Introduction
Horizontal gene transfer (HGT) involves the asexual movement of genetic material between disparate organisms and, in the process, the acquisition of traits typically absent from the inheriting organism. HGT has played a prominent role in the evolution of bacteria (Polz et al., Reference Polz, Alm and Hanage2013). Estimates as high as 81% have been proposed as the average number of prokaryotic genes involved at some point in HGT (Dagan et al., Reference Dagan, Artzy-Randrup and Martin2008). However, the importance of HGT in the evolution of eukaryotes remains in flux. As early as 1998, Keen and Roberts (Reference Keen and Roberts1998) hypothesized a relationship between the ability of nematodes to parasitize plants and the acquisition of genes from soil bacteria to facilitate that process. With the advance of genomics studies, HGT has been better implicated in the evolution of parasitism in plant nematodes (Mitreva et al., Reference Mitreva, Smant and Helder2009; Haegeman et al., Reference Haegeman, Jones and Danchin2011). Since that time, the database of putatively transferred genes in plant and free-living nematodes has increased substantially.
Habitat and opportunity have profound impacts on the potential for HGT, which is well documented in endosymbiotic relationships. However, HGT also occurs when there is a simple facultative, environmental association. Genome analysis of the necromenic nematode Pristionchus pacificus revealed functional cellulase genes of bacterial origin (Dieterich et al., Reference Dieterich, Clifton, Schuster, Chinwalla, Delehaunty, Dinkelacker, Fulton, Fulton, Godfrey, Minx, Mitreva, Roeseler, Tian, Witte, Yang, Wilson and Sommer2008). Further, Rödelsperger and Sommer (Reference Rödelsperger and Sommer2011) demonstrated that P. pacificus diapausin genes, typically associated with insects, were likely acquired via HGT from its necromenic associations with beetles. Consequently, evidence is mounting that HGT is not only common in the evolution of bacteria and in gene transmission between prokaryotes and eukaryotes, but also in the movement of functional genes between higher order organisms (Andersson, Reference Andersson2005; Richards et al., Reference Richards, Dacks, Jenkinson, Thornton and Talbot2006; Keeling and Palmer, Reference Keeling and Palmer2008).
Cyanase (cyanate hydratase; cyanate hydrolase; cyanate lyase) is an enzyme that catalyses bicarbonate-dependent degradation of cyanate to ammonia and carbon dioxide. Enzyme functionality is predicated on the formation of dimers which assemble into decamers; however, the active site is produced from residues of four adjacent subunits within the homodecamer (Walsh et al., Reference Walsh, Otwinowski, Perrakis, Anderson and Joachimiak Andrzej2000). Given that its presence is well disseminated among the ancestral lineages in the tree of life, it has been postulated that cyanase played a role in early evolution and the detoxification of environmental as well as metabolically generated cyanate (Ebbs, Reference Ebbs2004). In marine cyanobacteria, cyanase was originally thought only to detoxify cyanate produced from intracellular urea and/or carbamoyl phosphate (CP) decomposition; however, in more recent studies (Espie et al., Reference Espie, Jalali, Tong, Zacal and So2007; Maeda and Omata, Reference Maeda and Omata2009; Kamennaya and Post, Reference Kamennaya and Post2013), cyanase was found to be equally important in the generation of nitrogen from externally acquired cyanate for the growth and development of cyanobacteria.
In a recent study (Zarlenga et al., Reference Zarlenga, Wang and Mitreva2016), we used comparative genomics to investigate parasitism and adaptation within the clade I parasite Trichinella spiralis and identified a putative cyanase gene. Organisms of the genus Trichinella complete their life cycles within a single host and therefore have no free-living stages. Historically, cyanase has been linked only to organisms of plant, bacterial and fungal origins. However, recently, it has been found in insects (Wybouw et al., Reference Wybouw, Balabanidou, Ballhorn, Dermauw, Grbić, Vontas and Van Leeuwen2012, Reference Wybouw, Dermauw, Tirry, Stevens, Grbić, Feyereisen and Van Leeuwen2014) and nematodes (Opperman et al., Reference Opperman, Bird, Williamson, Rokhsar, Burke, Cohn, Cromer, Diener, Gajan, Graham, Houfek, Liu, Mitros, Schaff, Schaffer, Scholl, Sosinski, Thomas and Windham2008; Haegeman et al., Reference Haegeman, Jones and Danchin2011; Zarlenga et al., Reference Zarlenga, Wang and Mitreva2016), though its activity in nematodes has not been validated. Herein, we tested and validated functional activity of the cyanase gene present in T. spiralis. We further hypothesize independent origins for the cyanase found in clade I nematodes of the genera Trichinella, Trichuris and Soboliphyme relative to the cyanase found in other nematode groups. Results are discussed in the context of HGT, parasitism and functional repurposing of acquired genes.
Materials and methods
Parasite propagation and isolation
Swiss-Webster mice were infected orally with 500 muscle larvae (ML) of T. spiralis (ISS 4). Approximately 30 days post infection, ML were isolated from pepsin:HCl digestions of eviscerated carcasses as described previously (Zarlenga et al., Reference Zarlenga, Boyd, Lichtenfels, Hill and Gamble2002). Crude worm extract (CWE) was produced by homogenizing ML in phosphate-buffered saline (PBS), followed by centrifugation to remove debris. Adult worms and newborn larvae (NBL) were isolated essentially as described by Marti et al. (Reference Marti, Murrell and Gamble1987). Adult worms and NBL were each deemed >90% pure by microscopic examination. All animals were utilized and treated in accordance with an Animal Use Protocol (#15-003) approved through the Agricultural Research Service.
