Introduction
Sexual activity in sheep is strongly influenced by photoperiod, which is the main environmental factor responsible for the seasonality of reproduction of this species. Season also affects the availability of pasturage resources, producing in a particular year situations of penury and subnutrition, this circumstance being normal in sheep flocks under extensive farming systems, which widely depend on natural forage resources. It has been described that undernutrition affects sheep reproduction through changes in the hypothalamus–pituitary–gonadal axis (reviews: Boland et al., Reference Boland, Lonergan and O'Callaghan2001; Forcada & Abecia, Reference Forcada and Abecia2006) and in oocyte and embryo quality (Ashworth et al., Reference Ashworth, Toma and Hunter2009). Thus, together with season, undernutrition becomes one of the main environmental factors affecting lamb production. Subnutrition increases embryo mortality and reduces pregnancy rates in sheep (Abecia et al., Reference Abecia, Rhind, Bramley and McMillen1995, Reference Abecia, Lozano and Forcada1999; Lozano et al., Reference Lozano, Abecia, Forcada, Zarazaga and Alfaro1998) because of an inadequate oocyte quality and embryo development (for a review, see Abecia et al., Reference Abecia, Sosa, Forcada and Meikle2006).
The superovulated sheep model has been used to determine the effect of nutrition on the quality of oocytes and embryos. In the context of multiple ovulation and embryo transfer (MOET) programs, Borowczyk et al. (Reference Borowczyk, Caton, Redmer, Bilski, Weigl, Vonnahme, Borowicz, Kirsch, Kraft, Reynolds and Grazul-Bilska2006) reported that undernutrition of donor ewes has a negative effect on oocyte quality, which results in lower rates of cleavage and blastocyst formation, concluding that these specific animals may require a specific nutritional management to provide good quality oocytes for assisted reproductive technology. Thus, nutrition of donor animals seems to be a key component affecting development of oocytes and the preimplantation embryo. However, McEvoy et al. (Reference McEvoy, Robinson, Aitken, Findlay, Palmer and Robertson1995) showed that in the superovulated ewe the recommended high level of feeding before mating did increase ovulation rate. Similarly, Lozano et al. (Reference Lozano, Lonergan, Boland and O'Callaghan2003) reported that the cleavage rates of oocytes were negatively affected by an ad libitum diet compared with a half-maintenance (M) diet. Related to these results, Kakar et al. (Reference Kakar, Maddocks, Lorime, Kleemann, Rudiger, Hartwich and Walker2005) observed that consumption of the 0.5 × M diet compared with either the maintenance or 1.5 × M diet during the periconceptional period (18 days before until 6 days after the day of ovulation) increased the total number of cells in the blastocyst and that this increase occurred entirely within the trophectoderm lineage. Nutrition of ewes should be considered in cloning programs, so that the nutrition of the oocyte donor ewe has been studied to determine whether or not it influences the outcomes of somatic cell cloning (Peura et al., Reference Peura, Kleemann, Rudiger, Nattrass, McLaughlan and Walker2003).
The development of embryos during the preimplantation period is affected, in part, by various hormones, growth factors and their receptors (Kaye, Reference Kaye1997), such as plasma non-esterified fatty acid (NEFA), insulin, insulin-like growth factor (IGF)-I and leptin. They have been proposed as indices of metabolic status as well as metabolic signals to the reproductive system, so that they are believed to carry the ‘metabolic message’ to the reproductive system (Blache et al., Reference Blache, Chagas, Blackberry, Vercoe and Martin2000). Thus, exposure of maturing bovine oocytes to elevated NEFA concentrations has a negative impact on fertility, not only through a reduction in oocyte developmental capacity but through compromised early embryo quality, viability and metabolism (Van Hoeck et al., Reference Van Hoeck, Sturmey, Bermejo-Alvarez, Rizos, Gutierrez-Adan, Leese, Bols and Leroy2011). We have previously found that IGF-I and insulin, as well as glucose, presented fluctuations in their plasma concentrations that coincided with key events in the oestrous cycle of sheep and suggested that the reproductive system could also modulate the metabolic milieu (Sosa et al., Reference Sosa, Abecia, Forcada, Vinoles, Tasende, Valares, Palacin, Martin and Meikle2006; Reference Sosa, Abecia, Carriquiry, Forcada, Martin, Palacin and Meikle2009). IGF-I is produced locally in many organs of the body, including the reproductive tract of cyclic and pregnant ewes (Stevenson et al., Reference Stevenson, Gilmour and Wathes1994). In the uterus, it stimulates cell proliferation and differentiation of the early embryo and of endometrial cells (Kaye, Reference Kaye1997). Moreover, the adipose tissue secretes leptin and in ruminants the amount secreted directly reflects the mass of adipose tissue. This hormone serves as a signal of body fat reserves to the central nervous system, acting to decrease appetite and increase energy expenditure, thereby maintaining adiposity around a set point (Chilliard et al., Reference Chilliard, Delavaud and Bonnet2005). Besides, leptin has been shown to affect ovarian function (Smith et al., Reference Smith, Jackson and Foster2002) and there is accumulating evidence that leptin may be directly involved in preimplantation embryonic development (Herrid et al., Reference Herrid, Nguyen, Hinch and McFarlane2006), so this cytokine hormone is another candidate for linking the effects of undernutrition on uterine and embryonic function.
