INTRODUCTION
In Mexico, Leishmania mexicana parasites cause cutaneous leishmaniasis (CL), resulting in localized cutaneous leishmaniasis (LCL) or diffuse cutaneous leishmaniasis (DCL). This parasite is a digenetic protozoan that is transmitted to the mammalian host by sandflies of the genus Lutzomyia in the New World. In the alimentary tract of the insect vector, the parasites are found as extracellular flagellated promastigotes. During the insect blood meal, infective promastigotes are injected into the dermis of the mammalian host and phagocytosed by resident macrophages where they are differentiated into non-motile amastigotes that multiply within them. In addition to macrophages, other cells such as dendritic cells (DCs) and fibroblasts may also harbour parasites (Solbach and Laskay, Reference Solbach and Laskay2000).
Leishmania promastigotes are known to synthesize and traffic most surface molecules such as lipophosphoglycan (LPG), surface protease gp63 and acid phosphatases (McConville et al. Reference McConville, Mullin, Ilgoutz and Teasdale2002). LPG and gp63 have been widely studied and are considered Leishmania virulence factors (McConville and Blackwell, Reference McConville and Blackwell1991). Our group previously reported that L. mexicana LPG induced a differential production of cytokines in human DCs and monocytes (Argueta-Donohue et al. Reference Argueta-Donohue, Carrillo, Valdes-Reyes, Zentella, Aguirre-Garcia, Becker and Gutierrez-Kobeh2008). Additionally, we found that L. mexicana LPG activates ERK and p38 MAP kinase in macrophages and induces proinflammatory cytokine production through TLR2 and TLR4 signalling (Rojas-Bernabe et al. Reference Rojas-Bernabe, Garcia-Hernandez, Maldonado-Bernal, Delegado-Dominguez, Ortega, Gutierrez-Kobeh, Becker and Aguirre-Garcia2014).
It is well known that Leishmania species secrete proteins and other molecules that affect the host immune system facilitating the infection process (Santarem et al. Reference Santarem, Silvestre, Tavares, Silva, Cabral, Maciel and Cordeiro-da-Silva2007). In this regard, Silverman et al. identified 151 proteins from Leishmania donovani that were actively secreted and whose general properties allowed them to postulate potential mechanisms of secretion as well as functional roles within the infection (Silverman et al. Reference Silverman, Chan, Robinson, Dwyer, Nandan, Foster and Reiner2008).
Additionally, to these secreted proteins, other proteins of the sandfly saliva play an important role in the establishment of the infection (Rohousova et al. Reference Rohousova, Volf and Lipoldova2005; Santarem et al. Reference Santarem, Silvestre, Tavares, Silva, Cabral, Maciel and Cordeiro-da-Silva2007).
Other proteins present in Leishmania parasites are phosphatases, which have been identified as virulence factors in many pathogenic microorganisms such as virus, bacteria and parasites (Bliska et al. Reference Bliska, Guan, Dixon and Falkow1991; Shibata et al. Reference Shibata, Noda, Sawa and Watanabe1994; Green et al. Reference Green, Hartland, Robins-Browne and Phillips1995). Phosphatases are enzymes that remove phosphate groups from amino acid residues of proteins and are classified in terms of substrate specificity in: protein tyrosine phosphatases (PTPs) and serine/threonine phosphatases, which can be subdivided in phosphoprotein phosphatases (PPP) and metal-dependent protein phosphatases (PPM), which include protein phosphatase 2C (PP2C).
In Leishmania different PTPs have been reported to play a role in the pathogenesis of this parasite through the activation of PTPs of the host cells (Blanchette et al. Reference Blanchette, Racette, Faure, Siminovitch and Olivier1999).
Previously, we reported the presence of a membrane-bound PTP in Leishmania major promastigotes. We cloned and sequenced the gene, purified and characterized the protein phosphatase 2C and determined by immunolocalization that the molecule is present in the flagellum of promastigotes (manuscript in preparation). Also, we have reported that L. mexicana promastigotes are able to secrete a PTP activity in the culture medium (CM) (Escalona-Montaño et al. Reference Escalona-Montaño, Pardave-Alejandre, Cervantes-Sarabia, Garcia-Lopez, Gutierrez-Quiroz, Gutierrez-Kobeh, Becker-Fauser and Aguirre-Garcia2010).
The vast majority of studies involving Leishmania membrane-bound or secreted molecules have been done with the promastigote, although the amastigote is responsible for maintaining and spreading infection within the host.
In the present work, we give evidence that L. mexicana promastigotes and amastigotes are able to secrete a PTP and PP2C in the CM. The phosphatase activity secreted in the CM was higher in promastigotes as compared with the activity secreted by amastigotes. The phosphatase activity secreted by both morphological stages was inhibited by specific PTP inhibitors. On the other hand, the ultrastructural localization of PP2C in L. mexicana amastigotes showed that it is present in the flagellar pocket. Finally, we show that the secreted proteins of promastigotes and amastigotes induced the production of inflammatory cytokines in human macrophages.
