Introduction
The tomato psyllid, Bactericera cockerelli (Sulcer) (Hemiptera: Triozidae), is a key pest of several solanaceous crops in the United States, Mexico, Central America, and New Zealand (Munyaneza et al., Reference Munyaneza, Crosslin and Upton2007; Liefting et al., Reference Liefting, Southerland, Ward, Paice, Weir and Clover2009; Butler and Trumble, Reference Butler and Trumble2012). The most important damage caused by this pest, in addition to direct plant damage, is the transmission of the bacterium Candidatus Liberibacter solanacearum (alternatively, Ca. Liberibacter psyllaurous) (Hansen et al., Reference Hansen, Trumble, Stouthamer and Paine2008), which is associated with zebra chip disease in the potato (Solanum tuberosum L.), tomato (Solanum lycopersicum L.) (Liefting et al., Reference Liefting, Southerland, Ward, Paice, Weir and Clover2009), and pepper (Capsicum annuum L.) (Munyaneza et al., Reference Munyaneza, Sengoda, Crosslin, Garzón-Tiznado and Cardenas-Valenzuela2009) crops. Without control measures, B. cockerelli causes yield losses up to 100%, which result in monetary losses exceeding millions of dollars per year in the potato industry in the United States and Mexico (Butler and Trumble, Reference Butler and Trumble2012; Munyaneza, Reference Munyaneza2012).
Beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae), originally from Southeastern Asia, is a cosmopolitan insect that is also particularly abundant in North and Central America, Africa, Australia and Europe (Zheng et al., Reference Zheng, Cong, Wang and Lei2011; CAB International 2019a). This insect is one of the most important pests of various crops, such as the tomato, sweet pepper, bean, cucumber, alfalfa, cotton, and ornamentals (Zheng et al., Reference Zheng, Cong, Wang and Lei2011). The fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), is the most serious maize (Zea mays L.) pest throughout America (Nagoshi and Meagher, Reference Nagoshi and Meagher2008), including Mexico (Blanco et al., Reference Blanco, Pellegaud, Nava-Camberos, Lugo-Barrera, Vega-Aquino, Coello, Terán-Vargas and Vargas-Camplis2014). Recently, this pest has become a new invasive species in West and Central Africa where outbreaks were recorded for the first time in early 2016 (Goergen et al., Reference Goergen, Kumar, Sankung, Togola and Tamò2016; CAB International, 2019b).
In Mexico, growers typically use several applications of broad-spectrum insecticides per month throughout the growing season to control B. cockerelli (Vega-Gutiérrez et al., Reference Vega-Gutiérrez, Rodríguez, Díaz, Bujanos, Mota, Martínez, Lagunes and Garzón2008) as well as S. exigua (Osorio et al., Reference Osorio, Martínez, Schneider, Díaz, Corrales, Avilés and Pineda2008) and S. frugiperda (Blanco et al., Reference Blanco, Pellegaud, Nava-Camberos, Lugo-Barrera, Vega-Aquino, Coello, Terán-Vargas and Vargas-Camplis2014). However, the intensive use of these chemical compounds is costly and has led to the development of resistance in these pests towards many of the active substances designed for their control (Dávila et al., Reference Dávila, Cerna, Uribe, García, Ochoa, Gallegos and Landeros2012; Sayyed et al., Reference Sayyed, Naveed, Rafique and Arif2012; Wyckhuys et al., Reference Wyckhuys, Lu, Morales, Vazquez, Legaspi, Eliopoulos and Hernández2013). Thus, chemical control has been ineffective against all three insect pests, the natural predator species of various pest organisms are decimated and pesticide residues over the maximum residue level can be found in the harvested vegetables and fruits if pesticides have been incorrectly used (Bueno et al., Reference Bueno, Van Lenteren, Lins, Calixto, Montes, Silva, Santiago and Pérez2013).
