Published online by Cambridge University Press: 17 October 2003
The present studies on infections with Leishmania (Viannia) braziliensis in rhesus macaques were made to characterize the evolution of different parasite strains and the immune responses they elicited in this experimental host. A standardized inoculum of promastigotes was injected intradermally either above the eyelid or on the forearm of each monkey. Sixteen infected monkeys developed longstanding infections which lasted until the end of the observation period (33 months). The time required for lesion development was very variable, not only for the isolates showing molecular differences but also for individual animals in groups infected with the same parasite strain. The inocula produced lesions of variable severity, ranging from localized cutaneous leishmaniasis (CL) with a tendency to spontaneous healing to non-healing disease. One infected animal developed persistent metastatic skin and mucosal lesions. Anti-Leishmania antibodies and parasite-specific T-cell responses were induced by the experimental infections. As the granulomatous inflammatory response found at the lesions in L. (V.) braziliensis-infected M. mulatta was similar to that in patients with CL, this primate model could be useful for studying the pathophysiology and immunoregulatory events associated with disease evolution, as well as for the evaluation of new drugs or candidate vaccines.
Human leishmaniasis is a vector-borne infectious disease caused by related parasitic protozoa organisms belonging to the genus Leishmania (Kinetoplastida: Trypanosomatidae). Most of the New World Leishmania species appear capable of producing a variety of disease manifestations, depending on the immune status of the host and other external factors (Grimaldi & Tesh, 1993). Certain disease forms are, however, most often associated with specific organisms, such as L. (V.) braziliensis with mucosal leishmaniasis (ML), L. (L.) amazonensis with diffuse CL, and L. (L.) chagasi with visceral leishmaniasis (VL). Immunological studies have shown reversible defects in antigen-induced responses to be associated with diffuse cutaneous (Murray et al. 1984) and visceral (Carvalho et al. 1989) disease forms and hypersensitivity with ML (Saraiva et al. 1989). These findings provided evidence for the participation of parasite determinants and host factors in the expression of human disease.
Taxonomic studies have shown genetic polymorphism in natural populations of L. (V.) braziliensis (Cupolillo, Momen & Grimaldi, 1998). Whether or not strain variants of this parasite are responsible for a variety of clinical presentations is still to be determined. In many individuals L. (V.) braziliensis infection runs a subclinical course without symptoms (Jones et al. 1987), but this pathogen also causes a spectrum of diseases, such as (i) localized CL, which may heal spontaneously; (ii) non-healing disseminating CL; and (iii) ML, an hyperallergic, invasive, ulcerative form that progresses in the absence of any apparent cellular defect (Marsden, 1985; Carvalho et al. 1985, 1994).
The point in the infection when nasal metastasis occurs in patients with L. (V.) braziliensis CL and with what frequency is unknown. In a prospective field study, the prevalence of ML appeared to be less than 5% (Jones et al. 1987). The scarceness of parasites within these lesions does not correlate with the severity of disease (Saraiva et al. 1989). In fact, the magnitude of the parasite-specific T-cell responses tends to be greater in patients with ML than in cases with CL (Carvalho et al. 1985; Conceição-Silva et al. 1990). A better understanding of the virulence factors that contribute to the pathogenesis of L. (V.) braziliensis ML is needed, as are studies on the role of host factors in susceptibility to this form of the disease.
A generally accepted experimental model of L. (V.) braziliensis infection has yet to be defined. The use of murine models is limited, because mice are naturally resistant to this parasite species. New World primates were susceptible to infection with this pathogen, but none of them developed ML (Lainson & Shaw, 1977; Cuba Cuba et al. 1990). In our previous studies, the rhesus monkey (Macaca mulatta) was found to be readily infected with either L. (L.) amazonensis (Amaral et al. 1996) or L. (L.) major (Amaral et al. 2001), developed a human-like disease (namely, the self-healing CL and resistance to homologous challenges), and could be protected effectively by Leishmania vaccination (Kenney et al. 1999; Campos-Neto et al. 2001). The present studies on infections with L. (V.) braziliensis in rhesus monkeys were made in order to develop an animal model that would facilitate the study of ML, in particular to help understand the nature of parasite metastatic events and the immunopathology of this important human disease. Initially, we examined the susceptibility of M. mulatta to infections with different strains of this pathogen and the clinicopathological changes of the disease. Subsequently, the immunological profile of the host occurring during primary infections was studied.
A total of 19 laboratory-bred and -reared young adult (3–8 years old, weighing between 4100 and 10450 g) rhesus macaques (16 males and 3 females) were used. Their care and maintenance has been described previously (Amaral et al. 1996). The experiments were conducted using a protocol approved by the Institutional Committee of the Centre for Biological Evaluation and Care of Research Animals (CEUA-Fiocruz, Protocol no. P0048-00). Animals were immobilized (each monkey was given, intramuscularly, 10–40 mg/kg body weight of ketamine hydrochloride for anaesthesia) before infection and prior to each sampling or testing procedure.
