Introduction
The gastropod Ecphora is a large, abundant, and well-known component of many Neogene faunas from the Atlantic and Gulf Coastal Plain of the United States (Petuch Reference Petuch1988, Ward Reference Ward1992). Ecphora is likely a drilling predator (Petuch Reference Petuch1987, Vermeij Reference Vermeij1987, Petuch Reference Petuch1988, Campbell Reference Campbell1993, Vermeij Reference Vermeij1995) based on its systematic placement within the Muricidae, yet paleoecologists have regularly ignored its potential role as an important trace maker when evaluating the effects of naticid gastropod drilling predation on the evolution of infaunal molluscan prey (Kelley and Hansen Reference Kelley and Hansen2001, Kelley and Hansen Reference Kelley and Hansen2006). This tendency to ignore Ecphora is justified (more often implicitly than explicitly) on the assumption that Ecphora drillholes can be easily excluded from an analysis of naticid predator-prey interactions based on their small size or straight-sided morphology (the Oichnus simplex of Bromley Reference Bromley1981). The distinction is not always straightforward, however, due to the variability of drillhole morphology with factors such as differing prey shell thicknesses, predator sizes, and taphonomic processes (Kitchell et al. Reference Kitchell, Boggs, Kitchell and Rice1981, Kowalewski Reference Kowalewski1993, Reference Kowalewski2002, Reference Kowalewski2004, Dietl and Kelley Reference Dietl and Kelley2006). Recent work has cast further doubt on this assumption by demonstrating that large, extant muricids are capable of drilling large, naticid-like holes (Edward et al. Reference Edward, Ramesh and Ayakkannu1992, Gordillo and Amuchastegui Reference Gordillo and Amuchastegui1998, Dietl and Herbert Reference Dietl and Herbert2005, Tan Reference Tan2008) that are countersunk or parabolic in shape (the Oichnus paraboloides of Bromley Reference Bromley1981).
Confidence in predator identity is important because small differences between predators within the same functional group can lead to large changes in predatory interactions and community structure. Similar predators can exhibit variable strengths of predator-prey interactions that lead to disparate effects on prey biomass, abundance, survivorship, and diversity (Chalcraft and Resetarits Reference Chalcraft and Resetarits2003). In fact, even intraspecific variation can significantly alter the strength of subsequent trophic cascades (Post et al. Reference Post, Palkovacs, Schielke and Dodson2008). Predator identity also has important evolutionary consequences. For example, when using drilling gastropods to interpret escalation (Vermeil 1987) trends of evolving predator-prey lineages, confidence in predator identity is paramount for two main reasons. Firstly, if prey species are evolving in response to pressure from their predators then it is necessary to understand what selection pressures are involved. Even where the end result is the same—death of the prey due to drilling—the varied foraging behaviors of drilling predators could elicit different adaptive responses in the prey. Secondly, it is important to avoid confusion between evolutionary changes in predator behavior and a change in predator identity (Herbert and Dietl Reference Herbert and Dietl2002, Paul and Herbert Reference Paul and Herbert2014). A change in drill-hole patterns could simply be due to the introduction of a new predator and not due to a change in the drilling behavior of a single predator.
In this study, we present experimental, modern beach assemblage, and fossil evidence to evaluate the hypothesis that Ecphora was an important contributor of drilling traces among infaunal prey, specifically drillholes commonly found in large-shelled specimens of the venerid bivalve Mercenaria. If Ecphora drilled its prey in a similar manner to other confamilial taxa, stereotyped differences in naticid and muricid prey-handling behaviors can be used to create a series of testable predictions that would implicate its activity. Naticids primarily search for prey infaunally (Kelley and Hansen Reference Kelley and Hansen2003), then envelop and orient prey with the foot (mesopodium) for drilling (Kelley and Hansen Reference Kelley and Hansen2003). This behavior limits the size of prey that a naticid of a given size can drill and results in well-documented size selectivity of prey, with bigger predators selectively drilling bigger prey (Kitchell et al. Reference Kitchell, Boggs, Kitchell and Rice1981, Kabat Reference Kabat1990 and references therein). The manipulation of prey into a preferred orientation also produces a pattern of drillhole stereotypy in venerid prey (Kitchell Reference Kitchell1986, Alexander et al. Reference Alexander, Dietl and Farrell2007, Visaggi et al. Reference Visaggi, Dietl and Kelley2013), with holes drilled by naticids primarily located in the umbonal region of Mercenaria shells (Carriker Reference Carriker1951, Boggs et al. Reference Boggs, Rice, Kitchell and Kitchell1984, Visaggi et al. Reference Visaggi, Dietl and Kelley2013). In contrast, muricids that feed on infaunal bivalves search for prey epifaunally, exhume shallow, infaunal prey, and grasp the prey’s shell with the foot prior to initiating the drilling process. Because muricids do not completely envelop their prey they are not constrained to prey small enough to envelop completely. This lack of a size constraint leads to a wider range of predator:prey size combinations than is typical for naticids (Kojumdjeva Reference Kojumdjeva1974). In many cases muricids fail to show drillhole site stereotypy (Carriker and Yochelson Reference Carriker and Yochelson1968, Kowalewski Reference Kowalewski2004, Peharda and Morton Reference Peharda and Morton2006, Casey and Chattopadhyay Reference Casey and Chattopadhyay2008, Tan Reference Tan2008) or have lower drillhole stereotypy than naticids (Stump Reference Stump1975, Thomas Reference Thomas1976, Roopnarine and Willard Reference Roopnarine and Willard2001, Dietl et al. Reference Dietl, Herbert and Vermeij2004, Walker Reference Walker2007). In cases where muricid drillhole placement on Mercenaria prey has been described, hole placement was generally located in the center of the valve (Carriker Reference Carriker1957) or varied widely and included penetration between the valves (Carriker Reference Carriker1951, Reference Carriker1961).
Prediction 1: Due to differences in prey- handling behaviors, we expect predator size (indexed as outer borehole diameter (OBD); Kitchell et al. Reference Kitchell, Boggs, Kitchell and Rice1981) will be significantly, positively correlated with prey size for naticid- only assemblages but not for naticid + Ecphora or naticid + large muricid assemblages. Where both naticids and Ecphora are present there will be increased variability in the ratio of predator to prey size, eliminating the significant positive correlation of these variables prevalent in naticid-only assemblages.
