INTRODUCTION
The value of the radiocarbon bomb pulse as a biological tracer to study short-term processes was recognized in the mid-1970s, with the first publication appearing addressing the studies of metabolism and exchange rate of various tissues in vivo (Stenhouse and Baxter Reference Stenhouse and Baxter1977; Stenhouse Reference Stenhouse1979). Soon after, the method was applied to the study of the history of gallstone accretion in the human body, using the 14C/12C ratio determined in gallstone layers from core to surface and matching it to the atmospheric 14CO2 profile (Druffel and Mok Reference Druffel and Mok1983). With the appearance and widespread use of accelerator mass spectrometry (AMS) 14C measurements, and further developments allowing the sample size reduction to micrograms of carbon, bomb-pulse dating on in vivo biological compounds experienced a rebirth. 14C bomb-pulse dating, after establishing itself in geosciences (Hua Reference Hua2009) and forensic applications (Zoppi et al. Reference Zoppi, Skopec, Skopec, Jones, Fink, Hua, Jacobsen, Tuniz and Williams2004), has been recognized in the biosciences and successfully applied to examine the longevity of human tissues (such as tooth enamel; Spalding et al. Reference Spalding, Bhardwaj, Buchholz, Druid and Frisén2005a) and biomolecules such as proteins in the eye lens (Lynnerup et al. Reference Lynnerup, Kjeldsen, Heegaard, Jacobsen and Heinemeier2008) and DNA in adipocytes (Bhardwaj et al. Reference Bhardwaj, Curtis, Spalding, Buchholz, Fink, Björk-Eriksson, Nordborg, Gage, Druid, Eriksson and Frisén2006), neurons (Spalding et al. Reference Spalding, Buchholz, Bergman, Druid and Frisén2005b), and cardiomyocytes (Bergmann et al. Reference Bergmann, Bhardwaj, Bernard, Zdunek, Barnabe-Heider, Walsh, Zupicich, Alkaas, Buchholz, Druid, Jovinge and Frisen2009), as well as in eye lens lipids (Hughes et al. Reference Hughes, Levchenko, Blanksby, Mitchell, Williams and Truscott2015).
In developed countries, at the present time, upper urinary tract stones predominantly made of calcium oxalate and/or phosphate are already becoming a widespread medical condition and appear to be getting more so; therefore, they are the subject of considerable research. Currently, the growth rates and time histories of human kidney stones are still poorly known. Here, we report on an attempt to date a renal calculus by using bomb-pulse 14C. The aim of the current study is to test the applicability of the method for kidney stone growth research, and to develop a possible experimental approach to the task.
APPROACH
A kidney stone of calcium oxalate type, 6 mm across, with slightly irregular but almost round shape, was donated by one of the authors, and was used to test the feasibility of the study. The stone was first gamma sterilized (50 kGy) by the ANSTO Irradiation and Dosimetry Service. The step dissolution method to sample successive layers of material was employed, in a way reversing the depositional growth of the stone. Dissolutions were performed in ~5 mL of concentrated HCl acid at room temperature. After the size of the stone was visibly reduced, it was relocated to a second beaker, and later to a third beaker to dissolve the small central core. Thus, three samples were produced: the outer crust, middle shell, and inner core. After dissolution, the oxalate fraction was produced from the liquid phase by transferring the solution (leaving behind any undissolved solids) into a fresh beaker and carefully evaporating the solution down on a hot plate at ~50°C, in a fume cupboard. The concentrated samples were then glass pipetted into oxygen-baked quartz inner tubes (which were then dried down completely), Cu(II) oxide and silver wire were added. These inner tubes were then loaded into large-diameter quartz outer tubes, which were evacuated for 1 hr and then sealed using an oxygen/LPG gas torch. This method of combustion was used due to the high salt content present in the samples, which tends to damage quartz at elevated temperatures and creates potential cracks/leaks in the tube. The material was combusted at 900°C overnight, and the CO2 that was produced was then converted to graphite according to the standard ANSTO AMS protocol (Hua et al. Reference Hua, Zoppi, Williams and Smith2004). To investigate the possible origin of oxalate in the stone and explain the observed 14C levels, a vitamin supplement regularly taken by the donor, and samples of tomato and lettuce were also studied for their 14C content. One vitamin supplement sample was processed as is from the packaging and another with HCl treatment to remove possible carbonates used as a filler/binder. Vegetable samples were purchased at a local supermarket; a single tomato pip was cut out of a whole tomato and pieces of lettuce leaves were rinsed thoroughly. The samples were then combusted and converted to graphite following the usual ANSTO protocol (Hua et al. Reference Hua, Zoppi, Williams and Smith2004).
