage their adoption (2;12;25). For the purpose of international comparison, however, clinical advantages and disadvantages need not be considered, because they do not vary from country to country.
After a correlation analysis to discover covariation between individual factors, we conducted a multiple regression analysis to identify key correlates with the diffusion of CTs and MRIs. Our multiple regression analysis took the following form:
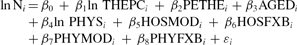
where ln Ni is the natural logarithm of the number of CTs and MRIs per million population for country i.
The explanatory variables in our statistical model are the natural logarithm of total health expenditure per capita in US$ at purchasing power parity (ln THEPCi), the share of public expenditure in THE (PETHEi), the percentage of population 65 years of age or older (AGEDi), the natural logarithm of the number of physicians per 1,000 population (ln PHYSi), moderate payment methods to hospitals (HOSMODi), flexible payment methods to hospitals (HOSFXBi), moderate payment methods to physicians (PHYMODi), and flexible payment methods to physicians (PHYFXBi). The constant β0 is the intercept, β1 through β8 are coefficients of independent variables, and εi is an error term. Note that independent variables representing fixed payment methods are not included in the equation, because they were designated as comparison groups for both hospitals and physicians.
Year 2000 data from all thirty OECD member countries was used in the analysis. OECD data was selected both because it represents the wide variation that exists among rich countries in the diffusion of CTs and MRIs and because it is readily accessible and of sufficient quality to allow meaningful international comparison. In cases where year 2000 data were not available, adjusted data from the most current available year was substituted. General information regarding factors selected to explain the diffusion of CTs and MRIs is shown in Table 2.

Information on payment systems was gathered from OECD Health Data 2003 (20) and other sources. (A complete list of references is available on request from the authors.) Because most payment systems are complex, it was frequently difficult to assign a country to a single payment category. For the purpose of our analysis though, a subjective, “best-fit” assignment was made. Hybrid payment systems that were too complex to categorize as either strict or flexible were designated as moderate.
Table 3 presents the results of correlation analysis. Three variables, THE per capita, aged population, and flexible payment methods to hospitals, show a statistically significant, positive correlation with the diffusion of both CTs and MRIs. In addition to these factors, the number of physicians per capita and moderate payment methods to hospitals show a correlation with the number of MRIs.

Results from the multiple regression analysis are presented in Table 4. In the case of CTs, it was found that THE per capita (p < .01) and flexible payment methods to hospitals (p < .05) were statistically significant (adjusted R2 = .477). THE per capita (p < .01) and flexible payment methods to hospitals (p < .05) also appear to be influential factors in the diffusion of MRIs (adjusted R2 = 0.656).

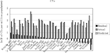
To assess the accuracy of our model, we examined outliers (9). As shown in Figure 2, the diffusion of CTs in Finland, Germany, the Czech Republic, and the Netherlands was accurately predicted by the regression model. Diffusion of MRIs was predicted with similar accuracy for Portugal, Germany, Poland, and Sweden, as shown in Figure 3.

The difference between predicted and actual numbers of computed tomography scans (CTs).