RNA isolation, cloning and PCR
Approximately 300 µl of settled ML (350 000 ML) were used for the purification of total RNA by proteinase K: SDS digestion, organic extraction and ethanol precipitation (Dame et al., Reference Dame, Murrell, Worley and Schad1987). Total RNA was isolated from NBL using Trizol Reagent (Invitrogen, Thermo Fisher, Carlsbad, CA, USA) (Zarlenga et al., Reference Zarlenga, Boyd, Lichtenfels, Hill and Gamble2002). RNA from adult worms remaining after culture and substantially devoid of NBL were also isolated by homogenizing in Trizol reagent. All RNA samples were treated with DNAse, followed by organic extraction and ethanol precipitation prior to cDNA synthesis.
Complementary DNA was synthesized at 42 °C using Superscript II reverse transcriptase (Invitrogen, Thermo Fisher), oligo dT primer and 5 µg of isolated RNA in a volume of 40 µl. The resultant cDNA was enzymatically amplified (94 °C 30 s; 60 °C 30 s; 72 °C 2 min; ×35 cycles) using ExTAQ DNA polymerase and cyanase-specific forward (#1440)
(5′-aaggatccgatgtcggtggtgtttagatt) and reverse (#1441) (5′-aaggtcgacctaatccagctgggttggt) primers complementary to the 5′ and 3′ ends of the gene, respectively. The primers contained Bam HI and Sal I restriction sites, respectively (underlined) for subsequent cloning for protein expression. The resultant product was cloned into the TA cloning vector TOPO Cr2.1 (Invitrogen, Thermo Fisher), according to the manufacturer's instructions, and used to transform Escherichia coli cells (DH5α strain). Clones were validated by colony PCR (Nishikawa et al., Reference Nishikawa, Fowlkes and Kay1989) and five were chosen for plasmid isolation and DNA sequencing. At least one clone, Tscyn-1(c13), exhibited 100% identity with the genomic DNA-derived sequence and was used for cloning and protein expression.
Stage-specific transcription was evaluated using forward (#1454) (5′-tagcagctacgttgaagcagcttg) and reverse (#1455) (5′-cattcgaatgacaacgcgtcgttct) cyanase-specific primers under the same conditions as described for cDNA amplification, except that random hexamers were used for cDNA synthesis. The primers targeted a 244 bp fragment within the full-length gene. Small subunit rRNA (cDNA) was amplified as a housekeeping gene using forward (#46) (5′-gctgaaacttaaaggaattgac) and reverse (#50) (5′-tcagtgtagcgcgcgtgc) primers. Amplifications were terminated within the linear portion of the amplification curve at 26 and 28 cycles (cyanase) and at 15 cycles (rRNA). Contamination of total RNA with parasite genomic DNA was evaluated by amplifying total RNA prior to reverse transcription with primers #46 and #50.
Bacterial expression and purification of cyanase
DNA constructs were made to generate His-tagged fusion proteins in two different expression vectors; pSUMO (LifeSensors) for high yield and antibody production, and pET28a (Novagen) for functional assays. To produce sufficient protein for antibody production, plasmid Tscyn-1(c13) was restriction enzyme-digested then cloned into the Bam HI/Sal I site of the pSUMO expression vector. One clone, Tscyn-1(c33A), was selected and validated by colony screening (Nishikawa et al., Reference Nishikawa, Fowlkes and Kay1989) and DNA sequencing. The expressed product [rCYN-1(c33A)] was present only within inclusion bodies and was not biologically functional. Consequently, a second expression construct was made in the Bam HI/Sal I site of vector pET28a using primers #1452 (5′-aatgggtcgcggatcctgtcggtggtgtttagat), #1453 (5′-ccgcaagcttgtcgacctaatccagctgggttggt) and the In-Fusion HD Cloning Kit as recommended by the manufacturer (Clontech); the underlined bases in primers 1452 and 1453 indicate Bam H1 and Sal I restriction sites, respectively. The resultant construct was transformed into E. coli ‘Stellar cells’ (Clontech). One clone, Tscyn-1(c94.1), was selected by colony screening (Nishikawa et al., Reference Nishikawa, Fowlkes and Kay1989) and exhibited 100% DNA sequence identity to the parent sequence.
For protein expression, purified plasmid preparations of Tscyn-1(c33A) and Tscyn-1(c94.1) were each transformed into BL21 DE3 cells (Invitrogen, Thermo Fisher). Overnight starter cultures were grown either in 100 µg/mL ampicillin [Tscyn-1(c33A)] or 50 µg/mL kanamycin [Tscyn-1(c94.1)] and then transferred to 500 mL of LB containing the appropriate antibiotic. The cells were grown to OD600 = 0.6, induced in 0.3 mm isopropyl β-D-1-thiogalactopyranoside for 5 h, and then centrifuged and treated for 20 min at 25 °C in 25 mL of 100 mm potassium phosphate buffer pH 7.0, containing 1 mg/mL lysozyme. The cells were then frozen overnight at −20 °C.
