INTRODUCTION
Sex differences in cognitive abilities have long been hypothesized with women performing better on tasks involving receptive and productive language and men excelling in visual–spatial abilities. The largest female advantage is found for executive speech tasks such as verbal fluency tasks (Hyde & Linn, 1988; Kimura, 1992, 1996; Weiss et al., 2003a). Verbal fluency is a widely used neuropsychological test of language production, requiring subjects to name as many words as possible beginning with a specified letter (letter fluency/phonemic fluency) or belonging to a certain semantic category (category naming/semantic fluency). Efficient fluency performance requires the generation of words within a subcategory (clustering) and switching to a new one when a subcategory is exhausted (Troyer et al., 1997). These components of fluency performance have been shown to be dissociable in healthy controls and in various neurological disorders. Clustering depends on processes such as verbal memory and verbal storage and is related to temporal lobe functioning, and impaired performance is seen in patients with temporal lobectomy (Troyer et al., 1998a) and Alzheimer disease (Troyer et al., 1998b). Switching, on the other hand, requires the ability to engage in strategic search processes such as initiation, cognitive flexibility and mental set shifting and decreases under conditions of divided attention (Troyer et al., 1997). Because it is related to frontoexecutive functioning, impaired performance was seen among patients with frontal lobe lesions (Troyer et al., 1998a), Parkinson's disease (Troster et al., 1998; Troyer et al., 1998b), Huntington's disease (Rich et al., 1999), multiple sclerosis (Troster et al., 1998), and schizophrenia (Kosmidis et al., 2005; Moelter et al., 2001; Robert et al., 1998).
Clinical and neuroimaging data suggest that phonemic and semantic fluency rely on distinct cognitive resources. For instance, semantic fluency is relatively more impaired in Alzheimer's disease, whereas phonetically cued fluency is more sensitive to frontal lobe lesions (Pendleton et al., 1982; Troyer et al., 1998a). These clinical findings are in line with numerous functional magnetic resonance imaging studies, demonstrating that phonemic verbal fluency was associated with extensive activation in the left frontal cortex (Curtis et al., 1998; Frost et al., 1999; Phelps et al., 1997; Pujol et al., 1996; Schlosser et al., 1998; Weiss et al., 2003b; Yetkin et al., 1995; Yurgelun-Todd et al., 1996), whereas semantic verbal fluency was associated with the activation of the temporal or retrosplenial areas of the left hemisphere (Mummery et al., 1996; Paulesu et al., 1997).
As to gender, a female superiority for phonemic verbal fluency has often been reported in studies carried out in normals (Bolla et al., 1990; Crossley et al., 1997; Weiss et al., 2003a), whereas in semantic verbal fluency, the available data do not suggest a female advantage (Capitani et al., 1998). However, some other studies failed to find sex differences regardless of task type (Cohen & Stanczak, 2000; Kempler et al., 1998; Tombaugh et al., 1999) or only found sex differences in specific categories that may reflect sociocultural factors (Kosmidis et al., 2004).
Whether sex differences emerge from neuroanatomical differences or from gender-specific behavior is still under debate. One view is that gender differences are related to differences in the cerebral organization of language function and to the structure of the language related cortex (for review, see Harshman, 1985; Hiscock et al., 1999). It has been proposed that language is more strongly lateralized in males than in females (Kansaku & Kitazawa, 2001; McGlone, 1977; Shaywitz et al., 1995; Strauss et al., 1992) and a more bilateral pattern of language representation is thought to result in better verbal skills for females. This theory is supported by findings from patient studies showing that males have a higher incidence of aphasia after lesions to the left hemisphere (Inglis & Lawson, 1981; McGlone, 1977). However, the available neuroimaging studies provide conflicting results, which can be attributed to the complexity of variables influencing cognitive sex differences.
Other studies attribute the female advantage in executive speech tests (such as verbal fluency tasks) to behavioral factors such as efficient processing strategies optimizing performance. The most commonly used score from verbal fluency tests is the total number of words generated. However, this score provides little information about the cognitive processes underlying fluency performance and does not answer the question as to why women perform at a higher level in phonemic verbal fluency tests. While the influence of clustering and switching on verbal fluency performance has been investigated more extensively in clinical populations (Moelter et al., 2001; Rich et al., 1999; Robert et al., 1998; Troster et al., 1998; Troyer et al., 1998b), only a few studies have investigated possible sex differences in these verbal fluency factors in healthy controls (Troyer et al., 1997; Troyer, 2000). Troyer et al. (1997) provided normative data for clustering and switching on verbal fluency tasks for healthy adults between 18 and 91 years of age. Sex showed only a minimal effect size as a predictor of every phonemic or semantic fluency variable.