As undernutrition has been determined as one of the main factors affecting oocyte and embryo development in naturally ovulated sheep, it is likely that a low plane of nutrition of donor ewes could affect embryo quality. Thus, the aim of this work was to determine the effect of a short-term undernutrition on embryo quality of superovulated ewes. In addition, metabolic and hormone concentrations were characterised to relate them to embryo development. We have used Rasa Aragonesa sheep, which is a Spanish genotype with a short seasonal anoestrous period (<100 days) between May and July (Forcada et al., Reference Forcada, Abecia and Sierra1992) and is well adapted for intensive production systems and an accelerated lambing rate (typically, three lambings in 2 years).
Materials and methods
The study was conducted at the experimental farm of the University of Zaragoza, Spain (latitude 41°N). All procedures were carried out under Project License PI05/10 approved by the in-house Ethic Committee for Animal Experiments from the University of Zaragoza. The care and use of animals were performed accordingly with the Spanish Policy for Animal Protection RD1201/05, which meets the European Union Directive 86/609 on the protection of animals used for experimental and other scientific purposes.
Animals and treatments
In mid-October, 45 mature Rasa Aragonesa ewes were housed in communal yards that had uncovered areas and offered a diet formulated to fulfil their maintenance requirements (Agricultural and Food Research Council, 1993) 1 month before the onset of the experimental procedure to allow adaptation to experimental conditions. The diet comprised 0.42 kg of pellets and 0.70 kg of barley straw per day, providing 7.8 MJ of metabolizable energy per ewe. The pelleted diet consisted of barley (85%) and soy bean (15%). The animals had unrestricted access to water and mineral supplement.
Oestrus (day 0) was synchronized in all ewes using intravaginal sponges that contained 30 mg fluorogestone acetate (Chronogest, MSD Salud Animal, Madrid, Spain), which were inserted for 14 days. To induce superovulation, ewes received 210 IU of pFSH (Follitropin; Bioniche Animal Health, Dublin, Ireland) and 500 IU eCG (Folligon, MSD Salud Animal, Madrid, Spain) in a single i.m. administration 48 h before the intravaginal sponge was removed. Fertile rams (one ram per four ewes) were placed with the ewes 24 h after pessary withdrawal and ewes were examined for evidence of oestrus every 8 h. At the time of sponge insertion, ewes were allocated to one of two groups to be fed diets that provided either 1.5 (control group (C); n = 20) or 0.5 (low group (L); n = 25) times the daily requirements for maintenance. Diets consisted of 0.8 or 0.5 kg of barley straw and 0.55 or 0.1 kg of pellets, for C and L groups, respectively. These regimens were maintained up to the day of embryo collection (day 7).
Live weight (LW) and body condition (BC) (over a scale of 0–5, where 0 = emaciated and 5 = obese; Russel et al., Reference Russel, Doney and Gunn1969) were determined at time of sponge insertion (day –14), withdrawal (day −1) and when animals were laparotomized (day 7).
Embryo recovery
Embryos from superovulatory treatments were collected through a mid-ventral laparotomy 7 days after the onset of oestrus. For surgical procedures, ewes were anesthetized using an i.m. administration of 0.4 ml 2% xylazine and, 5 min later, an intravenous (i.v.) injection of 10 ml sodium thiopental (20 mg/ml) (Thiobarbital, Braun Medical, Jaen, Spain). Uterine horns were exposed and, using a Foley catheter, flushed with a pre-warmed phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin (BSA; Sigma, St. Louis, MO, USA) and antibiotics (penicillin and streptomycin). To minimize the development of post-operative abdominal adhesions, before closure, reproductive tracts were flushed with a 2.5% heparin solution in saline. Ova and embryos were examined under a stereomicroscope (× 20–40 magnification) and classified based on their stage of development and morphology (Winterberger-Torres & Sevellec, Reference Winterberger-Torres and Sevellec1987).
