INTRODUCTION
Contemporary warfare increases the risk of various adverse and functionally relevant psychiatric and neuropsychiatric outcomes, including posttraumatic stress disorder (PTSD) (Eekhout, Reijnen, Vermetten, & Geuze, Reference Eekhout, Reijnen, Vermetten and Geuze2016; Vasterling et al., Reference Vasterling, Aslan, Proctor, Ko, Marx, Jakupcak and Concato2016) and neurocognitive alterations (Vasterling, Proctor, Amoroso, Kane, Heeren, et al., Reference Vasterling, Proctor, Amoroso, Kane, Heeren and White2006). The relationship of combat stress to PTSD is well documented (Ramchand, Rudavsky, Grant, Tanielian, & Jaycox, Reference Ramchand, Rudavsky, Grant, Tanielian and Jaycox2015), but the etiology of neurocognitive compromise in war veterans is less clear. Mild traumatic brain injury (mTBI), a prevalent injury of the wars in Iraq and Afghanistan, has been purported to be a potential source of neurocognitive impairment among warzone veterans. Although there is substantial evidence of acute effects of mTBI on neurocognition (Rabinowitz & Levin, Reference Rabinowitz and Levin2015), meta-analytic studies suggest that recovery typically occurs within 90 days for most milder TBI events (e.g., Karr, Areshenkoff, & Garcia-Berrera, Reference Karr, Areshenkoff and Garcia-Barrera2014). The longer-term impact of deployment-related mild TBI on neurocognitive functioning, however, has been debated, with some evidence suggesting that PTSD and other common psychiatric co-morbidities of deployment TBI may substantially contribute to neurocognitive compromise in warzone veterans with history of mild TBI (e.g., Neipert et al., Reference Neipert, Pastorek, Troyanskaya, Scheibel, Peterson and Levin2014; Vasterling et al., Reference Vasterling, Brailey, Proctor, Kane, Heeren and Franz2012; Verfaellie, Lafleche, Spiro, & Bousquet, Reference Verfaellie, Lafleche, Spiro and Bousquet2014).
Service members and military veterans may also engage in health risk behaviors (e.g., increased alcohol use, reckless driving) associated with PTSD (Kelley et al., Reference Kelley, Athy, Cho, Erickson, King and Cruz2012) that place them at risk for non-deployment TBI subsequent to their return from warzone participation (Regasa, Thomas, Gill, Marion, & Ivins, Reference Regasa, Thomas, Gill, Marion and Ivins2016), but the effects of non-deployment injuries on long-term psychiatric and neurocognitive outcomes among contemporary war zone veterans has received little attention.
The interplay among PTSD, TBI, and neurocognitive functioning is likely complex. As summarized in a recent review (Arnsten, Raskind, Taylor, & Connor, Reference Arnsten, Raskind, Taylor and Connor2015), the animal literature has suggested that the neurocognitive impairment observed in cross-sectional studies of PTSD in humans (Scott et al., Reference Scott, Matt, Wrocklage, Crnich, Jordan, Southwick and Schweinsburg2015) may be attributable to chronic neurobiological disturbances arising from exposure to extreme stress and maintained by PTSD. Evidence from prospective studies of trauma-exposed human populations, however, suggests that pre-exposure neurocognitive performance can moderate the effects of trauma exposure on PTSD development (Marx, Doron-Lamarca, Proctor, & Vasterling, Reference Marx, Doron-Lamarca, Proctor and Vasterling2009; Parslow & Jorm, Reference Parslow and Jorm2007).
Although the mechanisms are not as yet fully understood, greater neurocognitive integrity has been purported to buffer the effects of traumatic stress by enhanced cognitive control of trauma memories, stronger regulation of emotions, and the adaptive re-appraisal of trauma-related cognitions requiring both the inhibition of maladaptive thoughts and the generation of more constructive alternatives. Such bi-directional influences, if present, would potentially create a downward spiral, in which both neurocognitive impairment and PTSD symptoms increase over time. In addition, recent prospective studies of relatively short-term outcomes have indicated that deployment TBI increases risk of PTSD from 3 to 12 months after return from deployment (Mac Donald et al., Reference Mac Donald, Johnson, Wierzechowski, Kassner, Stewart, Nelson and Brody2017; Stein et al., Reference Stein, Kessler, Heeringa, Jain, Campbell-Sills and Cople2015).
Better understanding of longitudinal associations among common risk factors and emotional and neurocognitive outcomes of warzone deployment may inform points of intervention. Much of the existing research, however, has been cross-sectional, limiting the ability to make causal inferences. As part of Veterans Affairs (VA) Cooperative Studies Program #566 (CSP#566), we examined data gathered prospectively from U.S. Army soldiers before (2003–2005) and after (2004–2006) Iraq War deployment, and again at a long-term (>5-year) follow-up assessment (2010–2014). Our goal was to examine the longitudinal associations among PTSD, neurocognitive performance, and TBI following military deployment. Taking into account prior values of outcome variables and adjusting for age, education, race/ethnicity, marital status, military duty status, and combat severity, we hypothesized that for both post-deployment and long-term follow-up outcomes: (1) increases in PTSD symptom severity would be associated with less favorable neurocognitive outcomes; (2) more proficient neurocognitive performance at prior assessments would be associated with less severe PTSD symptoms at subsequent assessments; and (3) TBI (predominantly mild and uniformly post-acute in this sample) would be associated with more severe PTSD symptoms, but would not be associated with neurocognitive outcomes.
METHODS
Sampling and Recruitment
Human subjects approval was obtained from the VA Central Institutional Review Board. All participants provided written, in-person consent for neurocognitive data used in the current analyses; other data (e.g., details of TBI episodes) used in models were obtained previously via verbal, telephone consent.
