Introduction
Gastrointestinal (GI) parasites can give rise to major sub-clinical impacts such as decreased body condition, growth rate, production level and reproduction success, and sometimes to disease or death of their host, in numerous domestic and wild species of vertebrates (Gulland, Reference Gulland1992; Albon et al., Reference Albon, Stien, Irvine, Langvatn, Ropstad and Halvorsen2002; Tompkins et al., Reference Tompkins, Dobson, Arneberg, Begon, Cattadori, Greenman, Heesterbeek, Hudson, Newborn, Pugliese, Hudson, Rizzoli, Grenfell, Heesterbeek and Dobson2002; Craig et al., Reference Craig, Pilkington and Pemberton2006 Taylor et al., Reference Taylor, Coop and Wall2015). They might thus have consequences on host fitness and population dynamics and are major health and economic issues worldwide (Roeber et al., Reference Roeber, Jex and Gasser2013; Karrow et al., Reference Karrow, Goliboski, Stonos, Schenkel and Peregrine2014). The level of parasite infestation in a host population is often heterogeneous, with most of the hosts having weak parasite burdens, while a small proportion of them harbour the majority of parasites (Woolhouse et al., Reference Woolhouse, Dye, Etard, Smith, Charlwood, Garnett, Hagan, Hii, Ndhlovu and Quinnell1997; Wilson et al., Reference Wilson, Bjørnstad, Dobson, Merler, Poglayen, Randolph, Read and Skorping2002). The most parasitized and susceptible hosts strongly contribute to pathogen transmission, especially for digestive parasites emitted into the environment via faeces, because of their high infectiousness (i.e. magnitude of pathogen shedding); one of the three components of the transmission capacity (VanderWaal and Ezenwa, Reference VanderWaal and Ezenwa2016). Individual levels of infestations have been shown to depend on interactions among numerous individual, environmental and parasitical characteristics. Individual characteristics may, for instance, include gender, age, reproductive and social status, genetics or contact rate with others (Body et al., Reference Body, Ferté, Gaillard, Delorme, Klein and Gilot-Fromont2011; Hayward, Reference Hayward2013; Davidson et al., Reference Davidson, Ličina, Gorini and Milner2015; Habig and Archie, Reference Habig and Archie2015; Debeffe et al., Reference Debeffe, Mcloughlin, Medill, Stewart, Andres, Shury, Wagner, Jenkins, Gilleard and Poissant2016; Aleuy et al., Reference Aleuy, Ruckstuhl, Hoberg, Veitch, Simmons and Kutz2018; Portanier et al., Reference Portanier, Garel, Devillard, Maillard, Poissant, Galan, Benabed, Poirel, Duhayer, Itty and Bourgoin2019). Similarly, environmental features, such as resources availability and quality, density of hosts and temperature/humidity (Mbora and McPeek, Reference Mbora and McPeek2009; Body et al., Reference Body, Ferté, Gaillard, Delorme, Klein and Gilot-Fromont2011; Knutie et al., Reference Knutie, Wilkinson, Wu, Ortega and Rohr2017), and parasitical characteristics, such as means of transmission, spatial distribution, co-infections and genetic/virulence (Anderson and Gordon, Reference Anderson and Gordon1982; Telfer et al., Reference Telfer, Birtles, Bennett, Lambin, Paterson and Begon2008; Poulin, Reference Poulin2013), may influence individual levels of infestations. Understanding the individual heterogeneity of infestation and the consequences of parasites on host's health and ability of transmission is thus complex. It is nevertheless of primary importance for pathogen control strategies and long-term management of populations, due to the major negative consequences of parasites on individuals and population dynamics (Smith et al., Reference Smith, Acevedo-Whitehouse and Pedersen2009; Paull et al., Reference Paull, Song, McClure, Sackett, Kilpatrick and Johnson2012; Gervasi et al., Reference Gervasi, Civitello, Kilvitis and Martin2015; VanderWaal and Ezenwa, Reference VanderWaal and Ezenwa2016).
Several mechanisms have been suggested to explain the links between individual characteristics and parasite infestation level. First, because of physiological and behavioural variations occurring between individuals of different ages, age is recognized as one of the main driver of parasite infestation level. Higher levels of infestation are actually often reported in young and old individuals (Santín-Durán et al., Reference Santín-Durán, Alunda, Hoberg and de la Fuente2008; Hayward et al., Reference Hayward, Wilson, Pilkington, Pemberton and Kruuk2009; Body et al., Reference Body, Ferté, Gaillard, Delorme, Klein and Gilot-Fromont2011). One explanation is that age represents a proxy of hosts immune capacities. For instance, in ungulates, the exposure of young immunologically naïve animals to gastrointestinal strongyles (GIS) leads to the progressive mounting of an immune response limiting parasites development and reproduction, in addition to the innate immune response (McRae et al., Reference McRae, Stear, Good and Keane2015). In senescent individuals, these abilities to control parasitism may degrade with the decrease of immune functions, leading to an increase in parasite burdens (Nussey et al., Reference Nussey, Watt, Pilkington, Zamoyska and McNeilly2012; Cheynel et al., Reference Cheynel, Lemaître, Gaillard, Rey, Bourgoin, Ferté, Jégo, Débias, Pellerin, Jacob and Gilot-Fromont2017). The decrease of body condition in senescent individuals might also participate in increasing parasite burden (Mysterud et al., Reference Mysterud, Yoccoz, Stenseth and Langvatn2001; Weladji et al., Reference Weladji, Holand, Gaillard, Yoccoz, Mysterud, Nieminen and Stenseth2010; Nussey et al., Reference Nussey, Coulson, Delorme, Clutton-Brock, Pemberton, Festa-Bianchet and Gaillard2011). Body condition is indeed another important factor determining hosts susceptibility to infection (Beldomenico and Begon, Reference Beldomenico and Begon2010). Several studies thus highlighted higher parasite prevalence and infection intensity in individuals having a poor body condition compared to those of the same age class having a good body condition (e.g. Body et al., Reference Body, Ferté, Gaillard, Delorme, Klein and Gilot-Fromont2011; Davidson et al., Reference Davidson, Ličina, Gorini and Milner2015; Aleuy et al., Reference Aleuy, Ruckstuhl, Hoberg, Veitch, Simmons and Kutz2018). In line with this observation, populations facing poor environmental conditions may suffer from parasitism, and sometimes experience die-off (e.g. Soay sheep Ovis aries; Gulland, Reference Gulland1992; Craig et al., Reference Craig, Pilkington and Pemberton2006). Finally, the sex of individuals might also induce heterogeneity in parasite infestation levels. Sex-biased parasitism has been repeatedly observed in mammal species, and males often harbour higher parasite burden (Poulin, Reference Poulin1996; Klein, Reference Klein2004). This difference between sexes is thought to arise from not only different immune responses, resulting from direct (i.e. genes located on X or Y chromosomes) and indirect (e.g. sexual hormones; Folstad and Karter, Reference Folstad and Karter1992) effects of sex chromosomes, but also from different exposure probabilities to pathogens (Klein, Reference Klein2000; Markle and Fish, Reference Markle and Fish2014). The susceptibility of animals to pathogen infections may also greatly increase during reproductive periods as a consequence of various factors such as the increase of immunosuppressive hormones, stress and a negative energetic balance (Connan, Reference Connan1976; Hayward, Reference Hayward2013). Reproductive females thus commonly shed more parasite eggs (especially strongyles eggs) in their faeces during the periparturient period, in both domestic (Dunsmore, Reference Dunsmore1965) and wild/free-living species (Festa-Bianchet, Reference Festa-Bianchet1989; Wilson et al., Reference Wilson, Grenfell, Pilkington, Boyd, Gulland, Clutton-Brock and Pemberton2004; Leivesley et al., Reference Leivesley, Bussière, Pemberton, Pilkington, Wilson and Hayward2019), compared to non-reproductive females.
In free-living populations, quantifying parasites abundance and infectiousness of parasites at the individual level, and determining the relative importance of individual-related factors on parasite infestation is challenging because accessing to individual measurements from animals of known age is difficult. We thus aimed here at providing a new empirical contribution to this topic by focusing on a free-ranging population of Mediterranean mouflon from southern France with a unique long-term monitoring by capture-mark-recapture (>10 years) of a large number (n = 433) of known-age individuals. Despite the worldwide distribution of the Mediterranean mouflon (Ovis gmelini musimon × Ovis sp., Cassinello, Reference Cassinello2018; Weller, Reference Weller, Nahlik and Uloth2001) and its patrimonial (Chessa et al., Reference Chessa, Pereira, Arnaud, Amorim, Goyache, Mainland, Kao, Pemberton, Beraldi, Stear, Alberti, Pittau, Iannuzzi, Banabazi, Kazwala, Zhang, Arranz, Ali, Wang, Uzun, Dione, Olsaker, Holm, Saarma, Ahmad, Marzanov, Eythorsdottir, Holland, Ajmone-Marsan, Bruford, Kantanen, Spencer and Palmarini2009) and economical values (through hunting activities; Cugnasse, Reference Cugnasse1995), knowledge on the health status and eco-epidemiology of parasitism in this species is sparse and often focus on a low number of individuals, sometimes captive (e.g. Meana et al., Reference Meana, Luzón-Peña, Santiago-Moreno, De Bulnes and Gómez-Bautista1996; Magi et al., Reference Magi, Bertani, Dell'Omodarme, Prati and Poglayen2005; Balicka-Ramisz et al., Reference Balicka-Ramisz, Laurans, Jurczyk, Kwita and Ramisz2017; but see, e.g. Hille, Reference Hille2003; Portanier et al., Reference Portanier, Garel, Devillard, Maillard, Poissant, Galan, Benabed, Poirel, Duhayer, Itty and Bourgoin2019). In addition, continental populations of Mediterranean mouflon originate from the threatened and protected Corsican and Sardinian mouflon (Ovis gmelini musimon; e.g. European Habitat Directive Annexes II and IV), in which parasitism has been poorly studied (but see e.g. Deméautis, Reference Deméautis1981, Reference Deméautis1985; Poglayen et al., Reference Poglayen, Urbani, Modugno, Scala, Giannetto and Rossi2018). Our study thus also aimed at gathering knowledge about host–parasites relationship that could serve conservation of natural protected mouflon populations present on Mediterranean islands.
Using faecal samples, we first aimed to describe the diversity in GI parasites. Since they were the most abundant, investigations of the links between individual factors and parasite abundance were performed focusing on strongyles (GIS) and Eimeria spp. These parasites classes are also the most common classes of GI parasites in ruminants (Samuel et al., Reference Samuel, Pybus and Kocan2001; Taylor et al., Reference Taylor, Coop and Wall2015) and their abundance in faeces is known to be a good proxy of infectiousness and correlated with actual abundance or clinical expression in sheep (Cabaret et al., Reference Cabaret, Gasnier and Jacquiet1998; Chartier and Paraud, Reference Chartier and Paraud2012). We first expected a higher parasitism in young individuals as compared to mature individuals, as a consequence of their naïve immune status (McRae et al., Reference McRae, Stear, Good and Keane2015), and in males than in females (Poulin, Reference Poulin1996; Klein, Reference Klein2004), as a consequence of sex chromosomes and behaviour effects, and as previously observed in feral sheep (Soay sheep, Wilson et al., Reference Wilson, Grenfell, Pilkington, Boyd, Gulland, Clutton-Brock and Pemberton2004; Craig et al., Reference Craig, Pilkington and Pemberton2006). Second, in this population experiencing marked density-dependent effects on individual body mass (Garel et al., Reference Garel, Cugnasse, Maillard, Gaillard, Hewison and Dubray2007), we expected body condition to be related to parasite resistance and thus infestation level (Beldomenico et al., Reference Beldomenico, Telfer, Gebert, Lukomski, Bennett and Begon2008). More specifically, we expected to observe a higher parasitism in individuals with poor body condition as compared to individuals in good body condition (e.g. Body et al., Reference Body, Ferté, Gaillard, Delorme, Klein and Gilot-Fromont2011; Aleuy et al., Reference Aleuy, Ruckstuhl, Hoberg, Veitch, Simmons and Kutz2018). Similarly, due to the high energetic investment and the immunodepression occurring during the end of gestation – early lactation period (Lloyd, Reference Lloyd1983; Taylor et al., Reference Taylor, Coop and Wall2015; Carrau et al., Reference Carrau, Perez, Silva, Macías, Martínez-Carrasco, Taubert, Hermosilla and de Ybáñez2016), we expected higher parasite burden in reproductive females as compared to non-reproductive females and males.