Miridae is a hyperdiverse family containing more than 11,020 described species, which are commonly known as plant bugs and are found in all major biogeographic regions of the world (Cassis and Schuh, Reference Cassis and Schuh2012). They are phytophagous (Bryocorinae, Orthotylinae, Phylinae, and Deraeocorinae), mycetophagous (Cylapinae), carnivorous (Isometopinae and Deraeocorinae), and zoophytophagous (Bryocorinae: Dicyphini) (Cassis and Schuh, Reference Cassis and Schuh2012). The zoophytophagous species can have great economic impact as natural enemies (e.g., Nesidiocoris tenuis [Reuter], Dicyphus tamaninii Wagner, Dicyphus hesperus [Knight], and Macrolophus pygmaeus Rambur; Urbaneja et al., Reference Urbaneja, González-Cabrera, Arnó and Gabarra2012) but they can also become major pests of some food and fiber crops (e.g., tomato, potatoes, melon, tobacco, sesame, among other) at high population levels and scarcity of the prey (Alomar et al., Reference Alomar, Riudavets and Castañe2006; Calvo et al., Reference Calvo, Bolckmans, Stansly and Urbaneja2009; Castañé et al., Reference Castañé, Arnó, Gabarra and Alomar2011; Bhatt and Patel, Reference Bhatt and Patel2018).
Engytatus varians (Distant) (Hemiptera: Miridae) is also a zoophytophagous that feeds on plants and phytophagous insects living on them, such as aphids, whiteflies, pseudococcids, and lepidopterans (Bueno et al., Reference Bueno, Van Lenteren, Lins, Calixto, Montes, Silva, Santiago and Pérez2013; Silva et al., Reference Silva, Bueno, Montes and van Lenteren2016). This species is widely distributed in North (Madden and Chamberlin, Reference Madden and Chamberlin1945), Central (Maes, Reference Maes1998), and South America (Schuh, Reference Schuh1995). In Mexico, the presence of E. varians was reported for the first time in 2014 under greenhouse conditions feeding on B. cockerelli nymphs on tomato plants (Martínez et al., Reference Martínez, Baena, Figueroa, Del Estal, Medina, Lara and Pineda2014). Later, a study by Pineda et al. (Reference Pineda, Medina, Figueroa, Henry, Mena, Chavarrieta and Martínez2016) reported that, during the whole nymphal stage, E. varians consumed significantly the same number of B. cockerelli third instars (80–85) depending on whether it was fed only with third instars of the pest or with third instars + eggs of the grain moth Sitotroga cerealella Olivier (Lepidoptera: Gelechiidae). Also, Martínez et al. (Reference Martínez, Baena, Figueroa, Del Estal, Medina, Lara and Pineda2014) found that fourth instar of this predator preyed on 46% of the B. cockerelli third instars offered (9) during a 24 h period. In Brazil, other studies have determined that E. varians is an important predator of the tomato borer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). In this regard, Bueno et al. (Reference Bueno, Van Lenteren, Lins, Calixto, Montes, Silva, Santiago and Pérez2013) observed that E. varians consumed 92 eggs on average, over 24 h. By direct observations, these authors reported that this predator is capable of preying on larvae of this pest by stinging through the leaf epidermis into the larvae within the mine. Silva et al. (Reference Silva, Bueno, Montes and van Lenteren2016) observed that when E. varians fed on eggs and first instar larvae of T. absoluta, the survival rate of the predator was higher than 70%. However, the potential of predation of this mirid has been poorly studied on lepidopteran pests, and it is unknown on S. exigua and S. frugiperda.
In this study, the predation by E. varians adults on different developmental stages of B. cockerelli (egg, second, and third instar nymphs) and S. exigua and S. frugiperda (egg, first, and second larval instars) was recorded. The influence of E. varians age on the predation capacity was also analysed.
Materials and methods
Sources of insects
Bactericera cockerelli and E. varians were obtained from the colonies maintained in the Entomology Laboratory of the Instituto de Investigaciones Agropecuarias y Forestales (IIAF) of the Universidad Michoacana de San Nicolás de Hidalgo (UMSNH), El Trébol, Michoacán, Mexico. Nymphs and adults of B. cockerelli were reared on tomato plants (variety Saladette) (~30 cm in height with nine to ten fully expanded leaves) in three frame boxes (50 × 60 × 50 cm) entirely covered by a mesh screen. Tomato plants containing psyllid eggs were transferred to other insect-free frame boxes, and new host plants were supplied as needed. Bactericera cockerelli colony was maintained under laboratory conditions at ~25°C, 56% RH, and a photoperiod of 12:12 h (L:D) (Pineda et al., Reference Pineda, Medina, Figueroa, Henry, Mena, Chavarrieta and Martínez2016).