MATERIALS AND METHODS
Parasite culture
Leishmania mexicana strains MHOM/MX/92/UADY 68 and MHOM/MX/2011/Lacandona were used throughout experiments. MHOM/MX/92/UADY 68 strain was a generous gift from Dr Fernando Andrade, Centro de Investigaciones Regionales ‘Dr Hideyo Noguchi’ from Universidad Autónoma de Yucatán and culture conditions were previously reported (Escalona-Montaño et al. Reference Escalona-Montaño, Pardave-Alejandre, Cervantes-Sarabia, Garcia-Lopez, Gutierrez-Quiroz, Gutierrez-Kobeh, Becker-Fauser and Aguirre-Garcia2010). MHOM/MX/2011/Lacandona was used throughout the experiments. The strain was isolated from a Mexican patient diagnosed with LCL after returning from a trip to the Lacandona rainforest region of the Mexican State of Chiapas. Informed written consent was obtained from the patient and a 3 mm punch biopsy was taken from the active edge of the skin ulcer after previous asepsis and local anaesthesia administration. A fragment of the biopsy was homogenized in phosphate-buffered saline (PBS) by grinding the tissue with a pestle in a 1·5 mL conical tube to release amastigotes from infected cells. The resultant homogenate was inoculated subcutaneously in the right hind footpad of BALB/c mice. After lesion development, amastigotes were isolated from infected footpads and cultured as axenic amastigotes at 33 °C in Schneider's Drosophila insect cell CM (Sigma, St. Louis, MO, USA), pH 5·4, supplemented with 20% heat-inactivated fetal bovine serum (FBS), 100 U mL−1 penicillin G and 100 µg mL−1 streptomycin (Gibco-Life Technologies, Grand Island, NY, USA), as previously described (Wilkins-Rodriguez et al. Reference Wilkins-Rodriguez, Escalona-Montano, Aguirre-Garcia, Becker and Gutierrez-Kobeh2010). Promastigotes were transformed from footpad-derived amastigotes and cultured at 26 °C in medium 199, pH 7·2, supplemented with 10% FBS, 100 U mL−1 penicillin G, 100 µg mL−1 streptomycin, 2 mm l-glutamine and 1% BME vitamins (Gibco-Life Technologies). The isolated strain was identified as L. mexicana by PCR amplification of a L. mexicana-specific sequence of the internal transcribed spacer of the ribosomal RNA gene, as previously reported (Berzunza-Cruz et al. Reference Berzunza-Cruz, Bricaire, Salaiza Suazo, Perez-Montfort and Becker2009). Infectivity of the strain was maintained by regular passage through BALB/c mice.
Leishmania secreted proteins
Promastigotes
Promastigote secreted phosphatases were analysed according to Escalona-Montaño et al. (Reference Escalona-Montaño, Pardave-Alejandre, Cervantes-Sarabia, Garcia-Lopez, Gutierrez-Quiroz, Gutierrez-Kobeh, Becker-Fauser and Aguirre-Garcia2010). Briefly, L. mexicana promastigotes strain MHOM/MX/92/UADY 68 were cultured during 5 days, after which the parasites were washed two times with 199 medium without FBS. The parasites were incubated (50 × 106 parasites mL−1) in medium 199 free of serum, tyrosine and phosphate) for 1, 3, 5 and 7 h at 26 °C. After each time, parasites were harvested by centrifugation at 2000 g for 10 min at 4 °C. Supernatants were centrifuged at 11 000 g for 15 min and concentrated 10-fold by pressure ultrafiltration using an Amicon system with 10-kDa ultrafiltration regenerated cellulose membrane (Millipore Corporation, Billerica, MA, USA). Since L. mexicana strain 68 showed optimal secretion at 7 h, this time point was also used for the Lacandona strain in all the experiments with promastigotes. Concentrated medium is referred as Promastigotes Secretion Medium (PSM7).
Amastigotes
Leishmania mexicana strain MHOM/MX/2011/Lacandona amastigotes were cultured during seven days, after which the parasites were washed two times with Schneider's Drosophila medium (SDM), without FBS. The parasites were incubated (50 × 106 parasites 0·5 mL−1) in SDM free of serum, tyrosine and phosphate for 0·25, 1, 3 and 5 h at 33 °C. The parasites were harvested by centrifugation at 2000 g for 10 min at 4 °C in each time. Cell-free supernatant was concentrated 10-fold by pressure ultrafiltration using an Amicon system with 10-kDa ultrafiltration regenerated cellulose membranes (Millipore Corp.). This concentrated medium is referred as Amastigotes Secretion Medium (ASM1). PSM7 and ASM1 were used in the different assays. Viability of promastigotes and amastigotes was analysed by erythrosin B, a vital dye used to determine cell viability. Viable cells actively exclude the dye, while nonviable cells (those whose plasma membrane is damaged) take up the dye and appear red (Ruffolo et al. Reference Ruffolo, Cushion and Walzer1986). CM without parasites was used as negative control for all assays. The protein concentration of PSM7 and ASM1 was determined by the Bradford method with bovine serum albumin (BSA) as standard (Bradford, Reference Bradford1976).
Mice
BALB/c mice were purchased form Charles Rivers Laboratories (Wilmington, MA, USA) and bred at the animal facility of the Unidad de Investigación en Medicina Experimental de la Facultad de Medicina de la UNAM following the national guidelines for animal care. Mice were used at 8–10 weeks of age and all the procedures for the experiments were approved by the Ethics Committee of the Facultad de Medicina, UNAM.
Phosphatase activity assays
p-NPP substrate
Acid phosphatase activity was determined as described by Dissing et al. (Reference Dissing, Dahl and Svensmark1979). Briefly, 1 µg of PSM7 protein, obtained after 7 h secretion [PSM(1/7)] and 2 µg of ASM1 protein obtained after 1 h secretion [ASM(2/1)] were incubated in buffer (200 mm sodium acetate pH 5) + 10 mm of p-nitrophenyl phosphate [p-NPP] in a final volume of 100 µL for 60 min at 37 °C. Afterwards, the reaction was stopped with 20 µL of 2 N NaOH. The absorbance at 405 nm was read using a microtitre plate reader.
Phosphopeptides
Tyrosine and serine/threonine phosphatase activity was assayed using Promega's (non-radioactive tyrosine phosphatase assay system). The release of inorganic phosphate (P i), was monitored by measuring the absorbance of the molybdate–malachitegreen–phosphate complex. PSM(1/7) or ASM(2/1) were incubated in a total volume of 100 µL of assay buffer containing 200 mm sodium acetate pH 5. The reaction was started by adding 50 µ m Tyr phosphopeptide-1 substrate [END (pY) INASL], 50 µ m Thr [RRA (pT)VA] during 30 min at room temperature (RT) and was stopped with 50 µL molybdate dye/additive mixture. The optical density of the samples was read at 630 nm, using a curve of phosphates as standard (Aguirre-Garcia et al. Reference Aguirre-Garcia, Escalona-Montano, Bakalara, Perez-Torres, Gutierrez-Kobeh and Becker2006).
