INTRODUCTION
The reef ecosystem holds a high diversity of species living associated (Pennings, Reference Pennings and Birkeland1997; Sale, Reference Sale2002) and is known for their ecological, economic, and social importance (Bryant et al., Reference Bryant, Burke, Mcmanus and Spalding1998). Reefs represent hard substrates colonized by diverse coral fauna and can take the form of banks or sandstones reefs (beach rocks), algal reefs, volcanic islands, ship wrecks or coral reefs (Amaral et al., Reference Amaral, Broadhurst, Cairns and Schlenz2002, Reference Amaral, Hudson, Steiner and Ramos2007).
Despite the fact that there is not a high diversity of corals in Brazilian waters, most of the species can be considered endemic (Leão & Dominguez, Reference Leão and Dominguez2000). This fact characterizes Brazilian reef ecosystem as the one with higher rates of endemism in the world (Maida & Ferreira et al., Reference Maida and Ferreira1997). However, the structural complexity of reefs in the region is smaller compared to the Tropical Caribbean and Indo Pacific, except for the branched fire coral of the genus Millepora (Leão & Dominguez, Reference Leão and Dominguez2000).
Species from the genus Millepora are colonial polypoid hydrocorals (Boschma, Reference Boschma1948) found on coastal reefs throughout the world and considered the second most important reef formers, being surpassed only by hermatypic corals (Lewis, Reference Lewis1989). Four species of the genus Millepora have been identified along the Brazilian coast: Millepora alcicornis (Linnaeus, 1758), M. brasiliensis (Verrill, 1868), M. nitida (Verrill, 1868) and M. laboreli (Amaral et al., Reference Amaral, Steiner, Broadhurst and Cairns2008). Millepora alcicornis and M. braziliensis are considered abundant in Brazilian waters, especially in the north-east. Both occur at the edges of reefs, forming colonies that can reach 2 m in diameter. The habitat they occupy can be compared to the one used by the species of the genus Acropora, such as Acropora palmata and A. cervicornis in the Caribbean (Leão et al., Reference Leão, Kikuchi and Testa2003).
Millepora spp. form complex physical and biological systems, such as holes, crevices, and areas between branches that provide mutualism among species and offer refuge from predators. This structure increases the density, biomass and diversity of fish and invertebrates (Svane & Petersen, Reference Svane and Petersen2001; Garcia et al., Reference Garcia, Matthews-Cascon and Franklin-Junior2008; Rotjan & Lewis, Reference Rotjan and Lewis2008; Guichard et al., Reference Guichard, Bourget and Robert2001; Johnson et al., 2011; Pereira et al., Reference Pereira, Leal, Araújo and Souza2012), consequently reducing the predation risk (Leão & Dominguez, Reference Leão and Dominguez2000; Johnson et al., 2011).
Reef fish families (e.g. Tetraodontidae, Pomacentridae, Blennidae, Chaetodontidae and Scaridae) are known to establish close relationships with coral structure. This association can range from corallivorous feeding behaviour (Ciardelli, Reference Ciardelli1967; Rotjan & Lewis, Reference Rotjan and Lewis2008; Berumen & Rotjan, Reference Berumen and Rotjan2010; Pereira et al., Reference Pereira, Leal, Araújo and Souza2012) by using the spaces created by these colonies for shelter/protection or breeding (Munday et al., Reference Munday, Jones and Caley1997; Gibran et al., Reference Gibran, Santos, Santos and Sabino2004). In addition, some cryptobenthic species from the genus Gobiodon are highly dependent on branching corals living their whole post-settlement life associating exclusively with corals from the genus Acropora (Munday et al., Reference Munday, Harold and Winterbottom1999, Reference Munday2004).
Although branching fire-corals were previously recognized as important microhabitats for newly settled and juvenile fish in the Caribbean (Victor, Reference Victor1986; Nagelkerken & Nagelkerken, Reference Nagelkerken and Nagelkerken2004), few studies (e.g. Coni et al., Reference Coni, Ferreira, Moura, Meirelles, Kaufman and Francini-Filho2012) have addressed the structure of the ichthyofauna associated with the fire corals (Millepora spp.) in the Brazilian reefs.