The following information was recorded for each ewe: evidence of oestrus or ovulation indicated by the presence of corpora lutea (CL) that had a good morphological appearance in agreement with their age, the number of CL, the number of recovered oocytes and embryos, the number of fertilized embryos and the number of viable-transferable embryos (compacted morulae and early, expanded, or hatched blastocysts). We calculated rates of recovery (number of ova and embryos recovered per number of CL), fertilization (number of embryos recovered per number of ova and embryos recovered) and viability–transferability (number of viable embryos per number of ova and embryos recovered).
Blood sampling, hormone and metabolite assays
Blood samples were collected at days –14, 1 and 7 to determine plasma NEFA, insulin, IGF-I, leptin and progesterone concentrations (only day 7). NEFA concentration was determined by a commercial kit (NEFA-HR(2), Wako Chemicals Gmbh, Richmond, USA) and determined in an ELISA counter (Multiskan EX; Thermo Scientific, Waltham, MA, USA). The sensitivity of the assay was 0.01 mmol/l and the intra-assay CV was 1.7%.
Hormone determinations were as previously described (Fernández-Foren et al., Reference Fernández-Foren, Abecia, Vázquez, Forcada, Sartore, Carriquiry, Meikle and Sosa2011) and plasma samples were determined in only one assay. Progesterone and insulin concentrations were determined by a solid phase radioimmunoassay (RIA) utilizing commercial kits (Diagnostic Product Co., Los Angeles, CA, USA). For progesterone assay, the minimum detectable concentration was 0.02 ng/ml and for the intra-assay CV was 10.3%. For insulin assay, the minimum detectable concentration was 1.5 μIU/ml and for the intra-assay CV for low (2.2 μIU/ml) and high (12.6 μIU/ml) controls were 12.5 and 8.3 %, respectively. Concentrations of IGF-I were determined using an immunoradiometric assay (IGF-I-RIACT Cis Bio International, Gif-sur-Yvette, France). The sensitivity of the assay was 0.62 ng/ml and for the intra-assay CV for control 1 (46 ng/ml) and control 2 (268 ng/ml) were 6.7 and 9.5%, respectively. Leptin concentrations were determined by a liquid-phase RIA using a commercial comercial Multi-Species Leptin kit (Millipore, Billerica, MA, USA). The sensitivity of the assay was 0.7 ng/ml and for the intra-assay CV for control 1 (2.4 ng/ml) was 11.6% and for control 2 (13.5 ng/ml) was 6.4%.
The ratio progesterone/CL was calculated for each ewe as the division between plasma progesterone concentrations at embryo recovery and the number of CL in the ovaries.
Statistical analysis
The values expressed as percentages were compared between groups and among successive recoveries within each group using chi-squared or Fisher's Exact tests, as appropriate. To integrate the percentages in the model to determine whether they were influenced by the considered effects, individual proportions were arcsine-transformed before being subjected to statistical analysis. Results were expressed as mean ± standard error of the mean (SEM).
The rest of the variables were subjected to analysis of variance using a mixed model that included the fixed effects of nutritional treatment (low or control), day of cycle and their interactions. For the analysis of repeated measurements (LW, BC, NEFA, insulin, leptin and IGF-I), the covariance structure was modelled to consider the correlation between sequential observations on the same animal. Results were expressed as mean ± pooled standard error.
The probability level for statistical significance was set to P < 0.05 and trend to significance to P < 0.1.
Results
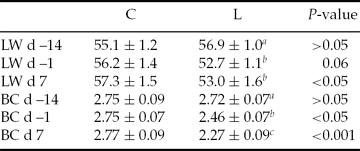
The low plane of energy in the diet significantly reduced (P < 0.001–0.06) LW and BC for the L group compared with the C group at sponge withdrawal and embryo recovery time (Table 1).
Table 1 Live weight (LW) and body condition (BC) (mean ± SEM) at sponge insertion (day –14), sponge withdrawal (day –1) and at embryo recovery (day 7) of Rasa Aragonesa ewes superovulated with 210 IU of pFSH and 500 IU eCG in a single i.m. administration 48 h before withdrawal of the intravaginal sponge and fed diets that provided either 1.5 (control group (C); n = 20) or 0.5 (low group (L); n = 25) times the daily requirements for maintenance from sponge insertion to embryo recovery (day 7)

Day 0 = day of oestrus. a,bDifferent superscripts in the same column indicates differences at P < 0.05.