Nymphs and adults of E. varians were originally collected in March 2013 from tomato plants grown in a greenhouse of the IIAF-UMSNH. They were reared on tomato plants infested with eggs and nymphs of B. cockerelli plus eggs of the S. cerealella as a supplementary food source. Using eggs and nymphs of the psyllid as diet is inexpensive and, additionally, it does not modify the predation rate of E. varians on third instars of B. cockerelli (Pineda et al., Reference Pineda, Medina, Figueroa, Henry, Mena, Chavarrieta and Martínez2016). The colony of E. varians was maintained under the same conditions as the prey colony.
The colony of S. frugiperda was started using larvae collected from the maize field at El Trébol, Municipality of Tarímbaro, Michoacán, Mexico. The colony of S. exigua was originally supplied by the Laboratorio de la Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, San Nicolás de Los Garza, Nuevo León, Mexico. The larvae of both lepidopteran species were reared on a wheat germ-based semi-synthetic diet (Poitout and Bues, Reference Poitout and Bues1974) in a growth chamber at 25 ± 2°C, with 70–80% RH and a photoperiod of 16:8 h (L:D). Adults were fed with a 15% honey solution. Brown paper was provided as a substrate for oviposition, which was replaced periodically, as required.
Prey consumption studies
The mirid predation on B. cockerelli and Spodoptera species was evaluated along its life span using 7, 9, 11, 13, 15, and 17 days old males and females except when B. cockerelli eggs were offered as prey. In this case, we had to stop the study when adults of the predator were 9 days old because of a temporary shortage in the number of psyllid eggs needed per days (1000–1500) to continue with the experiment for a longer period. In all experiment, 5 days old females and males (at this age they have already experience on mating and predation) were initially introduced in the cages and offered the selected prey one or two days later. Experiments with every prey were conducted under the same conditions described for the B. cockerelli rearing.
To obtain E. varians individuals of the same age for the tests, a tomato plant (~13 cm in height) with three to four fully expanded leaves, infested with a mixture of third, fourth, and fifth instar nymphs of B. cockerelli, was enclosed in a cylindrical plastic tube (11 cm in diameter, 15 cm in height) open at both ends. The top of the cylinder was covered with a fine mesh screen to permit air circulation and to prevent escape of the insects. Ten adult pairs (≤12 h old) were placed into the cylinder and S. cerealella eggs were dispersed on the tomato leaves as supplementary food. After 5 days, one of these females or males was taken randomly for the experiment and starved for 2 h before the bioassay to induce a higher feeding rate.
Prey consumption was assessed separately for females and males of E. varians on different types of prey: eggs or nymphs of B. cockerelli and eggs or larvae of S. frugiperda and S. exigua. Each experiment consisted of ten replicates per predator sex and developmental stage of the prey. In every case, ten tomato leaflets with eggs (n = 100–160) or nymphs (n = 40) of B. cockerelli and eggs (n = 50–55) or larvae (n = 20) of S. frugiperda and S. exigua without predator presence, were used as controls to observe natural and manipulation mortality, unless a different method is specifically detailed below.
Predation on eggs or nymphs of B. cockerelli
An excised tomato leaflet bearing 100–160 eggs (≤24 h old) of B. cockerelli was placed, with its adaxial side down, into a plastic Petri dish (9 cm diameter × 3 cm high), and then one female or one male (5 days old) was introduced to this Petri dish. The petiole of each leaflet was enveloped with a piece of moist cotton to delay dehydration. The tomato leaflets with B. cockerelli eggs were replaced every 24 h when females and males of E. varians were 6, 7, 8, and 9 days old. After each exposure, each leaflet was carefully examined using a stereoscopic microscope (40X; Zeiss Stemi DV4; Carl Zeiss, Berlin, Germany) to determine egg consumption. Consumed eggs were easily distinguished because they looked dehydrated, and no more yolk was left in them.
Predation by E. varians on B. cockerelli nymphs was evaluated separately for second- or third-instars of the psyllid. Tomato plants ~15 cm in height with four fully expanded leaves were used in these tests. On the adaxial surface of each leaf of the tomato plant, 10 second- or third-instar B. cockerelli (≤24 h old) (n = 40 for each instar/plant) were placed using a small brush. The tomato plant was enclosed into a ventilated cylindrical plastic tube as described above, and after that, one E. varians female or male with the same characteristics described above was introduced. Individualized 5 days old females or males of the predator were transferred to a new cylinder every 48 h when they were 7, 9, 11, 13, 15, and 17 days old. The predation by both sexes of E. varians at 15 and 17 days old on second instar B. cockerelli was not determined due to the low availability of individuals.
