INTRODUCTION
Microphytobenthos (MPB) is a vital component of most shallow marine ecosystems, contributing between 20% and 50% of annual primary production (Underwood & Kromkamp, Reference Underwood and Kromkamp1999; Guarini et al., Reference Guarini, Blanchard, Gros, Gouleau and Bacher2000; Underwood & Provot, Reference Underwood and Provot2000; Blanchard et al., Reference Blanchard, Simon and Guarini2002; Herlory et al., Reference Herlory, Guarini, Richard and Blanchard2004). MPB, which is usually dominated by diatoms, can attain a biomass of ~300 mg chlorophyll-a m−2 (Kromkamp et al., Reference Kromkamp, de Brouwer, Blanchard, Forster and Creach2006; Koh et al., Reference Koh, Khim, Araki, Yamanishi and Koga2007). Much of this biomass is concentrated in shallow water environments where it can be exposed to extremes of temperature at low tide from freezing in winter at less than 0°C to around 35°C in summer. There have been many studies of the effects of temperature on MPB, identifying it as one of the main environmental factors regulating production (Blanchard & Guarini, Reference Blanchard and Guarini1996; Barranguet et al., Reference Barranguet, Kromkamp and Peene1998; Du et al., Reference Du, Li, Li and Chung2012), but very few have investigated the effects of extreme temperatures. Responses to sub-zero temperatures in the northern hemisphere have been measured in macroalgae (Davison et al., Reference Davison, Dudgeon and Ruan1989; Dudgeon et al., Reference Dudgeon, Davison and Vadas1989, Reference Dudgeon, Davison and Vadas1990; Pearson & Davison, Reference Pearson and Davison1993) and microalgal studies have been undertaken in polar areas (Raymond, Reference Raymond2000; Raymond & Knight, Reference Raymond and Knight2003; Sabacka & Elster, Reference Sabacka and Elster2006; Bayer-Giraldi et al., Reference Bayer-Giraldi, Uhlig, John, Mock and Valentin2010; Bayer-Giraldi et al., Reference Bayer-Giraldi, Weikusat, Besir and Dieckmann2011; Foreman et al., Reference Foreman, Dieser, Greenwood, Cory, Laybourn-Parry, Lisle, Jaros, Miller, Chin and McKnight2011). This study is the first to investigate the effects of freezing temperatures on MPB from temperate regions of the southern hemisphere.
In many cool temperate areas, frost is likely to form in intertidal areas during winter under freezing temperatures. In these circumstances MPB use two freezing-tolerance mechanisms in response to freezing temperatures; freezing resistance and cryoprotection. Freezing resistance is strongly related to the ability of the plasmalemma to recover from freezing events (Davison et al., Reference Davison, Dudgeon and Ruan1989; Dudgeon et al., Reference Dudgeon, Davison and Vadas1989; Pearson & Davison, Reference Pearson and Davison1993). Loss of cell contents occurs following freezing events due to the breakdown of the plasmalemma. Release of amino acids indicates a breakdown of plasmalemma integrity (Davison et al., Reference Davison, Dudgeon and Ruan1989). Antifreeze production, or cryoprotection, is a mechanism providing freezing tolerance in some microalgae. In diatoms, cryoprotection is related to the occurrence and function of anti-freeze proteins (AFPs). AFPs are able to bind to ice and influence the formation of ice (Raymond, Reference Raymond2000; Bayer-Giraldi et al., Reference Bayer-Giraldi, Uhlig, John, Mock and Valentin2010, Reference Bayer-Giraldi, Weikusat, Besir and Dieckmann2011). The characteristic features of AFPs are thermal hysteresis, i.e. the reduction of the freezing point of a solution below melting point, and inhibition of recrystallization (Raymond, Reference Raymond2000; Bayer-Giraldi et al., Reference Bayer-Giraldi, Uhlig, John, Mock and Valentin2010, Reference Bayer-Giraldi, Weikusat, Besir and Dieckmann2011). Raymond & Knight (Reference Raymond and Knight2003) also found that in the sea ice diatoms, Berkeleya sp. and Navicula sp., there were substances functioning like AFPs, called the ice-active substances (IASs), with a function resembling that of glycoproteins in polar fish AFPs (Raymond et al., 1989; Bayer-Giraldi et al., Reference Bayer-Giraldi, Uhlig, John, Mock and Valentin2010, Reference Bayer-Giraldi, Weikusat, Besir and Dieckmann2011), but they were not able to lower the freezing point. Raymond & Knight (Reference Raymond and Knight2003) also showed that the presence of IASs in the temperate marine diatom, Navicula frustulum, greatly enhanced their freeze–thaw survival. The absence of free water molecules within the cell is also critical for the survival of algae under freezing conditions, as ice crystals can readily form when free water is present (Davey, Reference Davey1989; Harding et al., Reference Harding, Day, Lorenz, Timmermann, Friedl, Bremner and Benson2004). Active transfer of free water out of the cell provides another mechanism of cryoprotection in algae. Harding et al. (Reference Harding, Day, Lorenz, Timmermann, Friedl, Bremner and Benson2004) stated that this type of cryoprotection has colligative consequences, as the solute composition within the cells becomes increasingly concentrated, preventing the formation of ice crystals.