In this context, this is the first study to investigate the behaviour and ecological association between fish and coral structure, and the specific goals were: (1) determine the life phase that fish were associated with colonies of Millepora spp.; (2) analyse ecological and behavioural relationship between fish and coral colonies, as well as the habitat partitioning; and (3) determine reef fish foraging rates on the Millepora spp. coral colonies.
MATERIALS AND METHODS
Study area
The present study was conducted on coastal reefs located in the municipality of Tamandaré (8°44′54″S and 35°6′14″W), on the southern coast of Pernambuco state, north-east Brazil. The reef complex is located within the boundaries of the Coral Coast Marine Protect Area (Costa dos Corais MPA), that extends for 135 km just off the coast from Tamandaré to Paripueira up north of the Alagoas State. This MPA was the first federal conservation unit to include coastal reefs and is the largest marine protected area in Brazil (Ferreira et al., Reference Ferreira, Maida and Cava2001). The observations were conducted on the ‘Ilha do Norte’ reef, located about 300 m offshore; depth ranges from 0.5 to 6 m and the reef extends for approximately 1.5 km (Figure 1). Reef flats are covered with macroalgae species, hermatypic corals (Favia gravida (Verrill, 1868), Montastrea cavernosa (Linnaeus, 1766), Mussismilia spp. and Porites astreoides) (Lamarck, 1816), and large colonies of the fire corals Millepora braziliensis and M. alcicornis (Ferreira & Maida, Reference Ferreira and Maida2006).
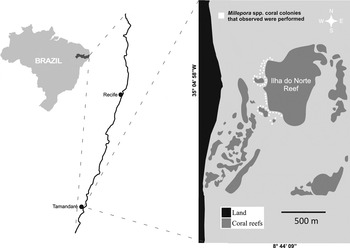
Fig. 1. Study area, Ilha do Norte reef in Tamandaré, indicating the Millepora spp. colonies that were examined.
Millepora braziliensis colonies are honeycombed, but can also be hemispheric, ramified, columnar, laminate, fan shaped, totally incrusting, or a mixture of these forms. However, Millepora alcicornis is normally ramified, but can also be incrusting, hemispheric, or a mixture of these forms (Amaral, Reference Amaral1997; Amaral et al., 2002). According to Amaral (Reference Amaral1997) the two species are so highly variable in shape that it is impossible to characterize them perfectly in this respect.
Censuses and behavioural observations
Underwater observations were made from September 2010 through to February 2011 during the regional dry season. In order to quantify the reef fish associated with the coral structure, as well as the agonistic integrations among fish, censuses were undertaken during free dives using the intensive search method. Censuses were carried out by searching for Millepora spp. colonies, where the researcher identified and quantified all fish species at the colony. In total, about 50 Millepora spp. coral colonies were surveyed during this research.
This methodology was chosen because it is more appropriate to measure quantitatively the fish communities, as well as being easy to apply and have low environmental interference (Bortone et al., Reference Bortone, Kimmel and Bundrick1989). Behavioural observations were also performed, using the focal animal method (Altmann, Reference Altmann1974). Individuals were classified as juveniles or adults according to their sizes and colour patterns (Humann & Deloach, Reference Humann and Deloach2002) to determine which life phases were most closely associated with the hydrocorals. The observation sessions were standardized as five minutes, except when individuals escaped. The observed behaviour patterns were classified into four categories: (1) sheltered/stationary: when fish were observed inside holes, crevices, and open areas within the branches of Millepora spp. colonies; (2) foraging: reef fish were observed feeding on the coral structure. The numbers of bites and the time spent performing this activity was noted in order to estimate their foraging rates; (3) swimming: when fish were seen actively moving in or near coral structures; and (4) breeding: when fish were observed in courting postures.
RESULTS
A total of 473 coral reef fish individuals belonging to 15 families, 23 genera and 27 species were recorded in association with the Millepora spp. coral colonies. Pomacentridae (187), Holocentridae (111), Serranidae (61) and Scaridae (21) were the most representative families in regards to abundance (Table 1). Juveniles were more commonly observed associated with the coral colonies (65%), in opposition to adults (35%), with the exception of Labrisomidae and Bleniidae families, the ones with a greater presence of adult compared with juveniles (Figure 2).

Fig. 2. The most representative fish families that occupy Millepora spp. colonies, classified according to their life phases.