Effect of inhibitors of phosphatases in PSM(1/7) and ASM(2/1)
The phosphatase activity of PSM(1/7) and ASM(2/1) was analysed in presence of specific PTP inhibitors such as 200 µ m sodium orthovanadate, 200 µ m ammonium molybdate and 200 µ m sodium tungstate. Additionally, serine/threonine phosphatase inhibitors were tested such as 100 µ m trifluoperazine and 1 µ m okadaic acid. Also, sanguinarine, a specific inhibitor for PP2C, was used at a concentration of 20 µ m. For the inhibition assays, 100 µL of the reaction mixture was pre-incubated for 15 min at RT before adding the p-NPP substrate (all reagents of Sigma-Aldrich) and were incubated thereafter for another 60 min at 37 °C. Afterwards, the reaction was stopped with 20 µL of 2 N NaOH. The absorbance at 405 nm was read using a microtitre plate reader.
Total extracts of promastigotes and amastigotes
After the secretion process 3·5 × 109 promastigotes and 2·5 × 106 amastigotes were harvested by centrifugation at 2000 g for 10 min and then washed three times with PBS. The pellet containing the parasites was suspended in cold lysis buffer (10 mm Imidazole pH 7·2, 2 µg mL−1 leupeptin, 10 µg mL−1 aprotinin, 2 mm benzamidine) and then sonicated [total extract (TE)]. The TE was centrifuged in order to separate a cytosolic fraction (CF) and a membrane fraction (MF). The protein contents of the TE, CF and MF was quantified by the Bradford method and adjusted to 10 µg of protein.
Western blot analysis
In our laboratory, a PP2C of L. major was cloned, the recombinant protein was purified (LmPP2C) and antibodies against the recombinant protein were generated (α-Lm PP2C). Both LmPP2C and α-Lm PP2C were used in this work (manuscript in preparation).
PSM7 and ASM1 were precipitated with acetone at a 1:10 ratio. Afterwards, 5 µg of the TE, SF, MF and 1 µg of Lm PP2C were used in this experiment. The samples were analysed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis in 10% acrylamide gels and then electrotransferred onto immobilon-P transfer membranes (Millipore, Billerica, MA, USA). Membranes were washed with TBS-T (20 mm Tris–HCl, 150 mm NaCl, 0·005% Tween-20) and membranes were blocked by treatment with 5% milk in TBS-T for 1 h. They were then immunoblotted with an antibody against the PP2C of L. major at a 1:1000 dilution in 1% milk in TBS-T overnight at 4 °C. After 1 h of washing with TBS-T, membranes were incubated with secondary HRP-conjugated goat anti-rabbit IgG (Cell Signaling, Danvers MA, USA) at a 1:5000 dilution in TBST with 3% of milk) and washed ten times in TBS-T. Bands were detected using enhanced chemiluminescent substrate (Super-Signal West Pico Chemiluminescent Substrate, Pierce, Rockford IL, USA), according to the manufacturer's instructions.
Macrophages differentiation from peripheral blood monocytes
Peripheral blood monocytes were obtained from voluntary healthy donors. Peripheral blood mononuclear cells were separated by using Ficoll–Hypaque (Sigma) density gradient centrifugation at 300 g for 20 min at 20 °C, and suspended in pyrogen-free and sterile PBS pH 7·2. They were then incubated with CD14 MACS microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) during 15 min and purified by magnetic sorting. CD14+ monocytes (1 × 106) were washed and left overnight in pyrogen-free and sterile RPMI-1640 medium (Life Technologies Laboratories, Gaithersburg, MA, USA) supplemented with 10% (v/v) FBS and 2 mm l-glutamine (Gibco Invitrogen Corporation, Carlsbad, CA, USA). They were then cultured in six-well tissue culture plates (Costar, Cambridge, MA, USA) for 5 days at 37 °C, with 5% CO2 in a humidified atmosphere. Cell viability was assessed using Alamar Blue (Gregoraszczuk et al. Reference Gregoraszczuk, Rak-Mardyla, Rys, Jakubowicz and Urbanski2015). After a 4 h-incubation of the cells with the dye, the absorbance of the medium was measured at 570 and 600 nm using a microplate reader (BioTek Instruments Inc., Winooski, VT, USA) (data not shown).
Cytokine assays
Human macrophages were plated at a concentration of 1 × 106 in 1 mL of RPMI-1640 medium supplemented with 10% heat-inactivated FBS during 24 h at 37 °C and 5% CO2 in 24-well culture plates (Corning Life Sciences, NY, USA). Macrophages were incubated under different conditions: with PSM7, ASM1 or RPMI medium (C) for 24 h. For positive controls, cells were stimulated with 100 ng mL−1 of lipopolysaccharide for 24 h. Cell-free culture supernatants were harvested and the concentrations of tumor necrosis factor alpha (TNF-α), IL-1β, IL-12p70 and IL-10 were determined by standard sandwich enzyme-linked immunosorbent assay (ELISA) according to BD-Pharmingen Cytokine ELISA Protocol. In brief, 96-well microtitre plates (Costar, Corning, NY, USA) were coated with an unconjugated anti-TNF-α capture antibody (clone Mab1, 6 µg mL−1), anti-IL-1β (clone Mab 1, 4 µg mL−1), anti-IL-10 capture antibody (clone JES3-19F1, 4 µg mL−1) and anti-IL-12 p70 (clone 20C2, 2 µg mL−1) in 100 mm Na2HPO4, pH 9 for 12 h at 4 °C, and blocked with PBS pH 7·4, supplemented with 5% casein, dissolved in 0·1 N NaOH. Cell supernatants and the standard curves prepared with recombinant hTNF-α, hIL-1β, hIL-10, hIL-12p70 were incubated in RPMI-1640 medium supplemented with 10% FBS for 2 h at RT. Bound human TNF-α, IL-1β, IL-12 p70 were detected using a biotinylated anti-mouse antibody in 1% BSA for 1 h. Human IL-10 was detected using a biotinylated rat anti-hIL-10. All antibodies and recombinant cytokines were from BD-Pharmingen (San Jose, CA, USA). The plate was developed using streptavidin-alkaline phosphatase conjugate with p-nitrophenyl phosphate 5·0 µg mL−1) (Life Technologies) as substrate. Plates were read at 405 nm using a microtiter (BioTek Instruments Inc.) and the concentrations were calculated from the standard curves. The concentration of each sample was calculated by regression analysis using the mean absorbance (based on the average of triplicates of each sample). After the time incubations, the viability of the human macrophages was tested by alamar blue (data not shown).