The current study is a component of a longitudinal cohort study (Neurocognition Deployment Health Study) (Vasterling, Proctor, Amoroso, Kane, Gackstetter, et al., Reference Vasterling, Proctor, Amoroso, Kane, Gackstetter, Ryan and Friedman2006) that has included pre-deployment, post-deployment, and long-term follow-up (CSP#566) assessments that were conducted in reference to an index Iraq War deployment for each participant. NDHS participants were sampled as U.S. Army soldiers at the military battalion level before their deployment to Iraq but, as a nationally dispersed cohort comprised of service members and military veterans at the time of long-term follow-up, were recruited individually for CSP#566. Sampling and recruitment for initial enrollment in Neurocognition Deployment Health Study and for CSP#566 core procedures are described elsewhere (Aslan et al., Reference Aslan, Concato, Peduzzi, Proctor, Schnurr, Marx and Vasterling2013; Vasterling et al., Reference Vasterling, Aslan, Proctor, Ko, Marx, Jakupcak and Concato2016).
Participants for the current study (Supplementary Figure S1) were recruited from 397 CSP#566 participants who had completed pre- and post-deployment neurocognitive testing, and who also scored ≥38, an established cutoff, on a performance validity index (Test of Memory Malingering [TOMM], trial 1) (O’Bryant, Engel, Kleiner, Vasterling, & Black, Reference O’Bryant, Engel, Kleiner, Vasterling and Black2007) at previous assessments. In addition, CSP#566 participants were not considered eligible if their responses on self-report measures suggested invalid responding. Specifically, if their responses were unidirectional on self-report measures in which the pathological response varied in direction (e.g., choosing all “5”s on a Likert scale when “5” could indicate maximum distress on some items but minimum distress on others).
Of this pool, enrollment closed before full recruitment procedures could be completed on 36 participants who were sent an initial invitation, 33 could not be reached, and 13 declined participation, resulting in a sample of 315 participants. Participants in the current study (n=43) were also excluded if, during the current assessment, they scored <38 on the TOMM, trial 1 (n=5) or were determined by consensus discussion to be notably disengaged from study procedures (e.g., not looking at the computer screen for extended periods during task administration) (n=2), if they reported deployment TBI history inconsistently across the post-deployment and long-term follow-up assessments (n=34), completed no questionnaires (n=1), or if they completed neurocognitive procedures >180 days after the phone interview (n=1), yielding an analytic sample of n=272.
Recruitment for the current study was conducted via phone after completion of the core (interview and questionnaire) CSP#566 procedures. Participants could opt to complete neurocognitive assessment procedures at a CSP#566 site (Boston, Seattle) (n=23), or request that a CSP#566 examiner travel to conduct the assessment within the participant’s home community (n=306). Tests were administered in private, quiet settings whether at the study site or within the participant’s community (e.g., rented hotel conference room).
Data Sources
Primary data from pre- and post-deployment assessments, occurring an average of 159.7 days (SD=139.1 days; range=2–589 days) before deployment and 100.3 days (SD=52.4 days; range=38–345 days) after return from deployment, were gathered in-person at military installations. TBI and PTSD data were derived from CSP#566 (long-term follow-up) phone interviews and mail surveys, occurring an average of 91.0 months (SD=11.4 months; or 7.6 years, after the post-deployment assessment; range=65.7–114.4 months). Details of the pre-deployment, post-deployment, and long-term follow-up assessments are described elsewhere (Vasterling, Proctor, Amoroso, Kane, Gacksetter, et al., Reference Vasterling, Proctor, Amoroso, Kane, Heeren and White2006; Aslan et al., Reference Aslan, Concato, Peduzzi, Proctor, Schnurr, Marx and Vasterling2013). Deployment information was verified by Defense Manpower Data Center records.
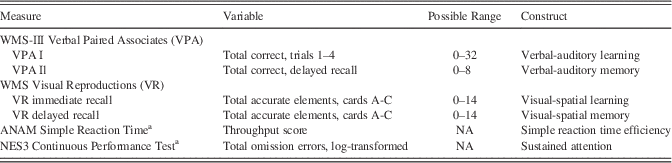
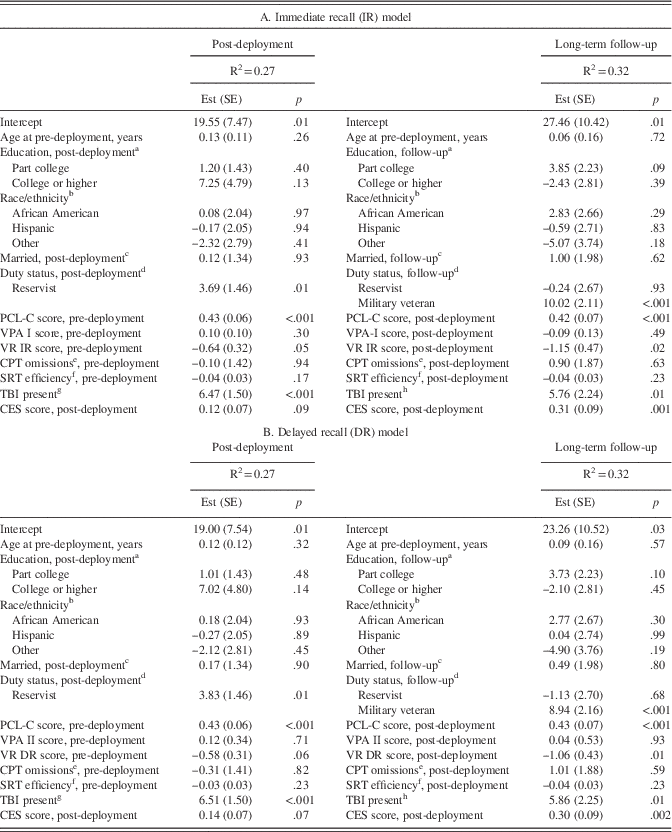
The neurocognitive battery at long-term follow-up, administered an average of 34.2 days (SD=25.2 days; range=0–177 days) after the CSP#566 core assessment, included standardized tests of verbal-auditory learning and memory (Wechsler Memory Scale, 3rd Edition, Verbal Paired Associates I and II, respectively) (Wechsler, Reference Wechsler1997), visual-spatial learning and memory (Wechsler Memory Scale Visual Reproductions, immediate and delayed recall, respectively) (Wechsler, Reference Wechsler1945), reaction time efficiency (Automated Neuropsychological Assessment Metrics Simple Reaction Time) (Reeves, Kane, Elsmore, Winter, & Bleiberg, Reference Reeves, Kane, Elsmore, Winter and Bleiberg2002), and sustained attention (Neurobehavioral Evaluation System, 3rd Edition., Continuous Performance Test) (Letz, Green, & Woodard, Reference Letz, Green and Woodard1996). These tests were selected on the basis of previous Neurocognition Deployment Health Study findings indicating deployment-related performance differences (Vasterling, Proctor, Amoroso, Kane, Heeren, et al., Reference Vasterling, Proctor, Amoroso, Kane, Heeren and White2006).