After each exposure, the number of consumed nymphs was recorded using a stereoscopic microscope. Bactericera cockerelli nymphs that had been preyed upon were distinguishable because no more haemolymph was left in the body and because of the presence of a little brown spot at their dorsum, indicating the place where the predator inserted its stylet for feeding.
Predation on eggs or larvae of S. frugiperda and S. exigua
Eggs of S. frugiperda or S. exigua (≤24 h old; n = 50–55) were placed on the adaxial side of a tomato leaflet using a small brush. After that, the tomato leaflet with its petiole wrapped in a piece of moist cotton to prevent dehydration was placed together with one female or one male predator into a plastic Petri dish. Previous tests have shown that egg mortality of both Spodoptera species resulting from this handling is minimal. Each female or male predator was transferred to a new Petri dish with a tomato leaflet with S. frugiperda or S. exigua eggs every 48 h for six d (when predators were 7, 9, 11, 13, 15, and 17 days old). After each exposure, leaflets were carefully examined using a stereoscopic microscope to determine egg consumption. Consumed eggs looked dehydrated and no more yolk was left in them.
For the bioassay of larvae consumption, tomato leaflets were used for both S. exigua and S. frugiperda, because tomato plants have been reported as natural host for both species (Andrews, Reference Andrews1988; Trumble and Alvarado-Rodríguez, Reference Trumble and Alvarado-Rodríguez1993; Casmuz et al., Reference Casmuz, Juárez, Murúa, Prieto, Medina, Willink and Gastaminza2010; Zheng et al., Reference Zheng, Cong, Wang and Lei2011). One tomato leaflet was placed inside a Petri dish, 20 first or second instar S. frugiperda or S. exigua (≤24 h old) larvae were added with a small brush, and one E. varians female or male was introduced. The tomato leaflet and larvae were replaced every 48 h for six days. After each change, each Petri dish was carefully examined using a stereoscopic microscope to determine larvae consumption. Preyed-upon larvae were easily distinguished because they were flabby and empty.
Statistical analysis
Data on the number of eggs and nymphs of B. cockerelli, as well as those of eggs and larvae of S. frugiperda and S. exigua consumed by E. varians adults, were subjected to a generalized linear models procedure (GLM) to determine the influence of the mirid sex and its life stage on the number of prey consumed. Data on the number of eggs and larvae of S. frugiperda or S. exigua consumed by E. varians adults were also compared between both noctuid species. To separate means, a least significant difference (LSD) multiple range test (P < 0.05) was used. All data were submitted to normality and homoscedasticity tests of accord with Anderson-Darling and Bartlett, respectively (Zar, Reference Zar2014). Differences among predation for each developmental stage of the prey (B. cockerelli, S. exigua or S. frugiperda) were tested through a repeated measures analysis of variance, with predator age (days) as the repeating factor, using the fixed effects model (MIXED) procedure. All data (mean ± standard error, SE) were analysed in the SAS/STAT programme, version 9.4 (SAS Institute, Cary, NC, EE. UU.).
Results
Predation on eggs and nymphs of B. cockerelli
No mortality by manipulation was observed on eggs or on second or third instar B. cockerelli in the negative controls. Engytatus varians females that were 6, 7, 8, and 9 days old consumed (ranging from 74 to 83%) significantly (P < 0.0001) more B. cockerelli eggs than males (ranging from 30 to 52%) (fig. 1). However, the predation of eggs by E. varians females was not significantly affected by the predator age (F 3,27 = 0.71; P = 0.55), while it decreased significantly in males (F 3,25 = 6.02; P = 0.003) (fig. 1).

Figure 1. Predation of Engytatus varians females and males of different ages on Bactericera cockerelli eggs (means ± SE) on tomato leaves. Within the same E. varians age, data followed by the same letter are not significantly different (P < 0.05; GLM, LSD). F 7,52 = 19.35, P < 0.001.
Engytatus varians females also consumed significantly more nymphs (second and third stage) than males (F 23,243 = 31.07, P < 0.0001) in all ages bioassayed. However, E. varians females that were 7, 9, 11, and 13 days old consumed between 76 and 86% second instars of B. cockerelli, while when they were 7, 9, 11, 13, 15, and 17 days old, they consumed between 63 and 79% third instars (table 1). Female predators consumed significantly more second (81–86% nymphs) than third instar (63 ± 3%) of B. cockerelli than males only when they were 9 and 11 days old. In the case of E. varians males, they consumed significantly less third instar B. cockerelli (10–27 nymphs) than second instar (34–38 nymphs) for all predator ages bioassayed (table 1).