Microphytobenthos can also respond behaviourally to extreme environmental conditions, by migrating vertically within the sediment, ensuring they do not experience photodamage (Guarini et al., Reference Blanchard, Simon and Guarini2002; Jordan et al., 2005). Motile or epipelic diatoms secrete a considerable percentage of photosynthetically fixed carbon as extracellular polymeric substances (EPS), which help in this migration within the sediment. EPS largely consists of carbohydrates, which allows diatoms to embed within the sediment by attaching themselves to sediment particles (Staats et al., 1999; De Brouwer et al., 2005). Several studies have shown that MPB migrated further into the sediment when the surface temperatures were unfavourable (Serodio & Catarino, Reference Serodio and Catarino2000; Blanchard et al., 2001, Reference Blanchard, Simon and Guarini2002, 2004; Jesus et al., 2006).
Fluorescence methods have become one of the main tools utilized to examine the environment responses of MPB (White & Critchley, Reference White and Critchley1999; Ralph & Gademann, Reference Ralph and Gademann2005; Jordan et al., Reference Jordan, McMinn and Thompson2010; Du et al., Reference Du, Li, Li and Chung2012). Here we use a fluorescence approach to examine the response of MPB to freezing temperatures in shallow marine environments of Southern Tasmania during periods of low tide. Our aim was to determine the physiological response of MPB to freezing temperatures; testing the hypothesis that freezing inhibits photosynthesis. This was undertaken both in the field and under laboratory conditions.
MATERIALS AND METHODS
Study sites
The photosynthetic response of MPB under ambient conditions was examined at two neighbouring sites, at a sandier site at Kings Beach, Sandy Bay (42°93′S 147°30 ′E) and at a muddier site at Browns River in Kingston Beach (42°96 ′S 147°51′E). Sampling occurred on frosty winter mornings at 6.00 am on 25 July 2011 at Browns River and on 5 August 2011 at Kings Beach. Sampling was performed before sunrise and during dawn at both sites during low tide period when the surface sediment is exposed. Ambient temperatures were −3°C with a thick frost (2 mm) on the sediment surface and the ambient light was approximately 90 µmol photons m−2 s−1.
Physical and chemical parameters of the study sites
The surface irradiance (PAR) at each sampling site was measured as µmol photons m−2 s−1 using a Biospherical QP radiometer with 2π sensor.
The vertical temperature profile within the sediment was measured by inserting a temperature probe (HI 766C) (K-type thermocouple thermometer, HI935005, Hanna Instruments, Woonsocket, Rhode Island, USA) vertically into the sediment until 120 mm deep. Triplicate measurements were determined at three distances (0 m, 1 m and 2 m) above the low tide mark. The same profiling approach was performed at both sampling sites.
Sediment grain size analysis was undertaken at each site. Only one sediment core was collected from each sampling site for the grain size analysis. Samples for the analysis were sieved at 2360 μm, 1400 μm, 710 μm, 500 μm, 335 μm, 250 μm, 180 μm, 90 μm and 62.5 μm using standard sieving methods (Folk, Reference Folk1974).
