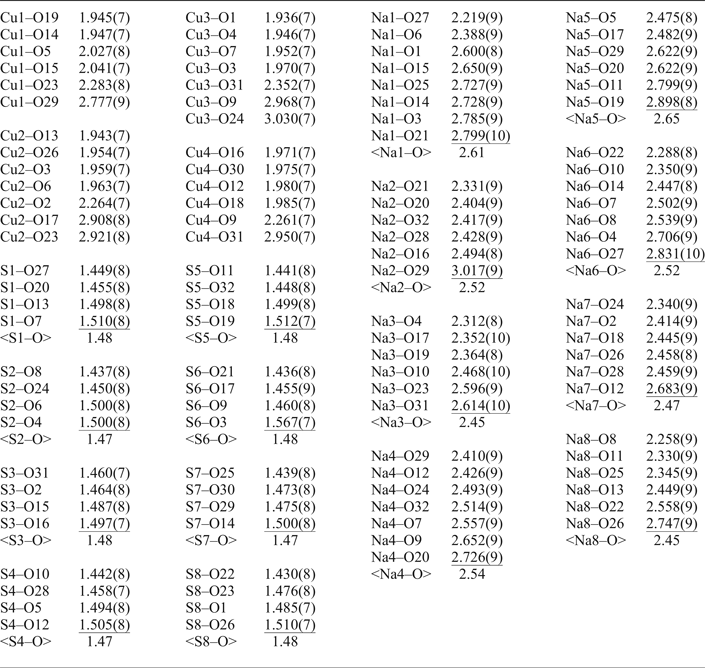Introduction
Sulfate-containing minerals constitute a rich family with more than 380 different species known to date. The formation of sulfate minerals in the oxidation zones of sulfide deposits represents an important factor affecting the redox conditions of the environment and the transport of secondary phases. Most known sulfate minerals are hydrated, but a significant number of anhydrous sulfate minerals formed as a result of high-temperature exhalative processes in the fumaroles of some volcanoes e.g. Vesuvius, Italy (Demartin et al., Reference Demartin, Campostrini, Castellano, Gramaccioli and Russo2012); Tolbachik, Russia (Siidra et al., Reference Siidra, Nazarchuk, Zaitsev, Lukina, Avdontseva, Vergasova, Vlasenko, Filatov, Turner and Karpov2017); and Eldfell, Iceland (Balić-Žunić et al., Reference Balić-Žunić, Garavelli, Acquafredda, Leonardsen and Jakobsson2009). Many of these minerals are unstable under ambient terrestrial conditions and the study of their structural and physicochemical properties is challenging.
The volcanogenic exhalation sulfate mineralization from fumaroles of the Tolbachik volcano is renowned for its rich mineral diversity (Vergasova and Filatov, Reference Vergasova and Filatov2016). Despite the large number of previous studies on different minerals discovered in this unique deposit, hydration and weathering processes of the primary volcanogenic mineral assemblages have not been studied in detail. However, it is clear that complex multistage physicochemical processes are involved which require detailed and rather elaborate studies to understand them.
During the past decade or so, the experimental mineralogy of sulfates has attracted considerable attention because remote sensing and direct sampling from satellites and rovers have resulted in the discovery of the various sulfate minerals on Mars (Peterson and Wang, Reference Peterson and Wang2006). As most Martian sulfate minerals have been found to be hydrated this observation suggests the existence of liquid water on Mars, at least in its early history (Vaniman et al., Reference Vaniman, Bish, Chipera, Fialips, Carey and Feldman2004; Szynkiewicz et al., Reference Szynkiewicz, Borrok and Vaniman2014), which opens the possibility that early Mars might have been habitable and life in some form might have existed. This explains in part the ongoing and planned exploration activities with regard to Mars (e.g. Vasavada, Reference Vasavada2017). As safe return of unaltered Martian samples to Earth is unlikely to be successful in the near future because of the instability of hydrous sulfates and their easy transformation into different phases, for instance as a result of changing temperatures, laboratory experiments performed on Earth have to simulate current and past conditions on the surface of Mars. Results of recent temperature-dependent X-ray diffraction (XRD) and Raman spectroscopy studies on hydrous and anhydrous sulfate minerals have been reported (e.g. Leftwich et al., Reference Leftwich, Bish and Chen2012; Wang and Zhou, Reference Wang and Zhou2014; Mills et al., Reference Mills, Wilson, Dipple and Raudsepp2010, Reference Mills, Nestola, Kahlenberg, Christy, Hejny and Redhammer2013).
Kröhnkite, Na2Cu(SO4)2(H2O)2 was first described 130 years ago (Domeyko, Reference Domeyko1879) from samples found in the famous Chuquicamata Mine in Chile. Kröhnkite crystals up to 5 cm long are known from the oxidized zones of copper deposits in very arid conditions in the Atacama Desert. The structure of kröhnkite was first determined by Dalman (Reference Dahlman1952) and later refined by Hawthorne and Ferguson (Reference Hawthorne and Ferguson1975). Its thermodynamic properties were studied recently by Majzlan et al. (Reference Majzlan, Zittlau, Grevel, Schliesser, Woodfield, Dachs, Števko, Chovan, Plášil, Sejkora and Milovská2016) who demonstrated the necessity of having high molality of Cu2+, alkali ions and (SO4)2– in the aqueous solution for kröhnkite to form. By means of high-temperature (HT) XRD, thermal analysis and emission infrared spectroscopy, Testasicca et al. (Reference Testasicca, Frost, Ruan, Lima, Belotti and Scholz2016) reported kröhnkite to decompose “into a complex mixture of sulfates below 500°C”. However, it should be noted that transformation and decomposition of kröhnkite revealed in the course of our HTXRD study is different as reported below. Kröhnkite-type compounds with various octahedrally coordinated cations and T 6+O4 (T 6+ = S, Cr and Se) groups are abundant in both minerals and synthetic compounds (Driscoll et al., Reference Driscoll, Kendrick, Wright and Slater2016; Marinova et al., Reference Marinova, Kostov, Nikolova, Kukeva, Zhecheva, Sendova-Vasileva and Stoyanova2015; Siidra et al., Reference Siidra, Vergasova, Kretser, Polekhovsky, Filatov and Krivovichev2014; Barpanda et al., Reference Barpanda, Oyama, Ling and Yamada2014; Saha et al., Reference Saha, Madras and Guru Row2011; Yang et al., Reference Yang, Jenkins, Downs, Evans and Tait2011; Stoilova et al., Reference Stoilova, Marinova, Wildner and Georgiev2009a,Reference Stoilova, Marinova and Georgievb; Wierzbicka-Wieczorek et al., Reference Wierzbicka-Wieczorek, Kolitsch and Tillmanns2008; Behera and Rao, Reference Behera and Rao2006; Kolitsch and Fleck, Reference Kolitsch and Fleck2005, Reference Kolitsch and Fleck2006; Fleck and Kolitsch, Reference Fleck and Kolitsch2003; Wildner and Stoilova, Reference Wildner and Stoilova2003; Pasha et al., Reference Pasha, Choudhury and Rao2003; Fleck et al., Reference Fleck, Kolitsch, Hertweck, Giester, Wildner, Prem and Wohlschläger2002a,Reference Fleck, Kolitsch and Hertweckb).
The present paper reports and discusses the results of HTXRD, thermal expansion and dehydration/hydration studies on kröhnkite from La Vendida mine, Antofagasta Region, Chile, and on the new mineral species saranchinaite, Na2Cu(SO4)2. The latter was discovered by O.I.S. and E.A.L. in a fumarole at the Naboko scoria cone of the Tolbachik Fissure eruption that occurred in 2012–2013. The structural characterization of saranchinaite presented here has allowed us to characterize the dehydration and rehydration behaviour of kröhnkite. The new exhalative mineral was named saranchinaite (Cyrillic: саранчинаит) in honour of Prof. Galina M. Saranchina (1911–2004), a Russian petrologist who worked at the Department of Petrology, St. Petersburg State University, Russia. Besides her many scientific achievements in the field of metamorphic petrology she was an outstanding lecturer of petrology at the SPbSU and taught many generations of geoscientists including four co-authors (O.I.S., E.V.N., A.E.Y. and S.K.F.) of this paper. Both, the new mineral and its name have been approved by the IMA Commission on New Minerals, Nomenclature and Classification (IMA2015-019). The type specimen is deposited in the collections of the Mineralogical Museum, St Petersburg State University, St Petersburg, Russia, catalogue number 19639.