The next day, both sets of cells were sonicated 3 × 1 min (22 Watts) and then centrifuged (25 000 × g). To purify recombinant CYN-1(c33A) [rCYN-1(c33A)], the pellet was washed three times for 1 h with 2% Triton X-100 in 100 mm potassium phosphate buffer, pH 7.2 (wash buffer), 1× in 2 M urea + wash buffer and one time in wash buffer. The cleaned inclusion bodies were solubilized in 8 mL 6 M guanidinium HCl overnight with rocking. The liquid was centrifuged and the cleared supernatant was mixed with 8 mL of a solution containing 20 mm β-mercaptoethanol, 500 mm L-arginine and 500 mm L-glutamine. The cleared lysate was incubated with 1 mL (settled volume) of Ni-NTA agarose (Macherey-Nagel) for 4 h with agitation, loaded on to a column and washed with 300 mm potassium phosphate containing 20 mm histidine. Bound protein was eluted with 400 mm histidine containing 0.3% sodium lauryl sarcosine (SLS) in wash buffer. The final product was dialysed against 50 mm potassium phosphate containing 0.2% SLS. For purifying rCYN-1(c94.1), the cleared lysate from sonicated bacteria was loaded directly onto a Ni-NTA agarose (Macherey-Nagel) column, washed with 300 mm potassium phosphate buffer, pH 7.2, and then eluted with 400 mm histidine in 300 mm potassium phosphate and 0.3% SLS. The final product was dialysed overnight against 50 mm potassium phosphate, and then used for activity analyses. Protein concentrations were determined using a BCA Protein Assay kit (Pierce/Thermo Fisher).
In vitro cyanase assay
Purified rCYN-1(c94.1) was assayed using sodium salicylate (Sigma), sodium nitroferricyanide (Sigma) and hypochlorite in a colorimetric assay for ammonia. A 100 µl mixture of 0.8 µg of rCYN-1(c94.1), potassium cyanate (2 mm) and bicarbonate (3 mm) in 50 mm potassium phosphate, pH 7.4, was incubated for 30 min at 42 °C. After incubation, the reaction was supplemented with 50 µl of hypochlorite solution (0.83% sodium hypochlorite, 0.13 M NaOH) and 50 µl of catalyst solution [0.6 M sodium salicylate, 1.34 mm sodium nitroferricyanide (III), 0.13 M NaOH], then incubated an additional 30 min at 42 °C; colour change was monitored at OD660. Since cyanases are present in most bacterial strains, a negative control-protein preparation was generated from column purified, bacterial lysates derived from an empty vector (pET28a). Enzyme activity was assayed in the presence of increasing concentrations of enzyme, increasing concentrations of sodium chloride (0–600 mm) and following heat inactivation (10 min 95 °C). All reactions were performed in quadruplicate. An ammonia standard was used as a reference.
Western blots and immunohistochemical staining
Mouse antibodies to purified rCYN-1(c33A) were produced as previously described (Zarlenga et al., Reference Zarlenga, Nisbet, Gasbarre and Garrett2011) with the addition of Emulsigen (MVP technologies) to the antigen/adjuvant mix to a final ratio of 20% (v/v). Recombinant proteins and CWE were separated on 8–16% SDS PAGE gels (Genscript), electrophoretically transferred to immobilon membranes and then blocked for 2 h in PBS containing 0.1% Tween-20 and 5% non-fat dried milk (PBST). Blots were incubated overnight (16 h) in primary mouse antibody diluted 1:200 in PBST, washed the next day and then incubated for 2 h in secondary antibody [affinity-purified goat anti-mouse IgG-horseradish peroxidase (HRP)] diluted 1:1000 in PBST. After washing in PBST, blots were stained in 4 chloro-1-naphthol/H2O2 solution and photographed.
For immunohistochemical staining, ML were fixed in 10% neutral formalin for 24 h, embedded in paraffin and sectioned at 5 µm (HistoServ Inc., Germantown, MD, USA). Sections were deparaffinized, quenched with 3% H2O2 and rehydrated. Antigen-retrieval was conducted by pepsin (0.4%)-HCl (0.01N) digestion at 37 °C for 15 min. The slides were washed twice with 0.75% Brij 35 in PBS (BRIJ-PBS), blocked with 0.5% sodium caseinate in BRIJ-PBS for 10 min, and then incubated for 30 min at room temperature (24 °C) with mouse anti-rCYN-1(c33A) serum (1:800) or with negative control mouse anti-rOos-APY-1 (1:600) (Zarlenga et al., Reference Zarlenga, Nisbet, Gasbarre and Garrett2011). After washing, goat anti-mouse IgG-HRP (Dako EnVision + System-HRP Labelled Polymer, Agilent) and Dako AEC substrate chromogen (Agilent) were used to detect antibody binding. Tissues were counterstained with haematoxylin, and micrographs were taken using a Zeiss Axioskope 2 Plus microscope and AxioVision software.