Many studies have investigated specific cognitive abilities that determine fluency performance such as word knowledge, information processing speed, executive processes of strategic retrieval search, and performance monitoring (Bryan & Luszcz, 2000; Bryan et al., 1997; Salthouse, 1993). Most importantly, verbal intelligence was found to be a discriminating factor in verbal fluency performance (Bolla et al., 1990; Cauthen, 1978; Miller, 1984). Other factors such as cognitive speed (Cauthen, 1978), cognitive flexibility (Perlmuter et al., 1987), and semantic memory (Martin & Fedio, 1983) were reported to be related to verbal fluency. Ruff et al. (1997) suggested that poor word fluency may result from deficient verbal attention, word knowledge, and/or verbal long-term memory. To our knowledge, no other study has investigated the influence of these cognitive factors on clustering and switching in verbal fluency.
We previously reported a significant female advantage in phonemic verbal fluency and a tendency in the same direction for semantic verbal fluency in a large sample of healthy university students (Weiss et al., 2003a). The primary object of the present study was to assess possible sex differences in clustering and switching in a semantic and phonemic verbal fluency test. Furthermore, the influence of cognitive factors such as speed of information processing, word knowledge, and/or verbal long-term memory on these verbal fluency factors will be examined.
METHODS
Data from 40 women and 40 men, matched for age and verbal IQ, who participated in a study of sex differences in cognitive functions, were analyzed. All participants were Austrian university students and native German speakers, who studied psychology or medicine and were right-handed according to the Edinburgh Handedness Scale (Oldfield, 1971). Details regarding the testing procedure have been published previously (Weiss et al., 2003a).
Verbal intelligence was measured with the Mehrfachwahl–Wortschatz test (Lehrl, 1989), which is a multiple-choice vocabulary intelligence test that assesses crystallized intelligence. The Digit Symbol test, a subtest of the Wechsler Adult Intelligence Scale test (Wechsler, 1981), was used to test accelerated visual–motor processing and attention. Verbal memory was evaluated by a modified version of the Verbal subtest of the Recognition Memory Test for Words (Warrington, 1984) after a delay of 20 minutes. The subtest Story Recall from the Rivermead Behavioral Memory Test (RBMT; Wilson et al., 1985) was also used, in which the subjects listen to a short passage of prose being read aloud and are required to write down as much of it as possible immediately afterward and again after a delay.
Phonemic (F, A, S) and semantic (animals) verbal fluency was assessed by the Controlled Oral Word Association test and the Category Fluency task (Spreen & Benton, 1977). A 60-s period was given for each letter and category. The dependent measures were total number of words produced (total score), total number of switches, and mean cluster size. Clustering and switching between clusters were coded according to previously published criteria (Troyer et al., 1997). As pointed out by Troyer and colleagues (1998a), the raw number of switches was used rather than correcting for the number of words generated (i.e., proportion score) to best represent the behavior of interest. To avoid gender-specific advantages for certain semantic categories (Capitani et al., 1999), the category of animals was chosen which is familiar to both genders.
Data Analyses
Demographic data (age and verbal intelligence) were compared using two-sample t tests. Neuropsychological data were compared using a nonparametric test (Mann–Whitney U Test). Spearman correlation coefficients were computed for the association between clustering and switching in phonemic fluency and semantic fluency for each gender separately. To test for differences in the correlations of the different cognitive tests (attention, verbal knowledge, and verbal memory) and the verbal fluency measures (total number of words, switches, and cluster size in phonemic fluency and semantic fluency) between men and women and across the cognitive tests, we used the analysis of variance (ANOVA)-like test for correlated correlations (CORANOVA) procedure (Bilker et al., 2004; for the SAS macro to perform the method see http://www.cceb.upenn.edu/main/people/docs/coranova.sas). The CORANOVA method tests three hypotheses, which are analogous to those in a two-way ANOVA with an interaction term, testing contrasts of the correlations rather than the means. The hypotheses on the correlations being tested in CORANOVA are the equality of the correlations between groups (the between-sex effect in our case), the equality of the correlations across the cognitive tests (within-cognitive test effect), and the interaction of cognitive test performance with gender (between × within effect interaction). The interaction hypothesis tests if the pattern of correlations between the cognitive tests and verbal fluency measure is the same for men and women. For each of these hypotheses, it is the strength of the linear relationship between the cognitive tests and verbal fluency measure that is being compared, as measured by the Pearson correlation coefficient. The CORANOVA procedure uses a bootstrap to estimate the covariance matrix of the correlations, and hypotheses are examined using permutation tests. These analyses used 1000 bootstraps and 1000 permutations. When a significant sex × cognitive factor interaction was found, we performed five pairwise comparisons, using a Bonferroni adjustment for multiple comparisons, comparing Spearman correlations between each of the five cognitive tests and verbal fluency measure for males versus females.