Occurrence and general appearance
Saranchinaite
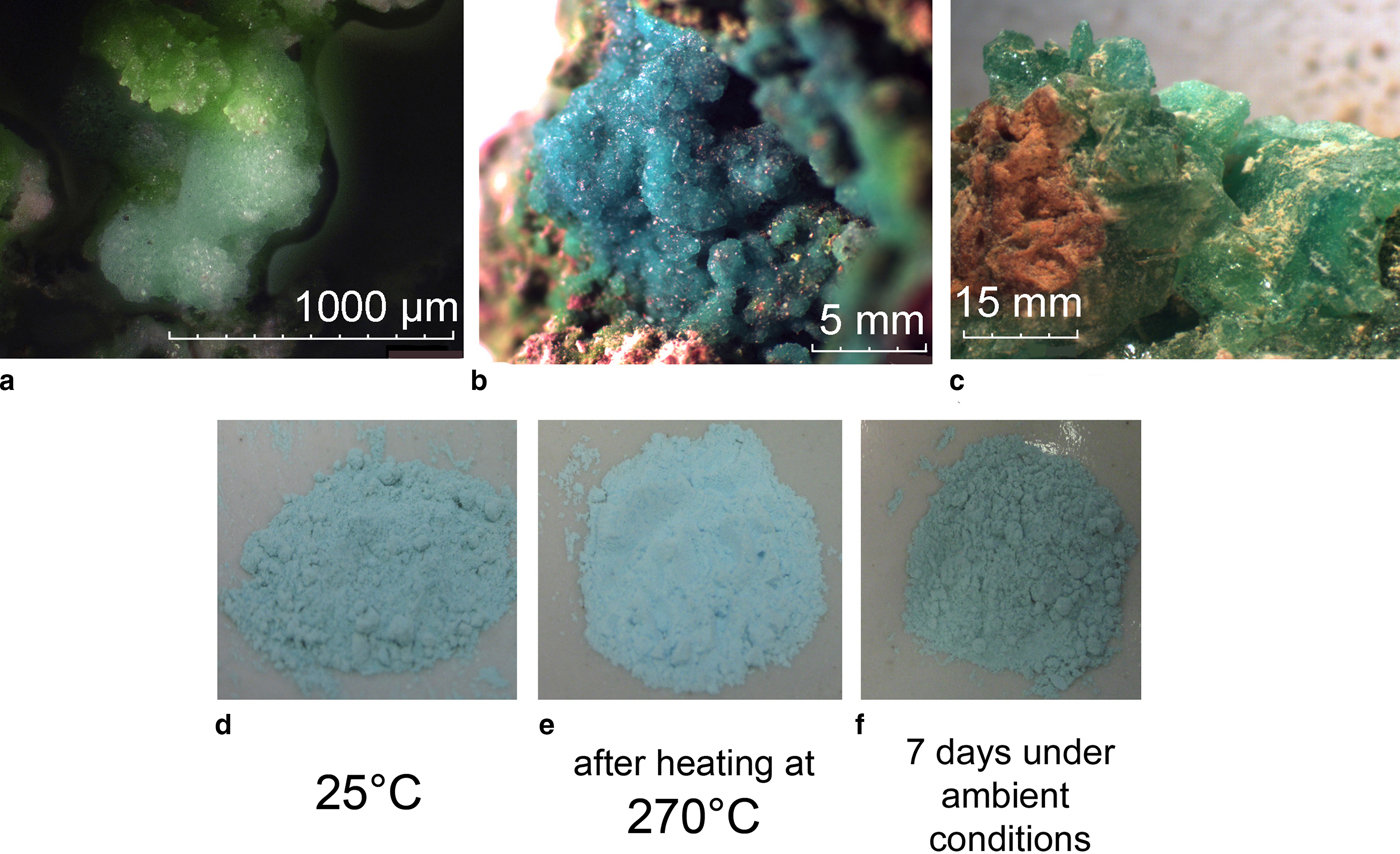
Saranchinaite is formed as a product of fumarolic activity, probably by direct deposition as sublimate from volcanic gases. The holotype material was collected in the fumarole located at the Naboko scoria cone (55°46′ N, 160°19′E, 1650 m a.s.l.) of the Tolbachik Fissure eruption that occurred in 2012–2013 on the south slope of the Ploskiy Tolbachik volcano, Kamchatka, Far-Eastern Region, Russia. The temperature of the gas at the sampling location was ~600°C. At the type locality saranchinaite is associated closely with euchlorine, KNaCu3(SO4)3O (Fig. 1a) and anhydrite. Other associated minerals are itelmenite Na4Mg3Cu3(SO4)8 (Nazarchuk et al., Reference Nazarchuk, Siidra, Agakhanov, Lukina, Avdontseva and Karpov2018), hermannjahnite CuZn(SO4)2 (Siidra et al., Reference Siidra, Nazarchuk, Agakhanov, Lukina, Zaitsev, Turner, Filatov, Pekov, Karpov and Yapaskurt2018), chalcocyanite CuSO4, thénardite Na2SO4, aphthitalite K3Na(SO4)2 and hematite Fe2O3. Euchlorine and, surprisingly, saranchinaite appeared to be the most abundant Cu-sulfate minerals in the fumarole of Naboko cone. Thus it was decided to give the name of ‘Saranchinaitovaya’ to this fumarole. After the discovery of saranchinaite many similar looking samples in Cu-sulfate associations were checked from fumaroles of the Second Scoria Cone and perfect crystalline aggregates of sky-blue colour (Fig. 1b) were found to be very common. The reason why anhydrous saranchinaite has not been determined previously in the extremely rich mineral diversity of the fumaroles of the Second Scoria Cone is most probably due to its indistinguishability from hydrous kröhnkite during the preliminary qualitative microprobe examination of mineral samples. Instead we used single-crystal X-ray analysis for the primary examination of crystalline material from the exhalative mineral assemblages and this enabled us to discriminate between hydrous kröhnkite and the anhydrous new mineral saranchinaite.
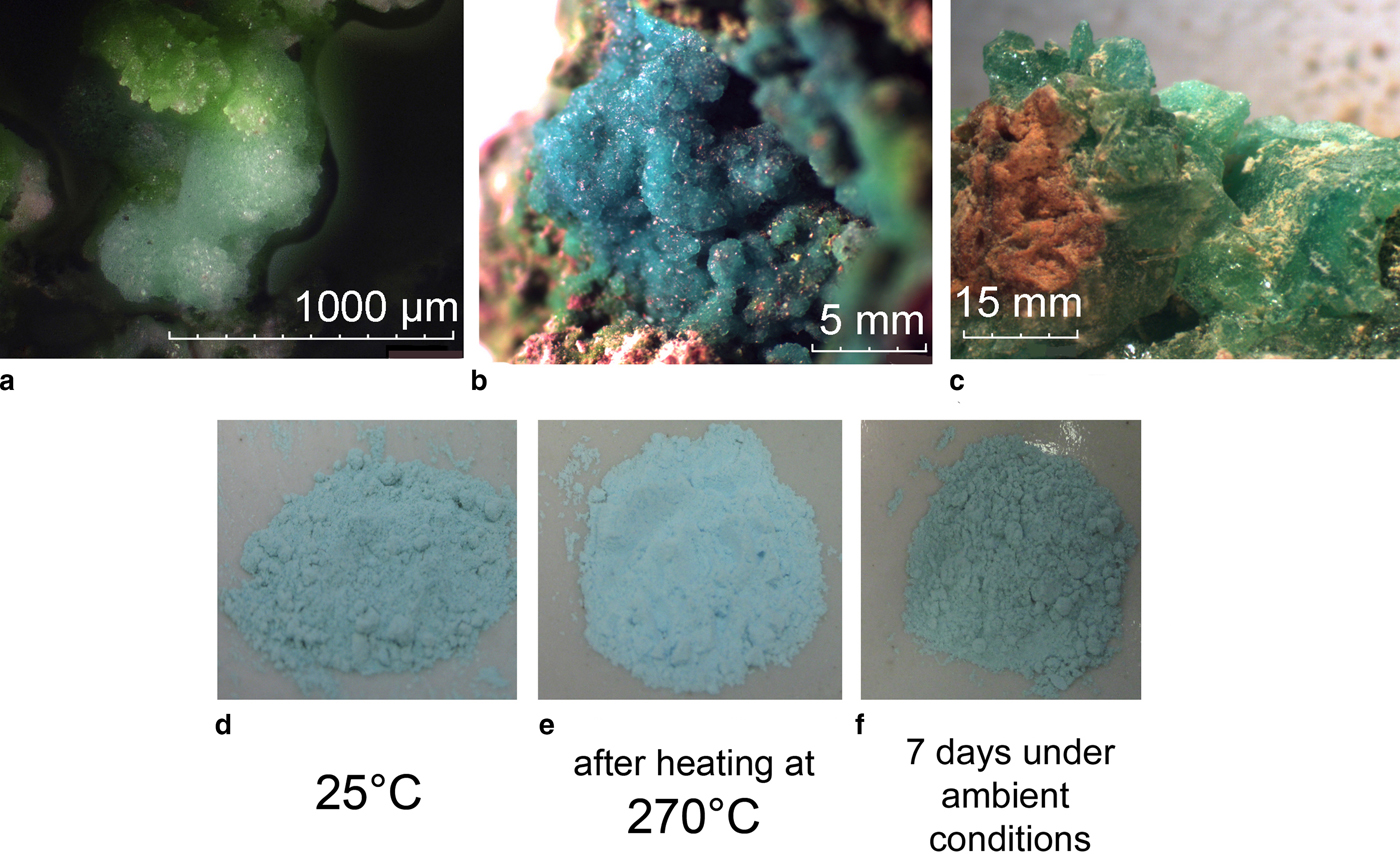
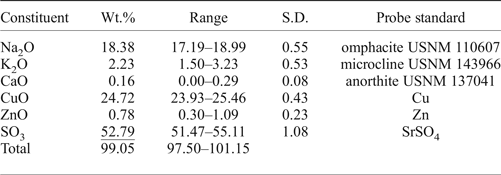
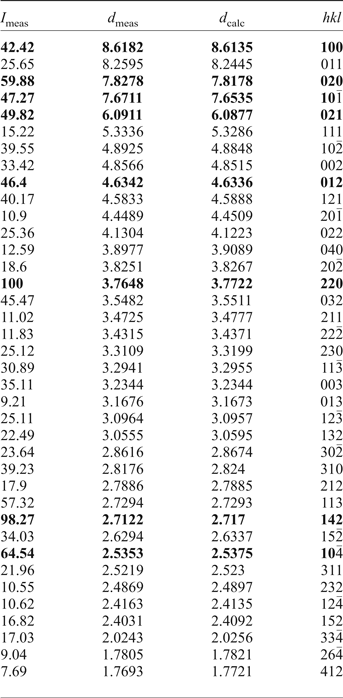
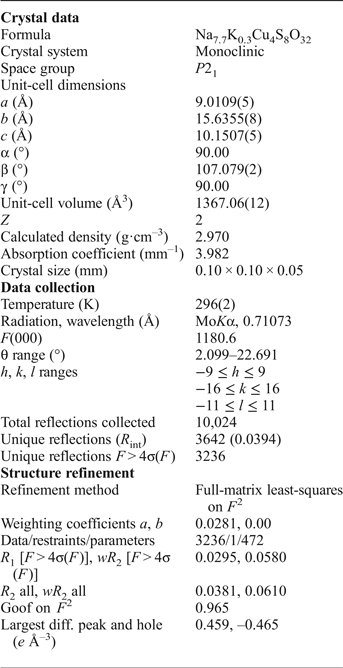
Fig. 1. (a) The holotype specimen of saranchinaite (light-blue) with euchlorine (green) from the Saranchinaitovaya fumarole, Naboko scoria cone, Tolbachik Fissure eruption; (b) the cotype specimen of saranchinaite from the Yadovitaya fumarole, Second scoria cone, Northern Breakthrough, Great Tolbachik Fissure eruption; (c) greenish-blue crystals of kröhnkite from La Vendida mine; (d) dehydration and transformation of polycrystalline sample of ground kröhnkite into saranchinaite by (e) heating to 270°C and (f) subsequent re-hydration back into kröhnkite after 7 days in air at relative humidity of 87% and 25°C.
Saranchinaite occurs typically as druses with crystals up to 0.1 mm, as spherolites or irregularly shaped grains, or in the form of microcrystalline masses. The colour of saranchinaite (Fig. 1a,b) is somewhat variable: very light-blue or nearly white in polycrystalline masses to sky-blue in crystalline aggregates. The streak is white. The lustre is vitreous. The mineral is transparent in individual grains and translucent in aggregates. Saranchinaite is brittle. Cleavage or parting has not been observed. The fracture is uneven. Hardness and density could not be measured because of the very small size of individual grains and the porosity of the aggregates. The density calculated on the basis of the empirical formula of the holotype is 2.937 g cm–3. Saranchinaite is optically biaxial (+), α = 1.517(2), β = 1.531(2) and γ = 1.559(2) (589 nm) with 2Vcalc. = 71.6°. Saranchinaite appears light grey and non-pleochroic under the microscope. The Gladstone-Dale compatibility index, 1 – (K p/K c) = –0.025, is excellent (Mandarino, Reference Mandarino1981).
Kröhnkite
Well-crystallized greenish-blue kröhnkite samples (Fig. 1c) used for the HTXRD studies were collected in 2016 at the abandoned open pit of the La Vendida copper mine situated ~3 km WNW of the Sierra Gorda village, Antofagasta Region, Atacama Desert, Chile. Kröhnkite is also a common mineral from various fumaroles of the Second Scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure eruption formed by alteration of Cu-sulfate mineral assemblages by air moisture and rain.