Phylogenetic analysis
Cyanase encoding sequences from 87 representatives of three Kingdoms, Animals (Nematoda), Plants and Eubacteria, were acquired from GenBank (https://www.ncbi.nlm.nih.gov) by independent searches (Supplementary Table S1). Full-length or near full-length amino acid sequences of individual proteins from all available nematodes were selected. A subset of plant and bacteria cyanases was chosen based on the completeness of the sequences and attaining a diverse representation of taxonomic subunits and constrained by best amino acid sequence similarity (BLASTp) matches to nematode queries within each Kingdom. Four of 37 nematode sequences that lacked canonical active site amino acids were not included in the alignment. Protein sequences were aligned using MAFFT, with default parameters as implemented in Geneious v.10.2.3 (BioMatters, Ltd, New Zealand). Following alignment, gaps were removed, leaving 135 homologous amino acids for tree-building. Maximum likelihood trees were built from alignments using PhyML (Guindon et al., Reference Guindon, Dufayard, Lefort, Anisimova, Hordijk and Gascuel2010). Trees were rooted using the bacterial sequence from Synechococcus, which was closest to the mid-point root of the tree. Of note, all sequences were derived from parasite genomes/transcriptomes. Among the filariods it is not known if these sequences were derived by HGT from Wolbachia endosymbionts or other bacterial sources.
Results
A gene encoding cyanase in the genus Trichinella was identified in a comparative genome study of T. spiralis with clade III, IV and V parasitic and free-living nematodes (Zarlenga et al., Reference Zarlenga, Wang and Mitreva2016). Phylogenetic analysis of amino acid sequences from currently available nematode cyanases, in conjunction with representative members of the Kingdoms Eubacteria, Plantae and Fungi, is presented in Fig. 1 (non-rooted tree) and Supplementary Fig. S1 (rooted tree). Cyanases from the clade I nematodes (Blaxter, Reference Blaxter2011; Blaxter et al., Reference Blaxter, Koutsovoulos, Jones, Kumar, Elsworth, Cotton, Hughes and Olson2014) Trichinella spp., Trichuris spp. and Soboliphyme baturini form a well-supported subclade with plant cyanases that is monophyletic, whereas all other nematode cyanases are in a clade with bacterial cyanases. A paraphyletic sister clade for basal nematodes and plant cyanases is comprised solely of fungal cyanases, which, in turn, is most closely related to cyanases from cyanobacteria. Tree topology was robust to amino acid substitution model (Dayhoff, JTT or BLOSUM62).

Fig. 1. Unrooted maximum likelihood tree (ln = −8051.23052) of cyanase from parasitic nematodes, bacteria, fungi and plants. Cyanase from clade I parasitic nematodes form a monophyletic clade sister to plant cyanases and more closely related to fungal cyanases than to bacterial cyanases. Cyanases from clade III and IV parasitic nematodes cluster with bacterial cyanases. Node support is indicated by the colour within the circle at each node, with red indicating strong support and purple weak support.
Aligning amino acid sequences within the putative active site revealed that the T. spiralis cyanase harboured all the key amino acids characteristic of a functional protein (Supplementary Fig. S2). To ascertain whether the DNA encodes an operative protein or represents a non-functional pseudogene, the full-length transcript was cloned, expressed and assayed for activity. The purified recombinant protein was found to be biochemically active, generating ammonia in the presence of potassium cyanate and bicarbonate in a dose-dependent manner (Fig. 2A). The activity of the recombinant protein was substantially reduced when incubated with increasing concentrations of NaCl (Fig. 2C), a known inhibitor of cyanases (Anderson and Little, Reference Anderson and Little1986), or when heated to 95 °C prior to activity testing (Fig. 2B). Cyanase activity was not observed when a sham protein prepared from an empty vector was assayed in a similar manner (Fig. 2A). Comparative PCR revealed that the cyanase mRNA was produced in all developmental stages studied; mRNA transcripts were dominant in the NBL and ML stages and least abundant in the adult stage (Fig. 3).

Fig. 2. Activity of T. spiralis recombinant cyanase. Affinity purified rCYN-1(c94.1) was used to assay; (A) cyanase activity (●) relative to affinity purified negative control antigen (■); (B) change in cyanase activity relative to NaCl concentration (0–600 mm); and (C) change in cyanase activity prior to and following heat inactivation (10 min 95 °C). The control is the reaction in the absence of recombinant protein.

Fig. 3. Stage-specific transcription of the T. spiralis cyanase gene. Random primer-derived cDNA from three developmental stages of T. spiralis (ML, NBL and adult) was amplified with cyanase-specific and 18S rRNA-specific primers at the defined cycle numbers. Amplification of rRNA within a total RNA preparation prior to reverse transcription was used as a control for gDNA contamination. Fragments were separated by agarose gel electrophoresis and stained with ethidium bromide. Cycle numbers were experimentally determined to be within the linear portion of the amplification curve. NBL = newborn larvae; ML = muscle larvae; Ad = adult worms.