Two strains of L. (V.) braziliensis isolated from patients with either CL (strain MHOM/BR/97/SIS) or ML (strain MHOM/BR/95/OSC) were used. The strains were typed by multilocus enzyme electrophoresis (Cupolillo et al. 1998) in our laboratory before being used for infection. The procedure for promastigote derivation and needle inoculation into experimental animals was as described (Amaral et al. 1996). Briefly, parasites were isolated from the patients in NNN blood agar medium, and then maintained by subcutaneous passage in hamsters. To obtain suspensions of promastigotes, tissue from chronically infected hamsters was cultured initially in NNN medium. When promastigotes appeared, the parasites were subinoculated into liquid Schneider's Drosophila medium (Sigma), supplemented with 20% heat-inactivated foetal calf serum, no more than 3 times before use for infection. Stationary-phase promastigotes were harvested, washed 3 times (by centrifugation at 1800 g at 4 °C for 10 min) in PBS, and adjusted to a concentration of 1×107 promastigotes/0·1 ml.
A study (Exp. 1) was first designed to provide information concerning the virulence of the infecting parasite. Groups of 5 monkeys were infected with either the MHOM/BR/97/SIS (group A) or MHOM/BR/95/OSC (group B) strain. A standardized inoculum of 1×107 promastigotes (Amaral et al. 1996) was injected intradermally above the left upper eyelid of each monkey. An additional study (Exp. 2) was designed to study the development of the infection with a distinct L. (V.) braziliensis genotype (Fig. 3), which was isolated from rhesus A5 affected mucosa at month 30 post-infection (p.i.). Groups of 3 healthy animals were injected (using the same inoculum/route) either above the left eyelid area or on the left forearm. Additionally, 3 naive monkeys were used as ‘normal’ controls. For each experiment, in parallel, hamsters were inoculated with identical numbers of promastigotes to determine parasite infectivity.
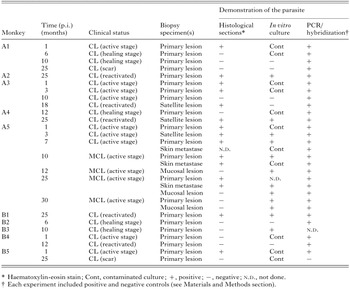
The size and appearance of leishmanial skin lesions, as well as examination for metastases were followed in all infected animals. Primary lesions were measured with a vernier caliper and lesion size estimated using the following formula: area(mm2)=Pi×greatest radius×least radius. Dermatological examination included the upper respiratory/digestive mucosae. Anterior rhinoscopy using an optical fibroscope (Storz, Mainz, Germany) was performed monthly for the monkey presenting lesions of the nasal mucosa. As indicated (Table 1), biopsy specimens were removed from cutaneous and/or mucosal disease at distinct stages of lesion development and then processed for paraffin embedding, haematoxylin and eosin stain, DNA isolation and/or culture in NNN medium. For PCR analysis, biopsy specimens from normal skin of either normal or infected monkeys were included as negative controls.
The methods followed for detection of Leishmania DNA in lesions by PCR and hybridization procedure were those as described (Schubach et al. 1998 a,b). Briefly, DNA purification from biopsy fragments was done using an anion-exchange chromatography spin-column (Pharmacia, Upsalla, Sweden) following the manufacturer's instructions. Next, samples were ethanol-precipited and resuspended in 10 μl of TE (10 mM Tris–HCl, pH 8·0; 1 mM EDTA, pH 8·0), and stored frozen at −20 °C until use. Aiming to amplify the conserved region of the minicircle molecule (kDNA), we used Leishmania genus-specific oligonucleotides (primer A: 5′-(G/C)(G/C)(C/G)CC(A/C)CTAT(A/T)TTACACCAACCCC; and primer B: 5′-GGGGAGGGGCGTTCTGCGAA) in a hot-start PCR procedure. The reactions were done with 200 ng of each oligonucleotide, 200 μM dNTP mixture, 1·5 mM MgCl2, 2·5 U of Taq polymerase in the buffer recommended by the enzyme manufacturer (Perkin-Elmer, Branchburg, NJ), and 5 μl of the extracted DNA, using 35 cycles of 94 °C, 50 °C, and 72 °C. Each experiment included a positive control (100 fg of Leishmania kDNA) and a negative control (no addition of DNA). The expected amplification products of 120 bp were analysed by agarose gel electrophoresis with ethidium bromide staining and visualized under UV light. All PCR-negative results were also tested for reaction inhibitors by adding 100 pg of total L. (V.) braziliensis DNA in a new amplification procedure, yielding then a positive signal. For hybridization procedure, all PCR products were denatured in 0·4 M NaOH, applied to nylon membranes with a dot-blot apparatus, and hybridized with a cloned Viannia minicircle as a molecular probe radio-isotope labelled with [α-32P]dATP by random hexamer priming. Filters were hybridized in Blotto at 65 °C, washed in 0·1× standard saline citrate at the same temperature, and exposed to radiographic films.