Immunolocalization of PP2C by immune electron microscopy (IEM)
Detection of the PP2C in the amastigote of L. mexicana was achieved by electron microscopy (Gomez de Leon et al. Reference Gomez de Leon, Diaz Martin, Mendoza Hernandez, Gonzalez Pozos, Ambrosio and Mondragon Flores2014). Briefly, amastigotes were washed with PBS and fixed with a mixture of 4% paraformaldehyde and 0·1% glutaraldehyde in PBS for 1 h at RT. Once washed, the amastigotes were gradually dehydrated in ethanol and then embedded in LR White resin (London Resin, Polysciences, Inc. USA). Amastigotes in resin were deposited in plastic moulds and were polymerized overnight, under ultraviolet light, at 4 °C. Thin sections of the blocks were obtained in an ultracut E ultramicrotome (Reichert Jung, Austria) and then mounted on Formvar-covered nickel grids. Immunogold labelling was carried out by flotation of the mounted sections on drops of the respective solutions; unspecific labelling was diminished by incubation with 1% skim milk and PBS-T (PBS and 0·05% Tween-20) for 30 min. Grids with the sections were incubated with the antibody against the PP2C of L. major at a 1:10 dilution in PBS-MT for 1 h at RT and overnight at 4 °C. Grids were washed with PBS-T and then incubated with a goat anti-rabbit polyclonal antibody coupled to 10 nm gold particles for 2 h at RT (Zymed, Thermo Scientific, PA, USA) (1:40 dilution in PBS-T). After thorough washings in PBS and distilled water, sections were contrasted with 2% uranyl acetate and a saturated solution of lead citrate and then examined with a transmission electron microscope (TEM, JEOL 1400×, JEOL Ltd., Japan). As negative control, sections were incubated with pre-immune rabbit serum diluted in PBS-T and then with the secondary antibody coupled to gold particles.
The identification of the subcellular structures positive for PP2C was performed using thin sections of parasites that were processed to preserve the ultrastructure as reported by (Gomez de Leon et al. Reference Gomez de Leon, Diaz Martin, Mendoza Hernandez, Gonzalez Pozos, Ambrosio and Mondragon Flores2014). Briefly, amastigotes were fixed for 1 h in 2·5% glutaraldehyde. After through rinsing in PBS, parasites were post fixed for 1 h in 1% OsO4 at 4 °C, rinsed, gradually dehydrated in ethanol, and finally embedded in Spurr's resin. Thin sections were obtained with an Ultracut E ultramicrotome and stained with uranyl acetate and lead citrate. Copper grids with the sections were examined in the TEM at 80 keV. Digital images were obtained and processed with Adobe Photoshop software (USA).
Statistical analysis
All data are expressed as mean ± SD (standard deviation). Statistical evaluation of the data was performed by the Mann–Whitney U-test. A value of P < 0·05 was considered statistically significant.
RESULTS
Phosphatase activity secreted by promastigotes and amastigotes from L. mexicana
According to our previous results with L. mexicana strain 68 (Escalona-Montaño et al. Reference Escalona-Montaño, Pardave-Alejandre, Cervantes-Sarabia, Garcia-Lopez, Gutierrez-Quiroz, Gutierrez-Kobeh, Becker-Fauser and Aguirre-Garcia2010), promastigotes showed optimal secretion of phosphatase activity after 7 h of incubation showing high phosphatase activity (Fig. 1A). The activity of the phosphatase secreted by amastigotes of L. mexicana 68 strain was highest after 1 h of incubation, yet it was much lower as compared with the activity found in promastigotes (Fig. 1B). Analysis of the phosphatase activity of PSM(1/7) and ASM(2/1) in the Lacandona strain showed that the enzymatic activity of PSM(1/7) was higher as compared with that observed in the ASM(2/1) (Fig. 1C). The secreted phosphatase activity was not due to parasite lysis, since the viability was analysed in both strains showing that the parasites were not affected (Fig. 1D).
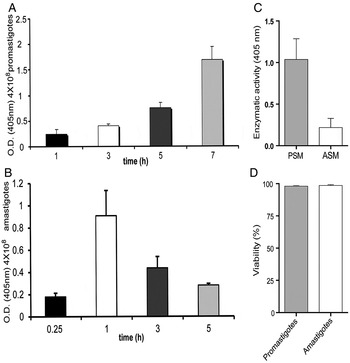
Fig. 1. Secretion of phosphatases by L. mexicana promastigotes and amastigotes to the culture medium. (A) Secretion of phosphatase by L. mexicana promastigotes 68 strain to the culture medium (PSM) at different times (1, 3, 5, and 7 h). This figure is reprinted with permission from Escalona-Montaño et al. (Reference Escalona-Montaño, Pardave-Alejandre, Cervantes-Sarabia, Garcia-Lopez, Gutierrez-Quiroz, Gutierrez-Kobeh, Becker-Fauser and Aguirre-Garcia2010); (B) Secretion of phosphatases by L. mexicana amastigotes 68 strain, ASM at different times (0.25, 1, 3, and 5 h); (C) Enzymatic activity of PSM(1/7) or ASM(2/1) of L. mexicana (Lacandona strain). All experiments were determined using p-NPP as substrate; (D) Cell viability in promastigotes and amastigotes after the secretion process was analyzed by eritrosine B. Bars represent the mean ± SE of three independent experiments.