Western blots (Fig. 4A) screened with mouse anti-rCYN-1(c33A) exhibited positive interactions with both the CYN-1(c94.1) (lane 1) and CYN-1(c33A) (lane 3), but showed no reactivity with protein isolated from cells harbouring the empty vector (lane 2). Lanes containing CWEs displayed multiple bands migrating at apparent molecular masses of 27, 50, 100 and 250 kDa. Bands migrating at 27 and 250 kDa were noticeably weaker relative to those migrating at 50 and 100 kDa. The functional protein expressed in the pET28a vector [CYN-1(c94.1)] appeared as a singlet migrating at 25 kDa, whereas the protein derived from inclusion bodies and expressed as a sumo-fusion protein [rCYN-1(c33A)] exhibited multiple immunoreactive bands migrating at 37 and 90 kDa, though only a singlet was observed in gels stained with Coomassie blue (Fig. 4B). The molecular mass calculated from the amino acid sequences including vector-derived sequences was 26 kDa (pET28a) and 33 kDa (pSUMO). No classic secretory signal was identified. Immunohistochemical staining of fixed ML tissue sections revealed that antibody binding at 1:800 dilution interacted strongly with the ML hypodermis beneath the cuticle and to a lesser extent with the muscle that lines the hypodermis and cell nuclei within the stichosome (Fig. 5A). No binding was observed to primordial reproductive cells or to tissues of the digestive system (Fig. 5B).

Fig. 4. Western blot of T. spiralis cyanase. Affinity-purified rCYN-1(c94.1), rCYN-1(c33A) and ML-CWE were separated on 8–16% denaturing polyacrylamide gels and either blotted with mouse rCYN-1(c33.A) antisera (gel A) or stained with Coomassie Blue (gel B). Pre-stained molecular weight standards (BioRad) were used as reference.
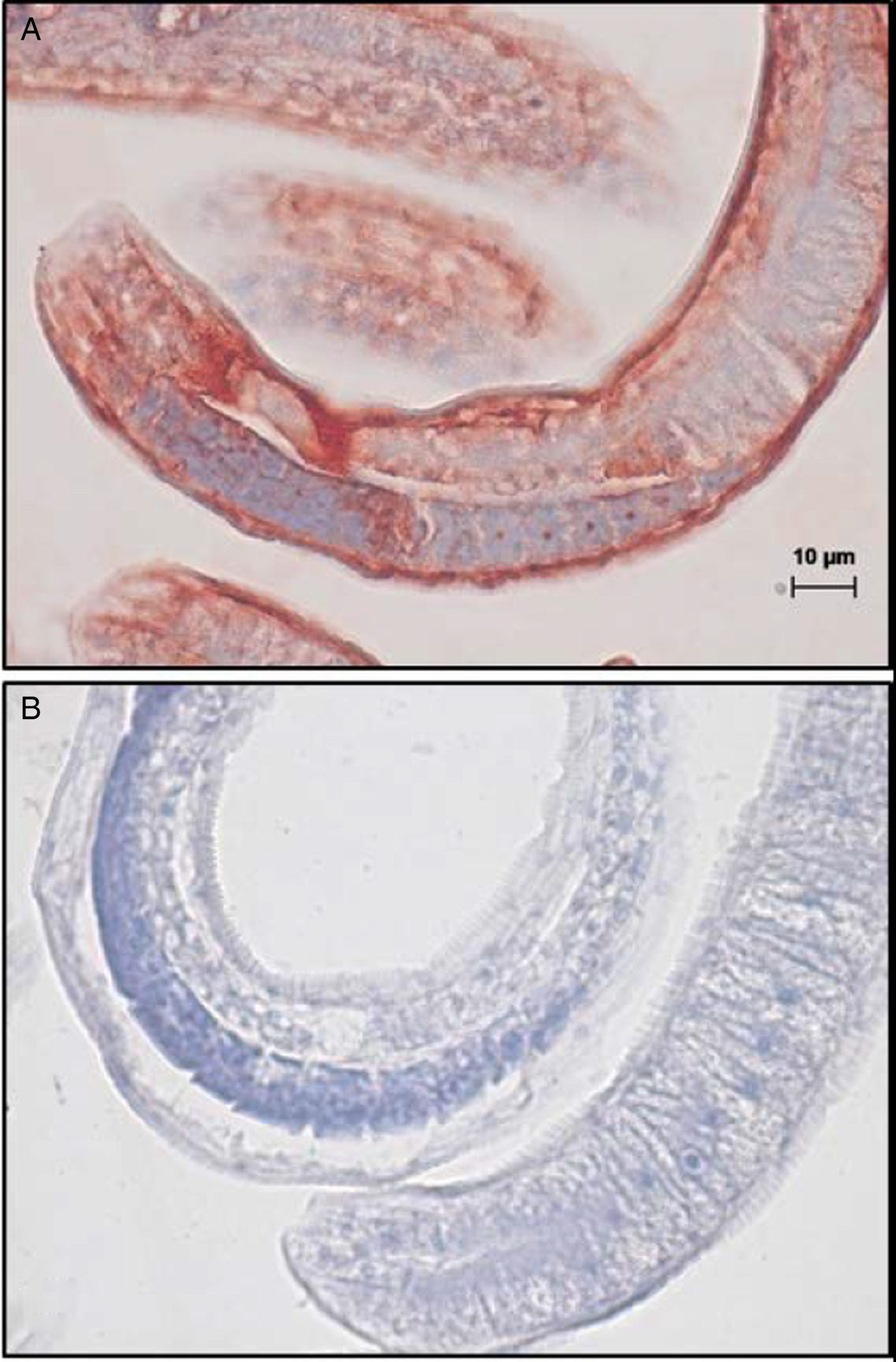
Fig. 5. Immunohistochemical localization of native cyanase in formalin-fixed, paraffin-embedded cross-sections of T. spiralis ML. Tissue sections were incubated with (A) mouse anti-rCYN-1 serum (1:800) or (B) a control mouse anti-recombinant Ostertagia apyrase serum (1:600) similarly produced. Antibody binding (red) was visualized by incubation with horseradish peroxidase-labelled anti-mouse IgG followed by Dako AEC substrate. Haematoxylin was used as counter-stain (blue). A scale bar of 10 µm is shown.