We examined the genetic variations of L. (V.) braziliensis parasites (among isolates from humans and experimentally infected rhesus) by extracting DNA promastigotes and analysing them with a PCR-based typing system we developed, termed intergenic region typing (IRT), which used restriction fragment analysis of the Leishmania internal transcribed spacers (ITS) between the SSU and LSU rRNAs, as described elsewhere (Cupolillo et al. 1995). The PCR products were digested with restriction enzymes (BstUI, EcoRI, HhaI, and HaeIII). The DNA fragments were separated through a 12% polyacrylamide gel using the Genephor® electrophoresis apparatus (Amersham Pharmacia Biotech). To differentiate strains further, a PCR fingerprinting (random-amplified polymorphic DNA, RAPD) method was used following the manufacturer's instructions (Amersham Pharmacia Biotech). The 4 most discriminatory primers (I, 5′-d[GGTGCGGGAA]-3′; II, 5′-d[GTTTCGCTCC]-3′; III, 5′-d[GTAGAC CCGT]-3′; and IV, 5′-d[AAGAGCCCGT]-3′) for Leishmania (Viannia) parasites (unpublished data) were used in this study.
Promastigotes of L. (V.) braziliensis (CL strain MHOM/BR/97/SIS) were the source of antigens. A preparation of soluble leishmanial antigens (SLA) was made as described (Amaral et al. 2001). The SLA was used at different concentrations for ELISA and in vitro blast transformation assays.
Animals were assessed for DTH reactions according to the method used for humans (leishmanin skin test, LST). The antigen (leishmanin), provided by the Fiocruz, Biomanguinhos Unit (Rio de Janeiro, Brazil), consisted of pooled heat-killed cross-species promastigotes suspended in PBS with 0·5% phenol. For the LST, a quantity of 0·1 ml containing macromolecules from 5×106 parasites was inoculated intradermally into the forearm, and the induration produced was measured in millimeters 72 h later. An induration diameter of equal or more than 5 mm was considered positive.
The cellular immune responses were studied in vitro by lymphocyte proliferative assays with SLA (10 μg protein/well) and mitogen (Phytohemagglutinin PHA-P at 12·5 μg/ml; Sigma). Basic experimental protocols have been reported elsewhere (Amaral et al. 1996). Cell proliferation was expressed as stimulatory index (SI) and was considered positive if >2·5. Levels of IFN-γ in supernatants of cultures of stimulated cells were measured by ELISA as described (Amaral et al. 2001). A rhesus monkey IFN-γ ELISA immunoassay kit was used following the manufacturer's instructions (Biosource International). IFN-γ concentrations for unknown samples and controls were read from the standard curve plotted.
The phenotype of responding T-cell subsets of infected animals was determined as described (Amaral et al. 1996). The following commercial monoclonal antibodies were employed: CD4 helper/inducer T cell subset (OKT4 antibody; Ortho Diagnostic Systems, Raritan, NJ), CD8 suppressor/cytotoxic T cell subset (Leu 2a antibody; Becton-Dickinson, San Jose, CA), and anti-CD25 (for IL-2 receptor; Becton-Dickinson). Briefly, samples from stimulated PBL cultures were washed using Hank's and incubated for 20 min with a FITC-conjugated monoclonal antibody at 4 °C. The cells were then washed and cytofluorographic analyses were performed using a fluorescence-activated cell sorter (EPIC 751, Coulter). Data are expressed as the percentage of positively staining cells.
Sera from the monkeys were analysed by adapting a standard ELISA technique (Campos-Neto et al. 2001) to detect parasite-specific antibodies (using a peroxidase-conjugated rabbit anti-monkey IgG; Sigma). Subclass-specific antibodies were detected using an anti-human IgG1 (clone 8c/6-39) biotinylated monoclonal antibody (Sigma) that cross-reacts with macaque IgG1. The reaction was revealed with biotin–avidin peroxidase system. The substrate consisted of 0·04% o-phenylenediamine (OPD) dihydrochloride and 0·012% hydrogen peroxidase in phosphate-citrate buffer, pH 5. All sera were tested in duplicate. A group of sera with known positive titres, as well as naïve rhesus controls, were included in each test. The lower limit of positivity (cut off) was determined by the mean of the negative control +2 S.D.
The immunoreactivity of serum antibodies to soluble antigens was determined by Western blot analysis as described (Leon et al. 1992). Briefly, the SLA extracts were resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) using 12% slab gels in non-reducing conditions, and electrophoretically transferred to nitrocellulose paper (Schleicher and Schuell, Keene, NH). The nitrocellulose strips were incubated overnight at 4 °C with sera obtained from infected primates, then with rabbit anti-monkey IgG-peroxidase conjugate. After rinsing, strips were developed in a saturated solution of 3,3′-diaminobenzidine in Tris buffer containing 0·01% H2O2.