Table 1. The fish species associated with the hydrocoral Millepora spp. classified in decreasing order of the total numbers of individuals encountered, citing their common names, life stage, and relative frequency of occurrence.

A total of 285 behavioural observation sessions were performed (lasting 5 min each), with a total of 1425 min (23.54 h) of direct observations. Among the four considered behavioural categories (based on the total number of individuals), sheltered/stationary was the most well-represented, (49.5%), followed by swimming (36.8%) and foraging (13.2%). Labrisomus nuchipinnis (Quoy & Gaimard, 1824) was the only associated species observed in a courting posture. The couple of Labrisomus nuchipinnis was observed upside down in its courting, near the coral base (Table 2).
Table 2. List of the fish species, classified in decreasing order of the total numbers of individuals encountered, and indicating their respective behaviours observed on the hydrocorals.

The highest foraging rate (bites/min) on the Millepora spp. coral colonies was observed for Microspathodon chrysurus (Cuvier, 1830) juveniles (1.16 ± 0.79 bites/min) followed by Stegastes fuscus juveniles (0.88/0.80 ± 0.36), Ophioblennius trinitatis (Miranda Ribeiro, 1919) juveniles had the juveniles (0.63 ± 0.07) and Scarus zelindae (Figueiredo & Sazima, 2001) juveniles (0.58 ± 0.21).
Stegastes fuscus individuals besides swimming and foraging on Millepora spp. colonies, were also observed performing agonistic behaviour. The highest number of recorded attacks was upon Ophioblennius trinitatis representing 40% of total number. Moreover, Labrisomus nuchipinnis (20%), Chaetodon striatus (20%), Diodon holocanthus (Linnaeus 1758) (13%) and Abudefduf saxatilis (Linnaeus 1765) (7%) were also targeted by the agonistic behaviour established by S. fuscus.
Habitat partitioning by some associated species with Millepora spp. colonies is illustrated in Figure 3. Species were observed at different locations in relation to the coral structure; whereas nocturnal and more cryptobenthic ones (e.g. Holocentrus adscensionis and Labrisomus spp.) were always recorded among the coral branches. In contrast, species with higher swimming capacity and active during the day were seen moving around the coral colonies (e.g. Chromis multilineata and Sparisoma axillare) (Figure 3).

Fig. 3. Representative sketch of a Millepora spp. colony indicating the positions and behaviours of the fish species observed. Breeding: Labrisomus nuchipinnis (1). Sheltered/stationary: Holocentrus adscensionis (2), Ophioblennius trinitatis (3) and Epinephelus adscensionis (7). Foraging: Chaetodon striatus (5) and Stegastes fuscus (6). Swimming: Chromis multilineata (4) and Sparisoma axillare (8).
Behavioural positions (foraging, swimming and sheltered/stationary) of some fish species, associated with the Millepora spp. coral colonies, were documented by unedited photography (Figure 4). This figure showed also the first record for Labrisomus kalisharae (Jordan, 1904) species in the State of Pernambuco.
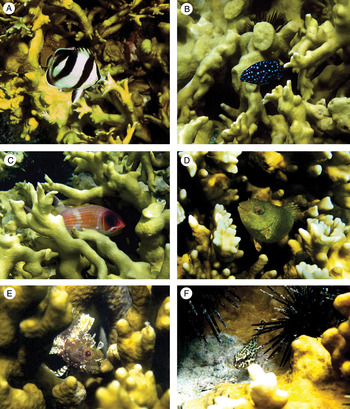
Fig. 4. Photographs of some of the fish observed on Millepora alcicornis colonies: (A) Chaetodon striatus (forraging); (B) Microspathodon chrysurus (swimming); (C) Holocentrus adscensionis (sheltered); (D) Sparisoma axillare (sheltered); (E) Labrisomus kalisherae (sheltered); (F) Epinephelus adscensionis (sheltered).
