Surgeons need heroes and C. Walton Lillehei is one of my heroes. I was born in Minneapolis, Minnesota, and hence I have been aware of the achievements of Walt Lillehei since I was a young boy. I first met Walt Lillehei in the fall of 1985 when he was the first invited lecturer to honour the late Dr Arthur DeBoer, the surgeon who performed the first open-heart surgery in the city of Chicago in 1956. At the time I met Walt, I was a resident in cardiothoracic surgery at the Feinberg School of Medicine, Northwestern University, and I was incredibly impressed with his achievements and his self-assured manner. Dr Lillehei gave me his business card, which I have kept to this day (Fig 1).

Figure 1 C. Walton Lillehei's business card given to me by Walt in 1985.
Walt Lillehei was born on October 23, 1918, in Edina, Minnesota.Reference Miller1 His grandparents had been born in Norway. As a teenager, he was both an Eagle Scout and a golf caddy. Walt's motto in life was, “work hard, play hard”. Walt obtained his Bachelor of Science, Doctor of Philosophy, and Medical Doctor degrees from the University of Minnesota. He was then a surgical resident at the University of Minnesota, completing his training under Owen Wangensteen in 1950. Less than 2 years later, on September 2, 1952, he was an assistant when F. John Lewis performed the first successful open-heart surgery, closing an atrial septal defect using hypothermia and inflow occlusion.Reference Lewis and Taufic2
On March 26, 1954, ![]() years later, when Walt was 36 years old, came the defining milestone in Walt's surgical career – the closing of a ventricular septal defect using the novel concept of cross-circulation on a boy who was 13 months old and weighed 6.9 kilograms.Reference Lillehei, Cohen, Warden, Ziegler and Varco3 The child's father was the “cardiopulmonary bypass pump”. This operation was accomplished with 15 minutes of cross-circulation (Fig 2). Walt's series of patients undergoing correction of congenital anomalies by controlled cross-circulation was published in Surgery in 1955.Reference Lillehei, Cohen, Warden and Varco4 The technique is illustrated in Figure 3. The essence of the operation was to cannulate the parent's femoral artery and vein and then use a small pump adapted from a milking machine to pump oxygenated blood from the parent into the child undergoing the surgery. The child's venous blood was routed to the parent's femoral vein to be oxygenated. The next milestone was to repair a patient with tetralogy of Fallot on August 31, 1954. Later that autumn, Walt performed the first atrioventricular septal defect repair (Fig 4). The repair looks remarkably similar to our current “modified single-patch repair”.Reference Nicholson, Nunn and Sholler5 On May 13, 1955, he performed the first open-heart surgical procedure – ventricular septal defect closure – using the bubble oxygenator for cardiopulmonary bypass support (Fig 5).Reference Lillehei, Dewall, Read, Warden and Varco6
years later, when Walt was 36 years old, came the defining milestone in Walt's surgical career – the closing of a ventricular septal defect using the novel concept of cross-circulation on a boy who was 13 months old and weighed 6.9 kilograms.Reference Lillehei, Cohen, Warden, Ziegler and Varco3 The child's father was the “cardiopulmonary bypass pump”. This operation was accomplished with 15 minutes of cross-circulation (Fig 2). Walt's series of patients undergoing correction of congenital anomalies by controlled cross-circulation was published in Surgery in 1955.Reference Lillehei, Cohen, Warden and Varco4 The technique is illustrated in Figure 3. The essence of the operation was to cannulate the parent's femoral artery and vein and then use a small pump adapted from a milking machine to pump oxygenated blood from the parent into the child undergoing the surgery. The child's venous blood was routed to the parent's femoral vein to be oxygenated. The next milestone was to repair a patient with tetralogy of Fallot on August 31, 1954. Later that autumn, Walt performed the first atrioventricular septal defect repair (Fig 4). The repair looks remarkably similar to our current “modified single-patch repair”.Reference Nicholson, Nunn and Sholler5 On May 13, 1955, he performed the first open-heart surgical procedure – ventricular septal defect closure – using the bubble oxygenator for cardiopulmonary bypass support (Fig 5).Reference Lillehei, Dewall, Read, Warden and Varco6

Figure 2 March 26, 1954: first ventricular septal defect closure with cross-circulation by Lillehei, University of Minnesota, Minneapolis, Minnesota (courtesy of the Lillehei Heart Institute).