Material and methods
Study area and species
The studied Mediterranean mouflon population inhabited the Caroux-Espinouse massif (43°38′N, 2°58′E, 17 000 ha, 150–1124 m a.s.l.), in southern France. This middle mountain area is composed of high plateaus separated by deep valleys. Summer are hot and dry [mean ± s.d. daily temperature = 16.4 ± 3.6°C and mean ± s.d. cumulative precipitation = 178.4 ± 68.5 mm in June–August 2010–2017; Meteo France weather station of Fraïsse-Murat (1041 m a.s.l., 13 km from the study area)], autumns wet (mean ± s.d. cumulative precipitation = 493.2 ± 161.8 mm in September–November 2010–2017) and winter fairly cold (mean ± s.d. daily temperature = 2.2 ± 4.1°C in December–February 2010–2018), with few days of snow fall. The vegetation is mostly composed of coniferous (Pinus sylvestris, P. nigra, Picea abies) and deciduous (beech Fagus sylvatica, chestnut trees Castanea sativa and evergreen oak Quercus ilex) forests, grass-rich areas [pastures, meadows and artificial cultures devoted to wildlife (Brachypodium sylvaticum, Festuca rubra, F. paniculata, F. ovina, Carex sp.); Baudière, Reference Baudière1970] and moorlands (heather Erica cinerea, Calluna vulgaris, blueberry Vaccinium myrtillus, broom Cytisus oromediterraneus, C. scoparius and fern Pteridium aquilinum), while rocky areas are mostly present on slopes (see Marchand et al., Reference Marchand, Garel, Bourgoin, Dubray, Maillard and Loison2015 for a detailed description).
Mediterranean mouflon is a gregarious species with a strong segregation between sexes occurring most of the year (Bourgoin et al., Reference Bourgoin, Marchand, Hewison, Ruckstuhl and Garel2018). Adult males and females have a body mass dimorphism of 40.1% [mean body mass in spring-summer (2002–2019 period): 24.5 ± 4.1 kg (⩾2 years old; n = 683) and 34.4 ± 5.5 kg (⩾4 years old; n = 222) for females and adult males, respectively]. Rut occurs from mid-October to December with the highest rut activity from November to mid-December (Bon et al., Reference Bon, Dardaillon and Estevez1993). Females ⩾2 years old give birth most of the time to one lamb (twinning rate <3%; Garel et al., Reference Garel, Cugnasse, Gaillard, Loison, Gibert, Douvre and Dubray2005) after 5 months of gestation, from late March to late May, with a peak of birth in April (Bon et al., Reference Bon, Dardaillon and Estevez1993).
Sample and data collection
Mouflon were baited with salt and captured during spring-summer (end of April to mid-July) from 2010 to 2019. For each individual, sex, live body weight and metatarsus length were recorded, and faecal samples were collected in the rectum for parasitological analyses. Only individuals of known age were included in the analyses, i.e. all males for which exact age can be determined by counting the horn growth annuli (Geist, Reference Geist1966) and females first captured at ⩽3 years old for which exact age can be estimated by counting the number of permanent incisors (Rieck, Reference Rieck1975).
Among sexually mature females (i.e. ⩾2 years old; Garel et al., Reference Garel, Cugnasse, Gaillard, Loison, Gibert, Douvre and Dubray2005), females were considered as reproductively active when observed pregnant, lactating or with a lamb at heel during capture, and/or when observed in the field at least twice with a lamb at heel from April to July. On the opposite, non-lactating females when captured, and without a lamb at heel when observed in the field from April to July, were considered as non-reproductively active females. An undetermined reproductive status was attributed to females for which the reproductive status was uncertain during capture and field observations, and to non-lactating females during capture but seen only once with a lamb at heel in the field.
Parasitological data
Faecal samples were stored at +4°C after sampling and sent to the parasitology laboratory of VetAgro Sup (Lyon, France) for analysis. A first macroscopical examination was done to detect potential presence of mature proglottids of Moniezia spp. We then performed parasite isolation and count using a modified McMaster protocol proposed by Raynaud et al. (Reference Raynaud, William and Brunault1970) with a solution of zinc sulphate (ZnSO4, density = 1.36). For each sample, we mixed 1–5 g of faeces in 14–70 mL of zinc sulphate (1/15 dilution). We then homogenized the solution and sampled 1 mL that was loaded on a McMaster slide with two chambers. After waiting a few minutes to allow eggs and oocysts to float at the surface of each chamber, we counted the number of parasite propagules inside the grid of each chamber (volume under grid of 0.15 mL; quantitative examination). We also filled a 14 mL tube with the remaining solution until we had a meniscus, and then placed a coverslip on the tube. After centrifugation (5 min at 1200 rpm), we recovered the coverslip and placed it on a microscope slide before to seek with a microscope (×40–400) for the presence of parasite propagules (‘control slide’; qualitative examination). The sensitivity of the McMaster is theoretically of 50 eggs/oocysts per gram (epg/opg) of faecal matter. We attributed the value of 25 epg/opg for parasites with no egg/oocyst observed on the McMaster slide, but at least one egg/oocyst observed on the control slide. Faecal count was performed for Eimeria spp. (Protozoa – Coccidia), GI strongyles (GIS; Nematoda), Nematodirus spp. (GI Nematoda; counted since 2014), Trichuris spp. (Nematoda), Moniezia spp. (Cestoda), Fasciola hepatica and Dicrocoelium dendriticum (Trematoda). For Giardia spp. (Protozoa; counted since 2011), we recorded their absence or presence (qualitative measure).