Variables extracted from these tests can be found in Table 1. All scoring was free of subjective judgment except for Visual Reproductions, which was scored according to set criteria. As with pre- and post-deployment assessments (Vasterling, Proctor, Amoroso, Kane, Heeren, et al., Reference Vasterling, Proctor, Amoroso, Kane, Heeren and White2006), long-term follow-up reliability ratings, performed on 12% of randomly selected drawings by a second rater blinded to other study data, indicated high inter-rater reliability [intraclass correlations (ICC)=0.94 for immediate recall; ICC=0.95 for delayed recall].
Table 1 Neuropsychological measures
WMS-III=Wechsler Memory Scale, 3rd edition. WMS=Wechsler Memory Scale. ANAM=Automated Neuropsychological Assessment Metric. NES3=Neurobehavioral Evaluation System, 3rd Edition. NA=non-applicable. aComputer-assisted administration. Scores are raw scores unless otherwise indicated.
The PTSD Checklist-Civilian version (PCL-C; Ruggiero, Del Ben, Scotti, & Rabalais, Reference Ruggiero, Del Ben, Scotti and Rabalais2003), yielding a summary score of 17–85, was administered at all time-points and was used as a self-report measure of Diagnostic and Statistical Manual, 4th Edition text revision (DSM-IV-TR; American Psychiatric Association, 2000) PTSD symptom severity for the primary analyses. At long-term follow-up, the PCL-C summary score was highly correlated (Pearson r=0.82; p<.0001) with the summary score of the Clinician Administered PTSD Scale (CAPS; Blake et al., Reference Blake, Weathers, Nagy, Kaloupek, Gusman, Charney and Keane1995), a highly reliable structured interview considered to be a benchmark instrument for DSM-IV PTSD assessment (Weathers, Keane, & Davidson, Reference Weathers, Keane and Davidson2001). The CAPS was administered only at long-term follow-up and, therefore, was not used in our longitudinal analyses. For the purposes of sample characterization only, the PCL-C was also used to determine probable PTSD cases at each assessment.
Congruent with other studies of Iraq deployment (Smith et al., Reference Smith, Ryan, Wingard, Slymen, Salis and Kritz-Silverstein2008; Sundin et al., Reference Sundin, Herrell, Hoge, Fear, Adler, Greenberg and Bliese2014), case definition was based on DSM-IV-TR symptom congruency and a cut-off of 50 on the summary score. Combat exposure was assessed using the Deployment Risk and Resilience Inventory (DRRI; Vogt, Proctor, King, King, & Vasterling, Reference Vogt, Proctor, King, King and Vasterling2008) Combat Experiences Scale (CES), modified to include a frequency-based response scale and an item pertaining to convoy participation. Archived CES data, obtained at the post-deployment assessment, measured combat exposure between pre- and post-deployment. CSP#566 participants who deployed at least once following their post-deployment assessment completed the CES again, by telephone interview, in reference to their most recent deployment. CSP#566 participants who did not deploy between post-deployment and follow-up assessment were assigned a CES score of 0 for the post-deployment to follow-up time period.
TBI during the index deployment was ascertained by structured in-person interview during the post-deployment assessment. Of note, at the time of the index deployment (2003–2005), TBI events in the war-zone were not systematically captured within military medical records. In the context of time constraints associated with conduct of the study at military installations, and consistent with the early focus of the study on environmental hazards and stress, the post-deployment interview documented only TBI events associated with outright loss of consciousness (i.e., “knocked out”) (Vasterling et al., Reference Vasterling, Brailey, Proctor, Kane, Heeren and Franz2012). This prioritization reflected attempts to capture those events with greater risk of adverse deployment-related clinical outcomes (Hoge et al., Reference Hoge, McGurk, Thomas, Cox, Engel and Castro2008; Luethcke et al., Reference Luethcke, Bryan, Morrow and Isler2011).
Reflecting developing knowledge over the course of this multi-year longitudinal study, and long-term follow-up assessments conducted individually outside the context of military duties, lifetime history of TBI events associated with either (a) alteration of consciousness (explained as “dazed” or loss of memory for “what was happening during, immediately before, or immediately after the injury”), or (b) loss of consciousness, was documented at long-term follow-up (see Alosco et al., Reference Alosco, Aslan, Du, Ko, Grande, Proctor and Vasterling2016 for further details). Long-term follow-up TBI interviews were conducted by a clinical psychologist using a structured phone interview.
Characteristics of the five most serious injuries for each participant were documented, including those experienced during the index deployment. Specifically, we used long-term follow-up report of TBI events with altered consciousness to supplement the post-deployment assessment report of TBI events with loss of consciousness during the index deployment. At both assessments, examiners clarified questions, as necessary, when participants were uncertain about their meaning. TBI classification required head injury or blast exposure (“injuries to your head or close exposures to explosive blasts”), with alteration or loss of consciousness. Each TBI event was categorized as mild or moderate-severe, based on commonly used categorizations for duration of loss of consciousness (mild:<30 min) and posttraumatic amnesia (mild:<24 hr).
Statistical Analyses
Data were analyzed using SAS v9.2 (SAS Institute Inc, Cary, NC) and Spotfire S+v8.2 (TIBCO Software Inc, Palo Alto, CA). Values for each missing PCL-C item, involving<5% of participants, were imputed case-wise, using the mean value of the PCL-C items completed during the relevant assessment episode for that person. The maximum number of missing items per individual case at each PCL-C administration was five (29% of 17 total items); no cases were missing>50% of the items relevant to each PTSD symptom cluster (DSM-IV-TR Criteria B, C, D). Values for missing CES items, also involving<5% of participants, were imputed case-wise using the mean value of the remaining completed items for that person. The maximum number of missing items per individual case at each CES administration was four (25% of 16 total items); three participants (1%) with multiple deployments were missing the entire CES on their most recent deployment.