Biochemical characterization of phosphatases secreted by promastigotes and amastigotes
Substrate
PSM(1/7) and ASM(2/1) were analysed for PTP activity using phosphotyrosine as a substrate. As shown in Fig. 2A, PSM(1/7) showed a higher dephosphorylation (55 p moles phosphate min−1 µg protein−1) in comparison with ASM(2/1), which only showed a minor dephosphorylation (7 p moles phosphate min−1 µg protein−1). However, after analysing the serine/threonine activity using phosphothreonine as a substrate, PSM(1/7)and ASM(2/1) showed minor dephosphorylation both cases:15 and 4 p moles phosphate min−1 µg protein−1, respectively (Fig. 2A).

Fig. 2. Biochemical characterization of the phosphatase activity in PSM(1/7) and ASM(2/1) of L. mexicana (Lacandona strain). (A) PTP and serine/threonine phosphatase activity was performed with Tyr phosphopeptide-1 substrate [END(pY) INASL] and Thr [RRA(pT)VA. (B) Effect of different PTP inhibitors on phosphatase activity of PSM(1/7) and ASM(2/1) of L. mexicana. (C) Effect of different serine/threonine inhibitors on phosphatase activity of PSM(1/7) and ASM(2/1). Bars represent the mean ± SE of three independent experiments.
Inhibitors for different PTPs and serine/threonine phosphatases
The effect of different PTP and serine/threonine phosphatase inhibitors on phosphatase activity present in PSM(1/7)and ASM(2/1) were tested. We observed that the phosphatase activity present in PSM(1/7) was completely inhibited by specific PTP inhibitors such as sodium orthovanadate (95%), sodium tungstate (96%) and ammonium molybdate (98%) (Fig. 2B). In contrast serine/threonine inhibitors such as okadaic acid, trifluoperazine and sanguinarine did not show any inhibitory effect (Fig. 2C). The percentages of inhibition of the phosphatase activity of ASM(2/1) were 45% for sodium orthovanadate, 24% for sodium tungstate and 26% for ammonium molybdate (Fig. 2B). On the other hand, serine/threonine inhibitors showed a very low effect on ASM(2/1) phosphatase activity (Fig. 2C).
Detection of PP2C in secreted proteins and extracts of L. mexicana promastigotes and amastigotes
Previously our group reported that a monoclonal antibody anti-human placental PTP1B recognized a 50 kDa molecule secreted by promastigotes to the CM (Escalona-Montaño et al. Reference Escalona-Montaño, Pardave-Alejandre, Cervantes-Sarabia, Garcia-Lopez, Gutierrez-Quiroz, Gutierrez-Kobeh, Becker-Fauser and Aguirre-Garcia2010). In this work, we used an antibody against the PP2C of L. major and it recognized a 70 kDa molecule secreted by PSM7 and ASM1 (Fig. 3A, lanes 4 and 8, respectively.

Fig. 3. Immunodetection of PP2C. (A) Immunodetection of PP2C in PSM7 and ASM1 and subcellular fractions of L. mexicana (Lacandona strain) promastigotes and amastigotes: Total extract (TE) of promastigotes and amastigotes (lanes 1 and 5), Cytosolic fraction (CF) of promastigotes and amastigotes (lanes 2 and 6), culture medium (CM) (lanes 3 and 7), PSM7and ASM1 (lanes 4 and 8). (B) Immunodetection of PP2C in extracts of L. mexicana amastigotes at different times of secretion. TE of amastigotes at 0, 1 and 5 h after secretion (a, d and g); CF at 0, 1 and 5 h after secretion (b, e, h); MF at 0, 1h after secretion (c, f). Recombinant PP2C of L. major was used as control (lane 9 in A and lane i in B).
TE of promastigotes and amastigotes were prepared after the secretion process and centrifuged in order to separate the CF from the MF. TE (Fig. 3A, lanes 1 and 5) and CF (Fig. 3A, lanes 2 and 6) from promastigotes and amastigotes were analysed with an antibody anti-L. major PP2C. In this cellular fraction, a 44·9 kDa molecule was identified. This molecule has a similar molecular weight as the recombinant protein PP2C from L. major (Fig. 3A, lane 9).
Amastigotes were left to secrete proteins to the CM for different times: 0, 1 and 5 h, afterwards a TE, CF and MF were prepared and analysed with the antibody anti- L. major PP2C that recognized a molecule of 44·9 kDa in TE (Fig. 3B, lanes d and g); CF (Fig. 3B, lane h) and MF (Fig. 3B, lane f). Interestingly, this molecule of 44·9 kDa molecule was not identified in the different amastigote fractions (Fig. 3B, lanes a, b and c). This molecule has a similar molecular weight as the recombinant protein PP2C from L. major that was used as control (Fig. 3B lane i).
Ultrastructural localization of PP2C in amastigotes of L. mexicana
The analysis of the distribution of the PP2C in amastigotes of L. mexicana was achieved by IEM. A characteristic structure found inside the pocket is the flagellum and its origin is clearly identified by the axonemal structure in transverse and longitudinal sections of the parasites (Fig. 4A–C, arrowheads). The immunostaining with the antibody against the PP2C in the amastigote form showed an abundant, specific and reproducible localization of the gold labelling within the flagellar pocket (Fig. 4E–G). Interestingly, the labelling was mostly associated with small vesicles and with a fuzzy material located within the flagellar pocket space (arrows) and a certain labelling was also detected at the flagelar membrane. Other structures and organelles of the amastigotes such as nuclei and mitochondria were not labelled, indicating the specificity of the antibody and the location of the PP2C. Other structures of the amastigotes seemed not to be labelled. The negative control with the pre-immune serum was negative for the flagellar pocket, which was virtually free of the gold labelling showing only few isolated particles found dispersed in the studied sections (Fig. 4d).

Fig. 4. Distribution of PP2C in amastigotes of L. mexicana (Lacandona strain) by IEM. Figures (A–C) correspond to amastigotes processed to preserve the ultrastructure in order to identify those structures positive to the labelling of PP2C. Micrograph (B) is a high magnification of the region marked in figure 4A; (D) Corresponds to the negative control of the labelling of amastigotes with the antibody against the PP2C; (F) is a high magnification of the flagellar pocket region delimited in figure 4E. Arrows indicate the presence of gold particles (10 nm) within the flagellar pocket, arrowheads indicate the presence of the flagella in transverse or longitudinal sections. Scale bars = 0.5 µm.