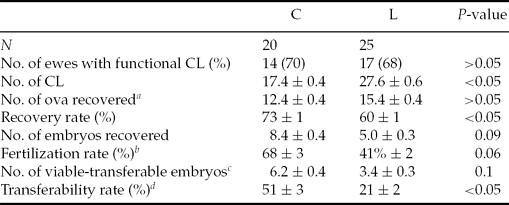
Thirty-one ewes (69%) responded to the superovulatory treatment presenting functional CL at embryo recovery, with no differences between groups (Table 2). Although L ewes presented a significantly (P < 0.05) higher ovulation rate in comparison with control ewes, recovery rate was 13% higher (P < 0.05) in the C group. The number of embryos collected per ewe and the number of viable-transferable embryos per ewe presented a trend to be significantly higher in C ewes (P = 0.09 and P = 0.1, respectively), so that undernourished ewes presented a trend to a significant lower fertilization rate (27%; P = 0.06) and yielded 30% lower transferability rate than C ewes (P < 0.05) (Table 2).
Table 2 Measurements of reproductive performances (mean ± SEM) of Rasa Aragonesa ewes superovulated with 210 IU of pFSH and 500 IU eCG in a single i.m. administration 48 h before withdrawal of the intravaginal sponge and fed diets that provided either 1.5 (control group (C); n = 20) or 0.5 (low group (L); n = 25) times the daily requirements for maintenance from sponge insertion to embryo recovery (7 days after oestrus)

aOocytes + embryos.
bNumber of embryos recovered per number of ova and embryos recovered.
cCompacted morulae and early, expanded, or hatched blastocysts.
dNumber of viable embryos per number of ova and embryos recovered.
CL: corpora lutea.
Plasma progesterone concentrations at day 7 (embryo recovery) were significantly higher in the L group (L: 62.7 ± 7.0; C: 44.7 ± 4.1 ng/ml; P < 0.05), although an opposite trend was observed for the ratio progesterone/CL (L: 2.00 ± 0.28; C: 2.71 ± 0.26; P = 0.08).
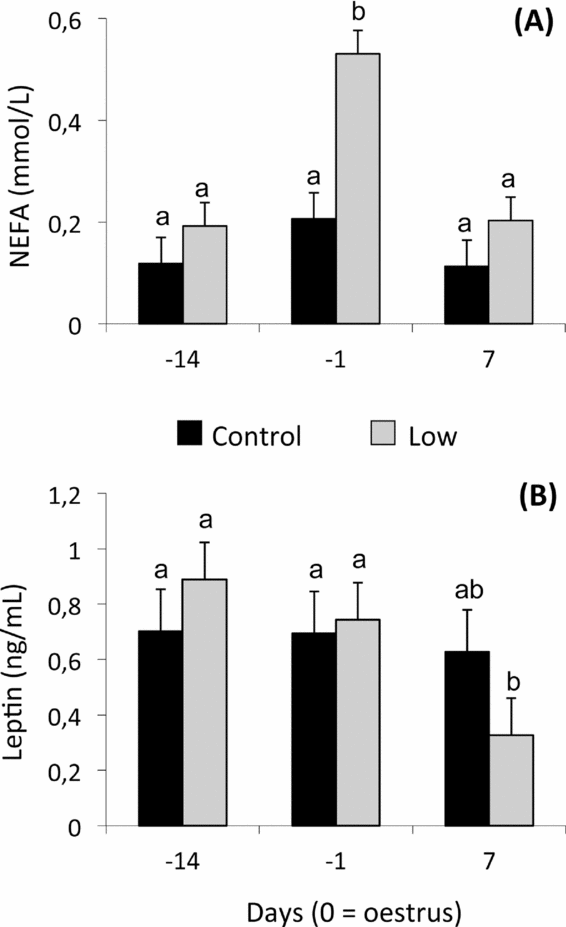
A significant effect of diet (P < 0.0001) and day of sampling (P < 0.0001) was observed for plasma NEFA concentrations (Fig. 1A). Significantly higher NEFA values were observed in L ewes compared with C ewes on day –1 (P < 0.0001). No effect of treatment was observed on plasma leptin concentrations; the effect of day tended to be significant (P = 0.07). L ewes presented a significant reduction (P < 0.05) of mean NEFA values on day 7 when compared with days –14 and –1 (Fig. 1B).