Discussion
Parasitic nematodes have undergone reductive genome evolution. This has been guided by adaptation to well-defined life cycles, homeostatic buffering of the host and constraints in host range. This phenomenon is no better exemplified than in the genus Trichinella which is characterized by high gene loss (Wang et al., Reference Wang, Zarlenga, Martin, Abubucker and Mitreva2012; Zarlenga et al., Reference Zarlenga, Wang and Mitreva2016). Similarities in gene set enrichment and duplications in remaining protein families suggest some overlap in the process of functional adaptation among parasites. However, the number of common protein families in disparate parasitic nematodes is limited and, thereby, consistent with an independent acquisition of parasitism. In the diversification of the genus Trichinella, at least 15 protein domains were born, 13 of which have been annotated to bacterial, viral or fungal origins (Wang et al., Reference Wang, Zarlenga, Martin, Abubucker and Mitreva2012). This information suggests that HGT played prominently in the evolutionary process.
Four characters have been proposed as key determinants when validating HGT: (1) extensive phylogenetic analyses; (2) distribution patterns in closely related organisms; (3) physical associations among putative donor and recipient organisms (Andersson, Reference Andersson2005); and (4) longevity and integration of the gene product into the biology of the recipient (Blaxter, Reference Blaxter2007). Through evolutionary genome-guided analyses, we previously identified a putative cyanase gene within the parasitic nematode T. spiralis (see Zarlenga et al., Reference Zarlenga, Wang and Mitreva2016). Given the gene was expressed most highly in a life stage within host striated muscle cells, the probability of this gene having been derived from contaminating plant or bacterial DNA is minimal. Although a physical association between the lineage giving rise to Trichinella and plants cannot be confirmed from extant organisms, we find no other plausible explanation for the presence of cyanase in Trichinella other than through HGT.
Phylogenetic analyses showed that the cyanase in Trichinella clustered more closely with those derived from plants than with those from bacteria. Monophyly of the Trichinella cyanase with plant cyanases had strong support based on phylogenetic analysis of full-length amino acid sequence datasets, and formed a subclade with other basal clade I nematodes that was sister to the larger plant-based group. This finding contrasts with prior work that holistically linked nematode cyanases to transient bacterial ancestry or to bacterial contamination (Gan et al., Reference Gan, Chua, Chua, Liu, Hii, Chong and Tan2002; Elleuche and Pöggeler, Reference Elleuche and Pöggeler2008; Schlachter et al., Reference Schlachter, Klapper, Wybouw, Radford, Van Leeuwen, Grbic and Chruszcz2017). The placement of Trichinella spp., Trichuris spp. and S. baturini within a subclade exclusive of plants suggests that the cyanase was acquired once by an ancient common ancestor of all three and passed down to extant taxa. The most recent common ancestor of these genera lived more than 400 million years ago (MYA) (McGill et al., Reference McGill, Fitzpatrick, Pisani and Burnell2017). In contrast, a broader examination of the Phylum Nematoda showed that cyanases among the Secernentea including filarioids, ascaridoids and strongyloids, formed a large monophyletic subclade within the bacterial cyanases. No homologues of cyanase have been identified in the entomopathogenic nematode Romanomermis culicivorax, the only other Adenophorea whose genome has been sequenced. Further, cyanase has neither been observed in free-living nematodes nor in the most recently diverged crown nematodes (clade V). A functional cyanase homologue has been identified as a product of HGT in numerous plant ectoparasites, such as the spider mite, Tetranychus urticae, for which data also support acquisition from plants (Wybouw et al., Reference Wybouw, Balabanidou, Ballhorn, Dermauw, Grbić, Vontas and Van Leeuwen2012, Reference Wybouw, Van Leeuwen and Dermauw2018).