To examine associations between the change in PTSD symptoms from pre- to post-deployment and post-deployment neurocognitive outcomes (hypothesis 1), we evaluated separate autoregressive linear regression models for each neurocognitive variable, adjusted for age at pre-deployment assessment. Age, pre-deployment (“autoregressive”) values of post-deployment outcomes, pre- to post-deployment PCL-C difference scores, and the presence or absence of any TBI during the index deployment, were entered simultaneously; CES scores were added in a second step. Associations between change in PTSD symptoms from post-deployment to long-term follow-up with neurocognitive outcomes at long-term follow-up were modeled similarly, but with values relevant to the (a) time period of interest, and (b) presence or absence of any TBI event experienced between the post-deployment and long-term follow-up assessments.
To examine associations between pre-deployment neurocognitive test performances (hypothesis 2) and TBI (hypothesis 3) with post-deployment PTSD symptom outcomes, as measured by PCL-C summary scores, we evaluated two autoregressive linear regression models. Both models adjusted for age at pre-deployment assessment. Model 1 included simultaneous entry of Visual Reproductions, immediate recall (visual-spatial learning), Verbal Paired Associates I (verbal-auditory learning), Simple Reaction Time throughput (reaction time efficiency), and Continuous Performance Test log-transformed omission errors (sustained attention) as neurocognitive predictors, as well as the presence or absence of TBI during the index deployment; age and the pre-deployment PCL-C summary score were also included.
CES scores were added in a second step to account for environments (i.e., high combat intensity) hypothesized to increase risk of both TBI and PTSD. Model 2 was identical to model 1, but consistent with prior research (Marx, Doron-Lamarca, et al., Reference Marx, Doron-Lamarca, Proctor and Vasterling2009), Visual Reproductions delayed recall and Verbal Paired Associates II (indices of visual and verbal memory, respectively) were substituted for Visual Reproductions immediate recall and Verbal Paired Associates I, respectively, due to high intercorrelations between learning and memory indices. Associations between post-deployment neurocognitive performances and long-term PTSD symptom severity outcomes (CSP#566 assessment) were modeled similarly, but with values relevant to the time period of interest (hypotheses 2 and 3).
RESULTS
Participants
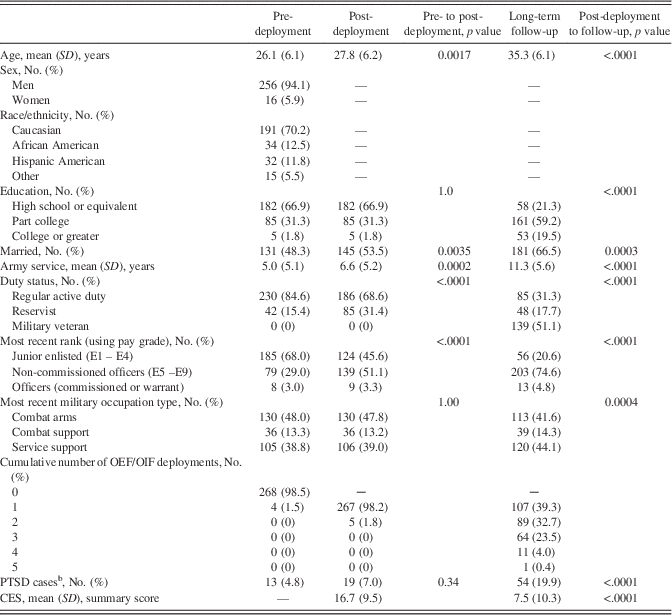
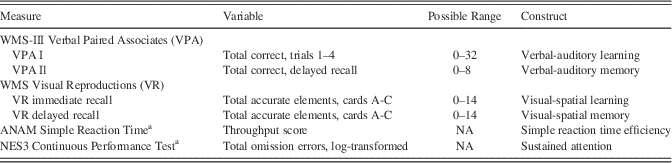
Sample characteristics are presented in Table 2. The majority of participants were men, Caucasian, and relatively young in age and without significant military experience at pre-deployment. Over time, an increasing number of participants obtained additional formal education, married, separated from military service, received military promotions, served in service support roles (with fewer in combat arms occupational specialties), deployed again, and were more likely to meet PTSD case definition (20% at long-term follow-up). TBI (Supplementary Table S1) was more prevalent between pre- and post-deployment assessment (n=73) than between post-deployment assessment and long-term follow-up (n=60). The majority of TBI events identified by participants as being the “most serious” were mild (89% of TBI events experienced between pre- and post-deployment assessment;>83% of TBI events experienced between post-deployment assessment and long-term follow-up). Almost all (>98%) of the most recent events during each interval were post-acute (>3 months) at the time of neurocognitive testing. Participants also more frequently reported one event (vs.>1 event) during both intervals.
Table 2 Participant characteristics across assessments (n=272a)
Data were tested with a McNemar test (categorical) or a paired t test (continuous). OEF=Operation Enduring Freedom. OIF=Operation Iraqi Freedom. PTSD=posttraumatic stress disorder. CES=Combat Experiences Scale. aVaries slightly across variables as a function of missing data. bPTSD cases were derived from the PTSD Checklist, civilian version.
In aggregate, PTSD symptom severity, as measured by PCL-C summary scores, increased from pre- to post-deployment, and again from post-deployment to long-term follow-up (Supplementary Table S2). Neurocognitive performances either did not change significantly or improved over time (Supplementary Table S2). The analytic sample (n=272) resembled the eligible participant pool (n=397) on all sample characteristics (Supplementary Table S3).