Table 1. Predation of Engytatus varians females and males of different ages on nymphal instars of Bactericera cockerelli (means ± SE) on tomato leaves

ND, not determined due to the low availability of individuals in B. cockerelli rearing.
Within the same column and Engytatus varians sex, data followed by the same letter are not significantly different (P < 0.05; GLM, LSD).
a Repeated measures ANOVA on the number of consumed preys by E. varians at different ages (*P < 0.05; **P < 0.01; NS = Not significant, P > 0.05).
b F 9,67 = 2.57, P < 0.05.
c F 9,70 = 5.55, P < 0.01.
The predation of B. cockerelli second nymphal instars by E. varians females was not significantly affected by predator age (table 1). In contrast, the maximum number of consumed B. cockerelli third instars (79 ± 4%) was recorded when E. varians females were 7 days old, and then it decreased significantly between 63 ± 3% and 66 ± 4% for the remaining evaluated ages (F 5,42 = 3.32; P = 0.013) of the predator. In the case of predation by E. varians males, the consumption of B. cockerelli third nymphal instars decreased significantly (F 5,44 = 3.83; P = 0.006) over the course of the study but not (F 3,26 = 0.2; P = 0.89) when they fed on second instars of the prey (table 1).
Predation on eggs and larvae of S. exigua and S. frugiperda
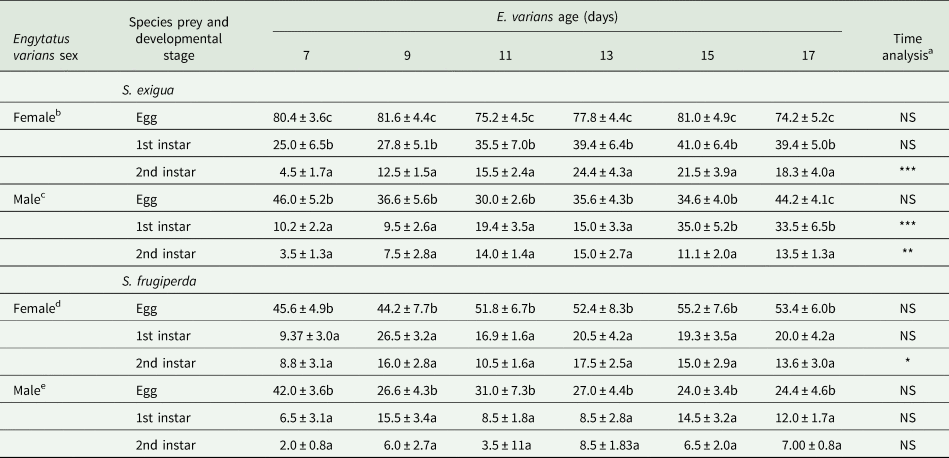
No mortality by manipulation was observed on eggs or on first or second instar S. frugiperda or S. exigua in the negative controls. Similar to the B. cockerelli experiment, E. varians females consumed a significant higher number of S. frugiperda eggs than males across all predator ages except when they were 7 days old (table 3). Additionally, E. varians females consumed significantly more first instar S. exigua than males, except the last two bioassayed ages of the predator females (15 and 17 days old). No significant differences were observed in the consumption between E. varians females and males when fed on second instar S. exigua or when both sexes consumed first and second instars S. frugiperda, except for E. varians females at 13 days old (table 2), which consumed significantly more first instar S. exigua than males.
Table 2. Influence of Engytatus varians sex on the predation rate on Spodoptera exigua and Spodoptera frugiperda (eggs, first and second instars) on tomato leaves at different predator ages

a F 35,308 = 32.63, P < 0.0001.
b F 35,314 = 14.58, P < 0.0001 (P < 0.05; GLM, LSD).
Table 3. Predation of Engytatus varians females and males of different ages on developmental stages of Spodoptera exigua and Spodoptera frugiperda (means ± SE) on tomato leaves

Within the same column and Engytatus varians sex, data followed by the same letter are not significantly different (P < 0.05; GLM, LSD).
a Repeated measures ANOVA on the number of consumed preys by E. varians at different ages (*P < 0.05; **P < 0.01; ***P < 0.01; NS = Not significant, P > 0.05).
b F 17,151 = 35.33, P < 0.01.
c F 17,157 = 13.82, P < 0.01.
d F 17,152 = 12.67, P < 0.01.
e F 17,162 = 11.42, P < 0.01.