Figure 1 Mean (± pooled standard error) plasma non-esterified fatty acid (NEFA) (A), and leptin (B) concentrations of Rasa Aragonesa ewes superovulated with 210 IU of Follitropin (pFSH) and 500 IU Folligon (eCG) in a single i.m. administration 48 h before the intravaginal sponge was removed (day –1) and fed diets that provided either 1.5 (control group (C); n = 20) or 0.5 (low group (L); n = 25) times the daily requirements for maintenance from sponge insertion (day –14) to embryo recovery (day 7). a,bDifferent superscripts among days and groups differ at least P < 0.05.
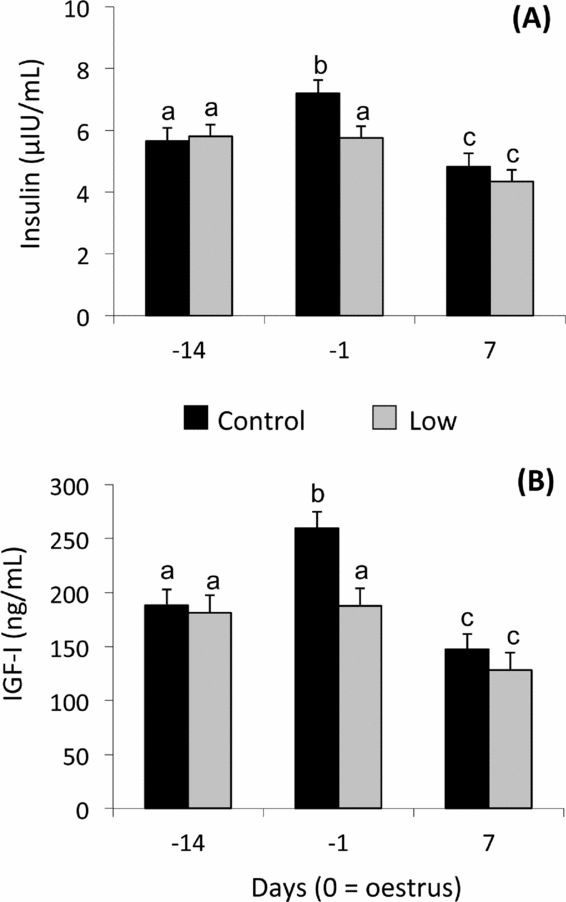
Plasma insulin levels were affected by dietary treatment (P < 0.01) and day (P < 0.0001). Higher insulin concentration was detected in the C than the L group on day –1 (P < 0.001) (Fig. 2A). Insulin concentrations increased from day –14 to day –1 in C ewes, but not in L ewes. Insulin concentrations in both groups decreased at day 7 (Fig. 2A).
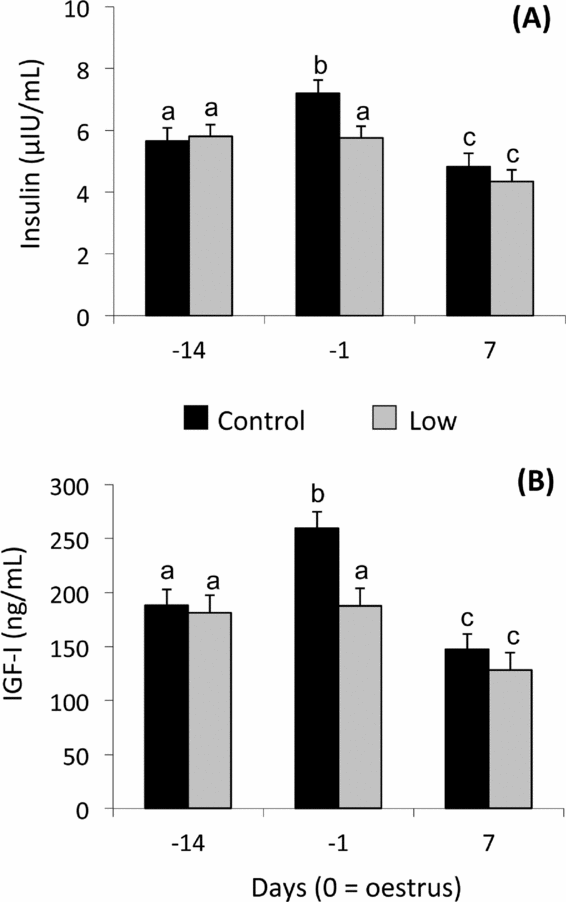
Figure 2 Mean (± pooled standard error) plasma insulin (A), and insulin-like growth factor (IGF)-I (B) concentrations of Rasa Aragonesa ewes superovulated with 210 IU of Follitropin (pFSH) and 500 IU Folligon (eCG) in a single i.m. administration 48 h before the intravaginal sponge was removed (day –1) and fed diets that provided either 1.5 (control group (C); n = 20) or 0.5 (low group (L); n = 25) times the daily requirements for maintenance from sponge insertion (day –14) to embryo recovery (day 7). a,bDifferent superscripts among days and groups differ at least P < 0.05.