Student's t-test was used in comparative analysis and a P value of <0·05 was considered significant. Concordance between in vivo and in vitro cellular immune responses was assessed as reported elsewhere (Amaral et al. 2001).
In Exp. 1, all of the monkeys inoculated intradermally above the left eyelid with virulent promastigotes of L. (V.) braziliensis strains MHM/BR/97/SIS and MHOM/BR/95/OSC developed chronic ulcerative CL that persisted from about 6 months to as long as 3 years. Lesion development (nodules varying between 40 mm2 to 300 mm2) progressed rapidly (Fig. 1), peaking at 4 weeks p.i. The initial lesion (an erythaematous papule) was first visible at 1–2 weeks p.i., became nodular and then ulcerated (Fig. 2A) after 3 to 6 weeks p.i. The ulcers persisted until covered by a crust (Fig. 2D), subsequently regressed but with cryptic parasitism as shown by detection of Leishmania DNA in scars of 5 monkeys with self-healing CL (Table 1).

Fig. 1. Course of skin lesion development in groups of 5 rhesus macaques following primary infection with 2 strains of Leishmania (V.) braziliensis (Exp. 1). The parasites were isolated from patients with either cutaneous (group A, strain MHOM/BR/97/SIS) or mucosal (group B, strain MHOM/BR/95/OSC) disease. Individual monkeys were inoculated intradermally on the upper eyelid area with 1×107 promastigotes of each parasite strain. All of the challenge-infected monkeys developed a lesion at the site of inoculation. Lesion sizes (diameter of the lesion in mm) are given as means±S.E. Progression of primary lesion is also indicated for the rhesus (A5) with non-healing mucocutaneous disease.

Fig. 2. Mucocutaneous leishmaniasis in Leishmania (V.) braziliensis-infected rhesus macaques. Clinical presentation of primary (A) Rh A1; (D) Rh A5; and (I) Rh C3 and metastatic ulcerated skin (F): Rh A5 and mucosal (D and G) Rh A5 lesions observed in monkeys over time p.i. Non-specific chronic inflammation (B) Rh A1 and tuberculoid-type granulomatous reaction (E and H) Rh A5 containing Langerhans cells (arrows) were the main histopathological manifestations of the disease (H & E stain; bar=80 μm). Also illustrated are a leishmanin skin test positive reaction detected (Rh A5) at 4 weeks p.i. (C) and dot-blot hybridization experiment of PCR amplified products of L. (V.) braziliensis (J) with Viannia-specific probe (K). In this case, each experiment included positive and negative controls (see Materials and Methods section).
The primary lesions in monkeys in group A persisted somewhat longer (14–33 months) than those of group B (6–18 months). The average (maximum) size of the skin lesions (90 mm2) in 4 of the 5 group A monkeys was not significantly different (P>0·05) from that in group B (70 mm2). However, satellite lesions peripheral to the primary nodule (Fig. 2A,D), and/or metastases in the extremities (Fig. 2F) also developed in monkeys (n=4) of group A, which persisted until the end of the observation period (33 months). One of these animals (A5) showed long-standing (Fig. 1) plaque-like infiltrative facial cutaneous lesions associated with coexistent mucosal disease (Fig. 2D,G). In 3 animals of group B spontaneous healing of their lesions was observed but with cryptic parasitism; in another infected monkey there was regression followed by reactivation of the primary lesions.
The granulomatous inflammation of primary and metastatic skin lesions were similar to each other, which consisted of macrophages (some of which contained a few amastigotes), lymphocytes, plasma cells, and occasional neutrophils and eosinophils. In the early phases of developing ulcerated skin lesions, indistinctly delimited and more or less differentiated macrophage accumulations were found (Fig. 2B), which evolved to the formation of granulomatous nodules containing giant cells (Fig. 2E). In the late stages (cutaneous scars), fibroblasts proliferated at the periphery of and finally invaded the granulomas with fibrotic substitution (data not shown). The mucosal lesions showed an exudative cellular reaction apparently associated with focal tissue necrosis, followed by poorly organized granulomata (Fig. 2H).
Leishmanial parasites were found in the lesions from all L. (V.) braziliensis-infected primates (Exp. 1). Table 1 summarizes the comparative results of conventional diagnostic methods and dot-blot hybridization experiment of PCR amplified products of L. (V.) braziliensis with Viannia-specific probe (Fig. 2J, K) of skin and mucosal lesions tested at different intervals p.i. Parasites were detected in 19 (82·6%) of 23 active lesions by conventional methods (using culture and/or direct microscopic evaluation), whereas PCR was positive in 23 (100%) of 23 active lesions. In addition, parasite DNA was detected in 5 (83·3%) of 6 skin lesions in the scarring phase (versus 14·3% (1/7) by conventional methods).