Hypothesis 1: Association of PTSD Symptom Increases with Neurocognitive Performances
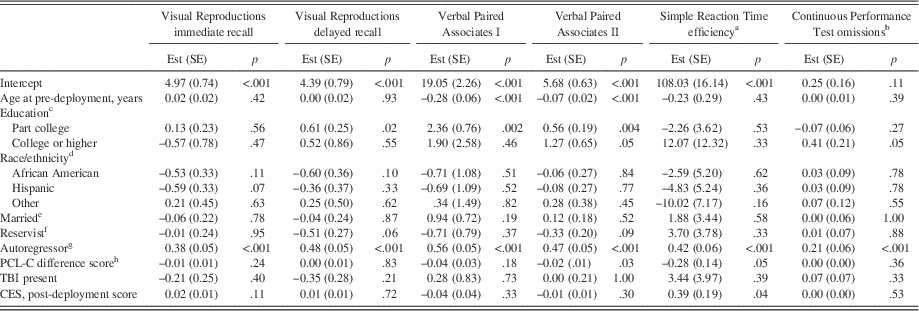
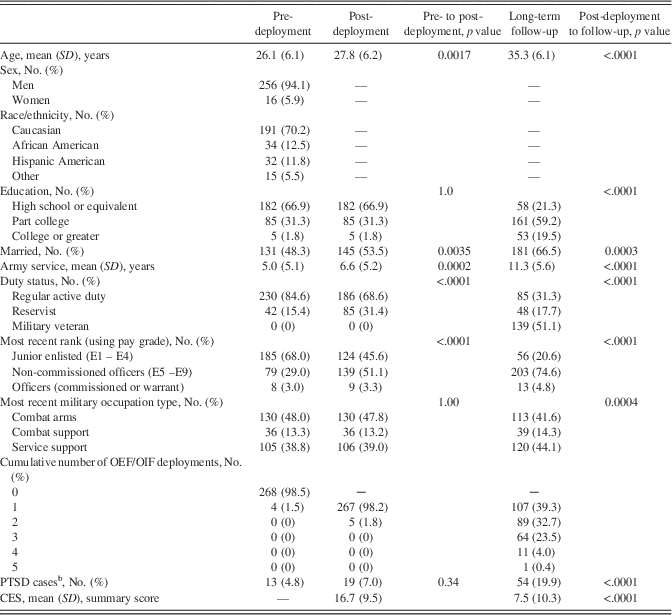
Examining neurocognitive performances as post-deployment outcomes (Table 3), fully adjusted linear regression models showed that increases in PTSD symptom severity from pre- to post-deployment were significantly associated with less proficient Verbal Paired Associates II and Simple Reaction Time throughput scores. In contrast, greater combat exposure was significantly associated with more proficient Simple Reaction Time throughput scores.
Table 3 Final regression models for neuropsychological performance at post-deployment
Higher neuropsychological scores indicate more proficient performance except for Continuous Performance Test omissions. All demographic and military variables pertain to post-deployment values unless otherwise indicated. R2 values=0.21, 0.27, 0.40, 0.41, 0.22, 0.07 for Visual Reproductions immediate recall, Visual Reproductions delayed recall, Verbal Paired Associates I, Verbal Paired Associates II, Simple Reaction Time efficiency, and Continuous Performance Test omissions, respectively. PTSD=posttraumatic stress disorder. TBI=traumatic brain injury. Est (SE)=estimate (standard error). PCL-C=PTSD Checklist, civilian version. CES=Combat Experiences Scale. aThroughput scores. bLog-transformed. cReference=high school or equivalent. dReference=Caucasian. eReference=not married. fReference=active duty. gDenotes the pre-deployment value of the corresponding neuropsychological outcome variable. hCalculated as (post-deployment PCL-C score – pre-deployment PCL-C score).
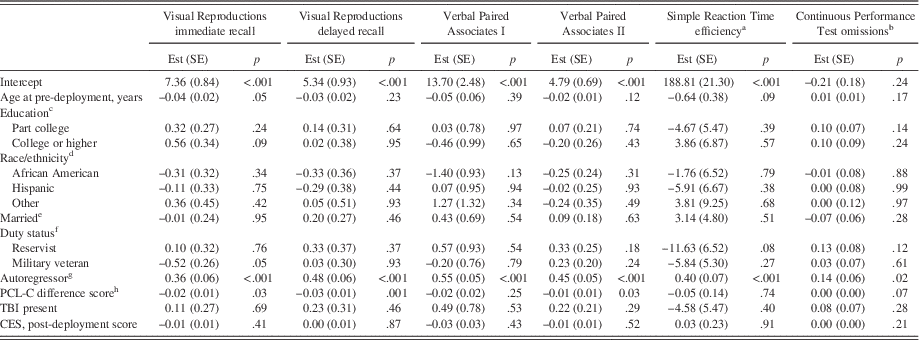
Fully adjusted models at long-term follow-up (Table 4) indicated that increases in PTSD symptom severity from post-deployment to long-term follow-up were significantly associated with less proficient scores on Visual Reproductions immediate and delayed recall, and Verbal Paired Associates II.
Table 4 Final regression models for neuropsychological performance at long-term follow-up
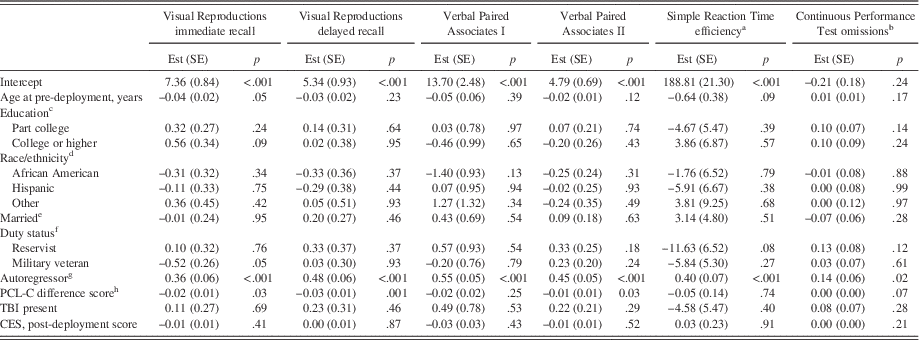
Higher neuropsychological scores indicate more proficient performance except for Continuous Performance Test omissions. All demographic and military variables pertain to long-term follow-up values unless otherwise indicated. R2 values=0.22, 0.31, 0.42, 0.33, 0.17, 0.08 for Visual Reproductions immediate recall, Visual Reproductions delayed recall, Verbal Paired Associates I, Verbal Paired Associates II, Simple Reaction Time efficiency, and Continuous Performance Test omissions, respectively. PTSD=posttraumatic stress disorder. TBI=traumatic brain injury. Est (SE)=estimate (standard error). PCL-C=PTSD Checklist, civilian version. CES=Combat Experiences Scale. aThroughput scores. bLog-transformed. cReference=high school or equivalent. dReference=Caucasian. eReference=not married. fReference=active duty. gDenotes the post-deployment value of the corresponding neuropsychological outcome variable. hCalculated as (long-term follow-up PCL-C score – post-deployment PCL-C score).