Statistical analyses
Faecal eggs counts (FEC) and faecal oocysts counts (FOC) tend to decrease as the time elapsed between faeces collection (i.e. sampling date) and coproscopic analyses increases (Drimtzia and Papadopoulos, Reference Drimtzia and Papadopoulos2016; Portanier et al., Reference Portanier, Garel, Devillard, Maillard, Poissant, Galan, Benabed, Poirel, Duhayer, Itty and Bourgoin2019), but the date in which coproscopic analyses were performed was nevertheless not available for all the samples. During the study period, the number of persons available to analyse the faecal samples varied. While between 2010 and 2015 only one person processed the samples, two persons did it between 2016 and 2019, allowing to significantly reduce the delay between the reception of samples and the coproscopic analyses. The initial dataset (n = 736; Table 1) was therefore divided in these two separate periods. For each period, we extrapolated the missing date of coproscopic analysis (n = 144/582 and n = 27/154 for periods 1 and 2, respectively) using the mean delay between the reception and analysis observed for samples for which both dates were available.
Table 1. Description of the data selected for analyses. Values correspond to the number of faecal samples analysed by age/sex category.

a Data selected for analyses included ⩾1-year-old individuals (see Materials and Methods), with known age, sex, body mass and metatarsus length.
b Data selected for testing the influence of the reproductive status of females included only ⩾2-year-old females with known reproductive status.
In subsequent analyses, only individuals from 1 year of age onwards with known sex, age, body mass and metatarsus length were considered (Table 1). Indeed, newborn lambs are mostly suckling milk and are therefore poorly exposed to free-living infective stages of parasites on the soil. In addtion, prepatent period of parasites may last several weeks (2–3 weeks for most GIS; Taylor et al., Reference Taylor, Coop and Wall2015). These two components have been found to generate high differences in fecal parasite counts between animals of few weeks or few months of age (e.g. Wilson et al., Reference Wilson, Grenfell, Pilkington, Boyd, Gulland, Clutton-Brock and Pemberton2004; Ozdal et al., Reference Ozdal, Tanritanir, Goz, Deger and Kozat2009). These marked differences cannot be controlled for without an accurate age estimate for first year animals (e.g. number of weeks since birth). However, this information was lacking here (we had no criteria to estimate age of lambs when captured) and we thus chose to remove lambs from our analysis. Very old individuals aged 10 years and older (14 females, 9 males) were grouped in a single class ‘10+’.
In order to assess the influence of age, body condition and sex on the parasitism of mouflon, we used linear mixed models. Indeed, some individuals (see Results) were sampled more than once during the study period which encompassed several years. The individual and yearly observations were thus expected to be correlated. We therefore used mixed models specifically designed to deal with such non-independent data, and explicitly took into account repeated measures by considering the individual identity and year of sampling as random effects. Alternative approach consisting of sampling randomly one observation for each individual would have led to strongly reduce the size of the dataset (by 24%) and, concomitantly, statistical power to detect some effects. Since they were the two most prevalent parasites in this population (see Results and Table 2) and may affect the health and the fitness of their host (Gulland, Reference Gulland1992; Taylor et al., Reference Taylor, Coop and Wall2015), our analyses focused on the FOC of Eimeria spp. and the FEC of GIS. In all analyses, FOC and FEC were log-transformed [log(FOC/FEC + 25)] to fit with a normal distribution and for stabilizing variance. Using the repeated measurements made on some individuals, we computed the intra-class correlation coefficients (Burdick et al., Reference Burdick, Quiroz and Iyer2006) to assess the repeatability of FOC/FEC, considering individual identity as a random effect (but without controlling for sampling design and individual factors – see below).
Table 2. Apparent prevalence and intensity values of propagules from the different parasite species observed on coproscopical examination of faeces from Mediterranean mouflon in the Caroux-Espinouse, France, in 2010–2019.

a 2014–2019 period.
b Only observed on control slide (i.e. <50 epg, noted 25).
c Abundance is not interpretable in these species, due to their intermittent elimination of proglottids containing the eggs.
d Not measured in 2010.
We tested effects of individual characteristics on mouflon parasitism while controlling for sampling design by using a two-step approach. In the first step, we tested for factors related to how faecal samples are sampled and that may potentially influence parasites burden measurements. According to the seasonality of parasitism (Wilson et al., Reference Wilson, Grenfell, Pilkington, Boyd, Gulland, Clutton-Brock and Pemberton2004; Magi et al., Reference Magi, Bertani, Dell'Omodarme, Prati and Poglayen2005; Balicka-Ramisz et al., Reference Balicka-Ramisz, Laurans, Jurczyk, Kwita and Ramisz2017), we expected to observe an influence of the sampling date on FEC and FOC and thus included the day of year of sampling in models (sampling date). We also tested for an influence of the time elapsed between faeces collection and coproscopic analyses (delay) (Drimtzia and Papadopoulos, Reference Drimtzia and Papadopoulos2016; Portanier et al., Reference Portanier, Garel, Devillard, Maillard, Poissant, Galan, Benabed, Poirel, Duhayer, Itty and Bourgoin2019). Only samples with a delay ⩽30 days were considered in the analyses. For each variable (delay and sampling date), we tested for linear and quadratic relationships. Models were compared with the Akaike's Information Criterion corrected for small sample size (AICc; Burnham and Anderson, Reference Burnham and Anderson2002). All the sampling variables included in the models within ΔAICc < 2 as compared to the best model (i.e. with the lowest AICc) were kept as correcting factors in subsequent analyses.