For plasma IGF-1, a tendency of the dietary treatment (P = 0.06) and interaction (P = 0.06) and a significant effect of day (P < 0.0001) were observed for plasma IGF-I concentrations (Fig. 2B). IGF-I profiles resembled insulin profiles in both groups: at day –1 C ewes had greater concentrations (P< 0.001) in comparison with L ewes. IGF-I concentrations increased from day –14 to day –1 in C ewes, but not in L ewes. IGF-I concentrations in both groups decreased at day 7 (Fig. 2B).
Discussion
A diet that provided half of the energetic requirements for maintenance in sheep has produced a significant decrease of embryo performances of superovulated donor ewes, undernourished ewes resulting in half of the number of viable-transferable embryos and a rate of transferability 30% lower than control ewes. The measurements of reproductive performance of the control group are comparable with or higher than those obtained by our group in other breeds but using the same methodology (simplified superovulatory treatment and natural mating). Thus, 13.8 or 14.5 CL; 5.4 or 8.6 embryos per ewe; 4.5 or 6.6 viable embryos per ewe and 53 or 68% viability rates have been observed in Corriedale (Simonetti et al., Reference Simonetti, Forcada, Rivera, Carou, Alberio, Abecia and Palacin2008) or Ojalada (Forcada et al., Reference Forcada, Ait-Amer-Meziane, Abecia, Maurel, Cebrián-Pérez, Muiño-Blanco, Asenjo, Vázquez and Casao2011) breeds, respectively.
Almost 30% of ovulations were non-functional in both groups, so that a premature regression of CL was observed. This regression is assumed as a failed response to superovulation and has also been reported by our group both in Rasa Aragonesa and Ojalada breeds (Forcada et al., Reference Forcada, Abecia, Lozano and Zúñiga2000, Reference Forcada, Ait-Amer-Meziane, Abecia, Maurel, Cebrián-Pérez, Muiño-Blanco, Asenjo, Vázquez and Casao2011). This regression after superovulatory treatments seems to be related to a lack of progesterone priming before treatment (Ryan et al., Reference Ryan, Hunton and Maxwell1991) and has not been affected by level of food intake.
As expected, in our study the lower reproductive performances reached by the undernourished group were accompanied by a significant reduction of both LW and BC. This finding is similar to that reported by Borowczyk et al. (Reference Borowczyk, Caton, Redmer, Bilski, Weigl, Vonnahme, Borowicz, Kirsch, Kraft, Reynolds and Grazul-Bilska2006), who observed that underfeeding resulted in lower LW and BC when compared with control ewes and oocytes derived from underfed ewes yielded fewer blastocysts and had lower rates of cleavage and blastocyst formation compared with oocytes derived from control ewes; it was suggested that BC could be used to predict successful embryonic development. Moreover, in the present study, the nutritional restriction increased NEFA and decreased leptin concentrations in the undernourished ewes, indicating an increase in the lipolytic activity. This result was similar to previous works by our group applying exactly the same experimental diets, 0.5 and 1.5 times the maintenance requirements (Sosa et al., Reference Sosa, Abecia, Forcada, Vinoles, Tasende, Valares, Palacin, Martin and Meikle2006, Reference Sosa, Abecia, Carriquiry, Forcada, Martin, Palacin and Meikle2009; Fernández-Foren et al., Reference Fernández-Foren, Abecia, Vázquez, Forcada, Sartore, Carriquiry, Meikle and Sosa2011). While the underlying mechanisms whereby nutrition affects reproduction are not fully elucidated, they are believed to involve nutritionally induced alterations in hormones and intermediary metabolites in the female body, which alter the biochemical properties of the ovarian follicle and reproductive tract where the oocyte and embryo, respectively, develop (Ashworth et al., Reference Ashworth, Toma and Hunter2009). Changes to the immediate environment surrounding the oocytes and embryos could alter the pattern of genes expressed by these structures, with consequences for both immediate and longer-term development. Peura et al. (Reference Peura, Kleemann, Rudiger, Nattrass, McLaughlan and Walker2003) observed that nutrition of the oocyte donor influences the outcome of nuclear transfer, given that significantly more pregnancies were established in the high nutrition group compared with the low nutrition group. In addition, significantly more transferred embryos implanted and developed to foetuses in the high group were observed. Pisani et al. (Reference Pisani, Antonini, Pocar, Ferrari, Brevini, Rhind and Gandolfi2008) reported that short-term food restriction of superovulated ewes alters the levels of a few specific transcripts in both oocyte and granulosa cells, indicating that genes involved in oocyte metabolic activity are affected, specifically and that this phenomenon is accompanied by the deregulation of the correct dynamic of gene expression in granulosa cells.