Prediction 2: Due to differences in prey- handling behaviors, the degree of drillhole stereotypy will be higher in naticid-only assemblages than for assemblages with both naticids and large muricids such as Ecphora. We predict that naticid-only assemblages will show clumping of drillhole sites near the umbo while naticid+large muricid and naticid+Ecphora assemblages will show a mix of umbonal and central drillholes.
If our predictions are correct, then: (1) Ecphora is the only predator capable of drilling Mercenaria specimens that are too large to have been suitable naticid prey in the naticid+Ecphora assemblages, and (2) Ecphora were frequent predators of large Mercenaria in the naticid+large muricid assemblages. Support for these predictions implies that Ecphora and other large muricids were important trace makers that cannot be excluded on the basis of drillhole morphology alone and must be explicitly accounted for in predation studies conducted in fossil assemblages where they occurred.
Materials and Methods
Fossil Samples
Samples of Mercenaria spp. shells that contained drillholes were examined from four Pliocene localities of the Atlantic and Gulf Coastal Plain of the United States (Table 1) — one from the Rushmere Member of the Yorktown Formation of Virginia (Lieutenant Run), one from the Moore House Member of the Yorktown Formation of Virginia (Chuckatuck), and two from the Buckingham Member (=Pinecrest unit 10 of Petuch Reference Petuch1982, =lower Tamiami Formation of Zullo and Harris Reference Zullo and Harris1992) of the Tamiami Formation of Florida (Quality Aggregates Phase 7A (QA) and APAC). Targeted samples (sensu Ottens et al. Reference Ottens, Dietl, Kelley and Stanford2012) for drillhole location analysis, in which only prey shells containing drillholes were collected, were obtained from the Lieutenant Run, Chuckatuck, and Quality Aggregates localities, thus precluding an analysis of the frequency of complete or incomplete drilling predation. These stratigraphic units consist of diverse and well-preserved shelly assemblages, with hundreds of mollusk species (Petuch Reference Petuch2004, Petuch and Roberts Reference Petuch and Roberts2007).
Table 1 Localities and stratigraphic unit of late Pliocene Mercenaria spp. samples used in drillhole analyses.

Samples of only the largest fossil Mercenaria spp. shells (Table 1), between ~50 and 175 mm in antero-posterior (A-P) width, were studied in order to limit potential drilling predators to a few candidates. We did not attempt to identify individual Mercenaria shells in our samples by species given the need for a systematic revision in the group (Campbell Reference Campbell1993). The dominant morphological form present at each locality, however, was noted and prey shell thickness at the drillhole site was measured to the nearest 0.1 mm as such variation may have influenced the foraging behavior of a drilling predator (Kabat Reference Kabat1990). Members of the genus Mercenaria that are abundant in the Pliocene Yorktown Formation units we sampled include both morphological forms belonging to M. corrugata, the thick- shelled, wavy “tridacnoides” form and the thin-shelled “rileyi” form (Wilson Reference Wilson1983), as well as M. campechiensis permagna, which is another relatively thin-shelled form (Campbell Reference Campbell1993). According to Petuch and Roberts (Reference Petuch and Roberts2007), Mercenaria species present in the Pliocene Buckingham Member of the Tamiami Formation include both forms of M. corrugata and the thick-shelled M. campechiensis ochlockoneensis. Although thin-shelled forms are present, the “tridacnoides” form of M. corrugata is most common in our samples at the Lieutenant Run and APAC localities; the “rileyi” form of M. corrugata and M. c. permagna are common in the Chuckatuck sample; and both the “rileyi” form and M. c. ochlockoneensis are common at the Quality Aggregates locality, where the “tridacnoides” form is absent (Table 1).
Of the potential naticid predators present in these stratigraphic units, only species of the genera Euspira, Neverita, and Naticarius were large enough to have potentially fed on Mercenaria prey >50 mm A-P width (E. Petuch personal communication 2015). The largest muricid genera in these assemblages include Ecphora and Phyllonotus (Petuch Reference Petuch2004; E. Petuch, personal communication 2015). Each of the assemblages assessed has a slightly different combination of the above listed potential predators. Only Neverita, Euspira, and Ecphora are present in the Yorktown Formation (Campbell Reference Campbell1993, Petuch Reference Petuch2004), making the naticid+Ecphora assemblages from Virginia the most straightforward study system. The large naticids, Neverita, Naticarius, and rarely Euspira, and the muricids, Ecphora and Phyllonotus, are present in the Buckingham Member of the Tamiami Formation (Petuch Reference Petuch2004) making issues of predator identity in the naticid+large muricid assemblages from Florida more complex. No occurrences of the large muricid Muricanthus have been recorded in the Buckingham Member of the Tamiami Formation (E. Petuch personal communication 2015). Furthermore, Muricanthus tends to attack large Mercenaria by grinding or drilling the edge of the prey’s shell (Wells Reference Wells1958, Dietl et al. Reference Dietl, Herbert and Vermeij2004), allowing it to be excluded from this analysis if present in these assemblages.
Laboratory Experiments
Species of Euspira, Neverita and Mercenaria are extant, which allows us to compare our fossil data with modern Mercenaria drilled under laboratory and field conditions. Laboratory experiments were conducted at the NOAA Northeast Fisheries Science Center, Milford, Connecticut, and the Paleontological Research Institution (PRI), Ithaca, New York, to determine the maximum size of Mercenaria prey potentially vulnerable to successful naticid predation. Specimens of Mercenaria mercenaria were purchased from local fish markets, and specimens of Euspira heros and Neverita duplicata were collected directly or by clam fishermen from localities along the Atlantic and Gulf coasts of the United States in order to maximize predator size. Fifteen E. heros, the largest naticid species co-occurring with Mercenaria, were obtained by fisherman off of the coast of Atlantic City, New Jersey. Five N. duplicata were collected from Alligator Harbor, Florida, where individuals reach sizes close to the species reported maximum. The naticids used in our experiment were thus all as large, or larger, than those found in the fossil assemblages, ranging in shell width (W), or whorl diameter, from ~60 mm to 80 mm for Euspira and ~50 mm to 65 mm for Neverita. Each predator was held in a ~38 liter tank with sea water—a closed, recirculating system at PRI and an open, flow-through system at the NOAA Northeast Fisheries Science Center—and a layer of coarse sand (~10 cm deep) maintained at a temperature of 18–20°C and salinity of ~30–35 ppt. Seawater in the recirculating tanks at PRI was made with Instant Ocean® sea salt and was changed every two weeks.