Figure 3 Schematic drawing of cross-circulation technique. The pump was adapted from a milking machine.

Figure 4 First atrioventricular septal defect repair. No patch material was used (reprinted with permission: Levy MJ, Cuello L, Tuna N, Lillehei CW. Atrioventricularis commuis: clinical aspects and surgical treatment; Am J Cardiol 1964; 14: 587–598).

Figure 5 First use of bubble oxygenator for open-heart surgery, May 13, 1955, University of Minnesota, Minneapolis, Minnesota (courtesy of the Lillehei Heart Institute).
On August 12, 1960, Walt performed his 1000th pump case at the University of Minnesota. He continued his wildly successful surgical career there until 1967 when John Najarian was appointed Chief of Surgery at the University of Minnesota. Disappointed by this selection, Lillehei left the University of Minnesota and moved to New York City. There he was made the Chairman of the Department of Surgery at Cornell University. His career there lasted only 6 years, and Walt performed his last open-heart surgery on December 30, 1973. At that time, he was 55 years old and had developed cataracts from radiation therapy. He moved back to Minnesota and became the Director of St. Jude Medical Inc. Walt died on July 5, 1999, at 80 years of age.
Often referred to as the “Father of Open-Heart Surgery”, Walt's list of accomplishments is truly amazing. Walt earned a Bronze Star in World War II participating in the Allied campaigns in North Africa and Italy. In 1955, he was awarded the prestigious Lasker Award. Walt trained over 150 cardiac surgeons from 40 different nations. These surgeons included Norman Shumway, Christian Barnard, and Vincent Gott. It is truly an honour for me to give the 12th Annual C. Walton Lillehei Memorial Lecture. Another testament to Walt's accomplishments is the distinguished nature of the previous surgeons who have given this lecture. They are listed in Figure 6.
Figure 6 List of previous C. Walton Lillehei's lecturers at the Annual Update on Pediatric and Congenital Cardiovascular Disease.
The topic that I have selected is the Fontan conversion procedure. We began performing this operation at Children's Memorial Hospital in 1994. At that time, Dr Constantine Mavroudis was the chief of our division and Dr Barbara J. Deal was the chief of paediatric electrophysiology. The Fontan operation was of course initially performed by another one of my surgical heroes, Francis Fontan.Reference Fontan and Baudet7 In Professor Fontan's original manuscript, he performed the “Fontan” operation successfully in two of three patients. In that manuscript, he made a remarkably prescient statement. In the discussion of those patients, he stated that “One element remains unpredictable – the hemodynamic consequences of eventual atrial rhythm disturbance such as atrial fibrillation or flutter”. As we now know, many – if not most – of the patients with an atriopulmonary connection for their Fontan procedure develop slowly progressive dilatation of the right atrium, thickening of the right atrial wall, and eventual atrial tachycardia and atrial fibrillation. The first surgeon to address these patients was Dr Hillel Laks. In 1994, he published an experience with three patients with atriopulmonary connections who developed late arrhythmias and atrial thrombus.Reference Kao, Alejos, Grant, Williams, Shannon and Laks8 All three patients were treated with surgical conversion of the atriopulmonary connection to a lateral tunnel cavopulmonary Fontan.
The contribution from our group at Children's Memorial Hospital was to add arrhythmia surgery consisting of either a right maze or a biatrial maze (Cox-maze III) to the cavopulmonary conversion.Reference Mavroudis, Backer, Deal, Johnsrude and Strasburger9 We also used the extracardiac Fontan rather than the lateral tunnel-type connection.Reference Backer, Deal, Kaushal, Russell, Tsao and Mavroudis10 Our series now totals 133 Fontan conversions, and the number of operations by year is shown in Figure 7. From a historical perspective, it is interesting to look at who the prior Fontan surgeon was. This is shown in Table 1. Some of those surgeons have given this lecture. The old operative reports are marvellous to read. One of the patients that we operated on was previously operated on by both Professor Fontan and Dr John Kirklin at the University of Alabama at Birmingham.
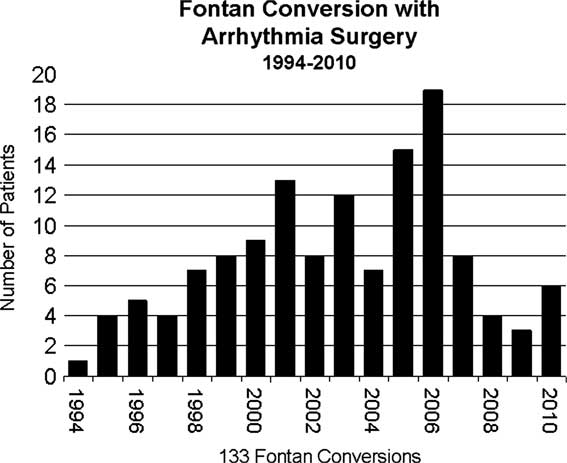
Figure 7 Fontan conversion patients by year of operation at Children's Memorial Hospital (1994–2010).
Table 1 Surgeon performing initial Fontan procedure – before Fontan conversion.