Relative species abundance was determined at each study site. One 150 mm long sediment core (diameter 45 mm) was collected from each sampling site, and the top 10 mm was removed and preserved in Lugol's iodine solution (10 ml) to stain the diatom frustules. Samples were cleaned in hydrogen peroxide (H2O2) for three days and mounted in Naphrax mounting medium for diatoms. The slides were examined with a compound microscope (Zeiss Axioskop, Jena, Germany) using a 100 ×, oil immersion objective. Photographs were taken using a Zeiss AxioCam digital camera and Zeiss Axiovision software. Species identification was carried out following Saunders et al. (Reference Saunders, Lane, Cook, McMinn, Hallegraeff, Hallegraeff, Bolch, Hill, Jameson, LeRoi, McMinn, Murray, de Salas and Saunders2010).
MPB sampling
Additional cores (45 mm diameter) were collected for fluorescence analysis of the sediment. Nine core samples were collected from each sampling site, with three replicate cores taken from three distances from the low tide mark (0 m, 1 m, 2 m above low water level). Clear polycarbonate tubes were manually pushed into the sediment and stoppered using a rubber bung and immediately returned to temporarily established working place. The top 10 mm of each core was sectioned on site into 2 mm intervals for pulse amplitude modulated (PAM) fluorometry analysis of the photosynthetic parameters of MPB. Each section was mixed with filtered seawater, the temperature of which was adjusted to be similar to the measured sediment temperatures at each depth, and transferred into a vial wrapped in aluminium foil to protect them from light and to dark-adapt the samples for 20 min. The samples were shaken vigorously and then allowed to settle for approximately 10 s before analysis. The chlorophyll fluorescence of the MPB was determined following Jordan & McMinn (Reference Jordan, McMinn and Wotherspoon2008) and Jordan et al. (Reference Jordan, McMinn and Thompson2010) using a PAM fluorometer (Water PAM; Walz, Effeltrich, Germany).
Fluorescence parameters and rapid light curves
Chlorophyll fluorescence was measured using a PAM fluorometer (Water-PAM, Waltz). The initial fluorescence (F) was measured by applying a weak measuring light (<1 µmol photons ms−2 s−1) and a saturating pulse (>3000 µmol photons m−2 s−1 for 0.8 s) was applied to determine the maximum fluorescence (F m ′). The ratio of the change in fluorescence (ΔF = F m ′ – F) and the maximal fluorescence, ΔF/F m ′, is a measure of the effective quantum yield of PSII in the illuminated sample. The relative photosynthetic electron transport rate (rETR) was calculated as the product of the effective quantum yield and quantum flux density of photosynthetically active radiation (PAR) (Genty et al., Reference Genty, Briantais and Baker1989).
To obtain the rapid light curves (RLC) samples were placed in the cuvette as quickly as possible. The RLC were obtained by illuminating the samples for 10 s before each ΔF/F m ′ measurement at each of a series of eight irradiances; 0, 83, 122, 186, 277, 393, 545, 890 and 1299 µmol photons m−2 s−1 (White & Critchley, Reference White and Critchley1999; Ralph & Gademann, Reference Ralph and Gademann2005). The rETR data generated by the rapid light curves were fitted to the following equation with a multiple non-linear regression (Platt et al., 1989):
rETR ma x represents the maximum potential rETR in the absence of photoinhibition. α is the initial slope of the light curve before the onset of saturation and represents the efficiency of light utilization. E d is the irradiance (in the general formula 400–700 nm). β is the parameter characterizing photoinhibition. In the absence of photoinhibition in the light curves, where β = 0, the function becomes:
where rETR max is the maximum rETR at light saturation and thus represents the photosynthetic capacity. Standard RLCs only generate nine points in their P vs E function, unlike traditional 14C-based P vs E functions, which typically have 20 or more data points (Lewis & Smith, Reference Lewis and Smith1983). This low number of data points makes correctly estimating both alpha and beta unreliable. Therefore, because none of the communities in this study were inhibited at their maximum ambient irradiance, we removed any ‘inhibited’ data points (rarely more than one per RLC) from the multiple non-linear regressions. In this way we achieved a more robust estimate of rETRmax and α. The PM-gain was adjusted to be between 5 and 15 before each measurement to keep the measurements consistent between samples. Red light-emitting diodes (LED) provided the measuring light, actinic light and saturating pulses used in the RLC.