In the second step, we tested for the influence of the individual variables [i.e. sex, body condition (linear) and age (linear and quadratic)] on mouflon parasitism. In this set of models, body condition of individuals was characterized by computing the scaled mass index (SMI; Peig and Green, Reference Peig and Green2009, Reference Peig and Green2010), using values of body mass and metatarsus length. The SMI aims to standardize the body mass values to a same body size, considering allometric relationship. We computed the SMI separately for each sex due to marked sexual dimorphism in this species (Garel et al., Reference Garel, Cugnasse, Maillard, Gaillard, Hewison and Dubray2007). Since the high investment in reproduction of females ⩾2 years old should influence the FOC/FEC (see below), two-way interactions between age and sex were included. Model selection was performed by comparing all the models including the different combination of variables. We retained the model with the lowest AICc value, and when two or more competing models had a ΔAICc < 2, we retained the simplest model according to the parsimony rule (Burnham and Anderson, Reference Burnham and Anderson2002). For each model set (controlling for sampling design and testing for individual characteristics), we also computed AICc weights to measure the relative likelihood of each model to be the best given the data and the set of candidate models (Burnham and Anderson, Reference Burnham and Anderson2002). We assessed the goodness-of-fit of the selected models by computing the conditional (R 2c; total variance explained by the model) and the marginal (R 2m; variance explained only by the fixed effects) R 2 and by plotting residuals.
Finally, since reproductively active females are expected to shed more parasite propagules in their faeces during the periparturient period (‘periparturient rise’; Dunsmore, Reference Dunsmore1965; Festa-Bianchet, Reference Festa-Bianchet1989; Wilson et al., Reference Wilson, Grenfell, Pilkington, Boyd, Gulland, Clutton-Brock and Pemberton2004), we performed a likelihood ratio test (LRT; χ 2 test) to test for the influence of the reproductive status of females (reproductive vs non-reproductive, see above) on FOC and FEC using the previously selected models for FOC and FEC on a data subset of sexually mature females (i.e. ⩾2 years old; Table 1).
At each step of the analyses, all continuous predictors were centred and scaled (mean = 0, s.d. = 1) so that their regression coefficient can be directly compared to assess the relative magnitude of their effect. All analyses and model plots were performed using libraries lme4 (Bates et al., Reference Bates, Mächler, Bolker and Walker2015), lmerTest (Kuznetsova et al., Reference Kuznetsova, Brockhoff and Christensen2017), MuMIn (Barton and Barton, Reference Barton and Barton2019) and visreg (Breheny and Burchett, Reference Breheny and Burchett2017) in R 4.0.0 (R Core Team, 2020).
Results
From 736 analysed faecal samples, 231 and 202 coproscopic analyses performed on 160 and 169 females and males, respectively, met the data quality and availability requirements (e.g. known age, ⩾1 year old, body mass, metatarsus length, delay between field sampling and lab analysis ⩽30 days, see Materials and Methods) and were thus considered in subsequent analyses (Table 1). Most of the females were captured once (n = 109), 37 were captured twice, 9 three times, 4 four times and 1 five times. Less males were trapped several times with 137 males captured only once, 31 twice and 1 three times. The median day of sampling was the day 139 of the year corresponding to the 19 May (25 April; 3 July)95%CI. The median delay between sampling and coproscopic analyses was 14 days (4; 27)95%CI.
The vast majority of mouflon were parasitized by Eimeria spp. and GIS [prevalence = 99.8% (98.7%; 100%)95%CI and 94.7% (92.1%; 96.6%)95%CI, respectively], with median intensity (i.e. number of parasites in infested hosts) of 1150 opg [(50; 11 056)95%CI] and 200 epg [(25; 2477)95%CI], respectively. Other parasites were far less often observed (prevalence = 0.5–15.8%) with low intensity (median = 25–75 epg; Table 2). The mean (±s.e.) repeatability was 0.34 ± 0.09 and 0.26 ± 0.09 for log-transformed FOC and FEC, respectively.
Faecal abundance of Eimeria spp.
The baseline model for sampling factors included the sampling date and its quadratic term as well as the delay between sampling and coproscopic analyses (Table S1). Both delay and the sampling date had a negative influence on the FOC, with the sharpest decrease during the first half of the sampling period (end of April – early May; Fig. S1).
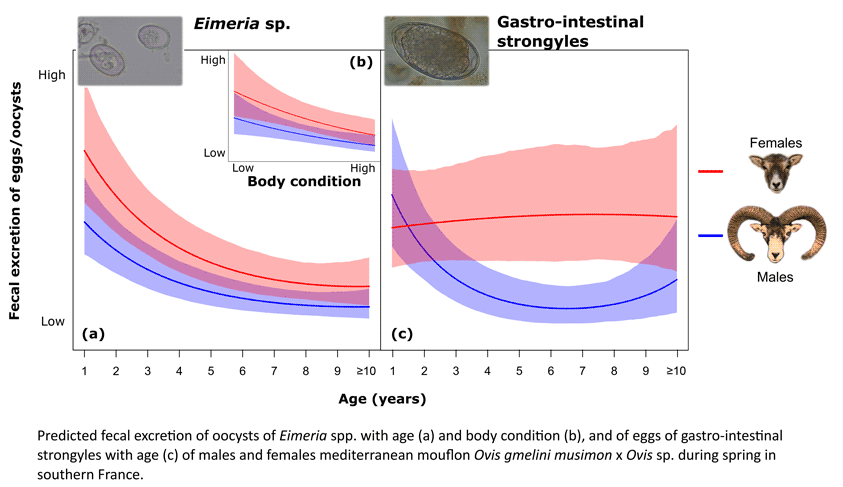
The final selected model (the most parsimonious model among the ones with ΔAICc < 2) included, in addition to the previously selected sampling factors, the quadratic influence of age, the sex and the SMI, but no interaction terms (Table 3). The FOC was the highest in young mouflon and decreased with age, especially during the first years of life (Table 4; Fig. 1a). FOC was 1.6 times higher in females than in males, at all ages (Fig. 1a), and between 2.3–2.4 (males) and 2.5–2.6 (females) times higher in individuals in poor (lowest SMI) than in good (highest SMI) body condition (Table 4; Fig. 1b).