Associations between increases in PTSD symptom severity and neurocognitive outcomes were similar in models unadjusted for combat severity (data not shown).
Hypothesis 2: Association of Neurocognitive Performance with PTSD Symptom Severity as an Outcome
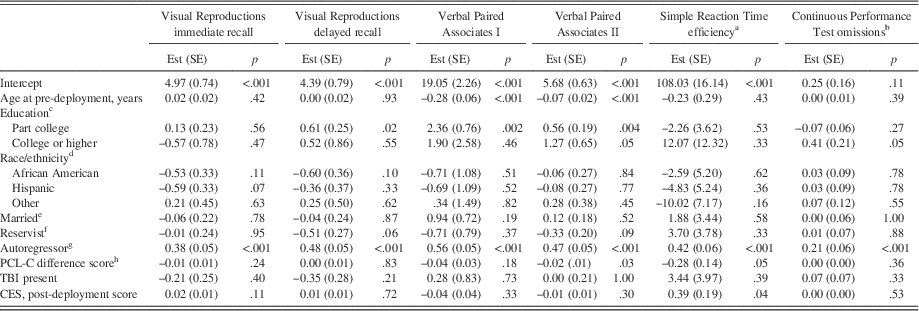
As shown in Table 5, fully adjusted linear regression models examining PTSD symptom severity as an outcome at post-deployment indicated an inverse relationship between performance on Visual Reproductions immediate recall at pre-deployment and post-deployment PTSD symptom severity.
Table 5 PCL-C outcomes at post-deployment and long-term follow-up, examining immediate (A) and delayed (B) recall scores separately
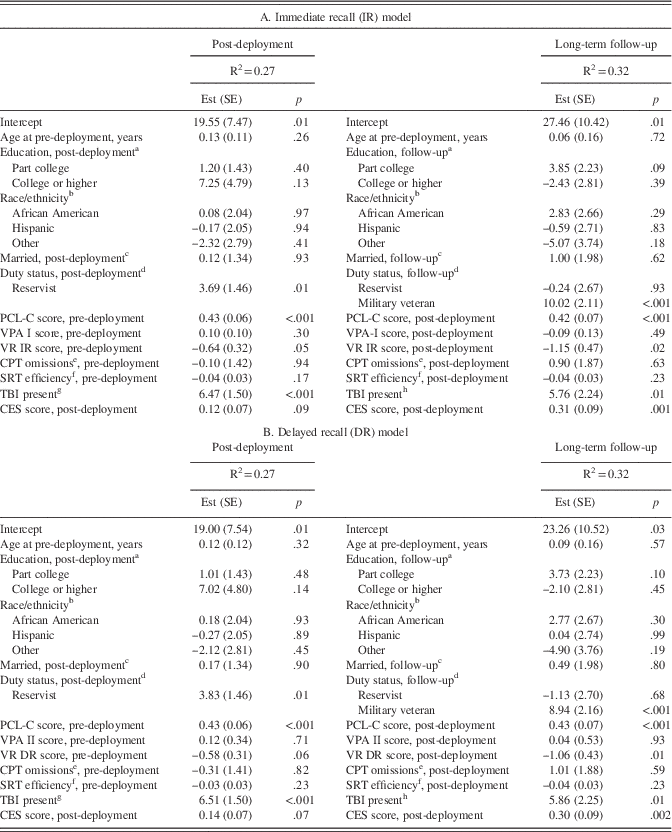
Higher scores on neuropsychological tests indicate more proficient performance except for Continuous Performance Test omissions. PCL-C=PTSD Checklist, civilian version. Est (SE)=estimate (standard error). VPA=Verbal Paired Associates. VR=Visual Reproductions. IR=immediate recall. DR=delayed recall. SRT=Simple Reaction Time. CPT=Continuous Performance Test. TBI=traumatic brain injury. CES=Combat Experiences Scale. aReference=high school or equivalent. bReference=Caucasian. cReference=not married. dReference=active duty. eLog-transformed. fThroughput scores. gPre-deployment to post-deployment assessment. hPost-deployment to long-term follow-up.
Also shown in Table 5, fully adjusted models examining PTSD symptom severity as an outcome at long-term follow-up showed that more proficient post-deployment Visual Reproductions immediate recall and delayed recall scores were associated with less severe PTSD symptoms at long-term follow-up.
The patterns of association were similar in models unadjusted for combat severity (data not shown).
Hypothesis 3: Association of TBI with Neurocognitive Outcomes and PTSD Symptom Severity
TBI was not significantly associated with any neurocognitive outcomes at either post-deployment or long-term follow-up in both models, either unadjusted for combat severity (data not shown) or in fully adjusted models (Tables 3 and 4). As shown in Table 5, fully adjusted linear regression models inclusive of pre-deployment neurocognitive scores, as predictors of post-deployment PTSD symptom severity, indicated that the presence of TBI between pre- and post-deployment assessments was independently associated with more severe post-deployment PTSD symptoms, in both immediate and delayed recall models. Similarly, the presence of TBI between post-deployment assessment and long-term follow-up assessment was independently associated with more severe PTSD symptoms at long-term follow-up for both immediate and delayed recall models.
DISCUSSION
In this prospective study of U.S. Army soldiers who deployed to the Iraq War, we examined longitudinal relationships among PTSD symptom severity, TBI, and neurocognitive functioning over almost a decade, including assessments conducted before and after an index deployment, and a long-term follow-up assessment. Our findings, adjusted for age, education, race/ethnicity, military duty status, marital status, baseline values of the outcome variables, and combat severity, indicated a complex pattern of associations among PTSD symptom severity, learning and memory performance, and TBI that may contribute to sustaining adverse mental health outcomes over time. This study is novel in examining long-term longitudinal associations, inclusive of baseline data, among these deployment-relevant variable domains.