Snails were offered large M. mercenaria prey, ranging in shell A-P width from ~40 mm to 95 mm for Euspira prey and from ~34 mm to 65 mm for Neverita prey. Each predator was initially given a Mercenaria prey of about 85% of its size. Tanks were checked daily and drilled prey were replaced with a similarly sized prey or slightly larger prey item (about a 5% increase) until the predator was unable to drill the prey. If a Mercenaria prey was still not drilled after a week, another prey of equal size was introduced. If, after repeating this procedure, no prey of this size class were drilled by the predator, a smaller Mercenaria was added to the tank. The experiment was completed when the smaller Mercenaria was drilled, confirming that the predator was not avoiding prey. The largest drilled prey shells by N. duplicata and E. heros observed in this experiment serve as conservative estimates of the upper size limits of what is functionally possible in the naticid-Mercenaria interaction, which can be compared with Mercenaria prey actually drilled by predators in our fossil samples.
Modern Beach Sample
The extant representatives of the naticid genera Euspira and Neverita also allow us to compare our fossil samples with prey shells collected from natural settings. Analysis of natural populations enables us to determine the range of prey sizes consumed by naticids that were: (1) free from potential laboratory tank effects, and (2) free to select Mercenaria prey from the natural size-frequency distributions they would normally encounter. To this end, modern M. mercenaria specimens were collected from the beach at Sandy Hook, New Jersey. These bivalves were likely drilled by N. duplicata and E. heros, which are both present in the area (Gosner Reference Gosner1979). The only muricid commonly found in the Sandy Hook area, the eastern oyster drill Urosalpinx cinerea (Gosner Reference Gosner1979), is too small to drill the largest Mercenaria thus making Sandy Hook a naticid-only sample for the purpose of this study. The maximum size of drilled prey from this sample was compared to similar naticid-only modern beach assemblages in the literature.
Maximum OBD Estimation
Shell width (W), or whorl diameter, of the largest complete specimens of Neverita spp. and Euspira spp. from the Rushmere and Moorehouse Members of the Yorktown Formation in Virginia and the Buckingham Member of the Tamiami Formation in Florida reported in the literature or present within the collections of the Virginia Museum of Natural History (VMNH), Florida Museum of Natural History (FLMNH), and the Paleontological Research Institution (PRI) were measured to the nearest 0.1 mm. These measurements were used to calculate estimated maximum OBD drilled by naticids in each sample using the regression equations in Dietl and Kelley (Reference Dietl and Kelley2006): for E. heros OBD=0.1151W+0.3501; and for N. duplicata OBD=0.0652W+0.6907.
Drillhole Placement
To determine drillhole placement, each of the prey valves from the experiments, modern beach, and fossil samples were placed on a flat surface and digitally photographed from above. The images were imported to the ImageJ Freeware program and A-P width in mm, Cartesian x-y coordinates of the drillholes, and x-y coordinates of five pseudo-landmarks were digitally collected. Images of the right valves were ‘mirrored’ horizontally to the left in order to make them directly comparable to left valves. The pseudo-landmarks were defined as points of maximum or minimum convexity in the following way (Fig. 1): (1) maximum curvature of dorsal shell margin (beak or umbo), (2) maximum curvature of the posterior margin, (3) maximum curvature of the ventral margin, (4) maximum curvature of the anterior margin, (5) minimum curvature below umbo (lunule), and (6) drillhole. Pseudo-landmarks 2 and 4 were set as a baseline and Bookstein shape coordinates (Bookstein Reference Bookstein1991, Roopnarine and Beussink Reference Roopnarine and Beussink1999, Kowalewski Reference Kowalewski2004) were obtained using the statistical software SAS (SAS Institute 2002–2008). This process was repeated ten times for a single shell as a means of judging operator error (Fig. 2H). This method allows direct comparison of drillhole placement on prey shells of different sizes.

Figure 1 Pseudo-landmarks collected for Bookstein analysis.