Chemical data for saranchinaite
Saranchinaite is water soluble and sensitive to moisture content in air. It transforms into kröhnkite after one week of exposure in open air at 87% relative humidity and 25°C. Therefore, all the samples were hermetically packed on-site immediately after collecting and isolated to avoid any contact with the atmosphere. The heating of the kröhnkite mineral sample up to 270°C was performed after the determination of the transformation temperature by HTXRD (see below). The transformation of kröhnkite powder samples into saranchinaite is accompanied by colour change from greenish-blue (Fig. 1d) to light-blue (Fig. 1e). No other intermediate phases are formed during this process as judged by powder XRD under controlled temperature conditions. In a humid atmosphere the obtained saranchinaite reverts back into kröhnkite (Fig. 1f).
Three crystals of saranchinaite checked previously by single-crystal XRD were mounted in epoxy resin and polished with oil suspension resulting in areas of ~40 µm × 40 µm. Ten spot analyses on the grains were obtained using a JEOL Superprobe 733 scanning electron microscope equipped with an Oxford Instruments INCA Energy Dispersive Spectrometer housed in the Fersman Mineralogical Museum, Russia. The electron beam accelerating voltage was 20 kV and the electron beam current 2 nA, measured with a Faraday cup. X-ray acquisition live-time was 30 s. System calibration was performed on Ni. The mineral is unstable under the focused electron beam with strong sodium loss during analysis and, therefore, a defocused beam (5 µm) was used for analyses. X-ray matrix correction was carried out using the XPP routine implemented in the INCA software of Oxford Instruments. No elements with Z ≥ 9 other than those reported in Table 1 were detected. The empirical formula calculated on the basis of 8 O atoms per formula unit is (Na1.81K0.14Ca0.01)∑1.96(Cu0.95Zn0.03)∑0.98S2.01O8. The simplified formula is Na2Cu(SO4)2.
Table 1. Analytical results (wt.%) for the holotype of saranchinaite from Saranchinaitovaya fumarole.
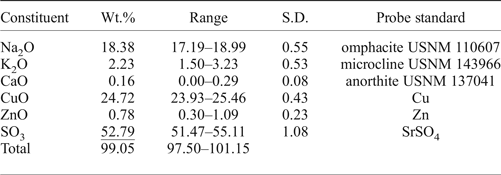
S.D.: standard deviation
X-ray crystallography and crystal structure of saranchinaite
Powder XRD data for the holotype of saranchinaite were obtained using a Rigaku Ultima IV diffractometer at the Department of Crystallography, St. Petersburg State University, Russia. Data (in Å for CuKα) are given in Table 2. The unit-cell parameters were refined in the monoclinic unit cell, space group P21, a = 8.995(3), b = 15.599(6), c = 10.159(3) Å, β = 107.07(11)° and V = 1363(1) Å3.
Table 2. Powder XRD data of saranchinaite (in Å for CuKα).
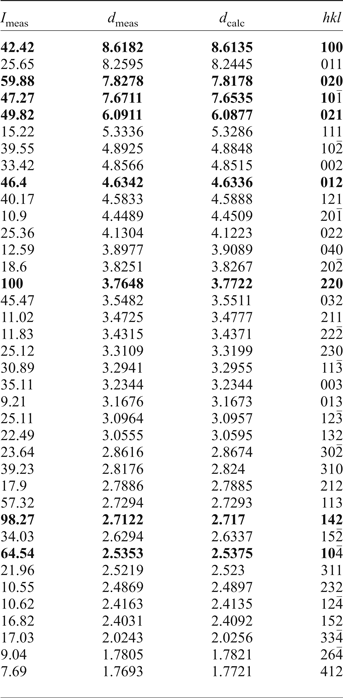
The strongest eight lines are given in bold
An equant crystal (Table 3) of saranchinaite was mounted on a Bruker APEX II DUO X-ray diffractometer operated at 50 kV and 40 mA and equipped with a micro-focus X-ray tube with an Mo anode at the Department of Crystallography, St. Petersburg State University, Russia. The data were integrated and corrected for absorption using a multi-scan type model with the Bruker programs APEX and SADABS (Bruker AXS Inc., Madison, Wisconsin, USA). More than a hemisphere of XRD data was collected with frame widths of 0.3° in ω, and with 15 s spent counting for each frame. The |E 2–1| parameter of 0.733 clearly indicated high probability of a non-centrosymmetric space group, which was confirmed by the subsequent structure solution and refinement. The structure of saranchinaite was solved in the space group P21 by direct methods. The crystal structure was refined to R 1 = 0.030 by means of the SHELX software package (Sheldrick, Reference Sheldrick2015) on the basis of 3236 independent reflections with F > 4σ(F). Atom coordinates and displacement parameters are given in Table 4 and selected interatomic distances in Table 5.
Table 3. Crystallographic data and refinement parameters for saranchinaite.

Table 4. Atomic coordinates and displacement parameters (Å2) for saranchinaite.
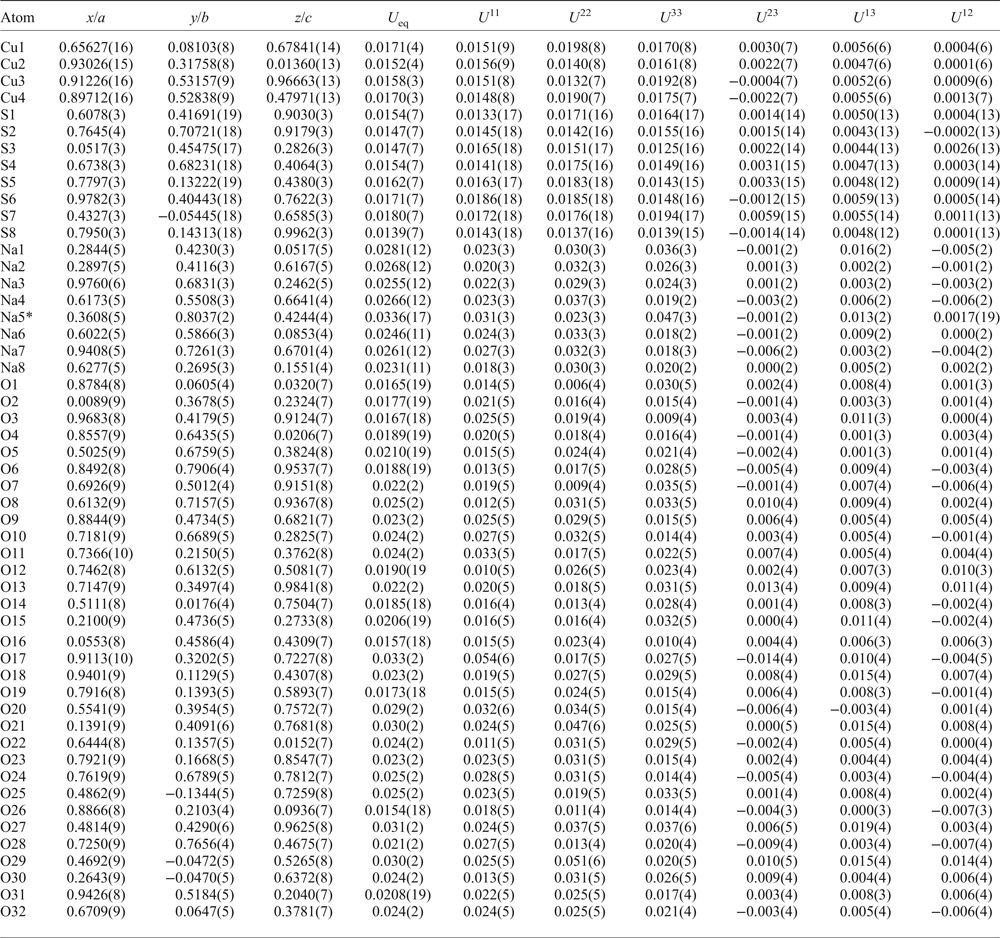
*Na0.71(2)K0.29(2)
Table 5. Selected interatomic distances in the structure of saranchinaite.