DISCUSSION
Reef fish are known to maintain direct relationship with coral colonies and many examples have been reported from the Pacific Ocean, which has the world's greatest diversity of corals (Patton, Reference Patton1994; Precht et al., Reference Precht, Aroson, Moody and Kaufman2010; Johnson et al., Reference Johnson, Holbrook, Schmitt and Brooks2011). Patton (Reference Patton1994) recorded a variety of fish in all analysed Acropora species on the Great Barrier Reef (Australia), and 85 species of fish belonging to 25 families were found associated with Acropora pulchra coral colonies (Brook, 1891) in French Polynesia (Johnson et al., Reference Johnson, Holbrook, Schmitt and Brooks2011). The present study described the reef fish community and their behaviour in association with Millepora spp. coral colonies, being that 27 fish species belonging to 15 families were identified. Branching corals are especially important by their capacity to provide structural complexity for a large number of reef fish (Holbrook et al., Reference Holbrook, Brooks and Schmitt2002a). Microhabitats such as holes, crevices, and open interior areas between branches are important aspects of habitat structure and can account for much of the variation in species richness and total abundance (Holbrook et al., Reference Holbrook, Brooks and Schmitt2002a, Reference Holbrook, Brooks and Schmittb; Brooks et al., Reference Brooks, Holbrook and Schmitt2007). The living corals create a variety of habitats for a large number of species, giving support for sedentary organisms and food or shelter for mobile ones (Holbrook et al., Reference Holbrook, Brooks and Schmitt2002a).
Among the four observed behaviour categories the most frequent was ‘sheltered/stationary’. Holocentrus adscensionis and Ephinephelus adscensionis (Osbeck, 1765) were recorded sheltering on coral colonies during all the observation sessions. These species are carnivorous, feeding on small crustaceans, gastropods, sea stars and other fish (Randall, Reference Randall1996; Greenfield, Reference Greenfield and Carpenter2002). Ephinephelus adscensionis and Labrisomus nuchipinnis displayed ‘sit and wait’ feeding behaviour, resting in holes or cracks in the coral and would rapidly flee if disturbed (Sazima et al., Reference Sazima, Gasparini and Moura2002). At night H. adscensionis feeds for crabs and other crustaceans living on sand and grass beds, hiding in reef crevices during the day (Greenfield, Reference Greenfield and Carpenter2002).
The second most frequent behavioural category was ‘swimming’. All registered species had direct contact with the Millepora spp. structure, except for Abudefduf saxatilis and Chromis multilineata (Guichenot, 1853). These two species were always observed swimming near the colonies’ crest. Chromis multilineata is a planktivore fish and several studies have found positive correlations between the abundance of planktivores and the water flow, a pattern explained by the higher influx of plankton in sites with higher water turbulence (e.g. Hobson & Chess, Reference Hobson and Chess1978; Hammer et al., Reference Hammer, Jones, Carleton, Hauri and Williams1988; Floeter et al., Reference Floeter, Krohling, Gasparini, Fereira and Zalmon2007). Abudefdub saxatilis is versatile forager that feed on a variety of food items (including algae) collected on water column (Randall, Reference Randall1967).
Stegastes fuscus individuals were recorded exhibiting a large amount of agonistic interactions towards another reef fish species around the coral colonies. Furthermore, we are able to assume that some S. fuscus individuals include Millepora spp. colonies within their territories (authors, personal observations). Species of the genus Stegastes (damselfish) are known to be strongly territorial and to actively compete for food and habitat with other reef fish (Haley & Muller, Reference Haley and Muller2002; Souza et al., Reference Souza, Ilarri and Rosa2011; Pereira & Ferreira, Reference Pereira and Ferreira2012). Aggressive behaviour by farming damselfishes is often used toward corallivores, herbivores and potential egg predators and largely ignored other carnivores, planktivores and omnivores (Johnson et al., Reference Johnson, Holbrook, Schmitt and Brooks2011). Fish of genus Stegastes can decrease the recruitment of surgeon fish and butterfly fish (Shulman, Reference Shulman1984), and modify the structure of fish communities associated with hard corals by their territorialism (Johnson et al., Reference Johnson, Holbrook, Schmitt and Brooks2011). Intraspecific competition for food and nest sites as well as nest defence has been implicated as underlying causes for this behaviour (Ebersole, Reference Ebersole1977; Haley & Muller, Reference Haley and Muller2002). However, research related to the agonistic behaviour of fish associated with the structure of Millepora spp. has not been conducted on the Brazilian coast, nevertheless this is essential for assessing the reef fish ecology during association with coral colonies.