Attempts to isolate parasites from lesions by the culture of biopsy specimens were successful in 5 animals (A2, A4, A5, B1, and B3). The experiments using DNA-based methods allowed us to examine polymorphisms among L. (V.) braziliensis isolates from humans and experimentally infected rhesus macaques. It was possible to characterize 4 genotypes, each representing parasites with unique ITS fragment profiles (Fig. 3A,B). It is of interest that the 2 isolates from patients with either CL (stock code L2199) or ML (stock code L2145) represented distinct genotypes (Fig. 3A). However, a clonal population contained 4 isolates (stock codes L2199a, L2199a(3), L2199c, and L2145a) from primates (A5, B1, and C3) that developed skin and/or mucosal lesions (Fig. 3A). Although the RAPD fingerprints (Fig. 3C) resulted in a very high degree of discrimination among those isolates, it was not possible to identify a pathogenic clone specifically associated with the development of ML using this primate model of the human disease.

Fig. 3. Genotyping analyses showing genetic variations among Leishmania (V.) braziliensis isolates from humans and experimentally infected rhesus monkeys. Shown are acrylamide gel electrophoresis comparison of ITS_rDNA fragment patterns, generated with the restriction enzyme BstUI (A) and HhaI (B), and RAPD fingerprints (C) of a representative selection of isolates. The apparent sizes of ITS fragments were estimated relative to molecular weight markers; their values (in bp) are at left. Stock codes indicated above the lanes were isolated from: L2199, human CL (MHOM/BR/97/SIS); L2199a, ML (Rh A5); L2199b, CL (Rh A1); L2199c, CL (Rh A5); L2199a(3), CL (Rh C3); L2145, human ML (MHOM/BR/95/OSC); L2145a, CL (Rh B1).
All the 6 rhesus in group C (Exp. 2) infected with L2199a (a stock that was isolated by cultivating a biopsy specimen of ML from rhesus A5 at 30 months p.i.) developed chronic ulcerative cutaneous lesions. The lesions at the left orbit on monkeys in that group were not different from those in group B in time of appearance or size. The lesions developed in monkeys infected at the left forearm (Fig. 2I) showed a similar clinical pattern to that of patients with CL caused by L. (V.) braziliensis. The primary lesions in group C monkeys persisted, but none of them was found with mucosal involvement until the end of the observation period (22 months).
A very high degree of variability was observed in the parasite-specific cell-mediated immune responses for individuals over time following infections with L. (V.) braziliensis MHOM/BR/97/SIS (group A) and MHOM/BR/95/OSC (group B) strains. Results showed that all experimental animals had positive DTH responses to the antigen as measured by skin induration. LST reactivity (Fig. 2C) was detected as early as 4 weeks p.i. and continued up to 33 months p.i. (Fig. 4). The reaction sizes (range 5–28 mm) found among positive animals were larger during active infection as compared with after self-cure. No statistical differences between infected animal groups (P=0·10) could be demonstrated in skin induration diameters (mean±S.E., A=9·9±3·6 mm and B=9·4±2·0 mm). In contrast, control naïve monkeys injected with leishmanial antigen developed either minimal skin induration (mean score, <5 mm) or nothing.

Fig. 4. DTH responses in rhesus monkeys following infections with Leishmania (V.) braziliensis MHOM/BR/97/SIS (group A) and MHOM/BR/95/OSC (group B) strains. Animals were measured at 72 h after injection of 0·1 ml containing 5×106 heat-killed promastigotes (leishmanin) into the shaven area of the right forearm and results are expressed as the diameter of skin induration in millimeters. Each point represents mean±S.D. of 5 monkeys per group.
The proliferative responses and the production of IFN-γ in the supernatants of PBL cultured with SLA or PHA were determined. The specific responses found in both groups of primates at different time-points following infection are shown in Fig. 5. The findings indicated that parasite-specific proliferative responses were negative in primates at the initiation of infection, and that PBL were comparably responsive to control mitogen PHA prior to and throughout infection (data not shown). PBL from all infected monkeys did proliferate (SI values ranged between 4·7 and 72) and produced IFN-γ (levels ranged between 0·11 and 1·35 ng/ml) in response to SLA. However, there was considerable variation between individual monkeys (as shown by some large S.D. values). Comparisons between groups did not show significant difference for SIs (mean±S.D., A=27·6±33·7 and B=14·7±13·3) or IFN-γ production (mean±S.D., A=0·7±0·3 and B=0·5±0·2) in response to SLA. Moreover, there was no concordance between the positive DTH reaction size values and the in vitro LPR (r=0·26; P=0·53) or IFN-γ levels produced by responding T cells (r=0·50; P=0·26) following Ag stimulation. It is of interest that positive and strong L. (V.) braziliensis-specific lymphoproliferation and IFN-γ responses were elicited in the rhesus (A5) with mucocutaneous disease. Again, strongest DTH responses during active infection were obtained in this animal as compared with other monkeys.