All samples were measured for 14C on the ANSTO AMS accelerator STAR (Fink et al. 2004), and results were normalized using the international 14C standards Ox I and Ox II. Raw data were corrected for isotopic fractionation and procedural blank (Hua et al. Reference Hua, Zoppi, Williams and Smith2004) and results are shown in Table 1. Vitamin samples returned statistically indistinguishable results, indicating that no calcium carbonate filler was used in this brand of tablets.
Table 1 Results of radiocarbon analyses.

RESULTS AND DISCUSSION
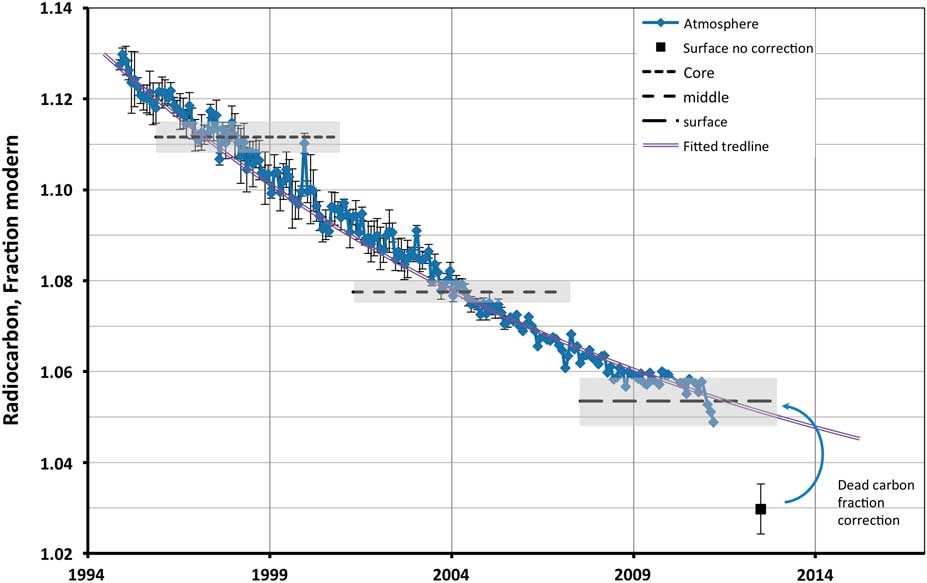
The 14C content of the kidney stone changed noticeably, decreasing in value from its core to the outer layers, demonstrating the significant period of growth despite its smallish size. The result for the outer shell of 102.97 pMC came initially as a surprise, since the value obtained was noticeably lower than the expected atmospheric level at the time of its extraction (mid-AD 2012). The latest published Southern Hemisphere atmospheric measurement of 104.9 pMC was for 2011.2 (Hua et al. Reference Hua, Barbetti and Rakowski2013). Exponential extrapolation of the atmospheric curve to mid-2012 produced a forecasted value of 105.1 pMC (see Figure 1). However, the value for the sample was expected to be even slightly higher, as material was averaging the input of at least a couple of years before extraction. The most common explanation of the observed deviation is the effect of the diet. Seafood is suggested as the traditional source of 14C-depleted food, when considering dietary 14C variation. However, as was demonstrated by Georgiadou and Stenström (Reference Georgiadou and Stenström2010), even 16% of seafood diet as a source of protein is not enough to alter the apparent bomb-pulse age for more than 3 yr, even less for SE Australia where our sample originated. And while the sample donor had a diet relatively rich in marine protein, it was less than 10% on average and by far not enough to explain the observed offset. On the other hand, protein is not the main source of urinary oxalate, and while some oxalate is a body metabolic product, up to 70% may come directly from diet and some food supplements like Vitamin C (Holmes and Kennedy Reference Holmes and Kennedy2000; Massey Reference Massey2003; Holmes and Assimos Reference Holmes and Assimos2004). The kidney stone donor was regularly taking a Vitamin C supplement of 120 mg daily in his diet, consistently of the same brand, and so it was tested as a possible source of the 14C-depleted oxalate in the stone. A 500-mg Vitamin C supplement tablet of the same manufacturer was processed and its 14C level was determined at 99.5±0.3 pMC (see Table 1). As was demonstrated by Massey (Reference Massey2003), 400 mg of ascorbate (Vitamin C) in a diet leads to an extra ~5 mg per day of urinary oxalate, which constitutes about 15% of usual daily excretion. Though the ascorbate supplement used by the kidney stone donor was found to be depleted in 14C, compared to the current ambient atmosphere, its intake, 14C content, and expected fraction of oxalate in urine from this source alone is not sufficient to explain the result of the outer stone shell.