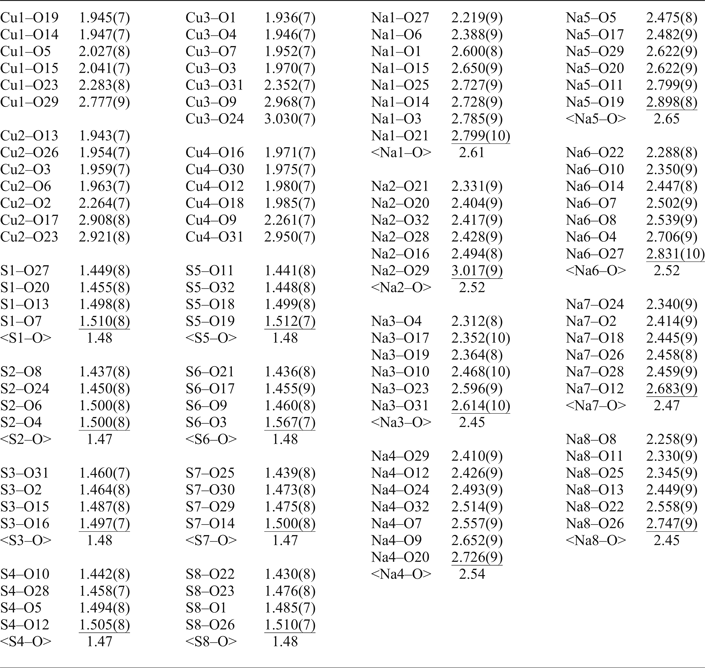
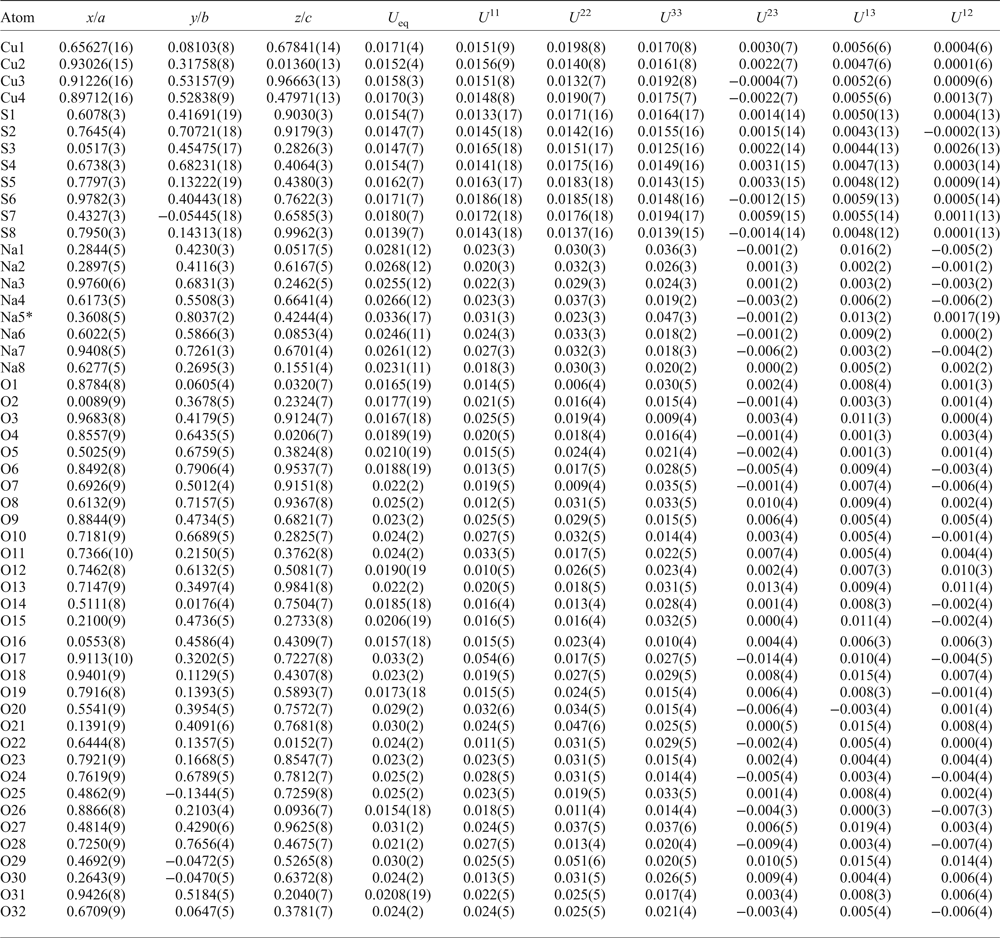
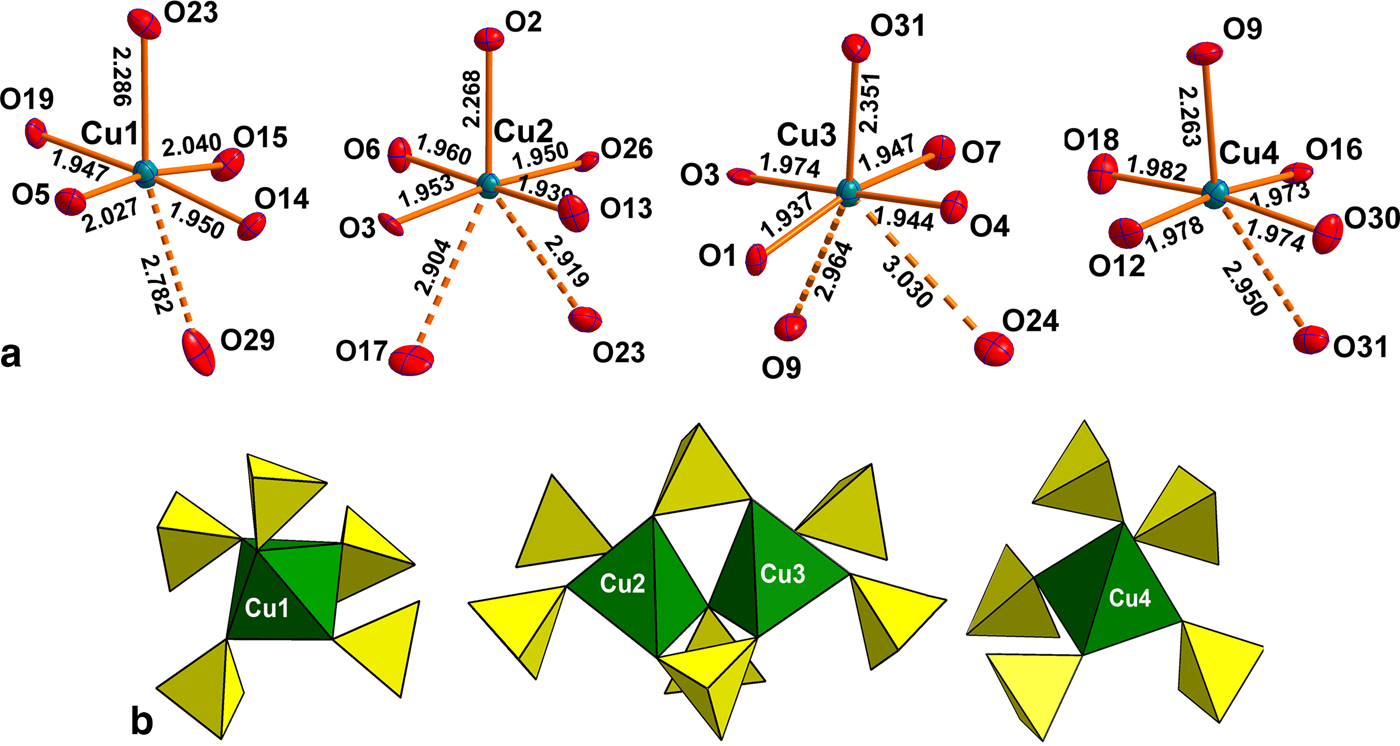
There are four symmetrically independent Cu2+ sites in the structure of saranchinaite (Fig. 2a). All Cu–O bonds <3.0 Å were taken into consideration. All Cu atoms form four very strong Cu–O bonds ≤ 2 Å resulting in CuO4 squares which are complemented by a fifth, longer bond of ~2.2–2.3 Å, thus forming CuO5 tetragonal pyramids. The Cu(1) and Cu(4) sites form one additional weaker bond resulting in [4+1+1] CuO6 octahedra strongly distorted by the Jahn-Teller effect. This type of coordination geometry of Cu2+ cations is rather common in minerals and inorganic materials (Burns and Hawthorne, Reference Burns and Hawthorne1995). By way of contrast, the Cu(2) and Cu(3) sites display a very unusual [4+1+2] Cu2+ coordination environment with two additional Cu–O bonds of ~2.9–3.0 Å, thus forming CuO7 polyhedra. To the best of our knowledge, only two other examples of heptacoordinated Cu2+ are known, viz. in the high pressure phase (II) of CuGeO3 (Yoshiasa et al., Reference Yoshiasa, Yagyu, Ito, Yamanaka and Nagai2000) and in a Cu-based complex polymer (Nadeem et al., Reference Nadeem, Bhadbhade, Bircher and Stride2010). Thus saranchinaite is unique as a mineral and as an inorganic compound housing heptacoordinated Cu2+ under ambient conditions. We plan to study this phenomenon subsequently with pure synthetic material. We hypothesize that the marked non-centrosymmetry of saranchinaite is related to the particularities of the Cu coordination. The Cu(1)-centred polyhedra are isolated (Fig. 3d) from other CuOn polyhedra, while Cu(2)- and Cu(3)-centred polyhedra share a common O(3) corner thus forming dimeric units. Cu(4)O6 polyhedra share common apical O(31) atoms with Cu(3)O7.
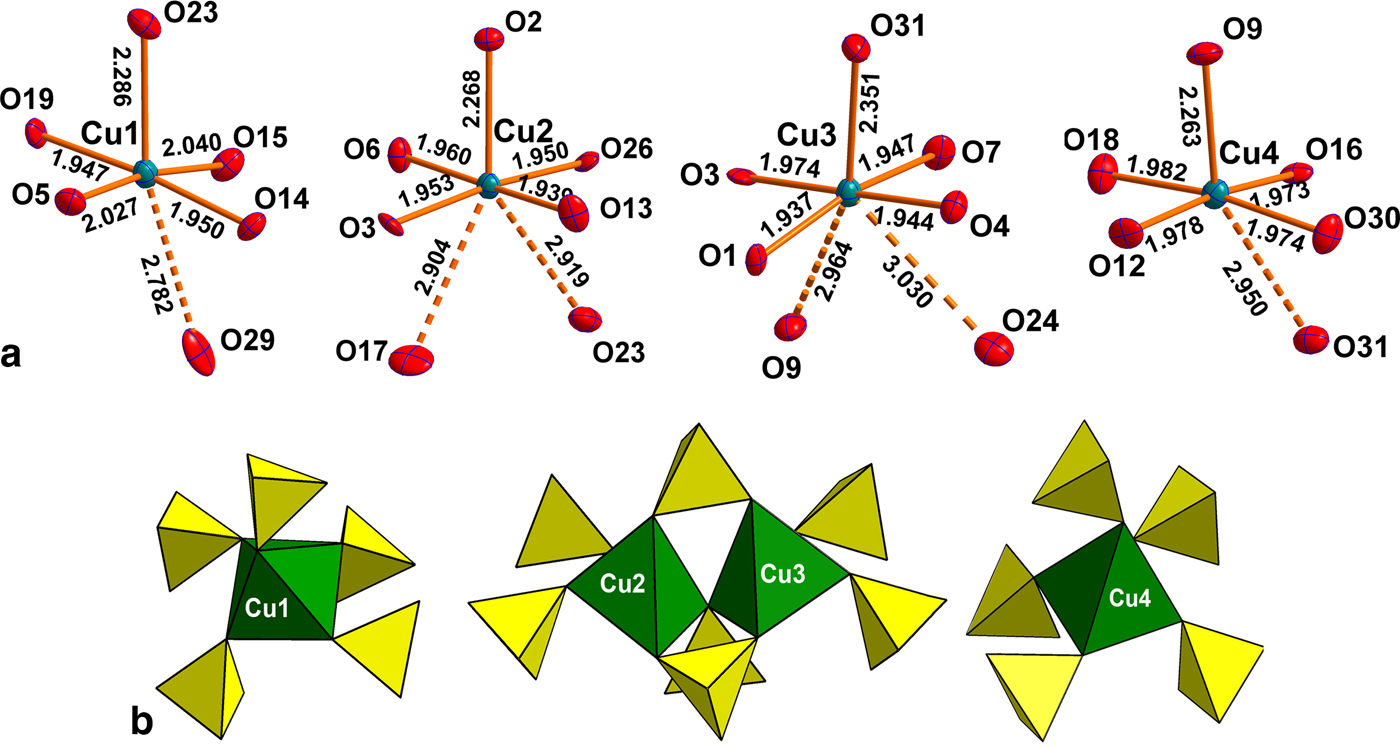
Fig. 2. (a) Coordination of Cu2+ cations and (b) interconnection of CuO5 pyramids (taking into account only strong Cu-O bonds) with sulfate tetrahedra in the structure of saranchinaite.