Four reef fish families (e.g. Pomacentridae, Scaridae, Blennidae and Chaetodontidae) were observed foraging on the coral colonies during the present study. Pratchett (Reference Pratchett2007) recorded fourteen species of the family Chaetodontidae consuming a total of 72 scleractinian corals on the Great Barrier Reef, Australia, and corallivorous fish of the families Tetradontidae and Labridae were reported foraging on Acropora spp. coral in the Red Sea (Berumen & Rotjan, Reference Berumen and Rotjan2010). During the present study, individuals of the Scaridae family were observed biting Millepora spp. colonies, often removing small pieces. Parrotfish are perhaps the best-known corallivorous group, consuming also the mucus that coats coral colonies, which can result in desiccation of the living surfaces (Rotjan & Lewis, Reference Rotjan and Lewis2008; Pereira et al., 2012). Parrotfish scrape or break off pieces of Millepora spp. colonies with their beaklike mouths (Lewis, Reference Lewis2006). Among all the analysed species foraging on the coral structure, juveniles of Microspathodon chrysurus and Stegastes fuscus showed the highest feeding frequency respectively. Feeding habits of M. chrysurus varies throughout its life; juveniles feed on copepods and other small crustaceans that live in Millepora spp. colonies and adults rely on green algae (cyanophytes) (Ciardelli, Reference Ciardelli1967). Ciardelli (Reference Ciardelli1967) found coral fragments and other hard material in the oral cavity of those fish, but not in the gastrointestinal cavity, suggesting the existence of a sorting mechanism that eliminates hard particles from food (Ciardelli, Reference Ciardelli1967). These data corroborate with the results of this survey, where only juveniles of Microspathadon chrysurus were seen foraging on the hydrocoral structure.
Stegastes fuscus are known as one of the main herbivores species from the family Pomacentridae (Ferreira et al., Reference Ferreira, Gonçalves, Coutinho and Peret1998); however, according to Feitosa et al. (Reference Feitosa, Cocentino, Teixeira and Ferreira2012) hydrozoans polyps were also found in their stomach contents. In the Indo-Pacific at least six damselfish species are recognized as coral specialized and several other species within this family rely mostly on corals for food (Wilson et al., Reference Wilson, Graham, Pratchett, Jones and Polunin2006, Reference Wilson, Burgess, Cheal, Emslie, Fisher, Miller, Polunin and Sweatman2008). However, another Pomacentridae, Plectroglyphidodon dickii (Liénard, 1839), actively kills coral polyps without consuming them, thus increasing the area for algal growth inside their coral-dominated territories (Kaufman, Reference Kaufman1977; Robertson et al., Reference Robertson, Hoffman and Sheldon1981; Jones et al., Reference Jones, Santana, McCook and McCormick2006). Damselfish cultivate and defend filamentous turfs of algae within territories (Mahoney, Reference Mahoney1981; Robertson, Reference Robertson1984; Hata & Kato Reference Hata and Kato2004; Precht et al., Reference Precht, Aroson, Moody and Kaufman2010). The settlement and growth of algae can occur on damaged or broken branches of Millepora spp. (Lewis, Reference Lewis2006). Therefore, it is important to emphasize the strong association between the family Pomacentridae and Millepora spp. coral colonies which have been considered as part of its territory, as highlighted in this study.
Large number of invertebrates from micro and macrofauna are found associated with Millepora spp. corals colonies (e.g. Protozoa, Mollusca, Crustacea, and Annelida) (Lewis, Reference Lewis1989; Amaral et al., Reference Amaral, Steiner, Broadhurst and Cairns2008; Garcia et al., Reference Garcia, Matthews-Cascon and Franklin-Junior2008). The presence of these animals likewise stimulates the foraging activity of different fish species. Most of these feeding events occurred in the bodies of the colonies or near their extremities, areas with more crustaceans and echinoderms (Garcia et al., Reference Garcia, Matthews-Cascon and Franklin-Junior2008).