Fig. 5. Parasite-specific lymphoproliferative responses (A) and IFN-γ levels (B) in rhesus monkeys following infections with Leishmania (V.) braziliensis MHOM/BR/97/SIS (group A) and MHOM/BR/95/OSC (group B) strains. Purified PBL were adjusted to 2×106 cells/ml in complete RPMI medium and stimulated with SLA (10 μg/well) for 96 h at 37 °C in 5% CO2. Then the cells were pulsed for the last 18 h of incubation with 0·5 μCi of [3H]thymidine. Cell proliferation was assessed by measuring [3H]thymidine incorporation; results are expressed as the stimulation index (SI, mean cpm stimulated cultures/mean cpm unstimulated cultures). Culture supernatants were collected from duplicate wells after 72 h of stimulation, and the concentration of IFN-γ in the supernatant was determined by ELISA. Data are means±S.D. of 5 monkeys per group.
Phenotypic characterization of CD4+ and CD8+ SLA-reactive T cells from primates (n=8) were assayed. Long-term follow-up of animals (during 9–33 months p.i.) showed higher proportions of CD8+ (55·8±18·2%) than CD4+ (34·7±7·7%) T cells. No significant variation (P>0·05) in the ratio of CD4+ to CD8+ responding (CD25+) T cells was found, however, in animals with active or healing lesions (CD4[ratio ]CD8 mean ratio, 0·7; range 0·3–2·1) as compared to uninfected controls (CD4[ratio ]CD8 mean ratio, 2·0; range 1·2–3·3).
The antibody responses were measured by ELISA. As shown (Fig. 6), animal groups had higher anti-L. (V.) braziliensis antibody levels at different time-points following infections when compared with pre-challenge background levels. The serum levels of Ag-specific antibodies (IgG and IgG1 isotypes) reached a plateau by 1 month after challenge, but the IgG response was consistently stronger than the IgG1 response. Interestingly, the rhesus with persistent metastatic skin and mucosal lesions also had heightened levels of Ag-specific antibodies. Western blot analyses of L. (V.) braziliensis promastigote antigens were performed employing immune sera (1[ratio ]150 was taken as the optimal titre, lowest dilution at which control animals gave no signal on the antigen profile) from monkeys at various times post-infection (data not shown). The reactions of serum antibodies (IgG) produced by infected animals recognized multiple bands ranging from 15 to 205 kDa. Animals giving higher ELISA antibody titres (e.g. rhesus A5) gave stronger reactions and recognized more antigens in Western blots (data not shown), when compared with animals with self-healing skin lesions (e.g. rhesus A1).

Fig. 6. Evolution of serum levels of Leishmania-specific antibodies (A, IgG and B, IgG1) in rhesus monkeys following infections with Leishmania (V.) braziliensis MHOM/BR/97/SIS (group A) and MHOM/BR/95/OSC (group B) strains. Each point represents the optical density value at 492 nm of sera from monkeys tested (1[ratio ]50 dilution) by ELISA. Data are means±S.E of monkeys/group. Individual humoral responses are also indicated for the monkey Rh A5 that developed persistent metastatic skin and mucosal lesions.
Non-human primates have varying degrees of susceptibility to Leishmania parasites. Marked variation in the outcome of the disease was observed when some species, including Saimiri sciureus, Cebus apella (Lainson & Shaw, 1977), and Callithrix penicillata (Cuba Cuba et al. 1990) were examined for susceptibility to L. (V.) braziliensis. Limited studies have been done on the susceptibility of M. mulatta to L. braziliensis s.l. (Marques da Cunha, 1944; Lainson & Bray, 1966). In our experiments the rhesus monkey was found to be susceptible to infections with different isolates of L. (V.) braziliensis. The time required for lesion development was very variable, not only for the parasites showing molecular differences but also for individual monkeys in groups infected with the same parasite strain/dose. However, the severity of the resulting lesions was greater compared to either L. (L.) major or L. (L.) amazonensis infection (Amaral et al. 1996, 2001). These observations suggest that an interplay exists between the host immune system and parasite pathogenic capabilities in the outcome of leishmanial infection in M. mulatta. Likewise, wide-ranging differences of clinical manifestations define Leishmania virulence (degree of pathogenicity) in human infection (Chang, Akman & Nielsen, 1999).