Laboratory experiments
A further six sediment cores, collected in the same way as those for the field measurements, were taken at each site for each experiment. Three cores served as the treatment group and three cores as the control group. The cores were collected on 22 August 2011 in Kings Beach and 17 August 2011 in Browns River at 7:00 am, when the sediment surface temperature was approximately 5°C and the ambient irradiance was ~100 µmol photons m−2 s−1. The treatment cores were placed in a chest freezer for two hours until the surface of the sediment core reached −5°C, a temperature sufficient to cause freezing of the MPB. A spot light (Osram Vialox NAV(SON)-E, 400 W, Munich, Germany) was used to apply a low intensity irradiance, similar to the irradiance at the time of collection (~90 µmol photons m−2 s−1) on the samples. The control cores were placed in an open polystyrene box inside the freezer where surface temperature was not allowed to drop below 0°C. The cores were then sectioned and the photosynthetic parameters measured. Photosynthetic parameters measuring protocols were the same as those used in the field component of the study.
Statistical analyses
The photosynthetic parameter data were compiled and organized in Microsoft Excel with statistical analysis performed with computing software, R (R Development Core Team, 2012). Three-way analysis of variances (ANOVA) was carried out to test the effect of the freezing treatment on each photosynthetic parameter. A significance level of P < 0.01 was considered to be strongly significant, while P < 0.05 was treated as significant. The analyses were undertaken on different spatial scales, with sampling sites, three distances from the low tide mark where the core samples were collected and sections of sediment at five different depths along core (2 mm per section down to 10 mm) as the fixed factors and core samples being collected as the random effects.
RESULTS
Environmental data
The surface irradiance before dawn at Kings Beach was 80 µmol photons m−2 s−1, whilst the irradiance at Browns River was 90 µmol photons m−2 s−1.
The sediment surface temperatures at Kings Beach were 2.400 ± 0.058°C at 0 m, 2.600 ± 0.058°C at 1 m and 2.467 ± 0.033°C at 2 m from the low tide mark, and increased with depth (Figure 1A).

Fig. 1. Sediment temperature profiles as a function of depth at Kings Beach (A) and Browns River (B); distance 1 (Loc 1) was 0 m (R 2 = 0.997 and 0.992, respectively), distance 2 (Loc 2) at 1 m (R 2 = 0.997 and 0.994, respectively), and distance 3 (Loc 3) at 2 m from the low tide mark (R 2 = 0.991 and 0.992, respectively).
At Browns River, sediment temperatures at the surface were below freezing, −1.444 ± 0.010°C at 0 m, −1.700 ± 0.100°C at 1 m, and −1.200 ± 0.100°C 2 m from the low tide mark (Figure 1B).
The grain size analyses of the sediment from Kings Beach and Browns River showed that they are mainly sandy clay, with 66.04% of sediments by weight <180 μm at Kings Beach and 71.47% at Browns River.
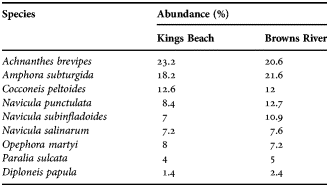
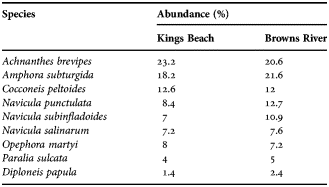
The diatom communities at Kings Beach and Browns River were similar and dominated by Achnanthes brevipes and Amphora subturgida (Table 1).
Table 1. Diatom species composition at Kings Beach and Browns River based on a count of 500 cells.
Photosynthetic parameters
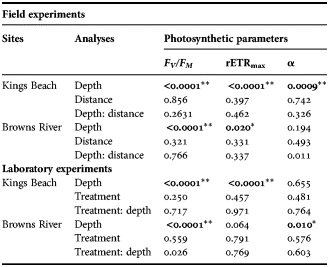
The F V /F M values of the MPB at the surface at Kings Beach differed significantly from those at depth, with the highest values at the sediment surface (Tables 2 & 3). There was no significant difference between surface F V /F M values with distance from the low tide mark, although a slight increase was observed moving further from the low tide mark (Tables 2 & 3).