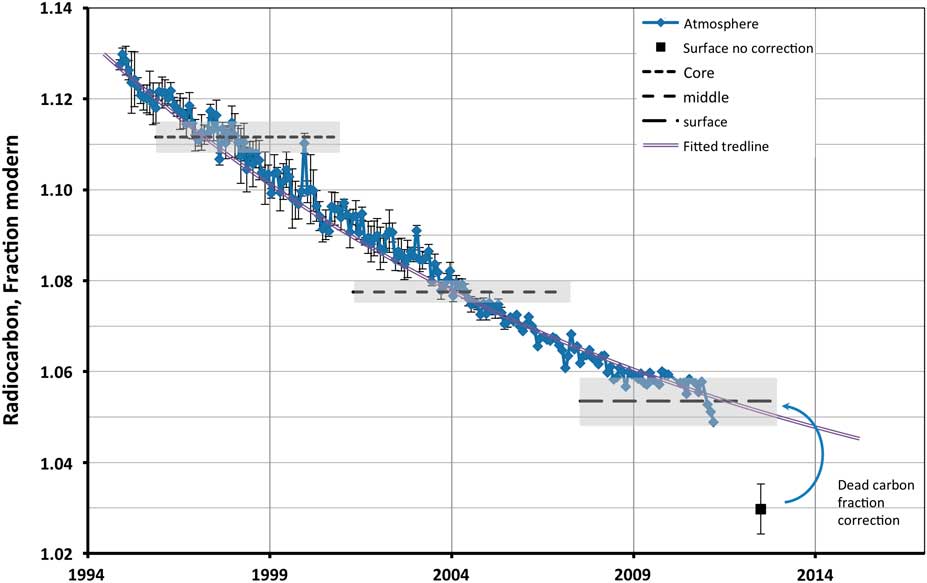
Figure 1 Radiocarbon content of outer, middle, and inner section of the kidney stone fitted to the Southern Hemisphere atmopsheric 14CO2 curve (Hua et al. Reference Hua, Barbetti and Rakowski2013). Shaded areas show 1σ range of determinations.
It is known that a significant part of dietary oxalate comes from vegetables, especially from beans, soy products, and green leafy vegetables (Krishnamurthy et al. Reference Krishnamurthy, Hruska and Chandhoke2003). Current industrialized food production produces a large percentage of these crops in controlled protected environments, including greenhouses and by using hydroponics. In Australia, up to 20% of total vegetable production comes from protected cropping, for some items like tomatoes and capsicums, reaching 60%, and for green leafy vegetables (like salad leaves), getting close to 90% (Taig Reference Taig2009). The use of fossil, i.e. 14C-depleted, CO2 for crop fertilization in greenhouses is well recognized by the 14C community. For example, van der Plicht and Beijers (Reference van der Plicht and Beijers2011) have noted fresh from the market tomatoes dated to 1300 yr ago corresponding to ~85 pMC, and similar values for greenhouse-grown flowers. With greenhouse CO2 concentration in air up to 1200 ppm (up to 3 times modern atmospheric levels), and usually achieved by natural gas or LPG combustion (Norén Reference Norén2002), one may expect the crops with 14C content even down to 30 to 50 pMC. Such levels of depletion and the fraction of dietary oxalate from plants in excreted oxalate are potentially more than sufficient to explain the observed result for the outer layers of the studied renal stone.