The majority of patients undergoing Fontan conversion had a diagnosis of either tricuspid atresia or double-inlet left ventricle. The diagnoses are illustrated in Table 2. The typical patient requiring a Fontan conversion has a very enlarged right atrium that often compresses the right pulmonary veins (Fig 8).
Table 2 Diagnosis of patients undergoing Fontan conversion.

PA-IVS = pulmonary atresia with intact ventricular septum; TGA = transposition of great arteries

Figure 8 Computerised tomography scan of the chest in a patient status post atriopulmonary Fontan. Axial image shows the tremendously dilated right atrium with compression of the right pulmonary veins.
The primary steps of the surgical Fontan conversion are as follows:
• resection of the majority of the large right atrium,
• extracardiac polytetrafluoroethylene tube graft from the inferior caval vein to the pulmonary artery,
• bidirectional superior cavopulmonary anastomosis,
• right or biatrial maze procedure with cryoablation,
• epicardial dual-chamber antitachycardia pacemaker.
For arrhythmia management, we have had two different strategies. For the patients with atrial re-entry tachycardia, the patients receive both a pre-operative and intra-operative electrophysiological study in the catheterisation laboratory. These patients then undergo a modified right atrial maze procedure. They have a post-operative electrophysiologic study again in the catheterisation laboratory. All patients with atrial fibrillation have the Cox-maze III or biatrial maze procedure. The patients with atrial fibrillation also receive post-operative aminodarone for 3 to 6 months.
Between 1994 and early 2011, 133 patients have undergone the Fontan conversion with arrhythmia surgery at Children's Memorial Hospital. The mean age at the time of the operation was 23.3 plus or minus 8 years. The mean age at the prior Fontan procedure was 7.0 plus or minus 5.0 years. The mean interval from Fontan operation to conversion was 16.3 plus or minus 5.4 years. The mean age at onset of supraventricular tachycardia was 15.5 plus or minus 8.0 years. Patients were on a mean of three anti-arrhythmic medications. Functional classification is shown in Table 3. The types of atrial arrhythmias are shown in Figure 9. The most common arrhythmia was atrial fibrillation followed by right atrial re-entry tachycardia.
Table 3 Fontan conversion with arrhythmia surgery – functional classification.