Associations between PTSD symptom severity and learning and memory performance in fully adjusted models (including adjustment for prior values of the outcome variables) suggest a possible bi-directional relationship between PTSD symptom severity and memory processes. Specifically, increases in PTSD symptom severity from pre- to post-deployment were associated with less proficient verbal memory; and, increases in PTSD symptom severity from post-deployment to long-term follow-up were associated with less proficient visual learning and memory in addition to less proficient verbal memory. Conversely, more proficient pre-deployment visual learning was associated with less severe post-deployment PTSD symptoms, with a similar pattern observed between post-deployment visual learning and memory performance and PTSD symptom severity at long-term follow-up.
A growing literature suggests that neurocognitive integrity, including learning and memory proficiency, may confer modest protection against PTSD development, or the severity of its expression, following trauma (Marx, Doron-Lamarca, et al., Reference Marx, Doron-Lamarca, Proctor and Vasterling2009; Parslow & Jorm, Reference Parslow and Jorm2007). Our findings additionally suggest that variation in visual learning and memory integrity may moderate the long-term course of PTSD symptoms subsequent to its initial development. Although some conceptualizations of trauma memory suggest that semantic memory enhances the reconstruction of trauma narratives in a constructive manner (Brewin, Gregory, Lipton, & Burgess, Reference Brewin, Gregory, Lipton and Burgess2010) and might predict that more proficient verbal memory, as compared with visual memory, would be more strongly associated with subsequent PTSD severity, it may also be that the ability to form a visual image facilitates rehearsal and habituation to the perceptual aspects of a trauma event, as well as their integration into a coherent narrative (Brewin et al., Reference Brewin, Gregory, Lipton and Burgess2010).
This notion is supported by studies indicating that visual imagery can be more effective than verbal processing in reducing anxiety (Holmes & Mathews, Reference Holmes and Mathews2005) and by findings showing that decreased visual input reduces the recollection of autobiographical events (Rubin, Burt, & Fifeld, Reference Rubin, Burt and Fifeld2003). Moreover, as suggested by Gilbertson et al. (Reference Gilbertson, Williston, Paulus, Lasko, Gurvits, Shenton and Orr2007), it may be that small hippocampal volume, possibly reflected in our study by relatively less proficient performance on visual learning and memory tasks, increases risk of greater subsequent PTSD severity through the failure to support visually-mediated extinction of conditioned emotional responses.
Consistent with conceptualizations of PTSD as a biopsychosocial disorder involving alterations in neural functioning (Pitman et al., Reference Pitman, Rasmusson, Koenen, Shin, Orr, Gilbertson and Liberzon2012), our findings also suggest that PTSD may subtly erode these potentially resilience-enhancing cognitive resources (i.e., learning and memory) as PTSD symptoms increase in severity. For example, neuroendocrine dysfunction, including dysregulation of glucocorticoids, is thought to be a core neurobiological feature of PTSD. Relevant to our findings, there are well-documented links between glucocorticoids and memory (Wingenfeld & Wolf, Reference Wingenfeld and Wolf2011). Our findings taken as a whole suggest a bi-directional relationship between memory and PTSD symptom severity, a relationship that we detected even in the absence of clinically significant (>15%) decline in learning and memory.
Also consistent with our previous findings using overlapping samples (Marx, Brailey, et al., Reference Marx, Brailey, Proctor, Macdonald, Graefe, Amoroso and Vasterling2009; Vasterling, Proctor, Amoroso, Kane, Heeren, et al., Reference Vasterling, Proctor, Amoroso, Kane, Heeren and White2006), we found that more extensive combat exposure was modestly associated with greater efficiency on a reaction time test (Simple Reaction Time) at post-deployment assessment. This finding can be interpreted as part of a broader unresolved stress response in which organisms prepare for survival in life-threatening contexts such as combat, in part via heightened arousal and in part by focusing attention more narrowly. Simple Reaction Time, which requires speeded responses to simple recurrent targets, could be hypothesized to capture this evolutionarily adaptive response. Conversely, increases in PTSD symptom severity from pre- to post-deployment were associated with less efficient reaction time, suggesting that the exposure to potential threat is dissociable from post-traumatic emotional responses in their effects on reaction time.
Regarding TBI, we found that the presence of TBI (predominantly mild in our sample) during each assessment interval (i.e., pre- to post-deployment; post-deployment to long-term follow-up) was associated with more severe PTSD symptoms at the conclusion of the interval. It has been hypothesized that the psychological trauma associated with deployment TBI, and the warzone context in which it occurs, accounts for the increased risk of PTSD in warzone veterans with history of mild TBI (Hoge et al., Reference Hoge, McGurk, Thomas, Cox, Engel and Castro2008). In contrast, our findings, which were adjusted for combat severity, suggest that mild TBI contributes to PTSD symptom expression independently of combat stress and are consistent with Yurgil et al. (Reference Yurgil, Barkauskas, Vasterling, Nievergelt, Larson and Schork2014), who found that deployment TBI predicted postdeployment PTSD after adjusting for combat severity.
Additionally, TBI events in our study were not limited to deployment, particularly during the post-deployment to long-term follow-up period. Consistent with a longitudinal study of civilians indicating that mild TBI experienced outside the context of combat was associated with poorer PTSD outcomes, both within the year after the injury (Bryant et al., Reference Bryant, O’Donnell, Creamer, McFarlane, Clark and Silove2010) and over 6 years later (Bryant et al., Reference Bryant, Nickerson, Creamer, O’Donnell, Forbes, Galatzer-Levy and Silove2015), our findings indicate that increased risk of PTSD following mild TBI in military populations is not restricted to deployment TBI. Other potential mechanisms explaining the association between TBI and PTSD, including increased disease burden via undocumented TBI co-morbidities (e.g., associated orthopedic injury) and acute TBI-related mental status changes affecting the formation of trauma memories in a manner precluding optimal subsequent processing of the memories (Vasterling, Verfaellie, & Sullivan, Reference Vasterling, Verfaellie and Sullivan2009), could not be tested by our study design.