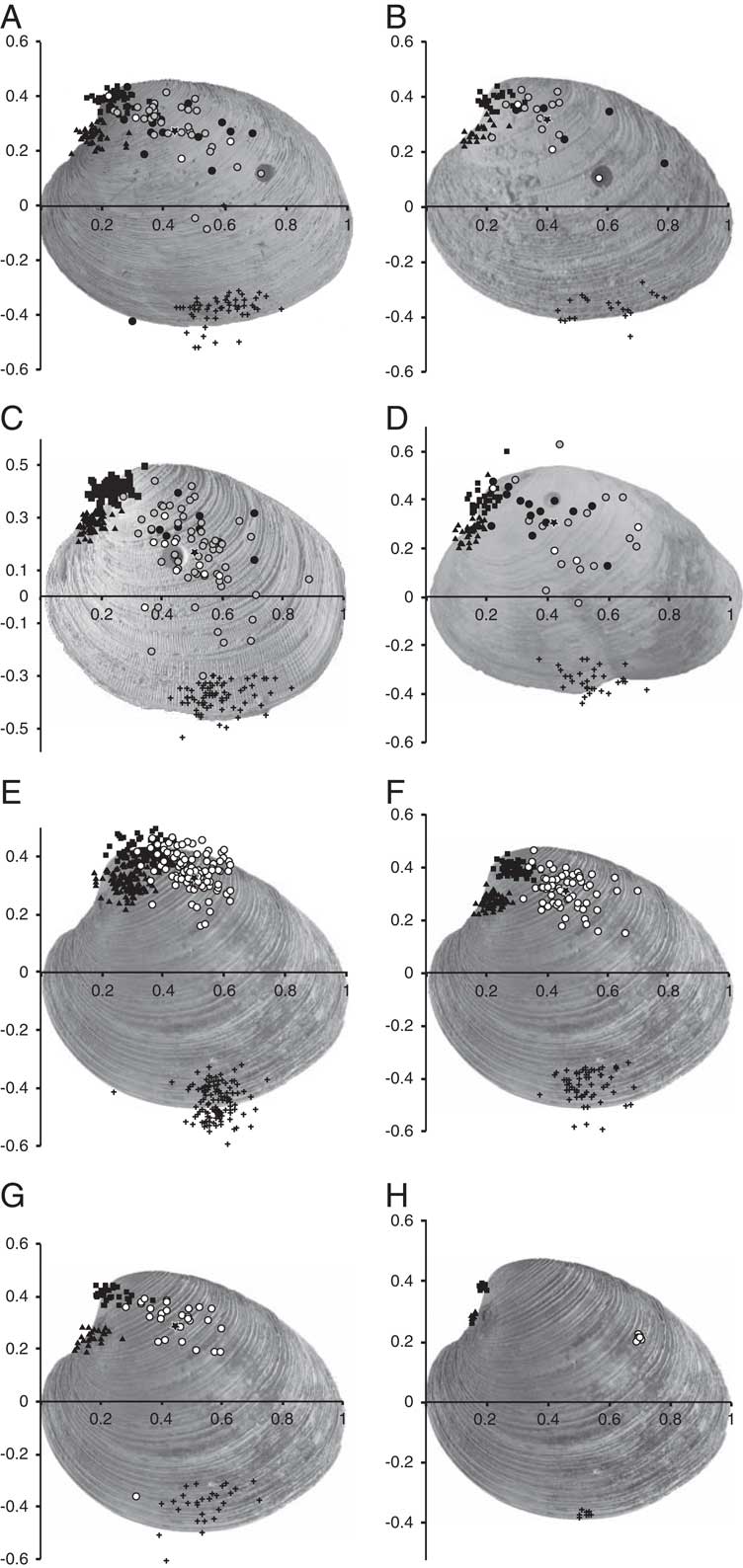
Figure 2 Bookstein plots showing placement of pseudo-landmarks as black squares, diamonds, pluses, and triangles. Drillhole placement indicated as circles color coded by prey size (antero-posterior width): prey within the Sandy Hook locality size range (<71.4 mm) in white; prey larger than those drilled at Sandy Hook but successfully handled by naticids in the laboratory experiments (71.4 mm>x<95.8 mm) in gray; and prey larger than any naticid-drilled modern shells (>95.8 mm) in black. Mean drillhole placement for each sample (A–G) indicated as stars. A, Chuckatuck (Moorehouse Member of the Yorktown Formation; Virginia). B, Lieutenant Run (Rushmere Member of the Yorktown Formation; Virginia). C, Quality Aggregates (Buckingham Member of the Tamiami Formation; Florida). D, APAC (Buckingham Member of the Tamiami Formation; Florida). E, Modern beach sample from Sandy Hook, New Jersey. F, Modern laboratory experiment containing N. duplicata. G, Modern laboratory experiment containing E. heros. H, Operator error showing ten digitizations of the same shell.
Statistical Tests
To evaluate prediction 1, outer borehole diameter (OBD) was used as a proxy for predator size, because it has been shown to be highly correlated with predator size (Kitchell et al. Reference Kitchell, Boggs, Kitchell and Rice1981, Carriker and Gruber Reference Carriker and Gruber1999) and is easily measured in the fossil shells. OBD was plotted against prey shell A-P width. All linear regression analyses were performed in PAST (Hammer et al. Reference Hammer, Harper and Ryan2001).
To evaluate prediction 2, differences in drill-hole placement and morphology of each sample were tested multiple ways. Canonical Variate Analysis (CVA) and Classificatory Discriminant Analysis were completed in SAS by analyzing drillhole Bookstein coordinates alone and in conjunction with variables quantifying drillhole morphology. Drillhole morphology was quantified using measurements of inner borehole diameter (IBD) and OBD, measured to the nearest 0.1 mm. Differences in mean drillhole placement of each sample were tested using 95% bootstrap confidence intervals around the mean Bookstein x- and y- coordinates computed in SAS. A nonparametric Mann-Whitney U test was used to test for significant differences in the median drillhole placement. This univariate test was applied separately for Bookstein x- and y- coordinates. Variation in drillhole placement was evaluated for each sample by calculating the standard deviation of the drillhole Bookstein x- and y-coordinates with bootstrap 95% confidence intervals using the statistical software SAS (SAS Institute 2002–2008); all SAS codes were written by M. Kowalewski.
Nearest neighbor analysis was conducted in PAST (Hammer et al. Reference Hammer, Harper and Ryan2001) in order to test the null hypothesis that drillholes in naticid-only, naticid + large muricid, and naticid+Ecphora samples are randomly distributed in Bookstein space (Alexander et al. Reference Alexander, Dietl and Farrell2007). Nearest neighbor analysis computes the nearest neighbor statistic R. When R=1.0, the distance between points is consistent with a random distribution. Values of R<1 indicate clumping of the data points (points are closer together than expected for a random distribution), whereas values of R>1 indicate overly dispersed points (Hammer and Harper Reference Hammer and Harper2006). All samples containing large muricids were pooled together and all samples with only naticids present were pooled together to meet the minimum sample sizes required to perform nearest neighbor analysis (n>50). Convex hulls were used for area estimation, no edge correction was used as edge drills are rare in nature (Chattopadhyay et al. Reference Chattopadhyay, Zuschin and Tomašových2014) and occur only once in this dataset as a laboratory artifact. Application of wrap around or Donnelly edge correction did not change the pattern of significance reported.
Results
The largest Mercenaria drilled in the laboratory experiment was 95.8 mm in A-P width; the largest Mercenaria found drilled on the beach at Sandy Hook was 71.4 mm A-P width. Maximum A-P width of drilled Mercenaria shells in the Pliocene fossil samples ranges between 109.7 mm at the Quality Aggregates locality and 171.5 mm at the APAC locality (Fig. 3A). The largest naticid drillhole recorded was 10.0 mm in OBD drilled by a laboratory Euspira (Fig. 3C). Drillholes greater than 10 mm in OBD are absent from all Pliocene fossil localities (Fig. 3C).