A relevant animal model of ML is urgently needed to help understand the nature of this important clinical outcome. Simian species more closely related to humans are the most likely to mimic accurately the human disease and the immune response to infection (Kennedy et al. 1997). In these studies we have shown that M. mulatta serves as a useful experimental model of the L. (V.) braziliensis CL in humans (Marsden, 1985; Saraiva et al. 1990). Infections reproduced the self-resolving or non-healing ulcerated skin lesions, as well as the chronic relapsing CL following reactivation of infection. Mucosal granulomata was observed in 1 rhesus (out of 16 infected). The primary lesion in this monkey was large and non-healing and contained many satellite and metastatic lesions in non-mucosal sites as well. This outcome appears to be more consistent with disseminating L. (V.) braziliensis CL (Carvalho et al. 1994). However, in the original model description (Marques da Cunha, 1944) ML was observed in 2 of 7 (28·5%) monkeys infected with L. braziliensis s.l., and when the changes extended to the mucous membranes, they persisted and evolved, whilst the skin changes observed on the same animal became regressive and tending towards a self-healing CL. These findings are significant, especially since they have not been reported in a number of New World monkey models of L. braziliensis infection (Lujan et al. 1986; Lainson & Shaw, 1977; Cuba Cuba et al. 1990).
Parasite persistence in active and/or healing lesions of L. (V.) braziliensis CL was documented in all rhesus macaques. Interestingly, our comparative results of conventional diagnostic procedures and PCR of lesions from primates confirmed the efficacy of DNA-based methods as a presumptive diagnosis of human leishmaniasis. Despite clinical healing, we were able to detect leishmanial DNA in skin scars of 5 (83·3%) recovered monkeys, indicating parasite persistence. Likewise, we have shown parasite DNA in L. (V.) braziliensis CL scars of 16 (80%) treated human patients (Schubach et al. 1998 a). In this case, cutaneous scars represented a site of parasite persistence and viability 11 years after antimonial therapy and clinical cure (Schubach et al. 1998 b).
Sensitization to the infection and/or the duration of the elicited immune responses, and their roles in acquired immunity or immunopathological consequences may vary in certain host-parasite combinations (Saraiva et al. 1989). The hyperergic response to L. (V.) braziliensis antigens encountered in the rhesus (A5) with active mucocutaneous disease was consistent with human data (Carvalho et al. 1985). Whether exposure to a particular parasite has led to the metastatic events and increased responses or, alternatively, whether hyperreactivity of the host has contributed to the mucosal granulomata could not be ascertained by this study. The data do not show either molecular differences between parasite strains or differences between immune responses to distinct L. (V.) braziliensis isolates apparently related with disease expression. It was possible to characterize one genotype (i.e. representing parasites with unique ITS fragment profiles) containing isolates from primates that developed skin and/or mucosal lesions.
The histopathological features of the lesions in this model were similar to human L. (V.) braziliensis CL (Marsden, 1985; Pirmez et al. 1990). The experimental animals presented scanty leishmanial organisms and a mononuclear cell infiltrate containing epithelioid cells in the chronic lesions, typical of an antigen-specific T cell-mediated response-induced granulomatous reaction (Lammas et al. 2002). In L. (L.) amazonensis CL of rhesus monkeys these structures showed a mixture of T-cell sub populations with the ratio of helper[ratio ]suppressor phenotypes less than 1 (Amaral et al. 2000). A pattern similar to the infiltrate in the skin of humans with CL (Lima et al. 1994). Different influences, such as pathogen virulence and host genotype, may affect the the granulomatous response (Murray, 2001). Clinical evidence suggests that T lymphocytes reactive to parasite antigens participate in the development of ML (Conceição-Silva et al. 1990; Pirmez et al. 1990).
A self-healing phenomenon in rhesus monkeys is indicative of the development of an acquired immunity. Protective immunity to Leishmania seems to require the development of a antigen-dependent Th1 immune response (Reed & Scott, 2000). Studies with the vervet monkey model for CL have indicated that IFN-γ secretion and positive DTH responses are associated with L. (L.) major infection and represent specific immunological correlates of protection (Olobo et al. 1992). Our findings indicate that parasite-specific type 1 responses develop in L. (V.) braziliensis-infected monkeys, but the levels of T-cell proliferative and IFN-γ responses and/or LST response do not always correlate with recovery from the skin lesions. Immunological assessments of patients with L. (V.) braziliensis infection have also provided evidence of a dissociation between immune parameters and acquired resistance (Saraiva et al. 1989). This was the situation in vaccinated monkeys as well, where neither positive DTH reactions nor increased production of IFN-γ were predictive of protection (Kenney et al. 1999; Gicheru et al. 2001). This fact raises a question on the current parameters that should be considered as a counterpart of expected protection induced by a vaccine.
With respect to humoral immunity, an increase in antigen-specific IgG and IgG1 subclass levels as detected by ELISA assays were observed in all experimental groups. In addition, immunoblot analyses showed that infected monkeys produced antibodies which bound to a number of Leishmania antigen components, consistent with human data (Leon et al. 1992). The presence of Leishmania-specific antibodies is a common feature of CL (Grimaldi & Tesh, 1993). Interestingly, the findings of increased antibody titres as well as heightened cutaneous DTH reactions detected in the rhesus (A5) with mucosal disease support a modulatory role for antibody found in human infection with L. (V.) braziliensis (Saraiva et al. 1989).