Fig. 1. Predicted faecal oocysts counts (FOC) for Eimeria spp. as a function of (a) the age and (b) the scaled mass index (SMI) of males and females mouflon based on the selected model (Table S1 and Table 3. Predictions were computed for null values of scaled delay (scaled number of days) between sampling and coproscopic analyses, and scaled sampling date, and (a) for null value of SMI, or (b) for 4-year-old mouflon. Lines represent predicted values and bands represent the 95% confidence interval. Points represent average partial residuals per (a) age and (b) SMI (values were grouped at 10% quantile intervals), with the size of the dots being proportional to the number of observations. Numbers on the top of the graph are the total number of females and males used to compute average partial residuals.
Table 3. Model selection of mixed-effects models based on corrected Akaike's Information Criterion (AICc) for testing the effects of the individual variables age, sex and scaled-mass index (SMI) on the faecal oocyst/egg count of Eimeria spp. and gastro-intestinal strongyles (FOC and FEC, respectively) in Mediterranean mouflon from the Caroux-Espinouse, France, in 2010–2019.

d.f. are the degree of freedom, weight are the Akaike weights. All models included the individual identity and the year of sampling as random effects, and the factors related to sampling design (EXT) previously selected (sampling date + sampling date2 + delay for FOC, and sampling date + delay for FEC; Table S1). Only the first ranked (ΔAICc <7) and baseline models are presented. The final selected model is shown in bold.
Table 4. Model estimates (β ± s.e.) of the selected models (Table S1 andTable 3) testing the influence of age, sex (F: females, M: males) and scaled mass index (SMI; centred and scaled for each sex) on the faecal oocyst/egg count of Eimeria spp. and gastro-intestinal strongyles (FOC and FEC, respectively) in Mediterranean mouflon from the Caroux-Espinouse, France, in 2010–2019.

Models included the individual identity and the year of sampling as random effects, and the factors related to sampling design previously selected (sampling date + sampling date2 + delay for FOC, and sampling date + delay for FEC, with sampling date and delay centred and scaled; Table S1). R 2m and R 2c are the marginal and conditional variance of the model, respectively.
*P < 0.05, **P < 0.01 and ***P < 0.001.
Faecal abundance of GIS
The sampling date and the delay between sampling and coproscopic analyses had linear negative influences on the FEC (Table S1; Fig. S2) and were included as correcting factors in the next models. Final model selection gave strong support to the model including the quadratic effect of age in interaction with sex (AICc weight = 0.545). We observed a quadratic decrease of FEC with age for males, with a marked decrease during the first years of life. For females, it tends to remain high and stable throughout life (Table 4; Fig. 2). FEC were not different between sexes in young individuals according to the model, while it reached a maximum difference of four times higher FEC in adult females than in adult males of 6 and 7 years of age. The second best model had also a good support (AICc weight = 0.400) and included a negative influence of the SMI as reported for FOC (β = −0.068 ± 0.056; P = 0.224). According to this model, FEC were 1.5 (females) and 1.5–1.6 (males) times higher in individuals in poor (lowest SMI) than in good (highest SMI) body condition.

Fig. 2. Predicted faecal egg count (FEC) for gastro-intestinal strongyles as a function of the age of males and females mouflon based on the selected model (Table S1 and Table 3). Predictions were computed for null values of scaled delay (scaled number of days) between sampling and coproscopic analyses and scaled sampling date. See Fig. 1 for details.
Reproductive status of females
We evaluated the effect of reproductive status of females by including this factor in baseline models built from covariates previously identified as influencing FOC and FEC in females (Tables 3 and 4). Analyses were restricted to a subset of data including only females ⩾2 years old (n = 179) with known reproductive status (n = 171 data from 120 females, including 144 reproductive and 27 non-reproductive statuses; Table 1). Age was entered in the baseline model for FOC as a three-level factor (2, 3 and ⩾4 years old), instead of as a continuous covariate, because almost all ⩾4-year-old females were reproductively active [92.4% (n = 97/105)]. The reproductive status of females did not influence the FOC (LRT χ 2 = 0.0006, d.f. = 1, P = 0.980), but the FEC was 2.5–2.7 times higher (β = 0.837 ± 0.257, P = 0.001) in reproductive than in non-reproductive females (LRT χ 2 = 524.6, d.f. = 1, P = 0.001).
Discussion
The objectives of the present study were to gain knowledge on GI parasitism in mouflon and on the individual characteristics determining the heterogeneity of infestation and infectiousness, since it is of prime importance for long-term management of populations (Paull et al., Reference Paull, Song, McClure, Sackett, Kilpatrick and Johnson2012; Gervasi et al., Reference Gervasi, Civitello, Kilvitis and Martin2015; VanderWaal and Ezenwa, Reference VanderWaal and Ezenwa2016). Studying wild Mediterranean mouflon, we highlighted that Eimeria spp. and GIS were the most prevalent while other parasites were present in <16% of the individuals with low values of faecal egg count. We thus focused on these two groups of parasites to investigate the factors determining parasite burdens and shedding. For all sex – group of parasites combination, there was a significant decrease of the propagule excretion from May to July and with age, except for GIS in females. Individuals in good body condition had lower values of faecal excretion than individuals in poor body condition. Finally, females had higher values of faecal excretion of both Eimeria spp. and GIS than males, with GIS excretion remaining high and stable with age and higher in reproductive than in non-reproductive females. As a consequence, young mouflon, individuals in poor body condition and females with a lamb at heels are the main spreaders of parasite propagules in this population during the spring−early summer period.