During the present study, a large number of juveniles were observed associated with the fire coral colonies. This same pattern was observed by Coni et al. (Reference Coni, Ferreira, Moura, Meirelles, Kaufman and Francini-Filho2012) in another reef complex on the Brazilian coast. This result indicates that the fire coral, the only conspicuous branching forms that occur in South-western Atlantic reefs, harbour a diverse fish assemblage (37% of the species pool known for the Abrolhos Bank (Coni et al., 2012) and 27% of the ichthyofauna of Tamandaré region (Ferreira et al., Reference Ferreira, Maida and Souza1995)). Most of them are constituted by small-bodied fish (cryptobenthic) and juveniles of large-bodied fish. Juvenile reef fish have higher predation rates (Abdulla, Reference Abdulla2004) and more intensive competitive interactions (Hobbs & Munday, Reference Hobbs and Munday2004) than adults, thus they need to remain in close association with a protective substratum (Faunce & Serafy, Reference Faunce and Serafy2007). In contrast, a predominance of adult individuals of the families Labrisomidae and Bleniidae was recorded in the present study. They represent small cryptobenthic fish that retain close associations with marine substratum throughout their entire life (Munday, Reference Munday2002; Depczynski & Bellwood, Reference Depczynski and Bellwood2004). It is likely that the smaller individuals of these taxa remained even more hidden than the adult, the ones which could have been underestimated during the present research.
Traditional models of competition (Diamond, Reference Diamond1978) propose that competing species coexist in the same area through resource partitioning. Habitat partitioning was observed for the Millepora spp. associated species, whereas more peripheral sites (around the colonies) were used by planktonic species and less habitat-specialized ones (Pratchett et al., Reference Pratchett, Coker, Jones and Munday2012). In contrast, species more associated with habitat, such as serranids and cryptobenthic species (Shpigel & Fishelson, Reference Shpigel and Fishelson1989; Munday et al., 1997) were always recorded inside the coral branches. Whenever a species decreases the common use of resources (Sale, Reference Sale1977), thereby specializing on a specific part of an available resource, this should result in reduced levels of competition between species than within species.
Coral reefs are threatened nowadays by both local and global human impacts (Carpenter et al., Reference Carpenter, Abrar, Aeby, Aronson, Banks, Bruckner, Chiriboga, Cortés, Delbeek and DeVantier2008; Hixon, Reference Hixon2011). On a local scale, pollution, eutrophication and overfishing represent the main cause of degradation; however, in an overall vision, ocean acidification and global warming are the major factors of habitat loss on reef ecosystems (Hixon, Reference Hixon2011). In addition, among a lot of coral taxa, the hydrocorals (Millepora spp.) was proved to be the most susceptible in regards to bleaching episodes in the Great Barrier Reef, Australia (Marshall & Baird, Reference Marshall and Baird2000). Biological effects of bleaching include reduced growth, reduced reproduction and increased mortality of corals (Gleason, Reference Gleason1993; Marshall & Baird, Reference Marshall and Baird2000). In Brazilian waters, the branching fire coral abundance has been declining over the past years (Amaral et al., Reference Amaral, Steiner, Broadhurst and Cairns2008). The species M. alcicornis has being exploited as a souvenir and ornamental feature in several areas of coast for at least two decades (Leão et al., Reference Leão, Hetzel and Castro1994). In Pernambuco State, there are records of intense trade of species from the genus Millepora, where the skeletons of their colonies and calcified hydroids are sold in markets and streets (Amaral et al., Reference Amaral, Steiner, Broadhurst and Cairns2008).
Millepora spp. hydrocorals are ecologically important species for both juvenile and adult reef fish belonging to various trophic guilds (Pereira et al., Reference Pereira, Leal, Araújo and Souza2012; Coni et al., Reference Coni, Ferreira, Moura, Meirelles, Kaufman and Francini-Filho2012). The complex structure of these coral colonies provide safe spaces for shelter, reproduction, many feeding opportunities due to the ample availability of associated micro and macrofauna, as well as important extensions of the territories of Stegastes fuscus. Therefore, the decline of this species can promote a reduction of abundance of associated organisms and also reduction of coral growth rates in Brazilian reefs (Oliveira et al., Reference Oliveira, Leão and Kikuchi2008).
ACKNOWLEDGEMENT
The authors would like to thank Elisabeth Cabral Silva-Falcão for helping with the manuscript.
FINANCIAL SUPPORT
The authors would like to thank CNPq, PIBIC-FACEPE, CAPES and IDEA WILD for financial and logistical support during this research.