RESULTS
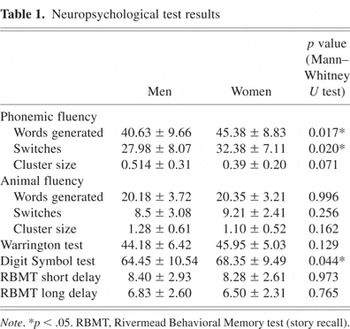
There were no statistically significant gender differences with regard to handedness (men [mean ± SD] = 81.75 ± 32.65; women = 87.38 ± 33.11; p = .447), age (men = 25.45 ± 2.43; women = 24.98 ± 3.59; p = .491), and verbal IQ (men = 116.03 ± 11.24; women = 112.28 ± 12.97; p = .171). Table 1 gives an overview of means and standard deviations of the cognitive variables for men and women in the cognitive task scores. The Mann–Whitney U Test, with sex as the independent variable, showed a significant effect of sex in the Digit Symbol test (p = .044), indicating that women performed at a significantly higher level than men. On phonemic fluency, women generated more words (p = .017) and made more switches (p = .020) than men. Additionally, there was a trend toward a larger cluster size in men (p = .071). On animal fluency, there was no significant difference in the total score, raw score of switches, and cluster size between both groups. Furthermore, there was no difference between men and women in the Warrington Recognition Memory Test and the RBMT (story recall) for short or long delay.
Neuropsychological test results
Correlation analysis indicated that clustering and switching were differentially related to phonemic and animal fluency (Table 2). In phonemic fluency, the total number of words generated was significantly correlated with number of switches for both sexes (p < .001 for both sexes). In animal fluency, the total number of words generated was significantly correlated with number of switches in men (p < .001) and there was a trend toward a significant correlation with switches (p = .087) and cluster size (p = .070) in women. Because of the time constraint, there was a negative correlation between clustering and switching, indicating that larger cluster sizes were associated with less switching and vice versa. However, both of these variables were positively correlated with the number of words generated in women; whereas in men, the cluster size was negatively correlated with the number of words generated in phonemic and semantic fluency.
Correlation coefficients for fluency variables

The CORANOVA showed a sex × cognitive factor interaction for the total number of words (p = .008) and the number of switches (p = .043) generated in phonemic fluency. It also showed a sex × cognitive factor interaction for total number of words generated in animal fluency (p = .014; see Table 3).
CORANOVA for main effect of sex (between effect), cognitive factor (within effect), and sex × cognitive factor interaction effect

Figure 1 shows the correlation between fluency variables and specific cognitive variables. Women showed higher correlations between number of words generated in the phonemic fluency test and the Warrington recognition memory test (p = .042), whereas men showed a higher correlation between number of words generated in the phonemic fluency test and the Digit Symbol test (p = .004; pairwise comparisons showed this last difference was statistically significant after Bonferroni adjustment, p = .020). Also, men showed a significantly higher correlation between number of switches in letter fluency and the Digit Symbol test (p = .006, Bonferroni adjusted p = .030). Furthermore, women had higher correlations between the total number of animals and story recall (short delay p = .041, Bonferroni adjusted p = .205; long delay p = .025, Bonferroni adjusted p = .125), whereas men had a higher correlation between the total number of animals and the Digit Symbol test (p = .032, Bonferroni adjusted p = .160). There were no significant sex differences, cognitive factor differences, or sex × cognitive factor interactions found for the phonemic cluster sizes, number of switches for animals, and animal cluster sizes.

CORANOVA: Pairwise comparison between letter/animal fluency and various cognitive factors. RBMT, Rivermead Behavioral Memory Test (story recall); DST, Digit Symbol test. *p < .01.