Figure 3 Box plots of data collected from drilled Mercenaria valves. For each box plot, center line represents the mean value, box terminates at the 25th and 75th percentiles, and error bars denote the minimum and maximum values. A, Size (antero-posterior width) of prey shells by sample. B, Thickness of prey shells by sample. C, Outer borehole diameter (OBD), a proxy for predator size, by sample.
Maximum OBD Estimation
We were unable to locate complete Neverita specimens from the Buckingham Member of the Tamiami Formation for the estimated OBD analysis. Therefore the largest Neverita specimen found within the upper units (Pinecrest and Fruitville Members; sensu Petuch and Richards 2007) of the Tamiami Formation was used as a conservative estimate of maximum OBD for the Buckingham Member. The largest Neverita from Florida exposures of the Tamiami Formation, UF 29167, measured 76.3 mm wide with an estimated OBD of 5.7 mm; the largest Euspira from the Buckingham Member of the Tamiami Formation, figured in Petuch (Reference Petuch1994), measured 26 mm wide with an estimated OBD of 3.0 mm; the largest Neverita from Virginia exposures of the Rushmere and Moorehouse Members of the Yorktown Formation, UF 250479, measured 57.6 mm wide with an estimated OBD of 4.4 mm; the largest Euspira from Virginia exposures from Rushmere and Moorehouse Members of the Yorktown Formation, VMNH uncatalogued Chuckatuck material, measured 60.1 mm wide with an estimated OBD of 7.3 mm.
Predator: Prey Size Ratio
Outer borehole diameter, a proxy for predator size, was significantly correlated with A-P prey width in the modern naticid-only locality (Sandy Hook; Table 4, Supplementary Fig. 1). None of the Pliocene fossil naticid+large muricid or naticid+Ecphora sites yielded significant correlations between A-P prey width and OBD (Table 4). Laboratory animals had access to a restricted range of prey sizes and, therefore, were not included in this analysis.
Drillhole Placement
Drillholes from samples containing Ecphora (Fig. 2A,B) or large muricid taxa (Fig. 2C,D) cover a larger area of the Bookstein plots than drillholes from the naticid laboratory experiments (Fig. 2E,F) and the naticid-only beach sample (Fig. 2G). The Bookstein plot of operator error (Fig. 2H) shows that the distance between a single drillhole analyzed multiple times is small relative to the distance between drillholes on different shells. Mean drillhole placement is consistently located in the umbonal region of the shell for all samples except the Quality Aggregates locality (Figs. 2, 4A), which shows no overlap of the bootstrap 95% confidence intervals (Fig. 4A).

Figure 4 Sample-level variation in drillhole placement for Lieutenant Run (Lt. Run), Chuckatuck (Chuck), Sandy Hook (SH), Quality Aggregates (QA), APAC, modern laboratory experiments using Euspira (E. lab), and modern laboratory experiments using Neverita (N. lab). A, Mean Bookstein drillhole placement. Error bars represent 95% bootstrap confidence intervals. B, Standard deviation of drillhole placement in terms of Bookstein x- and y- coordinates. Error bars represent 95% bootstrap confidence intervals.
Using only Bookstein coordinates for drill-hole placement, CVA recovered significant differences in squared Mahalanobis distances between sample means for all comparisons to the Quality Aggregates sample as well as between the modern beach sample at Sandy Hook and all samples except for laboratory Euspira (Table 2). Substantial overlap in the distributions of drillholes in both significant and nonsignificant comparisons, resulted in low accuracy of the resulting discriminant criterion; drillholes were incorrectly classified into their original sample 64.7% of the time. The addition of the variables IBD and OBD (Table 2) improved discrimination slightly. The four variable CVA recovered significantly different squared Mahalanobis distances between sample means for all comparisons but one (laboratory Neverita to Lieutenant Run). Observations were incorrectly classified into their original groups 54.0% of the time.
Table 2 Canonical Variate Analysis probability that squared Malhalanobis distance between locality means is equal. Shaded area represents Bookstein x- and y- coordinates for drillhole placement only; unshaded area represents Bookstein x- and y- coordinates for drillhole placement, IBD, and OBD. Significant values bold.

The median Bookstein x- and y- coordinates for the Quality Aggregates and Sandy Hook localities showed the most consistent significant differences in drillhole location. For the Quality Aggregates sample, median drillhole placement along the x-coordinate is significantly different than that of all samples except for lab Neverita and Sandy Hook (Table 3). For all remaining comparisons with the Quality Aggregates locality, median drillhole placement differs significantly (Table 3). This pattern is not associated with differences in shell thickness (the mean and maximum shell thicknesses at the Quality Aggregates site are comparable to the other fossil sites, Fig. 3B) or the presence of extreme Mercenaria spp. shell forms (i.e., no wavy “tridacnoides” forms are present). For the Sandy Hook sample, median drillhole placement along the y-coordinate is not significantly different than that of the Lieutenant Run and APAC localities (Table 3), whereas median drillhole placement along the x-coordinate is significantly different for all comparisons except that of the Quality Aggregates locality (Table 3).
Table 3 Significance values (p) for the non-parametric Mann-Whitney (Wilcoxon) test for equal median drillhole placement among localities: Bookstein x-coordinates shaded; Bookstein y-coordinates unshaded. Significant values bold.

Variation of drillhole placement, measured as standard deviation of drillhole Bookstein coordinates with bootstrap 95% confidence intervals, is significantly lower for naticid-only samples than for naticid+large muricid or naticid+Ecphora samples (Fig. 4B). However, the inclusion of the incomplete edge drill (Fig. 5) made by E. heros in the laboratory setting increases the variation sufficiently to make the laboratory standard deviation plot with the naticid+muricid fossil samples (Fig. 4B), possibly because of the low sample size (n=28) in the laboratory experiment. The increased variation in drillhole placement in the naticid+large muricid and naticid+Ecphora samples is not the result of edge drill-holes (those drilled on the commissure between the valves) but rather increased spread in the placement of wall drillholes (those drilled through the thicker part of the shell, away from the edge) (Fig. 2A–D). Pooled naticid-only samples showed significant clumping of drillholes (R=0.88, Z=−3.19, p=0.001), whereas pooled naticid + muricid samples failed to reject the null hypothesis that the distribution of drillholes is random (R=0.99, Z=−0.21, p=0.83).