Engytatus varians females consumed significantly more S. exigua eggs (ranging from 74 ± 5% to 82 ± 4%) than both first (ranging from 25 ± 6% to 41 ± 6%) and second instars (ranging from 4 ± 2% to 24 ± 4%) in all ages of the predator (table 3). This was also observed in males that preyed on more eggs (ranging from 30 ± 3% to 46 ± 5%) than both first (ranging from 9 ± 3% to 33 ± 6%) and second instars (ranging from 3 ± 1% to 15 ± 3%), with the exception of when they were 15 days old, where the number of consumed eggs and first instars was the same (table 3). In addition, no significant differences were observed in predation between first and second instars in four of the six ages tested (7–13 days old). Similarly, E. varians females and males consumed significantly more S. frugiperda eggs than both first and second instars, but no significant differences were observed between both larval instars (table 3).
In most of the cases, the predation by E. varians was not significantly affected by the predator age (table 3); however, in the cases where it was, for example, female predation on second instar S. exigua (F 5,42 = 6.32; P = 0.0002) and S. frugiperda (F 5,44 = 4.98; P = 0.001) and male predation on first (F 5,41 = 4.92; P = 0.000) and second instar S. exigua (F 5,44 = 4.98; P = 0.001), the prey consumption was, in general, positively affected.
Irrespective of the age tested, E. varians females consumed significantly more eggs (F 11,105 = 6.43; P = 0.001) and first instar S. exigua (F 11,91 = 4.1; P = 0.001) than S. frugiperda (table 3). In contrast, no differences in predation on second instar S. exigua and S. frugiperda were observed (P > 0.11 in all cases). Moreover, E. varians males consumed in general similar amounts of eggs as well as first and second instar of both noctuid species (P > 0.09 in all cases).
Discussion
It has been reported that E. varians can prey upon different developmental stages of several vegetable pests (Bueno et al., Reference Bueno, Van Lenteren, Lins, Calixto, Montes, Silva, Santiago and Pérez2013; Silva et al., Reference Silva, Bueno, Montes and van Lenteren2016), being, therefore an interesting candidate to be included in IPM programmes. In the present study, we determined that eggs of B. cockerelli, S. exigua and S. frugiperda and some nymphal or larval instars of these species can be added to the list of prey stages that can be consumed by this predator. The information available about predation on eggs of paurometabolous insects by zoophytophagous mirids is scarce. Deraeocoris ruber (L.) and Campylomma verbasci (Meyer-Dur) can prey on eggs of the psyllid Acizzia jamatonica (Kuwayama) in silk acacia trees (Albizia julibrissin Durazzini) in parks and private gardens of Southern Bulgaria, but not information on the predation rate is provided (Harizanova et al., Reference Harizanova, Stoeva and Mohamedova2012). Nesidiocoris tenuis females are very voracious and can prey 140 eggs of Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) of the 450 offered in 24 h (Baños et al., Reference Baños, Ruiz-Gil, del Toro-Benítez and Miranda-Babrera2016), whereas adults and nymphs of D. tamaninii and Macrolophus caliginosus Wagner prey a much lower number (Barnadas et al., Reference Barnadas, Gabarra and Albajes1998). To our knowledge, D. hesperus is the only mirid that has been reported feeding on B. cockerelli eggs, but at a low rate (5 eggs of the 10 offered in 24 h in a non-choice experiment) (Ramirez-Ahuja et al., Reference Ramirez-Ahuja, Rodríguez-Leyva, Lomeli-Flores, Torres-Ruiz and Guzmán-Franco2017). This information contrasts greatly with our results because E. varians females consumed between 95 and 109 B. cockerelli eggs of the 100–160 offered in 24 h, and therefore this species has a much higher potential as predator and could be an attractive biological control agent for decreasing the number of spring nymphs of this important pest.