The difference between predicted and actual numbers of magnetic resonance imaging scans (MRIs).
Our diffusion model of CTs and MRIs predicts the data well. Many studies are concerned about including Japan's extremely high numbers of CTs and MRIs in international comparisons; therefore, Japan is often excluded to avoid data distortion (14). If this were true, Japan would appear in the left-most position in Figures 2 and 3, with a large residual value. However, in both figures, Japan stands in the middle with well-predicted values. From this standpoint, it can be said that our model produced no outliers and that our model accounted well for all of the data.
These results can be interpreted to mean that the diffusion of technology is strongly related to economic power, as expressed by THE per capita, and economic incentives inherent in flexible payment methods, particularly to hospitals. The former is consistent with existing literature, except that, in previous studies, GDP per capita or share of THE in GDP was used rather than THE per capita (14;23).
The key findings from our analysis were that total health expenditure per capita and flexible payment methods to hospitals were equally influential factors in the diffusion of both CTs and MRIs and that none of the other coefficients included in the regression model were significant. There are several possible explanations for these results.
The observation that purchasing power, represented by total health expenditure per capita, is related to the diffusion of CTs and MRIs, is consistent with previous studies (14). Total health expenditure per capita is a better predictor of technology diffusion than more general metrics like GDP per capita or total health expenditure, because it more accurately represents the scale of investment in health care. As a result, in our model, coefficients of total health expenditure per capita for both CTs and MRIs are positive. Simply put, technology diffusion increases with economic power.
Other studies have highlighted the relationship between flexible payment systems and technology diffusion (23), but none have made the distinction between hospital and physician payment systems. Most studies considered payment methods to hospitals only. Where “big ticket” items like CTs and MRIs are concerned though, it makes sense that hospital and physician payment systems should impact diffusion differently. Physicians may play a role in driving utilization, but hospitals are more likely to have the resources necessary to support adoption of the technology.
Although physicians may often more accurately be described as users than adopters, they do have the capacity to stimulate demand by increasing utilization. Whereas physicians visibly contribute to demand by ordering CTs and MRIs, it is the hospital that actually purchases the equipment. For this reason, financial incentives to hospitals are the most direct driver of technology adoption. By representing payment methods to hospitals and physicians separately, we were able to highlight this important distinction in our analysis.
In most countries, hospitals account for the largest share of total health expenditure (19). If hospital payment methods can have a direct impact on technology diffusion, then careful consideration of payment methods would be an effective strategy for controlling health-care costs. However, policy-makers should take care not to loose site of the concept of appropriateness. Although the diffusion of new technologies in health care is not inherently bad, inefficient or inappropriate use of medical technology is. Policy-makers, therefore, must interpret diffusion in the context of utilization. If technologies are diffused in such a way that resources are not being used efficiently, corrective action should be taken; but limiting the diffusion of medical technology by itself will not solve problems of inefficiency.
The ratio of public spending on health to total health expenditure was included in our regression model to represent the scope of government regulation. Intuitively, one might assume that strict regulation of public expenditure on health care would be an effective way to control technology diffusion. However, under fee-for-service payment systems, where hospital owners have the ability to purchase CT or MRI machines without public support and recover costs by increasing utilization, public controls in financing will have little impact.
As a proxy for patient needs, the percentage of population over 65 years of age was included in our model, because it was assumed that a larger number of seniors would be associated with greater demand for health-care services. This assumption may be true, but patient needs are only a predisposing factor. In the absence of real purchasing power, patient needs cannot be translated into increased utilization or diffusion of technology. This finding explains both why age structure was not found to be significant and THE per capita was.
This study has obvious advantages over previous studies. First, it provides a systematic conceptual model of technology diffusion. There are many factors that are commonly assumed to be important to the diffusion of technology, which are difficult or impossible to compare across countries. Whereas it may not be possible to reveal their significance statistically, it is nevertheless important to accommodate these factors conceptually. In this study, we built a robust conceptual framework to explain the mechanism of technology diffusion by adapting a behavior adoption model of predisposing, enabling, and reinforcing factors. Our categorizations explaining technology diffusion were based on this systematic, robust, conceptual process.
The second advantage of this study is that more specific variables were used. Where previous studies included GDP per capita or THE in relationship to GDP (14;23), we have used THE per capita to represent specific health-care purchasing power. That economic power should drive technology diffusion is simple enough that it may be taken at face value. However, no previous study has succeeded in demonstrating a relationship between health-care–specific purchasing power and the diffusion of technology. Previous studies have not made the distinction between hospital and physician payment methods when analyzing the effect of payment systems on technology diffusion. Some have focused exclusively on hospital payment methods, but few have found empirical evidence to support a relationship between payment systems and technology diffusion.
A third advantage of this study is that our model accounted for all thirty OECD member countries without excluding any as outliers. In a previous empirical study of 24 countries (14), Japan was excluded due to its high number of CTs; and yet, our model predicted well the diffusion of CTs and MRIs in Japan. All of the countries included in this study are equally motivated to understand the mechanisms that drive technology diffusion.
A limitation of this study is that it relies on cross-sectional data. Our study focused on the state of CTs and MRIs in 2000 as a point in time, without considering history. The process of technology diffusion over time, however, deserves to be examined more closely. Further studies using panel data should be conducted to better explain the diffusion of technology.
Expensive technologies, such as CTs and MRIs, require a large capital investment to install. The purchase of these kinds of equipment requires financial resources that are typically only available to large facilities, as opposed to physicians in private practice. For this reason, payment methods to hospitals can be a direct incentive to promote technology adoption. At the same time, CTs and MRIs are relatively easy for physicians to use. Because of this characteristic, it is natural that professionals do not compete based on technical competency. Put together, hospitals are more sensitive to economic incentives, compared with physicians.
In addition, although many candidate factors have been proposed in the literature, this study found that the diffusion of CTs and MRIs was well explained by two variables: economic power at the macrolevel, and payment methods that are economic incentives for the adoption behavior of hospitals at the microlevel. It would not be practical to control the former, but consideration of payment methods could be incorporated in the process of decision-making at the policy level. In summary, payment methods to hospitals may be a critical dimension of health policy that seeks to manage the diffusion of health technologies that are capital-intensive and easy to operate, such as CTs and MRIs.
This study succeeded in constructing a model of technology diffusion that fit data from all thirty OECD countries well, without excluding any as outliers. To construct a concrete framework illustrating how technology diffusion is determined, we adapted the concepts of predisposing, enabling, and reinforcing factors, from a model used in health behavior diffusion. The implication of this study is that variation across countries in the diffusion of medical technology can be explained well by a logical mechanism with multiple variables, including purchasing power and economic incentives.
Our analysis of the determinants of diffusion for CTs and MRIs in thirty OECD countries revealed that purchasing power, represented by total health expenditure per capita, was positively related with technology diffusion. The more important finding is that economic incentives, in the form of payment methods, are an influential factor in determining the diffusion of technology.
To the extent that we have succeeded in presenting a systematically developed explanation of the mechanism governing technology diffusion, the results of this study hold profound implications for health policy regarding the adoption of innovations.
Eun-Hwan Oh, MPH, Doctoral Student (ehoh@pbh.med.kyoto-u.ac.jp), Yuichi Imanaka, MD, PhD, Professor (imanaka@pbh.kyoto-u.ac.jp), Edward Evans, BA, Master Student (ed.evans@gmail.com), Department of Healthcare Economics and Quality Management, Graduate School of Medicine, Kyoto University, Yoshida Konoe-cho, Sakyo-ku, Kyoto 606-8501, Japan
The results of this study were partly presented at the 1st HTAi Annual Meeting, May 30–June 2, 2004, Krakow, Poland.
This study was supported in part by the Grant-in-Aid for Scientific Research A [No. 16209019] from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
A proposed model of determinants of technology diffusion.

Categories of Payment Systems to Hospitals and Physicians in Terms of Strength of Regulation in OECD Countries (N = 30)

Distribution of Selected Variables in OECD Countries (N = 30)

Factors Associated with Technology Diffusion (Correlation Coefficients, N = 30)

Multiple Regression Analysis Results of Technology Diffusion and Selected Variables in OECD Countries (N = 30)
The difference between predicted and actual numbers of computed tomography scans (CTs).
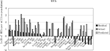
The difference between predicted and actual numbers of magnetic resonance imaging scans (MRIs).