Cytokine production induced by secreted proteins of promastigotes and amastigotes
The effect of PSM7 and ASM1 of L. mexicana on the production of IL-1β, TNF-α, IL-12p70 and IL-10 by human macrophages was tested. As shown in Fig. 5, the production of TNF-α after incubation with PSM7 increased 4-fold (212·166 pg mL−1), as compared with macrophages that were incubated in medium alone (50·5 pg mL−1). PSM7 also increased the production of IL-1β (139·25 pg mL−1, 3-fold), IL-12p70 (85·83 pg mL−1, 2·5-fold) and IL-10 (219 pg mL−1, 4-fold). While the production of TNF-α after incubation with ASM1 increased 3-fold (153·66 pg mL−1) as compared to macrophages that were incubated in medium alone (50·5 pg mL−1) and also increased the production IL-1β (147·75 pg mL−1, 3-fold), IL-12p70 (113·75 pg mL−1, 2·5-fold) and IL-10 (181·5 pg mL−1, 4-fold) as compared with macrophages that were incubated in medium alone. When comparing the effect of PSM7 and ASM1 on the production of cytokines with the values obtained from macrophages that were incubated in medium alone, only the production of IL-1β, IL-12p70 and IL-10 increased significantly (P < 0·05).

Fig. 5. Cytokine production induced by PSM7 and ASM1 of L. mexicana (Lacandona strain) in human macrophages. Macrophages (1 × 106 cells mL−1) were cultured in RPMI medium (C), in presence of LPS (100 ng mL−1), (PSM7) and (ASM1). After 24 h, TNF-α (A), IL-1β (B), IL-12p70 (C), and IL-10 (D) production was analysed by ELISA. The bars represent mean ± SD of four independent different experiments. Significant differences between PSM7 and ASM1 were calculated in relation to the control (C), while PSM7 and ASM1, were compared among themselves. Asterisk (*) is significant of (p < 0·05) and double asterisks (**) is significant of (p < 0·001).
DISCUSSION
Leishmania spp are known to synthesize and traffic most surface molecules such as LPG and gp63, along the classical endoplasmic reticulum-Golgi apparatus-plasma membrane pathway (McConville et al. Reference McConville, Mullin, Ilgoutz and Teasdale2002). As mentioned, these surface molecules are ultimately delivered to the flagellar pocket and it is thought that the pocket retains its role as the primary, if not sole, site of secretion in nonflagellated amastigotes (McConville et al. Reference McConville, Mullin, Ilgoutz and Teasdale2002). Also, it is known that Leishmania parasites use a classical amino-terminal signal sequence peptide to direct the export of most secreted proteins through the flagellar pocket. However, the vast majority of the characterized Leishmania secreted proteins have no identifiable secretion signal sequence and mostly lack an amino-terminal secretion signal sequence which suggests the existence of important non-classical pathways of secretion (Bates and Dwyer, Reference Bates and Dwyer1987; McConville et al. Reference McConville, Mullin, Ilgoutz and Teasdale2002). In this regard, non-conventional secretion pathways have been observed in Trypanosoma and Leishmania parasites (Cuervo et al. Reference Cuervo, De Jesus, Saboia-Vahia, Mendonca-Lima, Domont and Cupolillo2009; Trocoli Torrecilhas et al. Reference Trocoli Torrecilhas, Tonelli, Pavanelli, da Silva, Schumacher, de Souza, NC, de Almeida Abrahamsohn, Colli and Manso Alves2009; Geiger et al. Reference Geiger, Hirtz, Becue, Bellard, Centeno, Gargani, Rossignol, Cuny and Peltier2010). In the released secreted proteins by Leishmania (Viannia) braziliensis, 42 secreted proteins were identified that lack a classical secretion signal peptide, which has also been reported by Silverman et al. (Reference Silverman, Chan, Robinson, Dwyer, Nandan, Foster and Reiner2008). Their exoproteome analysis showed that only two proteins were possibly secreted through the classical pathway (Cuervo et al. Reference Cuervo, De Jesus, Saboia-Vahia, Mendonca-Lima, Domont and Cupolillo2009).
In different microorganisms, secreted proteins may function as an evasion mechanism against the immune response, thereby participating in the infection. Thus, Trypanosoma cruzi secrete vesicles having phosphatase activities, which increas parasite adhesion and infection of macrophages (Neves et al. Reference Neves, Fernandes, Meyer-Fernandes and Souto-Padron2014) Proteins secreted by Trypanosoma brucei inhibit the maturation of DCs and their ability to induce lymphocytic allogenic responses (Geiger et al. Reference Geiger, Hirtz, Becue, Bellard, Centeno, Gargani, Rossignol, Cuny and Peltier2010). In the case of Leishmania, virulence has been related to two different groups of parasite molecules: one composed of the secreted and surface molecules and the other by the intracellular molecules (Chang et al. Reference Chang, Reed, McGwire and Soong2003). Secreted molecules have a central role in the establishment of infection, protecting the parasite form the early action of the host immune system (Chang et al. Reference Chang, Reed, McGwire and Soong2003; Santarem et al. Reference Santarem, Silvestre, Tavares, Silva, Cabral, Maciel and Cordeiro-da-Silva2007).
Various phosphatases are present in several pathogenic microorganisms such as a Yersinia, Mycobacterium and Plasmodium, where they appear to be involved in signal transduction pathways of the host immune system.