Table 2. Photosynthetic parameters of microphytobenthos under ambient conditions from Kings Beach on 22 August 2011 and Browns River on 25 July 2011; mean ± standard error (N = 3). Distance is the horizontal distance from the high tide line. Depth is the depth within the sediment cores.

Table 3. Analysis of variance of microphytobenthos photosynthetic parameters under ambient conditions at Kings Beach on 22 August 2011 and Browns River on 25 July 2011 and variance between treatments in the laboratory experiment for both sampling sites; colon (:) indicating interaction. Significant differences are in bold with **indicates the significance of P < 0.01 and *indicating the significance of P < 0.05.
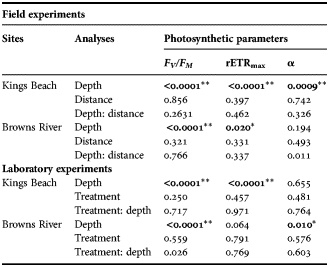
At Browns River there were significant differences in F V /F M between depths within the core at all three distances from the low tide mark (Tables 2 & 3). Maximum F V /F M values were observed at the sediment surface, and minimum F V /F M at a depth of 8 mm at 0 m from the low tide mark, and at 10 mm at 1 and 2 m from the low tide mark (Table 2).
rETRmax values at Kings Beach were highest at the sediment surface and decreased with depth. Minimum rETRmax values were observed at a depth of 10 mm and at all three distances from the shore (Table 2; Figure 2).

Fig. 2. Plots of rETR against PAR at Kings Beach at 0 m (A), 1 m (B), and 2 m (C) from the low tide mark and Browns River at 0 m (D), 1 m (E), and 2 m (F).
Browns River rETRmax values were highest at the surface, decreased with depth and were lowest at last section at 1 m and 2 m from the low water mark (Table 2). rETRmax at 0 m from the low tide mark was highest at the surface but the lowest rETRmax was at a depth of 8 mm (section 4); at the last section, it was 30% higher than at 8 mm (Table 2; Figure 2).
Alpha (α) values at Kings Beach did not change with increasing depth, although significant differences were observed between depth intervals (Table 2). The highest α values were consistently at a depth of 0.2–0.4 mm at all three distances from the low tide mark. The lowest α values were at a depth of 10 mm at 0 m and 1 m from the low tide mark, but this occurred in the first section of the core at 2 m from the low tide mark (Table 2).
Browns River α values did not change with increasing depth, although differences between depths were observed (Table 2). Additionally, the maximum and minimum α values fluctuated with depth at each of the three distances from the low tide mark (Table 2).
Laboratory studies
The surfaces of the sediment core collected from both sampling sites (Kings Beach, Browns River) were exposed to freezing temperatures of between −3°C and −5°C (Figure 3). The sediment temperature increased linearly with decreasing depth in both the control and freezing treatments (r 2= 0.929 and r 2 = 0.987, respectively).

Fig. 3. Plots of the sediment temperature profiles against depths in laboratory experiments of Kings Beach and Browns River under control (A) and freezing (B) treatments.
PHOTOSYNTHETIC PARAMETERS
There were no significant differences in F V /F M between the controls and the sub-zero temperature treatments in the cores collected from Kings Beach (Table 3). Maximum F V /F M values occurred at the sediment surface, while minimum values occurred at 10 mm depth in both the control and sub-zero temperature treatments (Table 4). However, F V /F M did vary significantly with depth in both treatments (Table 4).
Table 4. Photosynthetic parameters of freezing experiment on microphytobenthos for Kings Beach and Browns River; mean ± standard error (N = 3). Depth is the depth within the sediment cores.

There were no significant differences in the Browns River cores in F V /F M between treatments (Tables 3 & 4). However, F V /F M decreased significantly with depth in both the treatment and control cores (Table 3). The maximum F V /F M occurred at the surface in both control and sub-zero temperature treatments (Table 4). The minimum F V /F M in the control treatments occurred at a depth of 8 mm, while in the sub-zero treatment it occurred at a depth of 10 mm (Table 4).
rETRmax values of the Kings Beach cores were slightly higher in the sub-zero temperature treatment than in the control treatments, but the differences were not significant (Table 3). The highest values of rETRmax were measured in the surface layers and the lowest values at 10 mm in both control and sub-zero temperature treatments. The rETRmax values showed significant differences between depths (Tables 3 & 4). Furthermore, the rETRmax in the top two core sections were ~40% higher than those of the bottom sections (Figure 4).