Figure 5 Photograph of an incomplete edge drill from the Euspira laboratory experiment. Mercenaria prey specimen is ~69.7 mm antero-posterior width.
Discussion
Laboratory, field, and body fossil data supported both predictions 1 and 2, with samples showing increased variation in predator-prey size ratios and decreased site stereotypy of drillholes where Ecphora, with or without other large muricids, were present. The congruence of multiple types of data suggests it is unlikely that Pliocene Euspira or Neverita were capable of drilling the largest Pliocene Mercenaria. In spite of a laboratory set- up designed to maximize the size of prey consumed, laboratory specimens drilled by the naticids N. duplicata and E. heros did not approach those found drilled in Pliocene samples in terms of A-P width (Fig 3A) or thickness (Fig. 3B). Our experiment showed that relatively large extant Euspira are functionally capable of drilling large Mercenaria prey up to ~90 mm A-P width. Drilled Mercenaria valves collected from a natural setting, the beach at Sandy Hook, were even smaller (Fig. 3A,B). Under natural settings, Euspira do not feed on Mercenaria >50 mm A-P length (MacKenzie Reference MacKenzie1977, Kitchell et al. Reference Kitchell, Boggs, Kitchell and Rice1981, Bricelj Reference Bricelj1992, MacKenzie et al. Reference MacKenzie, Morrison, Taylor, Burrell, Arnold and Wakida-Kusunoki2002, Sandy Hook sample this study), when other prey are present. By restricting our analysis to the largest prey, the list of potential drilling predators was shortened, relative to the number of potential predators of small prey, thus simplifying the analysis. Even so, drillholes could only unambiguously be attributed to Ecphora where prey sizes exceeded naticid capabilities in the less diverse, naticid-only localities from Virginia. Regardless of our inability to attribute every drillhole to its trace maker, this study corroborates previous assertions that Ecphora was an important driller preying on large-bodied Mercenaria (Petuch Reference Petuch1987, Vermeij Reference Vermeij1987, Petuch Reference Petuch1988, Campbell Reference Campbell1993, Vermeij Reference Vermeij1995).
Size Selectivity, Prediction 1
The naticid-only sample from the beach at Sandy Hook showed signs of size selectivity (Table 4, Supplementary Fig. 1A). This positive correlation between predator and prey size is likely the result of limitations on the size of prey imposed on naticid predators by their engulfing and handling behavior. This pattern is consistent with other published results on naticid predation on Mercenaria under experimental conditions (Kitchell et al. Reference Kitchell, Boggs, Kitchell and Rice1981, Kabat Reference Kabat1990, Visaggi et al. Reference Visaggi, Dietl and Kelley2013). As predicted, naticid+Ecphora samples (Lieutenant Run and Chuckatuck) and naticid+large muricid samples (Quality Aggregates and APAC) did not show clear indications of prey size selection (Table 4, Supplementary Fig. 1B–E). We interpret this pattern as a reflection of the addition of muricid predators that have a different relationship between predator size and prey size, which dampens out the signal of naticid size selectivity with increased variation.
Table 4 Regression statistics for outer borehole diameter on antero-posterior prey shell width for each sample locality. Significant values bold.

Drillhole Stereotypy, Prediction 2
As predicted, the modern and experimental naticid-only samples showed stereotyped drill- hole placement in the umbonal region of the shell (Figs. 2 and 4A). This pattern is consistent with the prey-handling behavior of naticids (e.g., manipulating prey into a preferred orientation) and the findings of previous studies on naticid-Mercenaria interaction (Carriker Reference Carriker1951, Boggs et al. Reference Boggs, Rice, Kitchell and Kitchell1984, Visaggi et al. Reference Visaggi, Dietl and Kelley2013). Whereas the addition of Ecphora and other large muricids did not change average (mean or median) hole placement, the predicted increase in variability of drillhole placement of naticid+large muricid (Florida) and naticid+Ecphora (Virginia) samples was shown by the increased standard deviation (Fig. 4B) and lack of significant clumping in naticid+large muricid and naticid+Ecphora samples revealed by the nearest neighbor analysis.
When mean/median drillhole placement was significantly different from the naticid only samples, as in the case of the Quality Aggregates locality, it shifted away from the umbo and towards the center of the valve (Figs. 2 and 4A, Table 3). This shift in mean/median placement away from the umbo could be explained by the presence of relatively more muricid predators, which exhibit a lower degree of or different stereotyped handling behavior (Carriker Reference Carriker1981). As naticids are present in all assemblages, measures of central tendency would only be significantly affected where muricids were present in high relative abundance. The complex nature of the Florida assemblages—the presence of multiple large muricid taxa—prohibits the attribution of this difference in hole placement at the QA locality to the activity of Ecphora alone.
Based on the placement of boreholes drilled in prey too large for naticids (Fig. 2A–D, black dots) a number of drillholes that must have been made by Ecphora or other large muricids fall on the dorsal half of the shell. This pattern is surprising given the reports of other muricids, such as Urosalpinx, preferentially drilling the center of Mercenaria shells (Carriker Reference Carriker1957, Dietl et al. Reference Dietl, Herbert and Vermeij2004). This tendency to drill the umbo and central regions may be related to the differences in relative sizes of predator and prey. Ecphora feeding on large Mercenaria may have been forced to lie on the substrate next to prey in order to drill rather than crawling around on the surface of the prey shell as is common with small muricids (e.g., Urosalpinx and Eupleura) preying on relatively large prey (Carriker Reference Carriker1955, Reference Carriker1981).