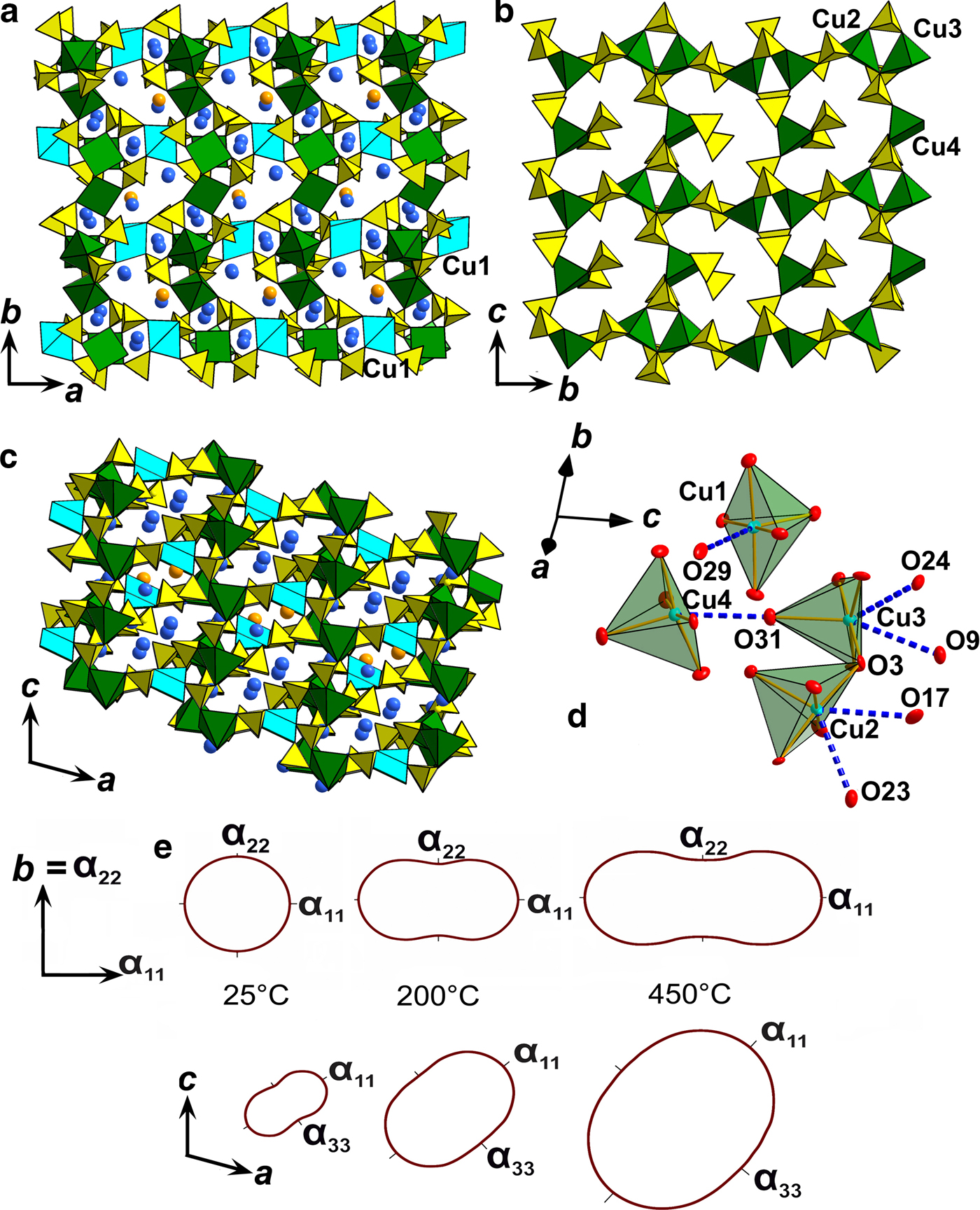
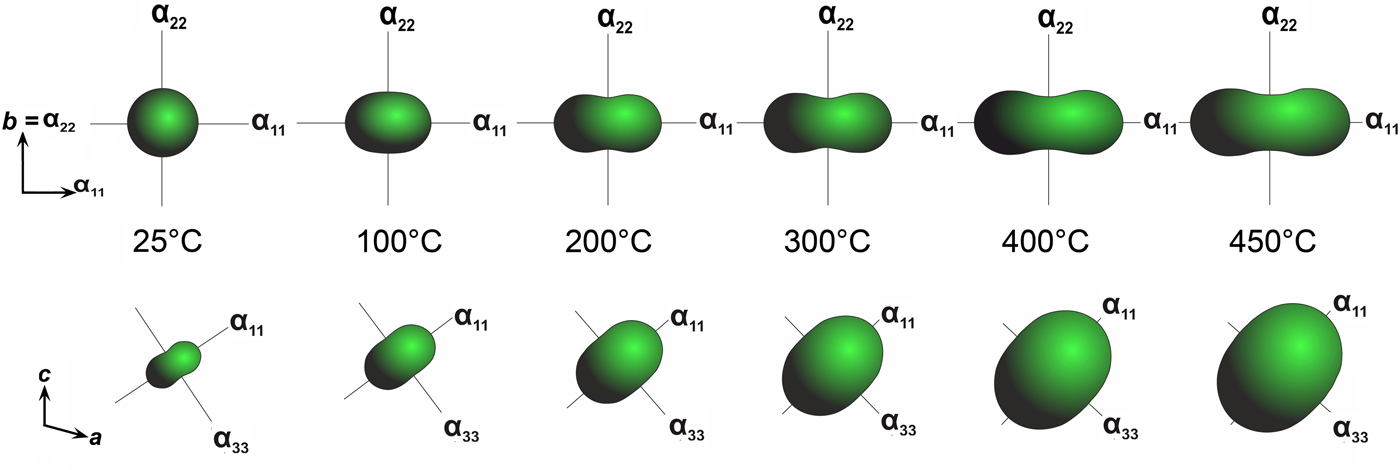
Fig. 3. General projection of the crystal structure of saranchinaite along the c (a) and a (b) and b (c) axis (Na5 sites are marked by orange and the remaining Na atoms are blue). The [Cu4(SO4)8]2– framework consists of layers (c) formed by Cu(2)O5, Cu(3)O5, Cu(4)O5 tetragonal pyramids and sulfate tetrahedra. Layers are interconnected via Cu(1)O5 (marked by sky blue) tetragonal pyramids. (d) Orientation of weak Cu–O bonds (blue dotted lines) in the structure of saranchinaite. (e) Pole figures of the thermal expansion coefficients of saranchinaite at different temperatures.
There are eight distinct S sites, each occupied by S6+ and coordinated tetrahedrally by four O atoms (Table 5). The average S–O bond-lengths, 1.47–1.48 Å, are consistent with the average value of 1.475 Å given for sulfate minerals in general (Hawthorne et al., Reference Hawthorne, Krivovichev, Burns, Alpers, Jambor and Nordstrom2000).
Each of the eight independent Na sites in the structure is fully occupied by Na+ cations except for Na(5) which is partly substituted for K+. The details on coordination of the Na sites can be found in Table 5. Coordination numbers of Na sites are different and the following polyhedra are formed: Na(1)O8, Na(2)O6, Na(3)O6, Na(4)O7, Na(5)O6, Na(6)O7, Na(7)O6 and Na(8)O6.
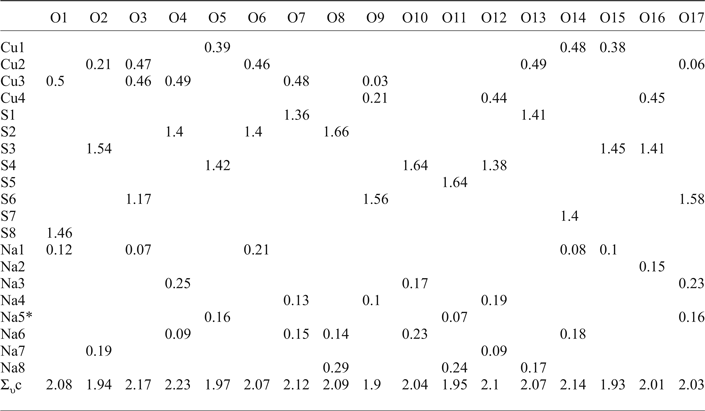
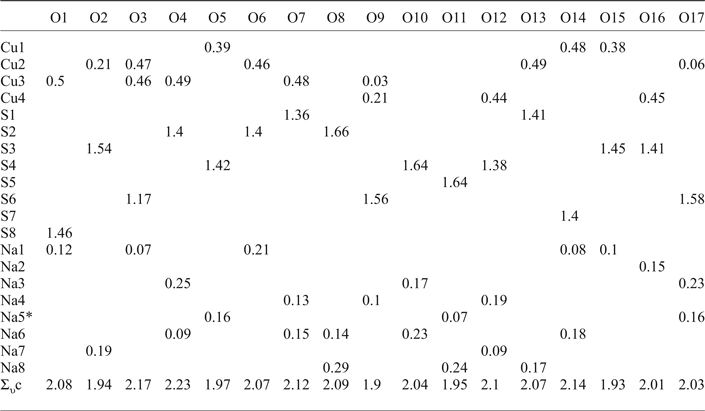
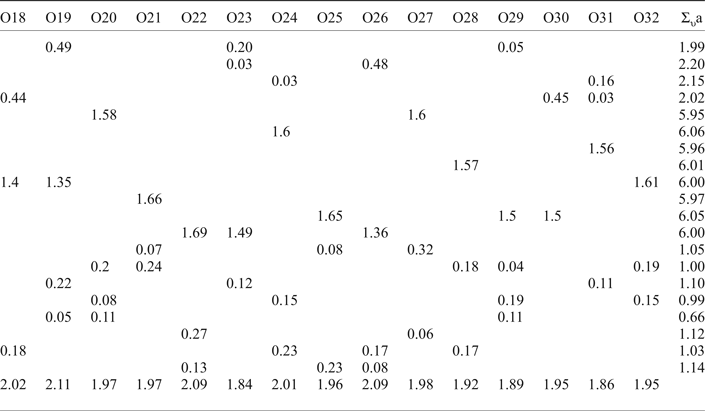
Bond-valence calculations were performed using bond-valence parameters taken from Brese and O'Keeffe (Reference Brese and O'Keeffe1991) for the Na–O, Cu–O and S–O bonds. The results are presented in Table 6. As can be seen, there is general agreement between the expected and calculated oxidation states for all atomic sites. Note, Cu(2) and Cu(3) sites appear to be slightly overbonded with the bond-valence sums of 2.20 and 2.15 vu (valence units), respectively.
Table 6. Bond-valence values (in valence units) for saranchinaite.
*Na0.71(2)K0.29(2).
The polyhedra Cu(2)O7, Cu(3)O7 and Cu(4)O6 share all of the oxygen apices with SO4 tetrahedra (Fig. 2b) thus forming slightly corrugated layers stacked along [100] (Fig. 3b). For the sake of clarity CuO5 (4+1) pyramids taking into account only the strongest Cu–O bonds are shown in Figs 2 and 3. Layers are linked into a rigid 3D [Cu4(SO4)8]8– framework by Cu(1)O5 pyramids acting as pillars (Fig. 3a). The complex 3D system of channels is occupied by Na (and minor K) atoms. In turn NaOn polyhedra share common edges and corners thus also forming a 3D architecture. The structural topology of the [Cu4(SO4)8]8– framework in saranchinaite is unique and has not been observed before.