Figure 9 Types of atrial arrhythmias in patients undergoing Fontan conversion.
The surgical technique is illustrated in the following series of illustrations (Figs 10–19). We begin with careful sternal re-entry and aortobicaval cardiopulmonary bypass (Fig 10). The connection of the inferior caval vein to the right atrium is transected, and the polytetrafluoroethylene tube graft – typically number 24 – is sutured to the orifice of the inferior caval vein. After the administration of cold blood antegrade cardioplegia, a significant portion of right atrium is excised (Fig 11). Atrial septectomy is performed. A right-sided maze procedure is performed in all patients (Fig 12). This consists of lesions from the inferior caval vein to the coronary sinus, inferior caval vein to the tricuspid valve annulus, lesions across the crista terminalis to the atrial septal defect, and a lesion from the resected right atrial appendage to the atrial septal defect. Finally, in the second half of the series, we have used a lesion from coronary sinus to the atrial septal defect referred to as the “Deal lesion”. The Deal lesion is illustrated in the photograph (Fig 13). The right-sided maze is modified depending on whether the patient has mitral atresia or tricuspid atresia. If the patient has atrial fibrillation, a Cox-maze III (biatrial maze) is performed (Fig 14). The pulmonary veins are completely encircled by cryoablation lesions (Fig 15). A linear lesion with a circle around the base of the orifice of the left atrial appendage is performed. A lesion is performed to the P3 site of the mitral valve. Finally, a lesion of 2 minutes is performed on the epicardial coronary sinus (Fig 14).

Figure 10 This illustrates the view of the heart from the surgeon's perspective. The patient has had aortic and bicaval venous cannulation for cardiopulmonary bypass. The dotted lines show the amount of enlarged right atrium that will be removed (reprinted with permission: Backer CL. Conversion of the failed Fontan circulation. Cardiol Young 2006; 16 (Suppl 1): 85–91).

Figure 11 This is an example of the amount of right atrium that is excised in these patients. This specimen is essentially 12 × 12 centimetres in size. Note also that the atrium is very thick, which makes transcatheter ablation of arrhythmias difficult in these patients (reprinted with permission: Backer CL, Tsao S, Deal BJ, Mavroudis C. Maze procedure in single ventricle patients. Pediatr Card Surg Annu 2008; 11: 44–48).

Figure 12 This illustration shows the cryoablation lesions (−160°C, 1 minute) for the right-sided maze performed in a patient who has had an atriopulmonary Fontan for tricuspid atresia (reprinted with permission: Mavroudis C, Backer CL, Deal BJ, Johnsrude C, Strasburger J. Total cavopulmonary conversion and maze procedure for patients with failure of the Fontan operation. J Thorac Cardiovasc Surg 2001; 122: 863–871).

Figure 13 The “Deal lesion” is a lesion from the coronary sinus to the surgically created atrial septal defect (reprinted with permission: Backer CL, Tsao S, Deal BJ, Mavroudis C. Maze procedure in single ventricle patients. Pediatr Card Surg Annu 2008; 11: 44–48).

Figure 14 This illustration demonstrates the lesions placed to complete the left side of the Cox-maze III. This is for patients with atrial fibrillation. In the patient illustrated here, the right-sided pulmonary veins and a portion of the left atrium have been opened surgically. The remaining lesions have been created with the cryoablation probe. The lesion on the coronary sinus is placed for 2 minutes (reprinted with permission: Backer CL, Deal BJ, Mavroudis C, Franklin WH, Stewart RD. Conversion of the failed Fontan circulation. Cardiol Young 2006; 16 (Suppl l): 85–91).

Figure 15 This illustrates the encircling lesion around the left pulmonary veins. This is a single 1-minute application with the cryoablation probe (−160°C; reprinted with permission: Backer CL, Tsao S, Deal BJ, Mavroudis C. Maze procedure in single ventricle patients. Pediatr Card Surg Annu 2008; 11: 44–48).

Figure 16 Evolution of pacemaker strategy over time.