We conclude that the rhesus monkey model could be useful for studying the interactions between parasite and host determinants for infection, disease (virulence) and cure in L. (V.) braziliensis CL, as well as for the evaluation of new drugs and candidate vaccines against the human disease.
We thank Dr Antonio da Mota Marinho and the staff in the Fiocruz Animal Care Facility for their assistance with daily care and procedures on the macaques. Financial support: Fiocruz; FAPERJ; PRONEX III/CNPq/MCT (Brazil).

Table 1. Comparative results of conventional diagnostic procedures and PCR of leishmaniasis lesions in Leishmania (V.) braziliensis-infected rhesus macaques

Fig. 1. Course of skin lesion development in groups of 5 rhesus macaques following primary infection with 2 strains of Leishmania (V.) braziliensis (Exp. 1). The parasites were isolated from patients with either cutaneous (group A, strain MHOM/BR/97/SIS) or mucosal (group B, strain MHOM/BR/95/OSC) disease. Individual monkeys were inoculated intradermally on the upper eyelid area with 1×107 promastigotes of each parasite strain. All of the challenge-infected monkeys developed a lesion at the site of inoculation. Lesion sizes (diameter of the lesion in mm) are given as means±S.E. Progression of primary lesion is also indicated for the rhesus (A5) with non-healing mucocutaneous disease.

Fig. 2. Mucocutaneous leishmaniasis in Leishmania (V.) braziliensis-infected rhesus macaques. Clinical presentation of primary (A) Rh A1; (D) Rh A5; and (I) Rh C3 and metastatic ulcerated skin (F): Rh A5 and mucosal (D and G) Rh A5 lesions observed in monkeys over time p.i. Non-specific chronic inflammation (B) Rh A1 and tuberculoid-type granulomatous reaction (E and H) Rh A5 containing Langerhans cells (arrows) were the main histopathological manifestations of the disease (H & E stain; bar=80 μm). Also illustrated are a leishmanin skin test positive reaction detected (Rh A5) at 4 weeks p.i. (C) and dot-blot hybridization experiment of PCR amplified products of L. (V.) braziliensis (J) with Viannia-specific probe (K). In this case, each experiment included positive and negative controls (see Materials and Methods section).

Fig. 3. Genotyping analyses showing genetic variations among Leishmania (V.) braziliensis isolates from humans and experimentally infected rhesus monkeys. Shown are acrylamide gel electrophoresis comparison of ITS_rDNA fragment patterns, generated with the restriction enzyme BstUI (A) and HhaI (B), and RAPD fingerprints (C) of a representative selection of isolates. The apparent sizes of ITS fragments were estimated relative to molecular weight markers; their values (in bp) are at left. Stock codes indicated above the lanes were isolated from: L2199, human CL (MHOM/BR/97/SIS); L2199a, ML (Rh A5); L2199b, CL (Rh A1); L2199c, CL (Rh A5); L2199a(3), CL (Rh C3); L2145, human ML (MHOM/BR/95/OSC); L2145a, CL (Rh B1).

Fig. 4. DTH responses in rhesus monkeys following infections with Leishmania (V.) braziliensis MHOM/BR/97/SIS (group A) and MHOM/BR/95/OSC (group B) strains. Animals were measured at 72 h after injection of 0·1 ml containing 5×106 heat-killed promastigotes (leishmanin) into the shaven area of the right forearm and results are expressed as the diameter of skin induration in millimeters. Each point represents mean±S.D. of 5 monkeys per group.

Fig. 5. Parasite-specific lymphoproliferative responses (A) and IFN-γ levels (B) in rhesus monkeys following infections with Leishmania (V.) braziliensis MHOM/BR/97/SIS (group A) and MHOM/BR/95/OSC (group B) strains. Purified PBL were adjusted to 2×106 cells/ml in complete RPMI medium and stimulated with SLA (10 μg/well) for 96 h at 37 °C in 5% CO2. Then the cells were pulsed for the last 18 h of incubation with 0·5 μCi of [3H]thymidine. Cell proliferation was assessed by measuring [3H]thymidine incorporation; results are expressed as the stimulation index (SI, mean cpm stimulated cultures/mean cpm unstimulated cultures). Culture supernatants were collected from duplicate wells after 72 h of stimulation, and the concentration of IFN-γ in the supernatant was determined by ELISA. Data are means±S.D. of 5 monkeys per group.

Fig. 6. Evolution of serum levels of Leishmania-specific antibodies (A, IgG and B, IgG1) in rhesus monkeys following infections with Leishmania (V.) braziliensis MHOM/BR/97/SIS (group A) and MHOM/BR/95/OSC (group B) strains. Each point represents the optical density value at 492 nm of sera from monkeys tested (1[ratio ]50 dilution) by ELISA. Data are means±S.E of monkeys/group. Individual humoral responses are also indicated for the monkey Rh A5 that developed persistent metastatic skin and mucosal lesions.