Behavioral Assumptions
The assumptions about the nature (behavior) of naticid and muricid predatory interactions with Mercenaria prey that underpin this study vary in degree of empirical support. Prediction 1 relies on the explicit assumption that different drilling predators would have different prey size selectivity patterns. This assumption is uncontroversial because it is widely known from empirical observations that naticid and muricid predators have species-specific size- structured relationships with their prey (Kojumdjeva Reference Kojumdjeva1974, Kitchell et al. Reference Kitchell, Boggs, Kitchell and Rice1981, Kabat Reference Kabat1990, Kelley and Hansen Reference Kelley and Hansen2003). Prediction 2, however, relies on the potentially problematic assumption that family-level differences in drillhole stereotypy are paramount, whereas differences between individual species within a family are less important. Although this assumption is likely sound for the naticids used in this study because they belong to extant, well-studied genera, one cannot be certain whether the drilling behavior of extinct Ecphora was the same as that observed by modern muricids preying on Mercenaria. If this assumption proves to be incorrect, then prediction 2 would be flawed. By using two parallel lines of evidence to test our hypothesis, however, we substantially increased the reliability of our conclusion. Results for prediction 1 do not depend on the validity of the assumption we made for prediction 2. In other words, the independent empirical support for prediction 1 that we found sustains the conclusion that Ecphora was a driller, even if our assumption for prediction 2 does not hold up.
Methods for Determining Predator Identity
Multivariate analysis using drillhole placement (Bookstein x- and y- coordinates) or a combination of drillhole placement and drillhole morphology (IBD and OBD) did not reliably differentiate naticid-only from naticid+muricid samples (Table 2). Poor performance of the discriminant criterion resulting from these analyses (high classification errors) prohibits the use of multivariate methods alone for distinguishing Ecphora and naticid holes in fossil assemblages. The tendency of Ecphora to drill umbonal holes makes it particularly difficult to differentiate Ecphora drillholes from traces of naticid predation in these assemblages. We were not able to employ variation in the shape of drillholes (e.g., IBD: OBD ratio and drillhole angle) (Dietl and Kelley Reference Dietl and Kelley2006, Grey et al. 2005, Kitchell et al. Reference Kitchell, Boggs, Kitchell and Rice1981) or ichnotaxonomy (Bromley Reference Bromley1981) both of which are affected by the thickness of the prey’s shell (Carriker and Yochelson Reference Carriker and Yochelson1968, Kitchell et al. Reference Kitchell, Boggs, Kitchell and Rice1981, Kowalewski Reference Kowalewski1993), because our Mercenaria samples lacked shells of overlapping thicknesses (Fig. 3B). Future work on differences in drillhole morphology that controls for shell thickness is needed to assess the reliability of these methods in identifying the trace maker. In addition, work to determine which aspects of prey morphology, habitat preference, or life habit are most likely to make prey vulnerable to predation by Ecphora, may yield insights useful in distinguishing likely naticid versus muricid prey.
Ecological Importance of Ecphora
The relative ecological importance of Ecphora can be estimated by determining the proportion of shells too large to have been preyed upon by naticids. Depending on the method we used to determine the maximum prey size that can be effectively drilled by naticids, between 85.7% and 92.2% (field-derived estimates) of drilled fossil Mercenaria or between 12.3% and 33.3% (laboratory-derived estimates) of drilled fossil Mercenaria in our samples are too large to be attributed to naticid predators. Using the estimated maximum OBD calculated based on the shell width of the largest complete predator shells available, between 4.8% and 34.2% of drillholes in our samples are too large to have been drilled by the largest Pliocene naticids. In the naticid+large muricid localities from Florida, where Ecphora and other large muricids, such as Phyllonotus, are present, 34.2% (Quality Aggregates) and 34.1% (APAC) of drillholes are too large to have been drilled by the naticids present. These estimates are based in part on the largest naticid specimen from the entire Tamiami Formation. If the largest Buckingham Member naticid was smaller than that reported for the Tamiami Formation, the proportion of drillholes present in the Florida assemblages too large to have been made by naticids would increase. The opposite would be the case if the largest Buckingham Member naticid was larger than that reported for the Tamiami Formation as a whole. In the naticid+Ecphora samples from Virginia, between 29.4% (Chuckatuck) and 4.8% (Lieutenant Run) of drillholes are too large to be attributable to Pliocene naticids. In these samples in particular, large Ecphora—which range between 80 and 100 mm in length, rarely exceeding 150 mm (Campbell Reference Campbell1993)—are the only predators present that were large enough to have attacked the largest prey and drilled the largest holes. In short, Ecphora is likely responsible for an unrecognized large proportion of the drillholes found in the shells of other infaunal prey and conspecifics in Neogene soft-sediment communities of the Atlantic Coastal Plain of the United States.
The absence of evidence indicating the occurrence of large, fossil naticids is not likely to be a taphonomic artifact. Any preservational bias that may have selectively removed shells of large predators would likely have removed both large muricids and large naticids from the record; thus, our ability to recover measurements of large muricids (80–100 mm) indicates that our search protocol would likely have found naticids larger than 50–76 mm if they were present in the stratigraphic units we sampled. Even if there was a preservational bias against large naticid shells, we would still expect to see correspondingly large drillholes left by these predators in their Mercenaria prey. Our fossil samples, however, lack drillholes that are comparable in size (OBD) to the largest holes drilled by laboratory Euspira—only the Chuckatuck range (max.=9.3 mm) overlaps with the laboratory Euspira mean (8.8 mm; Fig. 3C). It is highly unlikely that only prey shells with the largest drillholes, left by the largest naticid predators, would be selectively removed from the record.
In the absence of a taphonomic explanation, the commonness of inferred Ecphora-Mercenaria interactions implies that Ecphora was ecologically important. Calling attention to the prevalence of drillholes attributed to Ecphora creates the opportunity to ask questions that could not be asked before. Did Ecphora, naticids, and shell-chipping busyconine whelks compete for shared prey resources (Dietl and Kelley Reference Dietl and Kelley2004)? How did these competitors influence each other’s evolution? Limited evidence suggests that Ecphora may have restricted the size of co-occurring naticid predators with which it competed for shared prey resources. Once Ecphora became extinct in the late Pliocene, naticids increased in maximum body size (Vermeij Reference Vermeij2012). Perhaps this size increase reflects a competitive release of naticids wherein the absence of Ecphora facilitated expansion of the naticid niche to include the largest size classes of bivalve prey in temperate North American assemblages. After the extinction of Ecphora, however, drillholes in the largest size classes of Mercenaria disappear. In Pleistocene stratigraphic units of the Carolinas and Florida that follow Ecphora’s extinction, the largest size classes of Mercenaria lack drillholes (e.g., Dietl Reference Dietl2003). Perhaps large naticids were functionally incapable of manipulating the largest (and heaviest) Mercenaria, behaviorally avoided them in favor of thinner shelled prey, or faced intense competition from another predator, such as shell-chipping busyconine whelks that evolved the capacity to feed on large Mercenaria prey at this time (Dietl Reference Dietl2003).