Figure 17 Completed Fontan conversion procedure. The polytetrafluoroethylene tube graft from the inferior vena cava to the undersurface of the pulmonary artery is typically a 24 French size. A bidirectional superior cavopulmonary anastomosis has been created. The orifice of the prior atriopulmonary connection has been patched. Finally, atrial and ventricular pacing leads have been positioned. These are steroid eluting bipolar epicardial leads (reprinted with permission. Backer CL, Deal BJ, Mavroudis C, Franklin WH, Stewart RD. Conversion of the failed Fontan circulation. Cardiol Young 2006; 16 (Suppl l): 85–91).

Figure 18 Freedom from death after Fontan conversion.

Figure 19 Cox proportional–hazards risk factors for death or transplant.
The pacemaker strategy has changed over the years because of advances in the pacemaker technology.Reference Tsao, Deal, Backer, Ward, Franklin and Mavroudis11 This evolution is shown in Figure 16. The most common type of pacemaker placed in the last 8 years has been the dual-chamber atrial antitachycardia pacemaker with epicardial steroid eluting leads. An illustration of the completed Fontan conversion procedure is shown in Figure 17. Results are shown in Table 4.
Table 4 Fontan conversion with arrhythmia surgery – results (n = 132).

*Three late deaths post-transplant
The freedom from death is shown in Figure 18. Note that one patient died of sedation administration and one in a motor vehicle accident. At 10 years, the freedom from death was 85%. A previous analysis when we had 111 patients demonstrated the following Cox proportional–hazards risk factors for transplant or death. These are shown in Figure 19. Protein-losing enteropathy, long cardiopulmonary time (greater than 239 minutes), moderate to severe atrioventricular valve insufficiency, and right or ambiguous ventricle were associated with death or transplant. The freedom from arrhythmia recurrence is shown in Figure 20. All of the patients who had arrhythmia recurrence following attempted ablation of atrial fibrillation had atrial re-entry tachycardia, not atrial fibrillation. In nearly all cases, recurrent atrial tachycardia or atrial re-entry tachycardia after attempted surgical ablation of atrial fibrillation was easily controlled with one medication.

Figure 20 Freedom from arrhythmia recurrence after Fontan conversion. Two separate curves are shown, one for ART (atrial re-entry tachycardia) and the other for Afib (atrial fibrillation).
Nearly all patients have had a significant improvement in the New York Heart Association status. One of our patients from Spain expressed very well the improvement in the quality of her life following the Fontan conversion. She is a 34-year-old woman who had a Fontan operation in 1982 by Dr A. Pacifico and had her Fontan conversion in 2007:
I am here to tell you that having the surgery last year at Children's Memorial was the best thing I have done in my whole life. I couldn't imagine how ill I was until I had the surgery. Now I work 8 hours a day and I can do what everybody else can do. Having a Fontan conversion was the best thing I have ever done in my life. (January, 2011)
We have performed 133 Fontan conversions with arrhythmia surgery between 1994 and early 2011. The most common diagnoses were tricuspid atresia and double-inlet left ventricle, and most patients had a prior atriopulmonary connection. Our experience has been that this operation is highly successful for arrhythmia management and improved functional status. Patients have a better outcome if they are referred early before they develop protein-losing enteropathy or significant cardiac failure from arrhythmias and atrioventricular valve insufficiency. The key to success with these patients has been a remarkable collaborative effort between the cardiovascular surgery and electrophysiology teams at our hospital. It has been gratifying to see other institutions also reporting excellent results using this operation on these complex patients.Reference Takahashi, Fynn-Thompson, Cecchin, Khairy, del Nido and Triedman12–Reference Morales, Dibardino and Braud17
I would like to conclude this paper by recognising Dr C. Walton Lillehei as the father of open-heart surgery with a quote from Dr Michael E. DeBakey. Dr DeBakey commented on Dr Lillehei's paper after listening to his presentation on controlled cross-circulation read at the Society of University Surgeons meeting in February of 1955:
I rise to express tribute to Dr. Lillehei for a superb piece of work. He deserves a great deal of credit for his imagination…boldness, and demonstration of this…really and truly inspiring work.Reference Lillehei, Cohen, Warden and Varco4
Dr Lillehei is truly a surgical hero.


