Before ovulation (day –1), ewes presented lesser concentrations of insulin and IGF-I, as observed by Sosa et al. (Reference Sosa, Abecia, Forcada, Vinoles, Tasende, Valares, Palacin, Martin and Meikle2006), although this was less pronounced in undernourished ewes. Perhaps experimental procedures such as detection of oestrus (male introduction, presence of humans) could evoke a reflex release of insulin and glucagon, as well as peripheral insulin resistance, leading to reduced glucose uptake (Elsasser et al., Reference Elsasser, Klasing, Filipov, Thompson, Moberg and Mench2000). The rise in IGF-I at oestrus has also been observed previously in sheep (Viñoles et al., Reference Viñoles, Martin, Forsberg, Cajarville and Meikle2004; Sosa et al., Reference Sosa, Abecia, Forcada, Vinoles, Tasende, Valares, Palacin, Martin and Meikle2006) and could be explained by the coincident rise in insulin because this hormone increases liver concentrations of IGF-I mRNA and plasma IGF-I concentrations in cows (Butler et al., Reference Butler, Marr, Pelton, Radcliff and Lucy2003; Rhoads et al., Reference Rhoads, Kim, Leury, Baumgard, Segoale, Frank, Bauman and Boisclair2004). As a consequence, this could have affected the quality of oocytes, as nutritional changes can affect the intra-follicular IGF system and oocyte quality (Webb et al., Reference Webb, Garnsworthy, Gong and Armstrong2004; Muñoz-Gutiérrez et al., Reference Muñoz-Gutiérrez, Findlay, Adam, Wax, Campbell, Kendall, Khalid, Forsberg and Scaramuzzi2005). Moreover, it has been shown that IGF-I in uterine fluid increases around oestrous (Geisert et al., Reference Geisert, Lee, Simmen, Zavy, Fliss, Bazer and Simmen1991) and IGF-I mRNA endometrial synthesis is maximal on days 0–5 of the estrous cycle (Meikle et al., Reference Meikle, Sahlin, Ferraris, Masironi, Blanc, Rodríguez-Irazoqui, Rodríguez-Piñon, Kindahl and Forsberg2001; Sosa et al., Reference Sosa, Carriquiry, Chalar, Crespi, Sanguinetti, Cavestany and Meikle2010a), which are believed to play a role in early embryonic development.
There is a lack of leptin expression in the granulosa (Muñoz-Gutiérrez et al., Reference Muñoz-Gutiérrez, Findlay, Adam, Wax, Campbell, Kendall, Khalid, Forsberg and Scaramuzzi2005; Pisani et al., Reference Pisani, Antonini, Pocar, Ferrari, Brevini, Rhind and Gandolfi2008) but the presence of its receptors in the oocytes and in granulosa cells (Kawamura et al., Reference Kawamura, Sato, Fukuda, Kodama, Kumagai, Tanikawa, Nakamura and Tanaka2002; Muñoz-Gutiérrez et al., Reference Muñoz-Gutiérrez, Findlay, Adam, Wax, Campbell, Kendall, Khalid, Forsberg and Scaramuzzi2005) supports the role of this cytokine in follicular physiology and oocyte quality. Thus, the low leptin levels observed in the post-ovulatory period (day 7) in restricted animals could have been detrimental for oocyte quality. Regarding the role of leptin on embryo development, in embryo culture media, leptin has promoted the development of mouse embryos from the 2-cell stage to blastocysts, fully expanded blastocysts and hatched blastocysts (Kawamura et al., Reference Kawamura, Sato, Fukuda, Kodama, Kumagai, Tanikawa, Nakamura and Tanaka2002). However, exposure of porcine or ovine oocytes to leptin during in vitro maturation and subsequent embryo culture after IVF resulted in the formation of fewer blastocysts relative to the controls (Swain et al., Reference Swain, Bormann and Krisher2001). It remains to be explained whether or not the lower leptin concentrations of undernourished ewes have mediated in embryo quality observed in this experiment.