Within the concept of macroevolution, it has been proposed that ‘pre-adaptation’ (Bock, Reference Bock1959) is an early step in attaining or developing new structures in an organism. As this concept relates to parasitism, this proposal broadly correlates with the transition of an organism to a new environment or ecotropism. Dieterich and Sommer (Reference Dieterich and Sommer2009) hypothesized that the acquisition of detoxifying enzymes must occur during this pre-adaptation phase when a free-living organism transitions to a host environment. The authors cited, as an example, demonstrable increases in numerous detoxifying enzymes in the ‘intermediate’ necromenic nematode, P. pacificus, as precursors to a parasitic life style when compared with the free-living nematode, Caenorhabditis elegans. Evidence has also been presented that P. pacificus likely acquired insect-specific diapausin genes via HGT from its associations with the scarab beetle (Rödelsperger and Sommer, Reference Rödelsperger and Sommer2011). This evidence may be directly relevant to the presence of cyanase in Trichinella; however, whether the cyanase of Trichinella currently functions in this capacity, i.e. detoxification, is not yet known. Its unconventional and putative non-bacterial source raised reasonable doubt as to the activity of the cyanase gene of Trichinella and favoured that of an archaic pseudogene. To this end, a recombinant protein was generated from the cloned gene that was bioactive, heat-sensitive and predictably vulnerable to anion inhibitory effects. No activity was observed in the protein column purified from bacteria harbouring an empty vector, indicating that the observed activity was not of bacterial origin. Immunohistochemical staining of ML localized the protein to the epidermis and, to a lesser extent, to the proximal musculature and stichocyte nuclei. This location is consistent with the detoxification of cyanate or metabolites that may permeate the cuticle. However, active gene transcription was observed in all stages of development, including the NBL and ML, which exist within sterile environments, namely circulating blood and muscle cells, respectively. Thus, assuming that transcription equates to the translation of an active protein, functionality in extant organisms is not likely related to detoxification of externally acquired substrate but either to detoxification of internally generated products or to the parasites own metabolic needs. Reduced transcription in fully developed adult worms is consistent with this interpretation. Crisp et al. (Reference Crisp, Boschetti, Perry, Tunnacliffe and Micklem2015) discovered that most HGT genes expressed in animals are metabolically related and, during evolution, have likely contributed to a biochemical diversification of the organism.
The decomposition of cyanate by cyanase has been shown important for producing NH3 as an alternative nitrogen source (Kunz and Nagappan, Reference Kunz and Nagappan1989; Anderson et al., Reference Anderson, Sung and Fuchs1990) and CO2 for carbon utilization in photosynthetic cyanobacteria (Espie et al., Reference Espie, Jalali, Tong, Zacal and So2007). Given its life cycle, host niche and the ubiquitous transcription profile of Trichinella cyanase, cyanate degradation in extant Trichinella may be linked to pyrimidine or arginine biosynthesis and control of CP-derived cyanate production rather than the detoxification of host or environmentally acquired cyanate. Unlike most organisms, members of the genus Trichinella lack the prototypical CAD protein [CP synthetase 2 (CPS-2), aspartate transcarbamylase and dihydroorotase] that encodes the activities required to initiate pyrimidine biosynthesis. Absence of the CAD protein was also observed in most clade 8 (Spirurina; as defined by Holterman et al., Reference Holterman, van der Wurff, van den Elsen, van Megen, Bongers, Holovachov, Bakker and Helder2006) nematodes that harbour a cyanase gene (Supplementary Table S2). It is possible that the cyanase in these organisms is linked to unutilized substrate in pyrimidine biosynthesis.
Under physiological pH, cyanate is generated as both the primary decomposition product of CP (Guilloton and Karst, Reference Guilloton and Karst1987) and from the dissociation of urea. In the Trichinella genome (Mitreva et al., Reference Mitreva, Jasmer, Zarlenga, Wang, Abubucker, Martin, Taylor, Yin, Fulton, Minx, Yang, Warren, Fulton, Bhonagiri, Zhang, Hallsworth-Pepin, Clifton, McCarter, Appleton, Mardis and Wilson2011), we identified a gene corresponding only to the CPS-2 large subunit; the small subunit of CPS-2 that hydrolyses glutamine neither appeared in the genome nor did separate enzymes encoding aspartate transcarbamylase or dihydroorotase. It is possible that CP may be generated other than by the reciprocal linkage between the activities on the small and large subunits of the CPS protein from other hydrolytic activity capable of generating glutamate and free ammonia (Hewagama et al., Reference Hewagama, Guy, Vickrey and Evans1999). Precedence exists in nature for the existence of pyrimidine autotrophs primarily in protozoans (el Kouni, Reference el Kouni2017). Further, Entamoeba histolytica is capable of de novo pyrimidine synthesis in the absence of the archetypical biosynthetic pathway, even though none of the enzymes required for pyrimidine biosynthesis can be found within the genome (Anderson and Loftus, Reference Anderson and Loftus2005). A pathway for non-canonical pyrimidine biosynthesis in E. histolytica has not yet been characterized; however, it has been speculated that the pyrimidine degradation pathway might contribute to biosynthesis. Similar findings have been observed in cestodes and filaroids. Other possibilities are salvage pathways used by the parasite to acquire needed pyrimidines from the host. This is particularly interesting, given that parasites such as cestodes, filarids and, of course, Trichinella spp., Trichuris spp. and Soboliphyme spp. all have tissue-dwelling stages during development.
In aqueous solution, urea decomposes to ammonium ions and cyanate which further converts to CO2 and ammonia naturally or through the action of cyanase; however, the urea cycle is absent from most nematode species including Trichinella. Major constituents of Trichinella excretory/secretory products are ammonia and aliphatic amines in the form of methyl-, ethyl-, propyl-, butyl-, amyl- and heptylamine (Haskins and Weinstein, Reference Haskins and Weinstein1957; Gilbert et al., Reference Gilbert, Castro, Ferguson and Gorden1973). Ethylenediamine, cadaverine, ethanolamine and 1-amino-2-propanol are also by-products of Trichinella metabolism. The source of these amine products is not known; however, the ammonia likely originates in part, from the failed initial step in the mitochondrial-derived urea cycle because of the absence of CPS-1. It is also possible that cyanate and therefore ammonia are generated from host-acquired CP needed for pyrimidine and arginine biosynthesis via cyanase.