The Importance of Predator Identity
Whereas the end result for the prey is the same—death by drilling—behavioral differences between naticid and muricid predators may restrict the type of questions that can be asked in mixed-predator assemblages. When the predictions tested are the same for both groups, interpretations derived from mixing of muricid and naticid traces should be robust. For example, increased shell thickness is likely a general defensive adaptation, increasing prey effectiveness against predation by Ecphora (Vermeij Reference Vermeij1987, p. 311) and also by naticid predators (Kelley Reference Kelley1991) resulting in the same prediction in both cases (prey effectiveness will likely increase with increased shell thickness). Because the addition of muricids does not change the predicted outcome, functional group level investigations into the relationship between drilling predation pressure, prey shell thickness, and prey effectiveness are not necessarily confounded by the presence of different drilling predators. Functional group analysis will, however, preclude the attribution of observed adaptive changes in prey shell thickness to the selective pressure of a specific predator. In addition to situations with unchanged predictions, there is likely a scale of investigation (temporal and spatial) large enough that patterns are robust to the mixing of predator identities, similar to the way some assemblage-level trends have been shown to be robust to taxonomic standardization of prey species (Kelley and Hansen Reference Kelley and Hansen2006). More research will be necessary to determine the taxonomic and/or geographic scale(s) that this robustness to mixing predator identities emerges.
Certain topics are only suitable to situations in which confidence in predator identity is high (i.e., drilling traces can be reliably identified and assigned to a predator) because the predicted outcome differs based on the functional group or family to which the predator belongs (e.g., Paul and Herbert Reference Paul and Herbert2014). For instance, determining the size at which prey attain a size refuge from naticid predation will be complicated by the presence of large muricids; prey shell size increase is predicted in response to naticid predation but not necessarily in response to muricid predation. Thus evaluating the presence of a prey size refuge from a particular predatory group can only be accomplished where predator identity is certain. Lineage-level tests of the escalation hypothesis also require that selective agents be identified and ranked in terms of their effect on a particular species (Vermeij Reference Vermeij1987). The specificity of “selective agent” (a specific taxon vs. functional group) will depend on the generality of the prey defense under investigation. Examining bivalve prey species with multiple predators within the same functional group may complicate the ranking of predatory risks within and across guilds (e.g., drillers, shell crushers) making it more difficult to understand why one evolutionary pathway was taken by the prey over another. For example, we might observe a pattern of prey morphology fluctuating nondirectionally between different character states (e.g., a trait that effectively defends against naticids versus a trait that more effectively defends against Ecphora) and interpret it as a lack of support for escalation when in fact it is a trade-off between responses played out over the limited temporal or geographic scales at which prey are encountering different predators.
These examples are meant to illustrate the range of research topics that are studied in mixed-predator assemblages, which might benefit from a more explicit acknowledgment of predator identity. It is worth noting as well that any topic suitable to situations of low confidence in predator identity is likely also applicable in high-confidence situations. The issue of predator identity does not diminish the utility of drillhole data to investigate ecological or evolutionary questions; rather it acts as a constraint on the research topics available to empirical testing in a particular study system.
Lessons Learned and the Road Ahead
Our analysis of Ecphora predation on Mercenaria provides three key lessons: (1) high confidence in predator identity may be possible for other large prey taxa in the Pliocene of the mid-Atlantic region, where all drilled prey exceeding naticid handling capabilities in size can reasonably be attributed to Ecphora; (2) confidence in predator identity is more difficult to achieve in Pliocene localities in Florida where multiple large muricid predatory taxa are present, precluding identification of the trace maker to the genus level, but allowing for family-level attribution of the predator; and (3) as prey size, and not aspects of drillhole placement or morphology, was the only reliable indicator of predation by Ecphora on Mercenaria, it may not be possible to establish confidence in predatory identity when working with small prey taxa (those within the range of naticid handling capabilities in size). Because these “small” prey fall victim to so many muricid and naticid drilling taxa they make reliable identification of Ecphora as their predator problematic. These lessons provide a roadmap to future research with other prey taxa. To ensure biologically meaningful comparisons are made, researchers should be explicit about their assumptions related to predator identity and mindful of the limitations of drillhole data sets from mixed-predator assemblages.
Conclusion
The muricid gastropod Ecphora has frequently been overlooked as a potential tracemaker in naticid gastropod drilling studies conducted in the Neogene Atlantic Coastal Plain of the United States. Analysis of the differences in the predation signatures of naticid-only versus naticid+Ecphora samples, detected using the relationship of prey size to OBD (a proxy for predator size) and drillhole placement, supports claims that Ecphora was a driller. Prey size selectivity and stereotypy of drillhole placement near the umbo are indicators of naticid predation whereas increased variation in drillhole placement, the presence of numerous drillholes placed away from the shell’s umbo, and a lack of size selectivity are indicators of predation by Ecphora. Despite evidence supporting Ecphora as a drilling predator, we were unable to reliably recognize drillholes made by this predator based solely on drillhole morphology or placement. The inability to distinguish drillholes produced by Ecphora based on drillhole morphology or placement has implications for the types of questions we can reliably answer using drill- hole data. When investigating taxon-specific predictions where Ecphora is present, it may be important to limit the prey taxa, range of prey sizes, and range of prey morphologies examined. When testing taxonomically universal predictions or investigating the direction and magnitude of trends at the largest scales, standardization by predator type may be less critical. Researchers need to understand and address the limitations of data derived from mixed-predator assemblages along the Neogene Atlantic Coastal Plain of the United States.
Acknowledgments
Thanks to D. E. G. Briggs, J. Sawyer, E. Saupe, S. Dietl, J. Smith, S. Durham, R. Portell, E. Petuch, and reviewers G. Vermeij and C. Visaggi.
Supplementary Material
Supplemental materials deposited at Dryad: doi: 10.5061/dryad.m8413