High-temperature powder XRD study of kröhnkite and saranchinaite
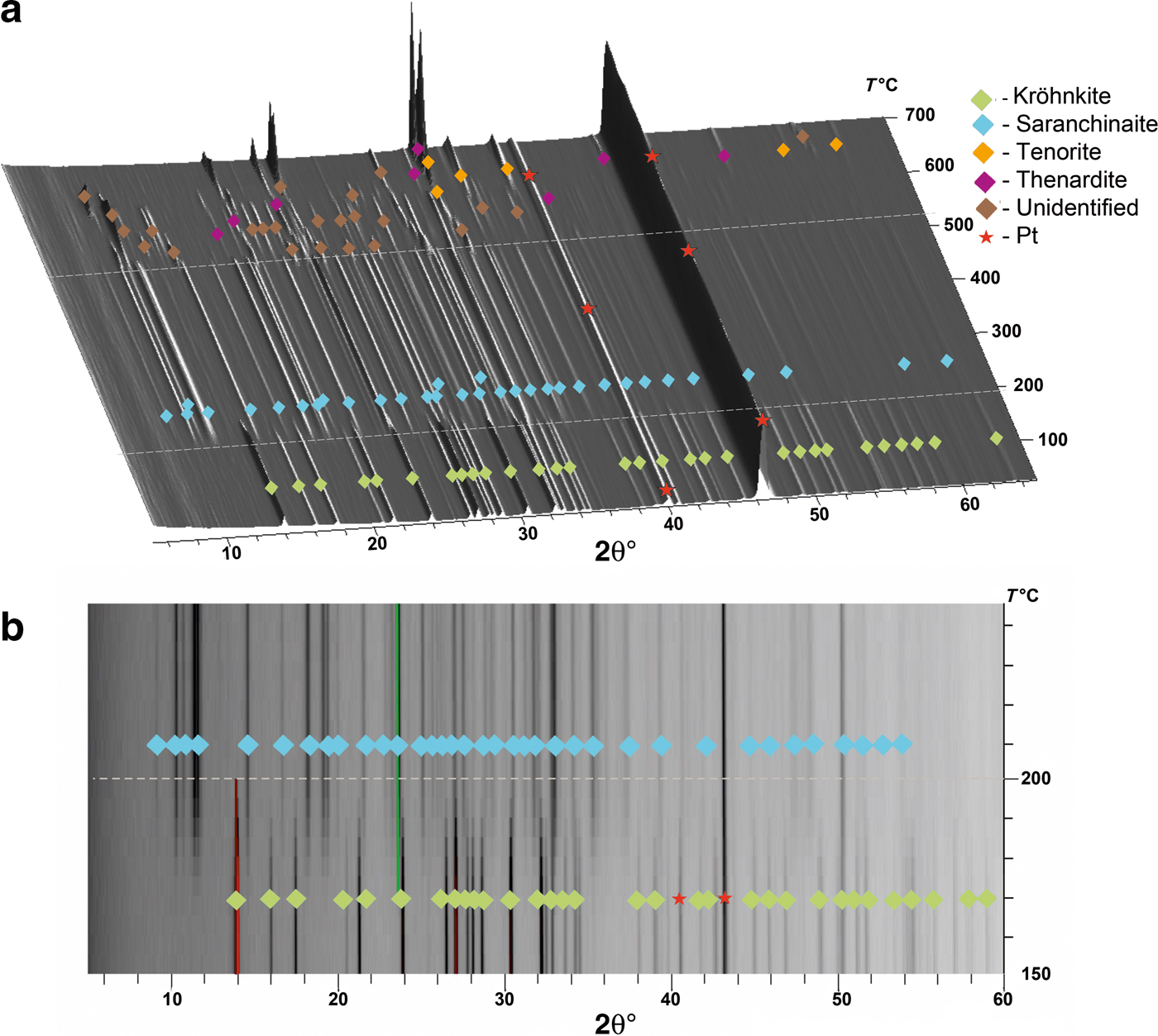
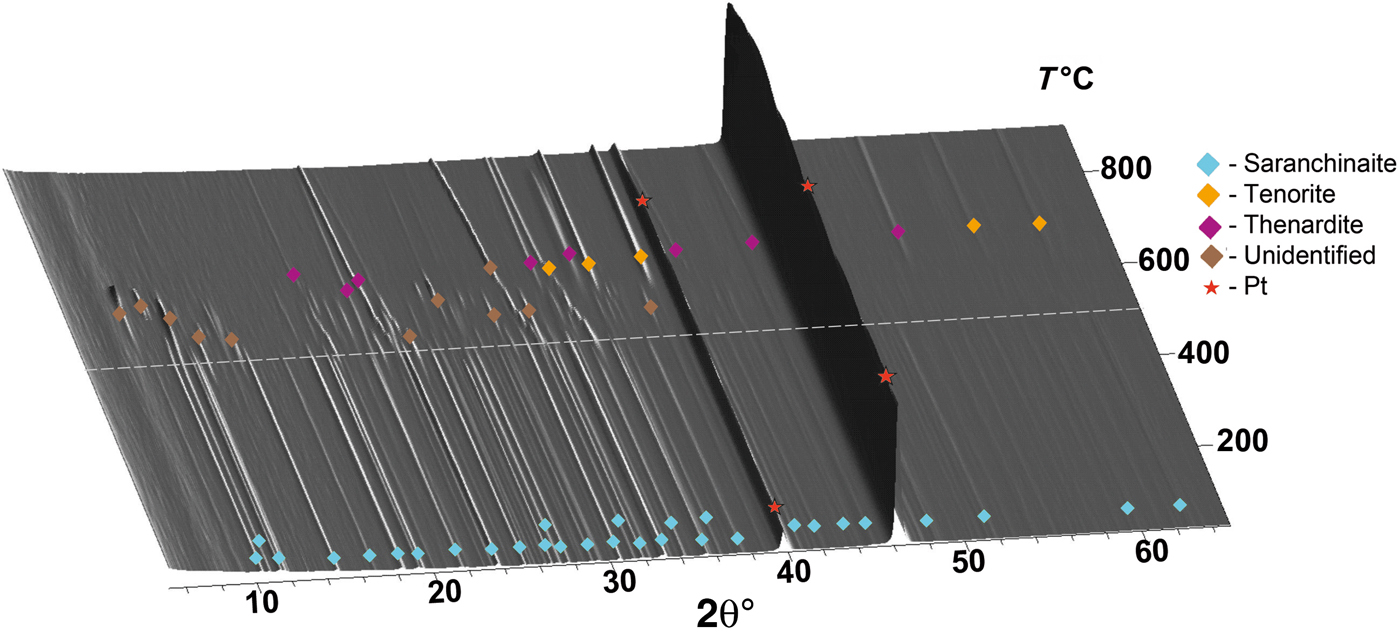
The thermal behaviour of kröhnkite (Fig. 4) and saranchinaite (Fig. 5) was studied in air by means of a Rigaku Ultima X-ray diffractometer (CuKα radiation) equipped with a high-temperature camera Rigaku HTA 1600. The samples were prepared from heptane suspension on Pt–Rh plates. Temperature steps were 25°C in the range 25–700°C (Fig. 4a) for the first full measurement of kröhnkite and detailed additional measurements were undertaken in the range 150–250°C with steps of 5°C (Fig. 4b). X-ray diffraction patterns for saranchinaite were obtained in the range 25–900°C with step size 25°C. Unit-cell parameters at different temperatures were refined by least-squares methods. The main coefficients of the thermal expansion tensor were determined using linear approximation of temperature dependences by the ThetaToTensor program (Firsova et al., Reference Firsova, Bubnova and Filatov2011). Temperatures of the decomposition of kröhnkite and saranchinaite were estimated as the mean temperature between the two corresponding HTXRD experiments where the diffraction pattern changes. i.e. where peaks of another phase appear.

Fig. 4. Three-dimensional perspective plot showing all diffractograms of kröhnkite over 5–60°2θ with increasing temperature. The dashed white line indicates transformation of kröhnkite into saranchinaite in the range of 170–200°C.

Fig. 5. Three-dimensional perspective plot showing all diffractograms of saranchinaite over 5–60°2θ with increasing temperature. The dashed white line indicates decomposition of saranchinaite into tenorite, thénardite and unidentified phase/phases at 520°C.
Kröhnkite
The evolution of the powder diffraction pattern of kröhnkite as function of temperature is shown in Fig. 4. With increasing temperature, the pattern does not undergo significant changes. The reflections of kröhnkite gradually start to disappear at ~170°C (Fig. 4b). At this temperature, peaks of saranchinaite appear indicating the dehydration of kröhnkite. Note that the chemical formulae of both minerals are identical except of H2O molecules in kröhnkite. Full dehydration of kröhnkite and transformation into saranchinaite is completed at 200°C. No peaks corresponding to other phases could be observed. The temperature dependence of the cell parameters (Supplementary Fig. 1S) of kröhnkite can be described by the following linear functions:
V t = 385.21(2) + 29(2) × 10–3t, where t is a temperature. Upon heating, the unit cell is expanding along all crystallographic axes.