In a previous study, E. varians females consumed more third (3.4), four (1.8), and fifth (0.7) instar B. cockerelli than males (≤0.42 for all three instar nymphal) in 24 h when five nymphs of each instar were offered on a tomato leaflet (Mena-Mocino, Reference Mena-Mocino2016). In agreement with this, in our trials, females of this mirid consumed more second (1.9- to 2.5-fold) and third (2.9 to 6.3-fold) instars B. cockerelli than males across all predator ages. The rule seems to be general in mirid species because females need getting more nutrients and energy to ensure the egg production and the development and fitness of their progeny, as stated by López et al. (Reference López, Arce, Villalba and Cagnotti2012) for N. tenuis. As such, Tupiocoris cucurbitaceus (Spinola) females consumed more third and fourth Trialeurodes vaporariorum (Westwood) (Hemiptera: Aleyrodidae) nymphs (López et al., Reference López, Arce, Villalba and Cagnotti2012) and females of M. pygmaeus and Macrolophus costalis Fierber preyed more first to third Myzus persicae (Sulzer) (Hemiptera: Aphididae) nymphs (Margaritopoulos et al., Reference Margaritopoulos, Tsitsipis and Perdikinds2003). Besides, in our study E. varians females of 9 and 10 d old consumed significantly more second than third B. cockerelli nymphs, while males always consumed younger instars (second better than third). The lower energy demand for handling younger instars, less mobile, and sclerotized than the older, could have accounted for such a result (Ramirez-Ahuja et al., Reference Ramirez-Ahuja, Rodríguez-Leyva, Lomeli-Flores, Torres-Ruiz and Guzmán-Franco2017). Similarly, to our findings, N. tenuis adults (the sex was not mentioned) feed more on a mixture of first and second instars T. vaporariorum than on third and four instars (Valderrama et al., Reference Valderrama, Granobles, Valencia and Sánchez2007).
Predation by mirids on Lepidoptera species has been previously documented, but most studies have focused on determining the potential of these predators for controlling T. absoluta (Urbaneja et al., Reference Urbaneja, Montón and Mollá2009; Desneux et al., Reference Desneux, Wajnberg, Wyckhuys, Burgio, Arpaia, Narváez-Vasquez, González-Cabrera, Catalán Ruescas, Tabone, Frandon, Pizzol, Poncet, Cabello and Urbaneja2010; Bueno et al., Reference Bueno, Van Lenteren, Lins, Calixto, Montes, Silva, Santiago and Pérez2013; van Lenteren et al., Reference van Lenteren, Hemerik, Lins and Bueno2016, Reference van Lenteren, Bueno, Smit, Soares, Calixto, Montes and De Jong2017), Helicoverpa armigera Hübner (Izquierdo et al., Reference Izquierdo, Solans and Vitalle1994), and Spodoptera litura Fabricius (Lepidoptera: Noctuidae) (Wei et al., Reference Wei, Xian, Zhou, Whang, Xinghua and Huang1997; Rim et al., Reference Rim, Uefune, Ozawata and Takabayashi2015). However, our study is the first contribution aiming at ascertaining if E. varians could be an interesting biological control agent of S. exigua or S. frugiperda. Similar to the results when using B. cockerelli as prey, E. varians females consumed in general, more S. exigua and S. frugiperda eggs than males across all predator ages. This pattern has been also reported for the mirids Hyanchloria denticornis Tsai Yu-Hsiao and N. tenuis females that consumed more eggs of Anomis texana Riley (Lepidoptera: Noctuidae) (Beingolea, Reference Beingolea1959) and S. exigua (Aragón-Sánchez, Reference Aragón-Sánchez2017) than males, respectively.
In addition, we also observed that predation by E. varians females and males were higher in eggs than in both first and second instars across all predator ages. Similarly, females and nymphs of N. tenuis fed more eggs (13.4 ± 0.6 and 8.4 ± 2 per day, respectively) than first instar S. exigua (7.25 ± 0.4 and 1.75 ± 0.3 per day, respectively) (Aragón-Sánchez, Reference Aragón-Sánchez2017). These results confirm that egg predation seems to be common in mirids and that Lepidopteran eggs offer high quality and nutritional value for E. varians development. In this sense, Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) and S. cerealella eggs have been used to mass rear several mirid species (Encalada and Viñas, Reference Encalada and Viñas1990; Urbaneja et al., Reference Urbaneja, Tapia and Stansly2005; Pérez-Aguilar et al., Reference Pérez-Aguilar, Araújo Soares, Clepf Passos, Martínez, Pineda and Carvalho2018).