On the other hand, O'Callaghan et al. (Reference O'Callaghan, Yaakub, Hyttel, Spicer and Boland2000) concluded that superovulation may profoundly affect the physiological environment within the follicle and that the oocyte morphology observed indicates that changes in the mRNA processing apparatus may occur at superovulation. Considering the differences between diets observed in the present experiment, it is likely that undernutrition exacerbated those changes provoked by superovulation, leading to lower embryo performances. It is known that the ovulatory response to commercial FSH preparations is related to the number of small antral follicles present in the ovaries and that the number of transferable embryos is negatively affected by the presence of large follicles at the start of the superovulatory treatment (Cognié et al., Reference Cognié, Baril, Poulin and Mermillod2003). As undernutrition has been related to the presence of large follicles in the static growing phase that, despite reaching ovulation, persists during the induced follicular phase (Sosa et al., Reference Sosa, Gonzalez-Bulnes, Abecia, Forcada and Meikle2010b), we hypothesize that this can be one of the mechanisms involved in the poorer response of undernourished ewes to superovulation in terms of transferability rates.
An interesting result of this experiment is the significantly lower ovulation rate presented by C ewes, in accordance with Lozano et al. (Reference Lozano, Lonergan, Boland and O'Callaghan2003), who reported that ewes offered a diet ad libitum had a lower ovulation rate than ewes offered the low or control diets. Staigmiller et al. (Reference Staigmiller, Short, Bellows and Carr1979) suggested that undernutrition produced changes of the metabolic clearance rate (MCR) of FSH in cows. Thus, an alternative possibility is that the higher ovulation rate of L ewes results from a change in their rate of hormonal MCR. However, Findlay & Cumming (Reference Findlay and Cumming1976) did not find any differences in the MCR of FSH between ewes on different planes of nutrition or between ewes with one and two ovulations and no significant differences were found in concentrations of FSH due to nutritional treatment. Similarly, FSH concentration has not been correlated with ovulation rate and although a relationship between ovulation rate and LW has been previously confirmed (Edey, Reference Edey1968; Findlay & Cumming, Reference Findlay and Cumming1976), this has not been observed in the present experiment. Thus, this opens a new discussion about the calculation of the doses of FSH and eCG under simplified superovulation protocols related to breed and/or body weight. The higher ovulation rate in the L group could also affect embryo quality, as it has been reported that fertilization failure and/or degeneration of embryos in donors prior to flushing accounts for lower recoveries of viable embryos from sheep, the incidence of both being greater in donors with higher ovulation rates (Armstrong & Evans, Reference Armstrong and Evans1983). High ovulation rate of donors also decreases percentage survival of sheep embryos after transfer; thus, the higher ovulation rate presented by L ewes, has indirectly and negatively affected embryo quality.
In spite of the worse embryo quality in the L group, plasma progesterone concentration was higher than in the C group which is in agreement with that reported by O'Callaghan et al. (Reference O'Callaghan, Yaakub, Hyttel, Spicer and Boland2000) and Lozano et al. (Reference Lozano, Lonergan, Boland and O'Callaghan2003), who observed significantly higher mean progesterone concentrations in superovulated underfed ewes when compared with ewes fed 2.0 M or ad libitum. It is well known that dietary intake is inversely related to progesterone concentrations in circulation. The blood flow rate in the portal vein has been related to dietary intake, suggesting that progesterone metabolization increased with high levels of food intake (Parr et al., Reference Parr, Davis, Miles and Squires1993). Many studies have shown that progesterone concentrations in the peripheral circulation and the rate of embryo loss or pregnancy failure are not positively related; indeed, low levels of food intake, which are associated with increased rates of embryo loss, have been shown to be associated with higher progesterone concentrations (Parr et al., Reference Parr, Davis, Fairclough and Miles1987; Rhind et al., Reference Rhind, McKelvey, McMillen, Gunn and Elston1989).
In conclusion, superovulated donor ewes fed half of their maintenance requirements during the period of superovulation and early embryonic development resulted in an increased number of ovulations, but reduced total and viable number of embryos. These effects might be mediated by disruption of endocrine homeostasis, oviduct environment and/or oocyte quality. Thus, nutrition of ewes in MOET or cloning programs should be carefully considered. The higher ovulation rate observed in the L group, which could be detrimental for ova fertilization, can be likely produced by a FSH dose-dependent mechanism and opens a new discussion about the calculation of the doses of hormones under this simplified superovulation protocol in terms of breed-body size.
Acknowledgements
This study was supported financially by grant AGL2010-15004/GAN from MICINN (Spain).