The acquisition of cyanase in clade I nematodes was probably associated with ancestral feeding habits. Trichinella and Trichuris are members of the order Trichinellida, and Soboliphyme belongs to the order Dioctophymatida. If acquired from a single HGT event, this would have occurred before the split, which is estimated to have transpired 400–470 MYA (McGill et al., Reference McGill, Fitzpatrick, Pisani and Burnell2017). In like manner, HGT of the plant-derived spider mite cyanase genes has been projected to have occurred before or shortly after the formation of the Acariformes which was >435 MYA (Dabert et al., Reference Dabert, Witalinski, Kazmierski, Olszanowski and Dabert2010). These timeframes approximate the period when aquatic plants diverged to embryophytes (land plants) (Sanderson et al., Reference Sanderson, Thorne, Wikstrom and Bremer2004; Rensing et al., Reference Rensing, Lang, Zimmer, Terry, Salamov, Shapiro, Nishiyama, Perroud, Lindquist, Kamisugi, Tanahashi, Sakakibara, Fujita, Oishi, Shin-I, Kuroki, Toyoda, Suzuki, Hashimoto, Yamaguchi, Sugano, Kohara, Fujiyama, Anterola, Aoki, Ashton, Barbazuk, Barker, Bennetzen, Blankenship, Cho, Dutcher, Estelle, Fawcett, Gundlach, Hanada, Heyl, Hicks, Hughes, Lohr, Mayer, Melkozernov, Murata, Nelson, Pils, Prigge, Reiss, Renner, Rombauts, Rushton, Sanderfoot, Schween, Shiu, Stueber, Theodoulou, Tu, Van de Peer, Verrier, Waters, Wood, Yang, Cove, Cuming, Hasebe, Lucas, Mishler, Reski, Grigoriev, Quatrano and Boore2008; Morris et al., Reference Morris, Puttick, Clark, Edwards, Kenrick, Pressel, Wellman, Yang, Schneider and Donoghue2018).
Within the clade I ancestry, current information does not differentiate between HGT followed by massive gene loss, or independent acquisition of cyanase followed by lineage-specific divergence. However, within the Phylum Nematoda, HGT of homologous cyanases, from two different Kingdoms is consistent with multiple, independent events. Independent HGT has been observed in cyst and root-knot nematodes in the acquisition of cellulase (Smant et al., Reference Smant, Stokkermans, Yan, de Boer, Baum, Wang, Hussey, Gommers, Henrissat, Davis, Helder, Schots and Bakker1998) and xylanase (Mitreva-Dautova et al., Reference Mitreva-Dautova, Roze, Overmars, de Graaff, Schots, Helder, Goverse, Bakker and Smant2006) genes from soil bacterial, and in the transfer of cyanase among some fungi (Elmore et al., Reference Elmore, McGary, Wisecaver, Slot, Geiser, Sink, O'Donnell and Rokas2015). Recently, Danchin et al. (Reference Danchin, Perfus-Barbeoch, Rancurel, Thorpe, Da Rocha, Bajew, Neilson, Guzeeva, Da Silva, Guy, Labadie, Esmenjaud, Helder, Jones and den Akker2017) showed that the transcriptomes of the clade I plant ectoparasites Xiphinema index and Longidorus elongates acquired GH12 cellulase from bacteria that may be linked to plant parasitism; however, it is not known whether this was a single or independent acquisition prior to divergence. To date, the acquisition of functionally homologous genes from different Kingdoms has not yet been observed.
In a molecular phylogenetic analysis of the Phylum Nematoda, Holterman et al. (Reference Holterman, van der Wurff, van den Elsen, van Megen, Bongers, Holovachov, Bakker and Helder2006) noted that the genus Teratocephalus coincides with secernentean radiation and is comprised predominantly of terrestrial bacterivores. In contrast, nematodes that feed predominantly on fungi are more closely associated with plant parasitic nematodes and support the notion that this group arose from fungivorous ancestors. Given that fungal cyanases form a monophyletic group with closer ties to the early-branching nematodes and plants than to bacteria, an association between the clade I nematodes and fungi cannot be excluded. Alternatively, clade I nematodes may have acquired the cyanase secondarily from a prior plant–fungal association. However, we believe that a delineation in feeding habits and associations among ancestral free-living nematodes account for the acquisition of cyanase from different kingdoms. This raises intriguing questions regarding HGT of cyanase in numerous parasite lineages and the evolution of parasitism.
In conclusion, although it is difficult to ascertain events that took place >450 MYA using only extant organisms, strong phylogenetic inference provides a pathway to explore deep evolutionary and ecological history (e.g. Brooks and McLennan, Reference Brooks and McLennan2002). Current data suggest that the early ancestors of Trichinella had an association with plants. They further indicate that the cyanase within Trichinella is functionally active in all development stages studied to date, suggesting if, at one time, the cyanase was acquired to detoxify environmentally acquired cyanate, this role has changed in extant organisms.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182018001701
Financial support
D.S.Z., P.T., W.T. and E.P.H. are funded by the US Department of Agriculture, Agricultural Research Service.
Conflicts of interest
None.
Ethical standards
Not applicable.