In our study E. varians females consumed more first than second instars S. exigua and this is in agreement with the behaviour of another mirid, N. tenuis, on the same prey (Aragón-Sánchez, Reference Aragón-Sánchez2017) and on S. litura (Rim et al., Reference Rim, Uefune, Ozawata and Takabayashi2015). Interestingly, Rim et al. (Reference Rim, Uefune, Ozawata and Takabayashi2015) reported that the preference of N. tenuis for young larvae was related to the total amount of volatiles detected in the host plant and to the host plant damage. Therefore, more studies are needed to determine if the intensity of E. varians attraction could change with the different lepidopteran instars present in the host plant. On the contrary, E. varians males, in most of ages analysed, consumed similar amounts of first and second larval instars S. exigua. As for the second lepidopteran studied as prey, S. frugiperda, both sexes of E. varians consumed of first and second larval instars.
In general, predation rates can be greatly influenced by several characteristics of the prey (e.g., density, activity, and distribution) or predator (e.g., stadium, age, and nutritional status) (Frechette et al., Reference Frechette, Dixon, Alauzet and Hemptinne2004; Lundgren, Reference Lundgren2011; Ramirez-Ahuja et al., Reference Ramirez-Ahuja, Rodríguez-Leyva, Lomeli-Flores, Torres-Ruiz and Guzmán-Franco2017). In previous studies, our group reported that the predation rates of E. varians nymphs were positively affected by the predator age (Pineda et al., Reference Pineda, Medina, Figueroa, Henry, Mena, Chavarrieta and Martínez2016). However, in the present study, this effect was not systematically observed for adults on any prey species. For example, only predation of third instar B. cockerelli declined over the course of the experiment, while predation of Lepidopteran larvae increased in four of the 12 cases analysed (table 3). To confirm these results, further laboratory tests along the entire E. varians adult lifespan are required, which was estimated as ~17 and 22 days for males and females, respectively, when the insects preyed on B. cockerelli third instars + S. cerealella eggs on tomato leaflets (Pineda et al., Reference Pineda, Medina, Figueroa, Henry, Mena, Chavarrieta and Martínez2016) and as ~26 and 32 days, respectively, when the insects preyed on eggs + first instar larvae of T. absoluta on the same plant host (Silva et al., Reference Silva, Bueno, Montes and van Lenteren2016).
Interestingly, only E. varians females consumed both more eggs and first instar larvae S. exigua than the same stages of S. frugiperda, which could indicate, again, that females are sensitive to the prey's particular characteristics (e.g., chorion architecture, nutritional quality of the egg, cuticle characteristics, among others). This behaviour has been suggested for D. tamaninii and M. caliginosus when both fed on T. vaporariorum and H. armigera eggs (Izquierdo et al., Reference Izquierdo, Solans and Vitalle1994). However, to confirm these results, choice experiments are needed to evaluate the mirid's preference when it is exposed to different developmental stages of different prey species. In general, the E. varians predation rates observed on eggs of S. exigua and S. frugiperda were lower or similar than those reported for this same predator (92 eggs per day, equivalent to 61% of predation) and other mirids such as Campyloneuropsis infumatus (Carvalho) and Macrolophus basicornis (Stål) on T. absoluta (51 and 101 eggs per day, equivalent to 34 and 67% of predation, respectively) (Bueno et al., Reference Bueno, Van Lenteren, Lins, Calixto, Montes, Silva, Santiago and Pérez2013). In addition, Mollá (Reference Mollá2013) reported that females of N. tenuis of <5 days old consumed ~80% eggs (n = 94) of T. absoluta in 24 h.
Several studies have supported that the mirid predators can offer advantages as biological control agents (Urbaneja et al., Reference Urbaneja, González-Cabrera, Arnó and Gabarra2012), as they are important generalist predators that regulate arthropod populations. Also, the mirids can establish on crops early in the growing season and can remain there when prey is scarce (Castañé et al., Reference Castañé, Arnó, Gabarra and Alomar2011), as it has been demonstrated with N. tenuis (Sánchez and Lacasa, Reference Sánchez and Lacasa2008; Sanchez, Reference Sanchez2009) and our predator species studied here (Pérez-Aguilar, Reference Pérez-Aguilar2016). In conclusion, we confirmed that E. varians is a potential candidate for biological control of B. cockerelli. On the other hand, further predation studies of this mirid must be performed under more realistic conditions before a final conclusion on its performance as a biological control agent against S. exigua and S. frugiperda can be reached.
Acknowledgements
This work was financially supported by the Coordinación de la Investigación Científica, Universidad Michoacana de San Nicolás de Hidalgo, México.