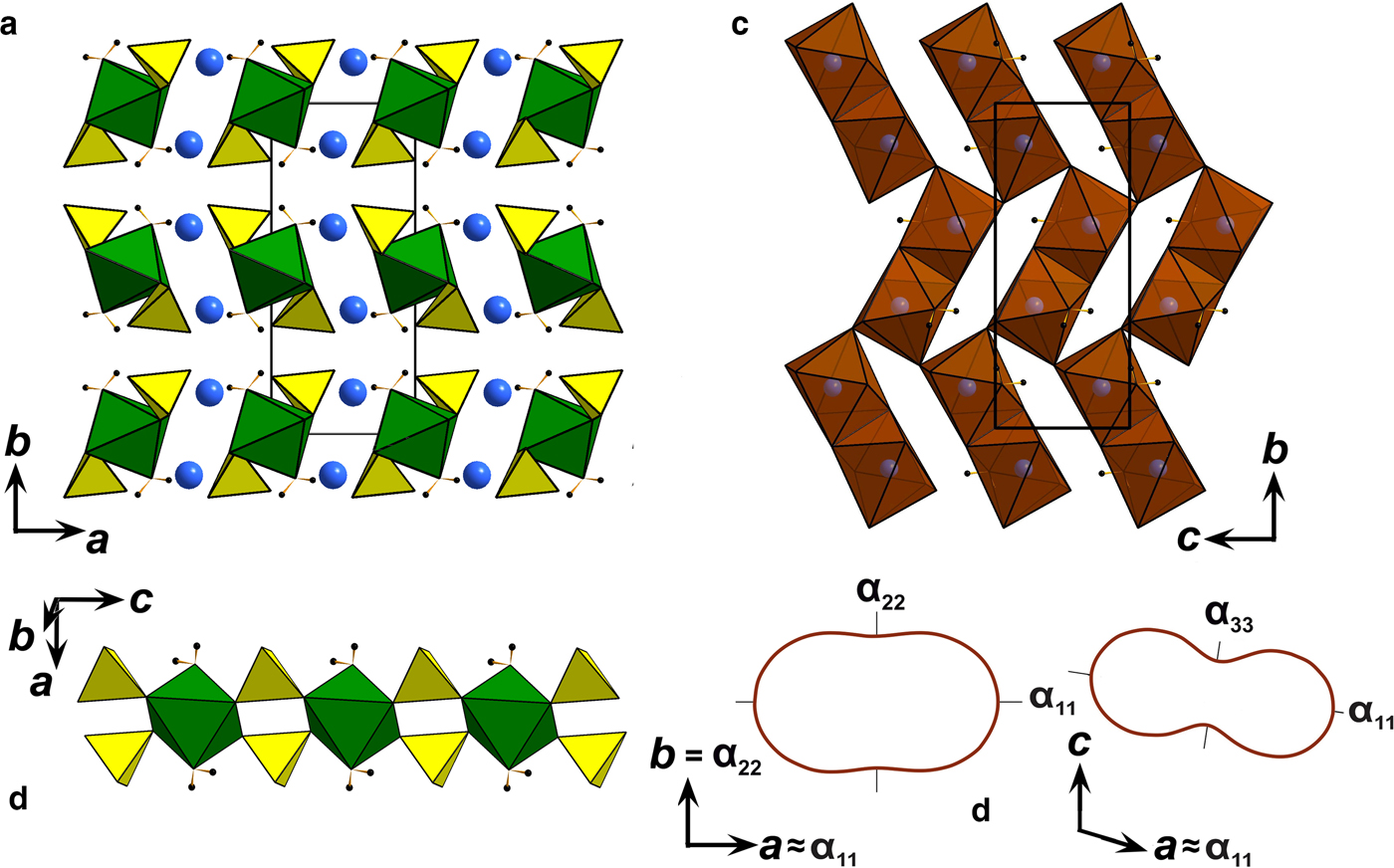
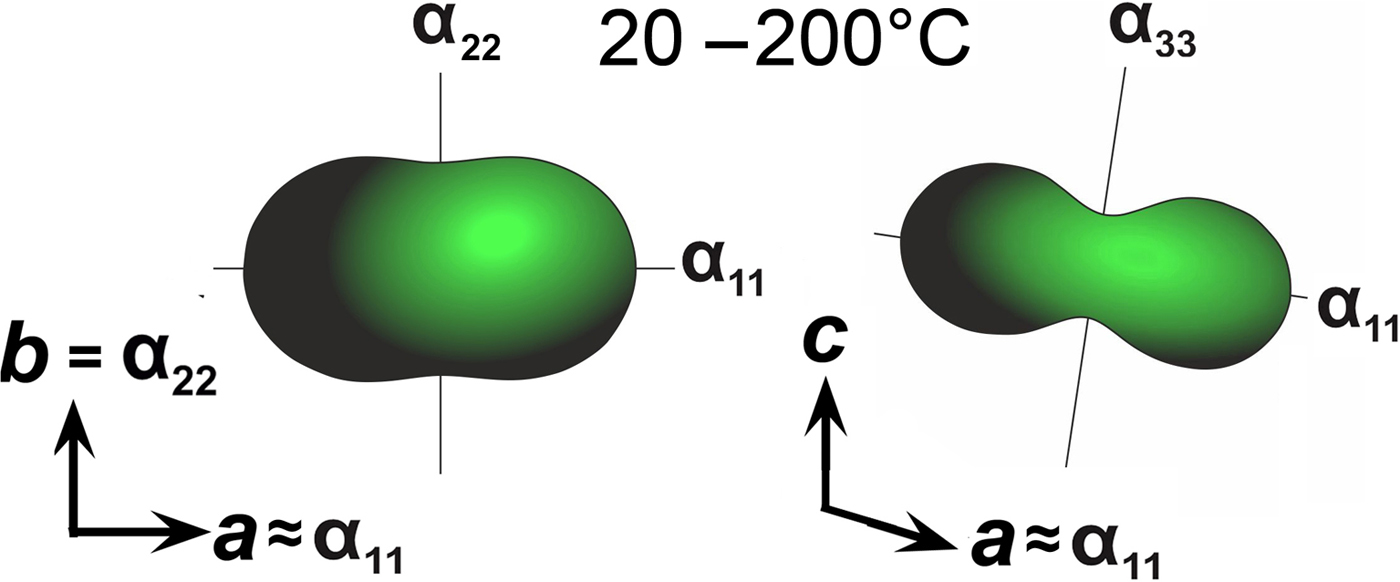
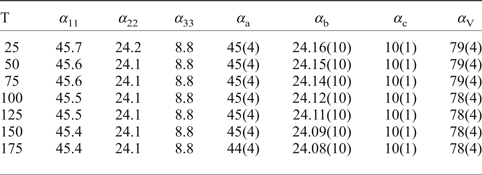
The following values of the principal eigenvalues of the thermal expansion tensor of a unit cell at 25°C were obtained: αa ≈ α11 = 44.6 ×10–6 °С−1, αb = α22 = 24.2 ×10–6 °С−1, αc ≈ α33 = 6.4 ×10–6 °С−1, αV = 75.4 ×10–6 °С−1 and μ (α11^a) = 8.9°. The structure of kröhnkite (Hawthorne and Ferguson, Reference Hawthorne and Ferguson1975) (Fig. 6) is based on chains of SO4 tetrahedra and CuO4(H2O)2 octahedra extending along the c axis. The chains are linked by Na+ cations each coordinated by seven O atoms. The CuO4(H2O)2 octahedra show typical Jahn-Teller distorted [4+2] geometry. NaO7 polyhedra share common edges thus forming dimers (Fig. 6c) which are interconnected eventually into layers via common corners. The strongly anisotropic character (Fig. 7) of the thermal expansion of kröhnkite remains essentially unchanged up to its decomposition (Table 7). The highest αa expansion is observed perpendicular to the Na interlayer (Fig. 6a), whereas minimal αc thermal expansion occurs in the direction of the rigid [Cu(SO4)2(H2O)2]2– chains (Fig. 6b). Rather strong bonding between Na-centred polyhedra in the interlayer is reflected in the αb expansion value.
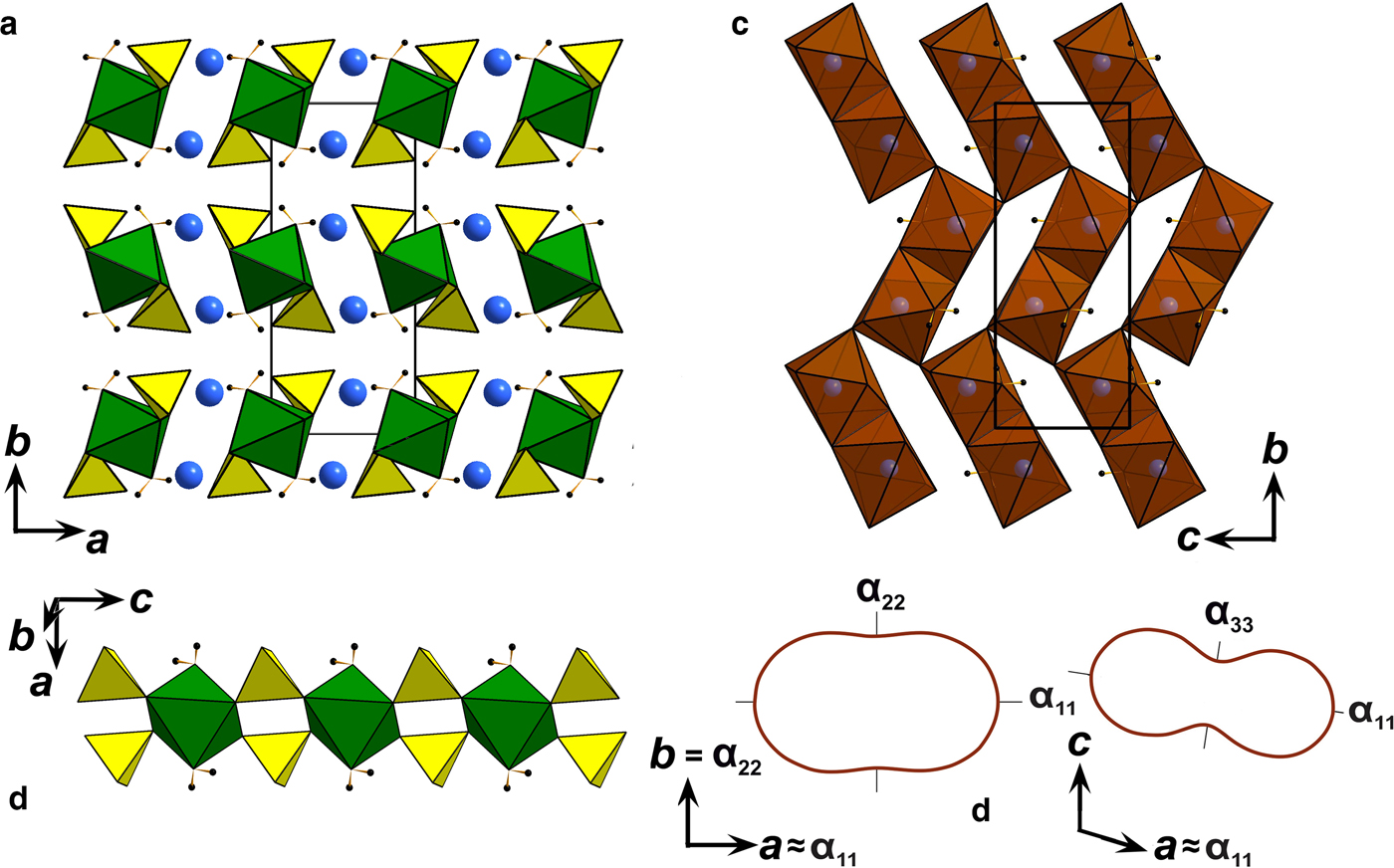
Fig. 6. (a) General projection of the crystal structure of kröhnkite (after Hawthorne and Ferguson, Reference Hawthorne and Ferguson1975) along the c axis (Na sites are marked by blue balls and H atoms are black). (b) [Cu(SO4)2(H2O)2]2– chains in the structure of kröhnkite. (c) Arrangement of NaO7 polyhedra in the interlayer. (d) Pole figures of the thermal expansion coefficients of kröhnkite in ba and ca planes.