The secreted acid phosphatase (SAcP), relased from the flagellar pocket of leishmania promastigotes and amastigotes, has been previously characterized and found implicated in dephosphorylating organic substrates (Bates and Dwyer, Reference Bates and Dwyer1987; Bates et al. Reference Bates, Hermes and Dwyer1989). Yet the role of SAcP in leishmania pathogenesis has not been characterized. It has been suggested that Leishmania amazonenesis PKC activity may modulate the host cell infection via SAcP (Vannier-Santos et al. Reference Vannier-Santos, Martiny, Meyer-Fernandes and de Souza1995). Furthermore, activities of Leishmania ecto-phosphatases can participate in nutrition, growth and ROS (reactive oxygen species) sensing (Cosentino-Gomes and Meyer-Fernandes, Reference Cosentino-Gomes and Meyer-Fernandes2011).
In L. donovani it has been shown that a phosphatase from promastigotes correlates with the degree of virulence (Singla et al. Reference Singla, Khuller and Vinayak1992). Also, in L. amazonensis several secreted phosphatase activities have been characterized (Fernandes et al. Reference Fernandes, Soares, Saraiva, Meyer-Fernandes and Souto-Padron2013). Recently, we have shown that L. mexicana promastigotes secrete a PTP to the CM (Escalona-Montaño et al. Reference Escalona-Montaño, Pardave-Alejandre, Cervantes-Sarabia, Garcia-Lopez, Gutierrez-Quiroz, Gutierrez-Kobeh, Becker-Fauser and Aguirre-Garcia2010). Interestingly, also host PTPs have been implicated in Leishmania pathogenesis. In particular, PTP1B and T cell PTP have been shown to be activated and post-translationally modified in L. mexicana-infected macrophages (Gomez et al. Reference Gomez, Contreras, Halle, Tremblay, McMaster and Olivier2009).
In the present work, we extended the analysis of the phosphatases secreted by both morphological stages of Leishmania and found that L. mexicana promastigotes and amastigotes secrete a protein with PTP activity to the CM. In promastigotes the maximal phosphatase activity was secreted after 7 h of incubation in conditioned medium, whereas in amastigotes the maximal phosphatase activity was secreted at 1 h. After the secretion process the viability of the parasites was determined and showed to be optimal, which proved that the presence of the phosphatase activity was the result of an active secretion process and not due to parasite lysis. The enzymatic activity was higher in PSM than in ASM, although for amastigotes the concentration of protein analysed was the double the amount as that of the protein analysed for promastigotes. The lower secretion of phosphatase activity in amastigotes, as compared with promastigotes, correlates with that of other molecules that can be secreted such as LPG which has been shown to be produced in low quantity by amastigotes as compared with promastigotes (McConville and Blackwell, Reference McConville and Blackwell1991). The fact that promastigotes and amastigotes differ in their surface and secreted molecules may correlate with other differential capacities. It has been shown that L. mexicana promastigotes and amastigotes differ in their ability to alter macrophage signalling and functions as is exemplified by the fact that in this Leishmania species promastigotes, but not amastigotes, were able to rapidly activate host PTP1B (Abu-Dayyeh et al. Reference Abu-Dayyeh, Hassani, Westra, Mottram and Olivier2010).
We found that the phosphatase activity present in PSM dephosphorylate in a major extent phosphotyrosine residues as compared to phosphothreonine, whereas the enzyme present in ASM showed a low dephosphorylation of both substrates. The use of specific inhibitors for PTP activity only inhibited the phosphatase activity present in PSM, but not in ASM. Due to the fact that orthovanadate has been shown to be an irreversible inhibitor of PTPs, it is expected that the inhibition of phosphatase present in PSM and ASM by PTP inhibitors as has been reported for phosphatases present in other microorganism (Catta-Preta et al. Reference Catta-Preta, Nascimento, Garcia, Saraiva, Motta and Meyer-Fernandes2013). On the other hand, serine/threonine phosphatase inhibitors showed no effect. The characterization of phosphatases with these compounds has been reported in different systems (Aguirre-Garcia et al. Reference Aguirre-Garcia, Anaya-Ruiz and Talamas-Rohana2003, Reference Aguirre-Garcia, Escalona-Montano, Bakalara, Perez-Torres, Gutierrez-Kobeh and Becker2006; Escalona-Montaño et al. Reference Escalona-Montaño, Pardave-Alejandre, Cervantes-Sarabia, Garcia-Lopez, Gutierrez-Quiroz, Gutierrez-Kobeh, Becker-Fauser and Aguirre-Garcia2010).
A further characterization of the phosphatase activity secreted by promastigotes and amastigotes was performed by Western blot using an antibody raised against a recombinant PP2C of L. major. We detected a protein phosphatase PP2C secreted by L. mexicana promastigotes and amastigotes. This antibody reacted with 44·9 kDa protein present in the TE and CF of promastigotes and amastigotes, respectively, while in PSM and ASM reacted with a 72 kDa protein.
The Leishmania secretome has been analysed in search of potential virulence factors (Silverman et al. Reference Silverman, Chan, Robinson, Dwyer, Nandan, Foster and Reiner2008). Candidate virulence factors have been divided into four categories: proteins putatively involved in intracellular survival, proteins with known immunosupressive functions, proteins involved in signal transduction, and proteins involved in transport processes. In the group of proteins involved in signal transduction a protein phosphatase type 2C (LmjF25·0750) has been reported and when analysed in GeneDB and shown to be a PP2C similar to a Leishmania chagasi type 2C serine/threonine protein phosphatase, LcPP2C. LcPP2C was shown to be present as a 42-kDa protein in L. chagasi and L. amazonensis promastigotes and tissue amastigotes of (Burns et al. Reference Burns, Parsons, Rosman and Reed1993).