To test this hypothesis, we measured the 14C content of three samples fresh from a local supermarket: a pip from a tomato and pieces of hydroponically grown lettuce leaves of different varieties (Table 1). The tomato result was in perfect agreement with the expected atmospheric value for early 2015 (see Figure 1). The lettuce leaves demonstrated some depletion of about 3% compared to the tomato, though not on the level observed by van der Plicht and Beijers (Reference van der Plicht and Beijers2011), or expected from full-scale CO2 fertilization. This, in our opinion, could be explained by the lesser need for greenhouse heating in Australia using fossil fuels, and consequent carbon dioxide fertilization, compared to that in Europe.
The slope of atmospheric 14CO2 in the years before stone extraction was rather gentle. So, for the period of averaging from 1 to 4 yr, the average value changes only from 105.1 to 105.6 pMC. We took as the mean the value of 105.35 pMC, considering it a good approximation of expected sample 14C content. This corresponds to 2 yr of averaging. With this value, the dead carbon fraction in the outer stone layers can be determined to be 2.3%. The origin of this depletion in the food source seems plausible, assuming that most of the dietary oxalate originated in green leafy vegetables and vitamin supplements as discussed above. The sample donor had on average a relatively stable diet over a long period, so all results were corrected for this dead carbon fraction, finally giving 105.35, 107.75, and 111.16 pMC for outer, middle, and core layers, respectively. The calendar dates for each subsample were determined by fitting results to the atmospheric curve (see Figure 1) by using CALIBomb online software with the Southern Hemisphere data sets for the bomb pulse (http://calib.qub.ac.uk/CALIBomb/).
According to the calibration, the oldest core part of the stone has a 1σ average age range of AD 1996.6 to 1998.7, the average 1σ age range of the middle layers is 2003.4 to 2005.3, and the outer layer has 1σ age range from 2008.4 to the limit of calibration curve at 2011.21. The real physical extraction, and consequently end of growth, occurred at AD 2012.5. The dates show that the stone growth was possibly rather uniform, though one needs more samples with better resolution to properly study the growth rate variation. If we assume that the growth was indeed uniform, i.e. the mass of deposited material was steady in unit time, we may determine the starting time more accurately. The mass fraction of each subsample (see Table 1), and consequently the deposition period, would constitute 29, 58, and 13% of total growth time for the core, middle, and outer subsample, respectively. Each subsample represents the averaged 14C atmospheric signal over the period of its deposition with the end date for the whole fixed on the extraction time at AD 2012.5. This simple model produces the best fit of expected 14C values in the studied subsamples, to the measured results, if the growth had started about AD 1994.9. That gives the age of the stone of about 17.6 yr, over which it grew to ~6 mm across in size.
CONCLUSION
The bomb-pulse dating approach has proven its usefulness in determining the growth history of human kidney stones in vivo. To the best of our knowledge, it is the first determination of the longevity of kidney stone growth. The tested sampling and preparation method suits small stones with more or less round shapes, ideally growing in free-floating form. In principle, it allows multiple consecutive subsamples with the possibility of better depositional resolution to study the growth history. AMS 14C laboratories with experience working with sub-100 μg samples could be required for 14C determinations in this case. Non-centrally symmetrical stones may require physical cutting and sampling following the growth pattern.
The presence is detected of a noticeable dead carbon fraction in the diet and metabolic products of an inhabitant of a modern developed country. Potentially, this effect may cause problems in applying the bomb-pulse 14C determinations for in vivo biomedical studies. Thus, determining its level in every individual case and possible correction is important.
ACKNOWLEDGMENTS
The authors wish to thank the ANSTO AMS 14C chemistry team for their work related to the samples used in this experiment, and Dr Alistair Cameron-Strange, MBChB FRCSE FRACS (Urology), Senior Surgeon at The Sutherland and The President Private Hospitals, NSW, Australia for obtaining and saving the sample for study.