Age had a strong negative influence on both FEC/FOC for males and on FOC for females. Young individuals (1–2 years old) of both sexes were the main source of environmental contamination by parasites. Similar effects of young age have been reported elsewhere for several host–parasite systems (e.g. females Soay sheep – GIS, Wilson et al., Reference Wilson, Grenfell, Pilkington, Boyd, Gulland, Clutton-Brock and Pemberton2004; roe deer Capreolus capreolus – GIS and Trichuris sp., Body et al., Reference Body, Ferté, Gaillard, Delorme, Klein and Gilot-Fromont2011; feral horses – GIS, Debeffe et al., Reference Debeffe, Mcloughlin, Medill, Stewart, Andres, Shury, Wagner, Jenkins, Gilleard and Poissant2016), including eimerian infections (e.g. gemsbok Oryx gazelle & blue wildebeest Connochaetes taurinus, Turner and Getz, Reference Turner and Getz2010). Such negative relationship during the first years of life and the relative stability afterwards can be explained by the acquisition of immunity; juveniles being immunologically naïve and immune response reaching its maximum for adults which have been exposed to more infections during their life (Wilson et al., Reference Wilson, Bjørnstad, Dobson, Merler, Poglayen, Randolph, Read and Skorping2002; Turner and Getz, Reference Turner and Getz2010; Body et al., Reference Body, Ferté, Gaillard, Delorme, Klein and Gilot-Fromont2011). The decrease of parasite load with age could also partly result from a higher mortality of highly susceptible and infected young individuals leading to a higher proportion of resistant adult individuals (viability selection; Monaghan et al., Reference Monaghan, Charmantier, Nussey and Ricklefs2008; Benavides et al., Reference Benavides, Huchard, Pettorelli, King, Brown, Archer, Appleton, Raymond and Cowlishaw2012; Debeffe et al., Reference Debeffe, Mcloughlin, Medill, Stewart, Andres, Shury, Wagner, Jenkins, Gilleard and Poissant2016).
Such differential mortality between individuals of different age classes might also explain why we did not detect an increase of parasitism in old individuals. Indeed, parasitism is expected to increase in old individuals as a consequence of immunosenescence (i.e. decreased immunocompetence; e.g. red deer: Santín-Durán et al., Reference Santín-Durán, Alunda, Hoberg and de la Fuente2008; roe deer: Body et al., Reference Body, Ferté, Gaillard, Delorme, Klein and Gilot-Fromont2011; Cheynel et al., Reference Cheynel, Lemaître, Gaillard, Rey, Bourgoin, Ferté, Jégo, Débias, Pellerin, Jacob and Gilot-Fromont2017; Soay sheep: Hayward et al., Reference Hayward, Wilson, Pilkington, Pemberton and Kruuk2009; Froy et al., Reference Froy, Sparks, Watt, Sinclair, Bach, Pilkington, Pemberton, McNeilly and Nussey2019). We observed such a trend here, but only for FEC in males. In other sex – group of parasites combination, the immunosenescence pattern in old mouflon might be hidden by a higher mortality of immunosenescent individuals (Froy et al., Reference Froy, Sparks, Watt, Sinclair, Bach, Pilkington, Pemberton, McNeilly and Nussey2019).
Finally, we did not detect any influence of age on FEC <for females. However, in spring, most of the females of 2 years old onwards are reproductively active in our population and, as a consequence, might shed more GIS eggs in their faeces (‘periparturient rise’; see also our results on FEC for reproductive and non-reproductive females and below). This seasonally increase of FEC in females might not allow to detect any influence of age. Further studies outside the gestation–lactating period will thus be necessary to disentangle the influence of reproduction and age in females.
Interactions among body condition, resources, immune function and pathogens are diverse and vary according to host and parasite species (Cressler et al., Reference Cressler, Nelson, Day and McCauley2014). Since immunocompetence of individuals relies in part on nutrients they ingest, well-nourished and in good body condition individuals are expected to be able to better cope with infections, having more resources to allocate to costly immune responses (Chandra, Reference Chandra1996; Beldomenico and Begon, Reference Beldomenico and Begon2010). Parasites can also negatively impact the body condition. Accordingly, in several wild ungulate species, parasite load, estimated either by coproscopic analysis or by autopsy, is often negatively related to body condition (e.g. reindeer Rangifer tarandus, Stien et al., Reference Stien, Irvine, Ropstad, Halvorsen, Langvatn and Albon2002; red deer Cervus elaphus, Irvine et al., Reference Irvine, Corbishley, Pilkington and Albon2006; roe deer, Body et al., Reference Body, Ferté, Gaillard, Delorme, Klein and Gilot-Fromont2011; moose, Davidson et al., Reference Davidson, Ličina, Gorini and Milner2015; feral horses, Debeffe et al., Reference Debeffe, Mcloughlin, Medill, Stewart, Andres, Shury, Wagner, Jenkins, Gilleard and Poissant2016; Dall's sheep Ovis dalli dalli, Aleuy et al., Reference Aleuy, Ruckstuhl, Hoberg, Veitch, Simmons and Kutz2018). In agreement with these previous results, we observed a negative relationship between body condition and parasite load in Mediterranean mouflon, especially for Eimeria spp.