TBI was not significantly associated with any measure of neurocognitive functioning during any time period, either before (data not shown) or after adjustment for combat severity. This finding, in the context of predominantly mild TBI experienced over three months before pre-deployment testing and over a year before long-term follow-up testing in most of our sample, is consistent with meta-analytic research suggesting that acute neurocognitive decrements following mild TBI tend to resolve within 90 days in most people (Karr et al., Reference Karr, Areshenkoff and Garcia-Barrera2014). Recent studies, however, suggest that white matter abnormalities may mediate the relationship of mild deployment TBI to neurocognitive performance (Clark et al., Reference Clark, Sorg, Schiehser, Luc, Bondi, Sanderson and Delano-Wood2016; Hayes, Miller, Lafleche, Salat, & Verfaellie, Reference Hayes, Miller, Lafleche, Salat and Verfaellie2015). Thus, it remains unclear whether our results and other findings relying on aggregate data potentially mask poor performance in subgroups for whom deficits do not resolve as quickly as in the majority of those experiencing mild TBI (Belanger, Curtiss, Demery, Lebowitz, & Vanderploeg, Reference Belanger, Curtiss, Demery, Lebowitz and Vanderploeg2005; Pertab, James, & Bigler, Reference Pertab, James and Bigler2009).
Our findings should be interpreted in the context of the study’s methodological strengths and limitations. Although our TBI interview administered at long-term follow-up has been shown to have high inter-rater reliability and was conducted by doctoral level clinicians (Alosco et al., Reference Alosco, Aslan, Du, Ko, Grande, Proctor and Vasterling2016), retrospective report of TBI events may be subject to memory and other reporting biases. To minimize this possibility, we used data from our post-deployment TBI interview to document TBI with loss of consciousness and excluded participants who reported deployment interval TBI inconsistently across post-deployment and long-term follow-up assessments. Because we did not capture TBI with altered consciousness at the post-deployment assessment, however, we relied on long-term follow-up accounts of these events to supplement our post-deployment assessment of TBI with loss of consciousness.
Findings should also be interpreted in the context of the TBI events captured. Although most were mild, a small proportion (11.0% occurring between pre- and post-deployment assessments; 16.7% of those occurring between post-deployment and long-term follow-up assessments) were categorized as greater than mild, thereby introducing some heterogeneity of severity. We additionally did not capture the mechanism of injury (e.g., blast, blunt trauma, etc.), although there is not yet clear evidence that mechanism of injury strongly influences neuropsychological outcomes in military populations (Belanger, Kretzmer, Yoash-Gantz, Pickett, & Tupler, Reference Belanger, Kretzmer, Yoash-Gantz, Pickett and Tupler2009; Mac Donald et al., Reference Mac Donald, Johnson, Wierzechowski, Kassner, Stewart, Nelson and Brody2014). Finally, we examined non-deployment-related TBI in addition to deployment TBI. The inclusion of both deployment and non-deployment-related TBI, however, enhances the generalization of findings within the military and military veteran population, a population at risk for TBI events in civilian as well as military contexts (Armistead-Jehle, Soble, Cooper, & Belanger, Reference Armistead-Jehle, Soble, Cooper and Belanger2017; Regasa et al., Reference Regasa, Thomas, Gill, Marion and Ivins2016).
We assessed a relatively limited range of neurocognitive functions and could not feasibly capture other neurobiologically relevant data (e.g., neuroimaging, biological assays), but our measures were selected based on their sensitivity to deployment-related effects and included a performance based validity index as an eligibility criterion, a feature that has been only infrequently included in studies of PTSD and neurocognition. Furthermore, our use of performance-based tests individually administered in person, in the context of a longitudinal study of a nationally-dispersed sample, is a rare methodological strength. PTSD symptom severity was measured via a face valid self-report instrument (i.e., PCL-C), but the PCL-C, designed to measure symptom severity, has strong psychometric properties (Wilkins, Lang, & Norman, Reference Wilkins, Lang and Norman2011) and showed strong correlations with CAPS symptom severity scores when analyzed as a severity measure. Notably, a major methodological strength of our study was our ability to account for prior values of both PTSD symptom and neuropsychological outcomes, including pre-deployments levels.
It is possible that conditions that are commonly co-morbid to PTSD and/or TBI but that were not captured in this study (e.g., depression, chronic pain) contributed to neuropsychological decrements. Of note, there were only a small number of women in the sample, thereby precluding examination of any gender effects. Finally, because a comparison sample of non-deployed soldiers was no longer plausible within the NDHS cohort, we were unable to examine the longer-term effects of deployment (vs. non-deployment).
In conclusion, although our sample displayed relatively robust neural health, as indicated by neurocognitive performances, findings indicate specific risk factors for relative neurocognitive performance decrements and suggest that the constellation of PTSD, TBI, and relative neurocognitive decrements may contribute, via their longitudinal associations, to sustaining emotional and neurocognitive symptoms over time. More generally, these novel findings help elucidate potential bi-directional relationships between neurocognition, as a behavioral and mechanistically relevant indicator of neural health, and PTSD in trauma-exposed populations and highlight neurocognition as both a predictor and outcome of emotional distress following trauma.
ACKNOWLEDGMENTS
Funding was provided by the Department of Veterans Affairs (VA) Cooperative Studies Program (CSP#566). Support for previous collection of archived data used in this study’s longitudinal analyses was provided by the U.S. Army Medical Research and Materiel Command (DAMD 17-03-0020) and VA Clinical Sciences Research and Development. The funding agency (VA) provided general guidance and policies for conducting human subjects research. The manuscript underwent administrative review at the U.S. Army Research Institute of Environmental Medicine. The views expressed in this article are those of the authors and do not reflect official policy or position of the U.S. Army, Department of Defense, VA, or U.S. government. The authors have no conflicts of interest to declare. We appreciate the support of the Defense Manpower Data Center in obtaining military service status data. We also thank the VACSP#566 team, including Drs. Helen MacDonald and Christopher Harte for their leadership of clinical data collection, Patricia Crutchfield and Joseph Turner for their organizational and administrative support, Drs. Matthew Jakupcak, Brian Marx, and Miles McFall for their roles as site investigators, our dedicated team of study psychologists, Drs. Grant Huang and Theresa Gleason for their support of the study, and the VACSP#566 Executive Committee for their on-going guidance. Finally, we are grateful to the Soldiers and military veterans who comprise the Neurocognition Deployment Health Study cohort for volunteering their time to participate in the study, and also for their military service.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617717001059