The antibodies against the PP2C of L. major used in this work were generated against a recombinant protein PP2C from L. major that has a molecular weight 44·9 kDa that corresponds with the molecular weight detected. However, the fact that it also recognized a protein with a molecule weight of 72 kDa in PSM and ASM possibly indicates that this molecule is the result of a glycosylation process or the classical amino-terminal signal sequence peptide to direct the export of most secreted proteins through the flagellar pocket used by Leishmania. Additionally, we analysed ET, CF and MF of amastigotes after different times of secretion and interestingly found that the antibodies against the PP2C of L. major recognized a 44·9 kDa molecule only in amastigotes after 1 and 5 h of secretion. Antibodies against the PP2C of L. major did not detect any molecule in ET of amastigotes at different protein concentration (data not shown). This result is important because this L. mexicana PP2C could be activated as a result of a stress response of amastigotes due to the secretion process. Similar events have been observed that PP2Cs of plants, representing the major group of protein phosphatases. The recent discovery of novel abscisic acid (ABA) receptors (ABARs) has placed the PP2Cs at the centre stage of the major signalling pathway regulating plant responses to stresses and plant development (Mitula et al. Reference Mitula, Tajdel, Ciesla, Kasprowicz-Maluski, Kulik, Babula-Skowronska, Michalak, Dobrowolska, Sadowski and Ludwikow2015; Singh et al. Reference Singh, Pandey, Srivastava, Tran and Pandey2015).
Once we determined that L. mexicana amastigotes secrete a PP2C, we were also interested in determining its localization in this morphological stage by IEM. The abundant, specific and reproducible localization of the immunogold labelling within the flagellar pocket and its particular association with vesicles and a fuzzy material, suggest that the enzyme could be secreted from the flagellar pocket to the extracellular space. It remains to be determined whether the enzyme is secreted in a soluble form or is associated to secretory vesicles. Similar results have been observed in L. major promastigotes by IEM analysis, where in promastigotes it was shown that the antibody against the PP2C of L. major specifically labelled abundantly the flagellum since the beginning of the structure at the level of the flagellar pocket, but also along the whole length of this motile organelle (manuscript in preparation).
We then analysed the effect of PSM and ASM on cytokine production by human macrophages. We found that cytokines are produced by macrophages after the incubation with PSM and ASM. In regard to the production of TNF-α, IL-12 p70 and IL-10 we observed differences when macrophages were incubated with PSM or ASM. It is expected that cytokines production by human macrophages is driven by molecules secreted by promastigotes and amastigotes; albeit it is not clear whether secreted phosphatase are involvement in this process.
The effect that Leishmania secreted molecules exert on the immune response has been analysed for different Leishmania species. Such is the case of L. donovani, secreted vesicles and exosomes have been shown to alter the cytokine response of human monocytes to Leishmania infection and interferon (IFN)-γ treatment. The inhibition of IL-8 and TNF-α production combined with augmented production IL-10 due to L. donovani secreted vesicles and exosomes favoured an anti-inflammatory response. These results suggest that secretion of exosomes by Leishmania likely plays major role in the pathogenesis of these organisms and led us to speculate that exosomes may be a mechanism of immune modulation used more generally by intracellular and extracellular pathogens (Silverman et al. Reference Silverman, Clos, de'Oliveira, Shirvani, Fang, Wang, Foster and Reiner2010).
It has previously been shown that L. major secreted antigens supress in vitro proliferation of lymphocytes in BALB/c mice. Additionally, semi-purified secreted antigens were shown to suppress 60% of lymphocyte proliferation and prevented the stimulation of lymphocytes. These fractions decreased IFN-γ production and increased IL-4 in lymphocytes, and downregulated nitric oxide production in activated macrophages. These results suggest that L. major secreted proteins could function as immunosuppressive factors that downregulate the immune system (Tabatabaee et al. Reference Tabatabaee, Abolhassani, Mahdavi, Nahrevanian and Azadmanesh2011). Conversely in Leishmania infantum the immunomodulatory effect of excreted/secreted proteins has been analysed in differentiation and maturation of human DC (Markikou-Ouni et al. Reference Markikou-Ouni, Drini, Bahi-Jaber, Chenik and Meddeb-Garnaoui2015). Furthermore, L. donovani excretory–secretory antigens (LESAs) released by promastigotes to the CM, have shown an immunomodulatory role. Fractions from LESAs containing proteins of different molecular weights were found to be highly immunogenic, as they significantly induced NADPH oxidase (reduced nicotinamide adenine dinucleotide phosphate-oxidase) and SOD (superoxide dismutase) activities, as well as NO (nitric oxide), TNF-α, IFN-γ and IL-12 production in stimulated RAW 264·7 macrophages. These results strongly suggest the potential role of LESAs in the modulation of macrophage effector functions and Th1 response that gives a hope for the development of a vaccine for visceral leishmaniasis (Gour et al. Reference Gour, Kumar, Singh, Bajpai, Pandey and Singh2012). Similar results were reported by Kumar et al. Reference Kumar, Samant, Misra, Khare, Sundar, Garg and Dube2015, who found a potential immunostimulatory effect when using soluble exogenous antigens of L. donovani, suggesting that these immunostimulatory molecules may be further exploited for developing a subunit vaccine against visceral leishmaniasis (Kumar et al. Reference Kumar, Samant, Misra, Khare, Sundar, Garg and Dube2015).
The fact that we also observed that the production of cytokines in macrophages was differentially induced by proteins secreted from L. mexicana promastigotes and amastigotes opens novel lines of research. The analysis of L. mexicana secreted proteins, especially from parasites isolated from patients with different disease severity as observed with LCL and DCL, will possibly shed new light on their role as immunomodulators. Additionally, it will be very interesting to explore the participation of secreted protein phosphatases PTP and PP2C in different signal transduction pathways of the host cell.
ACKNOWLEDGEMENTS
Alma Reyna Escalona Montaño is a student of Posgrado en Ciencias Biológicas, Universidad Nacional Autónoma de México and was supported by a fellowship from CONACyT México (fellowship165409, CVU 165409). Authors express their gratitude to Marco Gudiño Zayas, Rocely Cervantes Sarabia, Miriam Berzunza Cruz, Carlos Ramírez Álvarez, Omar Agni García-Hernández and Jesús Argueta-Donohué for technical assistance. Authors are also grateful to Mónica Mondragón and Sirenia González from the Biochemistry Department and Electron Microscopy Facility-LANSE, respectively, at CINVESTAV, México, for their technical support.
FINANCIAL SUPPORT
This work was supported by grants 152433 from CONACyT, IN218412 from DGAPA-PAPIIT (UNAM) to M.M.A-G.