Sex of individuals is also often reported as a factor influencing parasite burden, with males being frequently more susceptible than females to parasite infections in several ungulate species (e.g. Soay sheep, Wilson et al., Reference Wilson, Grenfell, Pilkington, Boyd, Gulland, Clutton-Brock and Pemberton2004; Craig et al., Reference Craig, Pilkington and Pemberton2006; Alpine chamois Rupicapra r. rupicapra, Citterio et al., Reference Citterio, Caslini, Milani, Sala, Ferrari and Lanfranchi2006; roe deer, Body et al., Reference Body, Ferté, Gaillard, Delorme, Klein and Gilot-Fromont2011; Pyrenean chamois Rupicapra pyrenaica pyrenaica, Martínez-Guijosa et al., Reference Martínez-Guijosa, Martínez-Carrasco, López-Olvera, Fernández-Aguilar, Colom-Cadena, Cabezón, Mentaberre, Ferrer, Velarde, Gassó, Garel, Rossi, Lavín and Serrano2015), although opposite results or no difference between sexes have also been reported (e.g. mouflon, Dyk and Chroust, Reference Dyk and Chroust1973; African buffalo Syncerus caffer, Gorsich et al., Reference Gorsich, Ezenwa and Jolles2014). In the present study, and contrary to our expectation, a significantly higher intensity of GIS and Eimeria spp. was observed in females than in males. This result could be related to the sampling period that corresponds to the end of gestation and lactation periods in females (see Material and methods; Garel et al., Reference Garel, Cugnasse, Gaillard, Loison, Gibert, Douvre and Dubray2005). During this period most (>80%) of the females of 2 years old onwards reproduce in our population (see Results and Garel et al., Reference Garel, Cugnasse, Gaillard, Loison, Gibert, Douvre and Dubray2005) and reproductive ewes have been found to have an increased excretion of nematode eggs (e.g. domestic sheep, Dunsmore, Reference Dunsmore1965; bighorn sheep Ovis canadensis, Festa-Bianchet, Reference Festa-Bianchet1989; Soay sheep, Gulland and Fox, Reference Gulland and Fox1992; Leivesley et al., Reference Leivesley, Bussière, Pemberton, Pilkington, Wilson and Hayward2019).
This periparturient rise is mostly explained by a relaxation of immunity, resulting from the increase of immunosuppressive hormones, stress, and a negative energetic balance due to the high energetic costs of gestation and lactation (Connan, Reference Connan1976; Clutton-Brock et al., Reference Clutton-Brock, Albon and Guinness1989; Houdijk, Reference Houdijk2008). Complementary explanations might come from the spatial and social segregation occurring between males and females’ mouflon during the lambing period (Marchand et al., Reference Marchand, Garel, Bourgoin, Dubray, Maillard and Loison2015; Bourgoin et al., Reference Bourgoin, Marchand, Hewison, Ruckstuhl and Garel2018). Reproductive females segregate from other age and sex classes and select areas perceived as safe, likely at the detriment of foraging quality and quantity (Marchand et al., Reference Marchand, Garel, Bourgoin, Dubray, Maillard and Loison2015). This selection of habitat, associated with the elevated costs of lactation, might favour a higher sensitivity to parasites. In addition, maternal groups are generally composed of an adult female, her newborn lamb and a lamb from the previous year (yearling, Bon et al., Reference Bon, Cugnasse, Dubray, Gibert, Houard and Rigaud1991). Since the young individuals are the most sensitive to parasites, they are also super-spreaders that heavily contaminate the environment they share with their mother, increasing her exposure to parasites.
Altogether, these results revealed strong influences of both sampling and individual factors on the magnitude of Eimeria spp. and GIS propagule shedding. While, because of their high infectiousness that contaminates the environment and other sensitive animals, the main contributors to GI parasite transmission at individual level were reproductively active females, young mouflon and individuals in poor body condition; their relative contribution may change between seasons and in the future. Indeed, in the current context of global change, the modification of environmental conditions (e.g. climate and habitat changes; Hoberg et al., Reference Hoberg, Polley, Jenkins and Kutz2008; Mbora and McPeek, Reference Mbora and McPeek2009; Brearley et al., Reference Brearley, Rhodes, Bradley, Baxter, Seabrook, Lunney, Liu and McAlpine2013; Rose et al., Reference Rose, Hoar, Kutz and Morgan2014) might impact numerous factors known to be associated with parasitism. These include, for instance, resource availability, reproduction phenology, individual behaviour, parasite free-living stages survival on the soil, which can modify both hosts (e.g. Vors and Boyce, Reference Vors and Boyce2009; Descamps et al., Reference Descamps, Aars, Fuglei, Kovacs, Lydersen, Pavlova, Pedersen, Ravolainen and Strøm2017) and parasites dynamics (Morgan and van Dijk, Reference Morgan and van Dijk2012; Rose et al., Reference Rose, Hoar, Kutz and Morgan2014, Reference Rose, Wang, van Dijk and Morgan2015) and thus host–parasite interactions (Hoberg et al., Reference Hoberg, Polley, Jenkins and Kutz2008; Mbora and McPeek, Reference Mbora and McPeek2009; Brearley et al., Reference Brearley, Rhodes, Bradley, Baxter, Seabrook, Lunney, Liu and McAlpine2013; Cable et al., Reference Cable, Barber, Boag, Ellison, Morgan, Murray, Pascoe, Sait, Wilson and Booth2017). Researches on the complex and moving host–parasite–environment relationships should be performed to anticipate changes and improve management policies for population health (Sutherst, Reference Sutherst2001).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021000329.
Acknowledgements
The authors warmly thank all the professionals from the Office Français de la Biodiversité (formerly Office National de la Chasse et de la Faune Sauvage) and all the trainees for data collection. G. Bourgoin, J. Duhayer, M-T Poirel, S. Benabed and M-P Callait-Cardinal are part of the French Laboratory of Excellence project ECOFECT (ANR-11-LABX-0048). The authors are grateful to the two anonymous reviewers for their help in improving the manuscript.
Author contributions
GB and MG conceived and designed the study; GB, EP, MPCC and MG performed statistical analyses and wrote the article; MTP, CI, JD, SB and AC collected and analysed data.
Financial support
This work was supported by the Office Français de la Biodiversité and VetAgro Sup – LBBE – Université Lyon 1.
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical standards
The study of the mouflon population was carried out with the approval of the Préfecture de l'Hérault and the Préfecture de Paris, in agreement with the French environmental code (Art. R421-15 to 421-31 and R422-92 to 422-94-1).