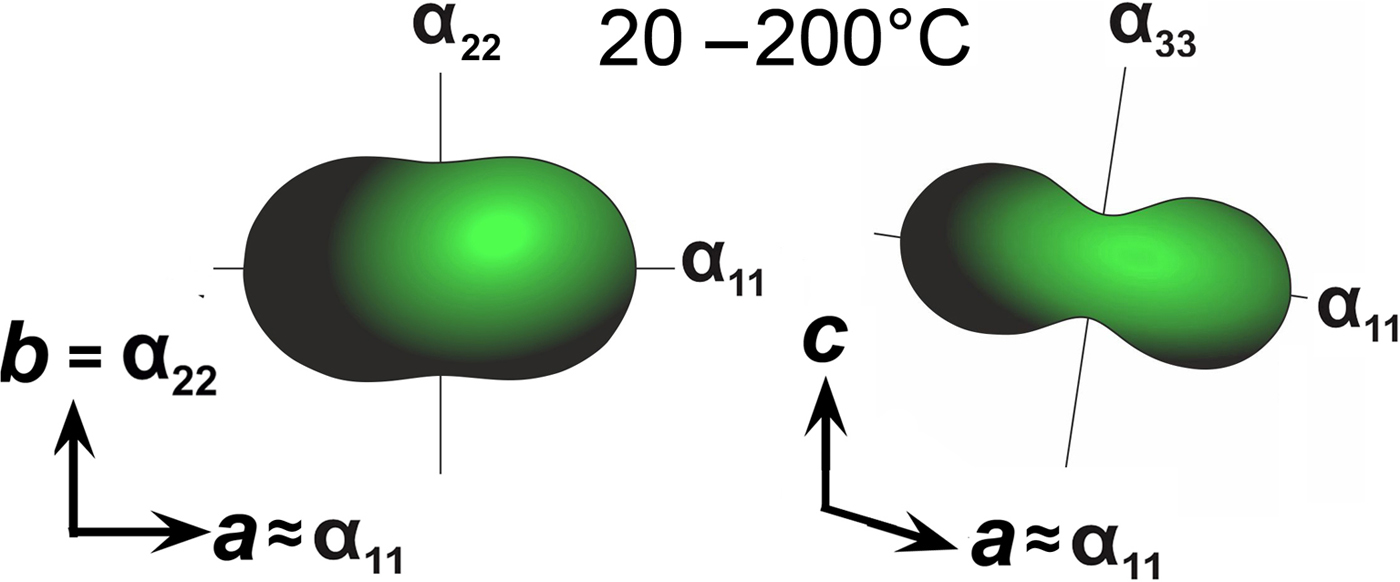
Fig. 7. Thermal expansion tensors for kröhnkite (see Table 8) in the range of 20–200°C.
Table 7. Values (×10–6 °С−1) of the principal eigenvalues of thermal expansion tensor of the unit cell of kröhnkite at different temperatures (T°C).
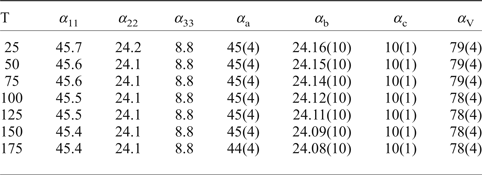
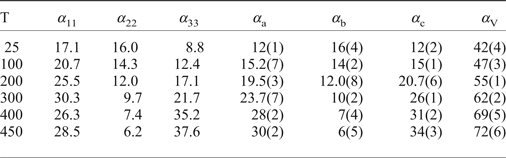
Table 8. Values (×10–6 °С−1) of the principal eigenvalues of thermal expansion tensor of the unit cell of saranchinaite at different temperatures (T°C).

Saranchinaite
The thermal expansion and decomposition of saranchinaite (Table 8; Figs 3, 8) is significantly more complex than that of kröhnkite. It is identical for both saranchinaite from Tolbachik and saranchinaite obtained by dehydration of kröhnkite. The results reported below were obtained from saranchinaite found in the Saranchinaitovaya fumarole. Saranchinaite is stable up to 475°C when it starts to decompose into tenorite, thénardite and an unidentified phase.
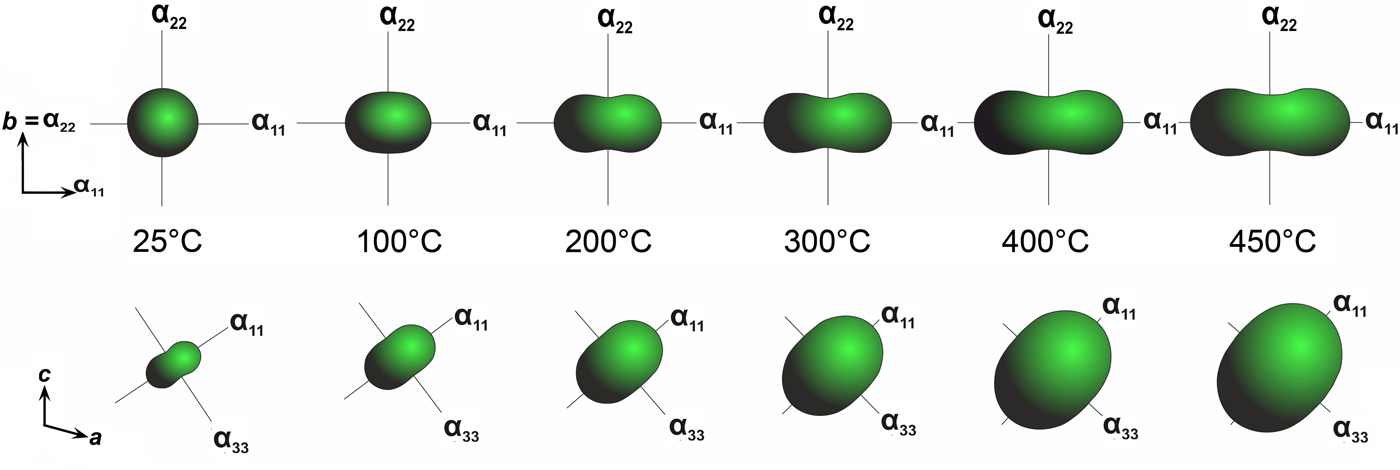
Fig. 8. Evolution of thermal expansion tensors of saranchinaite at different temperatures.
There is a continuous expansion of the unit-cell volume (Supplementary Fig. 2S), associated with a volume thermal expansion coefficient (Table 8). The expansion of saranchinaite is strongly anisotropic, such that the a, b and c axes expand with increasing temperature:
V t = 1357.75(4) + 87(2) × 10–3t, where t is a temperature.
At 25°C α11 and α22 are very similar but expansion behaviour changes with the temperature rise. The strongest expansion of saranchinaite is observed in the direction of the bisector of the β angle (corresponding to α11), whereas less expansion occurs in the direction of the perpendicular diagonal of the unit cell (α33). This thermal behaviour can be explained on the basis of the considerations of the respective rigidity of structural units in saranchinaite. SO4 tetrahedra and CuOn polyhedra are the most rigid building units. The b axis is nearly parallel to the virtual Cu(2)–Cu(3) axis in dimers (Fig. 2b). These dimers are fixed rigidly by sulfate tetrahedra in between them. α22 expansion of the structure decreases significantly (Fig. 8, Table 8) with the temperature. α11 occurs in a direction nearly perpendicular to the Cu-sulfate layers formed by Cu(2)O7, Cu(3)O7 and Cu(4)O6 (Fig. 3).
Final remarks
Saranchinaite represents a new structure type and has no direct synthetic analogues. Anhydrous compounds of composition A 2Cu(SO4)2 (A = Na, K, Rb and Cs) are unknown. The structural topologies of the high-temperature anhydrous mineral saranchinaite and of the low-temperature hydrated kröhnkite are unrelated; saranchinaite is significantly more complex than kröhnkite. Natrochalcite, NaCu2(SO4)2(OH)(H2O) (Chevrier et al., Reference Chevrier, Giester and Zemann1993) is again structurally unrelated to saranchinaite, but is based on kröhnkite-type chains. ‘Pure’ Na–Cu sulfate minerals sensu stricto (i.e. minerals with independent crystallographic positions of Na and Cu) without additional cations and anions are unknown. The majority of Cu-containing sulfates of exhalative origin are K-dominant (Siidra et al., Reference Siidra, Nazarchuk, Zaitsev, Lukina, Avdontseva, Vergasova, Vlasenko, Filatov, Turner and Karpov2017). Saranchinaite is the second Na-dominant Cu-sulfate mineral observed in fumaroles of scoria cones on Tolbachik volcano. Recently, we have described another Cu sulfate which, however, contains additional oxygen atoms, viz. puninite, Na2Cu3O(SO4)3 from fumaroles of the Second Scoria cone. Kröhnkite is a very abundant mineral in altered mineral crusts of the Second Scoria cone and most probably the hydration product of saranchinaite.
High-temperature powder XRD studies show that kröhnkite starts decomposing at 170°C and at ~200°C full transformation into saranchinaite is achieved by losing all of the water content. Notably, during this transformation the ‘Na2Cu(SO4)2’ main part of the chemical formula is retained, despite the lack of direct structural relations between kröhnkite and saranchinaite. Kröhnkite crystals (Fig. 1c) have a greenish tint whereas saranchinaite is blue (Fig. 1a,b). The difference in colour may be the result of different coordination environments of Cu2+ cations in both crystal structures: [4+1+1] and [4+1+2] in saranchinaite, but [4+2] in kröhnkite.
The discovery of saranchinaite and the successful determination of its crystal structure may facilitate the structure determination of various kröhnkite-type materials (Driscoll et al., Reference Driscoll, Kendrick, Wright and Slater2016; Barpanda et al., Reference Barpanda, Oyama, Ling and Yamada2014; Saha et al., Reference Saha, Madras and Guru Row2011; Behera and Rao, Reference Behera and Rao2006; Pasha et al., Reference Pasha, Choudhury and Rao2003) from powder samples and the investigation of the physical properties of products of decomposition of others (Driscoll et al., Reference Driscoll, Kendrick, Wright and Slater2016; Barpanda et al., Reference Barpanda, Oyama, Ling and Yamada2014; Saha et al., Reference Saha, Madras and Guru Row2011; Behera and Rao, Reference Behera and Rao2006; Pasha et al., Reference Pasha, Choudhury and Rao2003). It should be noted that recently the transformation of kröhnkite-type Na2Mn(SO4)3·2H2O into an alluaudite-type structure has been reported (Marinova et al., Reference Marinova, Kostov, Nikolova, Kukeva, Zhecheva, Sendova-Vasileva and Stoyanova2015). This lends itself to the speculation that the transformation of kröhnkite-type materials into saranchinaite-type phases may be restricted to Cu-based compounds due to the Jahn-Teller effect on Cu2+.
The observed complex character of thermal expansion in saranchinaite might be of special interest, given the high scientific interest in this phenomenon and its potential for practical applications in controlled thermal expansion composites. We have successfully synthesized the K-free Na2Cu(SO4)2 analogue of saranchinaite. Magnetic properties as well as measurements of electrochemical and non-linear optical properties are planned for the near future.
Acknowledgements
We are grateful to Edward Grew, Peter Leverett, Anna Garavelli and an anonymous reviewer for valuable comments. This work was supported financially by the Russian Science Foundation through the grant 16-17-10085. Technical support by the SPbSU X-ray Diffraction and Microscopy and Microanalysis Resource Centres are gratefully acknowledged.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/minmag.2017.081.037