DISCUSSION
The aim of the present study was to investigate gender differences in clustering and switching in a semantic and phonemic verbal fluency test. The results showed that women switched more often between categories in the phonemic fluency test, whereas men showed a trend toward a larger cluster size. Our data indicate that men and women are using different processing strategies for phonemic verbal fluency tests to optimize verbal fluency task performance. Women adopted a more successful strategy of balancing clustering and switching in the phonemic verbal fluency task. Males on the other hand, switched less frequently and tended to produce larger clusters leading to a smaller total number of words generated. The female advantage in the phonemic verbal fluency task is consistent with the results of previous studies (Bolla et al., 1990; Capitani et al., 1998; Crossley et al., 1997; Weiss et al., 2003a), which also showed sex differences only in phonemic verbal fluency tests but not in semantic verbal fluency. Rende et al. (2002) suggested that letter fluency performance relies on the phonological loop of the working memory, whereas category fluency relies on the visuospatial sketchpad, therefore enabling participants to effectively implement visualization strategies. Evidence from functional neuroimaging studies support the finding that phonemic verbal fluency and semantic verbal fluency are distributed and partially distinct functions that rely on different component processes of the word retrieval system (Curtis et al., 1998; Frost et al., 1999; Mummery et al., 1996; Paulesu et al., 1997; Phelps et al., 1997; Pujol et al., 1996; Schlosser et al., 1998; Weiss et al., 2003b; Yetkin et al., 1995; Yurgelun-Todd et al., 1996). Semantic fluency depends strongly on access to and integrity of semantic stores, where activation of an initial and highly prototypical exemplar leads to automatic activation of closely related semantic neighbors (Leggio et al., 2000; Martin et al., 1994; Rosser & Hodges, 1994). By contrast, phonemic fluency requires the processing of the phonemic characteristics of words according to a given rule (i.e., same first letter). The search process is less automatic and necessitates the active generation of a new strategy. More than semantic fluency tasks, the phonemic fluency task requires participants to make correct selections, to inhibit intrusions, and to keep a constant level of focused attention (Martin et al., 1994).
Correlation analyses showed that switching was highly correlated with total number of words generated on phonemic fluency in both groups, indicating that switching is more important for optimal performance on this task than clustering. In semantic fluency, switching was only correlated with total number of words in men. Neither switching nor clustering was correlated with total number of words in women. We also found sex differences in the effect of verbal attention, cognitive speed, and verbal memory on verbal fluency performance. Verbal fluency requires focused attention, development and implementation of a search strategy, working memory, and episodic memory. Bolla et al. (1990) reported that, although cognitive speed and flexibility influence phonemic fluency performance, level of verbal intelligence and strategic thinking (good organizational skills) as used in different memory tasks appear to play more crucial roles. Memory tests require the spontaneous formulation of effective encoding and recall strategies to make associations between unrelated words for future retrieval. In the current study, there was a sex-difference between memory performance and fluency task performance, insofar as better memory performance in women was associated with better performance on the phonemic verbal fluency test, although this result could not withstand a Bonferroni correction. Furthermore, our study showed a significant sex difference in the correlation of the Digit Symbol test with the total number of words and number of switches in letter fluency insofar as higher performance on the Digit Symbol test was associated with better performance on the verbal fluency test in men, but not women. The Digit Symbol test is thought to be an indicator of working memory, cognitive flexibility, and cognitive speed. Consistent with previous findings, women tended to outperform men on this test, almost reaching ceiling level. Thus, the correlation between the Digit Symbol test and phonemic fluency performance was higher in men than in women, who already performed at a very high level in this specific function. One may speculate that lower scores in the Digit Symbol test in men stem from lower flexibility. Additionally, it may be hypothesized that, to exploit good memory and intelligence functions, mental flexibility is required and possibly men are disadvantaged in cognitive flexibility under time pressure (as indicated by the Digit Symbol test).
Troyer et al. (1998a) suggested that differences in the cluster size in phonological fluency may reflect differences in vocabulary size. A more extensive vocabulary may provide participants with a larger pool from which to draw phonemically related words, so that more words are generated before a phonemic subcategory is exhausted. Despite the trend toward gender differences in cluster size in phonemic fluency, no significant gender difference was found in vocabulary in our study. Nevertheless, it should be kept in mind that our population tended to be average to above average in their level of verbal intelligence.
In summary, our findings suggest that men and women are using different processing strategies on phonemic verbal fluency tests, with an optimal balance between clustering and switching in women. On the other hand, male participants switched less frequently and tended to produce larger clusters on phonemic fluency, leading to a smaller total number of words generated. These results point out the importance of examining gender as a moderator variable in future clinical studies.