Fig. 4. Plots of rETR against PAR of laboratory experiments of Kings Beach under control (A) and freezing (B) treatments, and Browns River under control (C) and freezing (D) treatments.
At Browns River, there was no significant difference in rETRmax between treatments (Tables 3 & 4). Maximum rETRmax occurred at the sediment surface in both control and sub-zero temperature treatments, whilst the minimum rETRmax of control treatment was observed at the depth of 10 mm and that of sub-zero temperature treatment was observed at the depth of 6 mm (Table 4).
There were no significant differences in α between treatment cores or between depths in the Kings Beach samples (Table 3). Maximum α values were observed at the depth of 6 mm in the control and the sub-zero temperature treatments. Minimum value was observed at the second section of the core in the control treatment, while that of sub-zero temperature treatment was observed at the depth of 10 mm (Table 4).
Significant difference in α between depth sections was observed at Browns River in both control and sub-zero treatments (Table 3). Maximum α were observed at the sediment surface in both control and sub-zero temperature treatments (Table 4). The minimum α occurred at the depth of 10 mm in the control treatment, while that of sub-zero temperature treatment occurred at the depth of 8 mm (Table 4).
DISCUSSION
Freezing had little impact on most of the photosynthetic parameters of the MPB measured in the field. An exception was F V /F M , which was significantly lower at Browns River, which experienced lower temperatures than Kings Beach. Other photosynthetic parameters did not show significant differences. While it is difficult to determine whether the lower F V /F M values of MPB at Browns River were due to the direct effect of freezing or merely the result of lower temperatures, the laboratory experiments, in which there were no significant differences between treatment and control in any photosynthetic parameters, suggest that the MPB have high freezing tolerance. This implies that the MPB were capable of withstanding short term sub-zero temperatures with minimal impact on photosynthetic capacity. Studies on sea ice algae by Ralph et al. (Reference Ralph, McMinn, Ryan and Ashworth2005), which were also mostly pennate diatoms, reported somewhat similar results, with the microalgal cells able to photosynthesize normally at temperatures down to −5°C. They only displayed lower photosynthetic responses when the temperature went down to −10°C (Ralph et al., Reference Ralph, McMinn, Ryan and Ashworth2005). The ability of algae to withstand freezing conditions has been seen in a number of other studies. The red algae, Chondrus crispus, had the ability to acclimate to freezing conditions lasting up to three hours per day at −5°C, for 30 days; this occurred through the closure of reactions centres and an increase in the photosynthetic rate following the freezing events (Dudgeon et al., Reference Dudgeon, Davison and Vadas1990). This process enabled the acclimated fronds to maintain higher photosynthetic rates than the non-acclimated fronds. Similar work undertaken with another red alga, Mastocarpus stellatus, showed there was no significant effect on photosynthesis, indicating that M. stellatus has a greater freezing tolerance than Chondrus crispus (Dudgeon et al., Reference Dudgeon, Davison and Vadas1990).
Observation on the effects of freezing on the brown seaweeds, Fucus spiralis and Fucus edentates. showed that the photosynthetic rate of F. spiralis was unaffected, but that of F. edentatus was reduced by 97% after three hours at −20°C (Davison et al., Reference Davison, Dudgeon and Ruan1989; Pearson & Davison Reference Pearson and Davison1993). These variations in freezing tolerance resulted in a vertical zonation, where the most freezing tolerant species (M. stellatus and F. spiralis) were located in the upper intertidal zones, whilst those with less freezing tolerance (C. crispus, F. edentatus and F. evanescens) were located in the lower intertidal zones (Davison et al., Reference Davison, Dudgeon and Ruan1989; Dudgeon et al., Reference Dudgeon, Davison and Vadas1990; Pearson & Davison, Reference Pearson and Davison1993). Greater freezing tolerance in these seaweeds was mainly due to their ability to resume photosynthesis immediately following the freezing events (Davison et al., Reference Davison, Dudgeon and Ruan1989; Dudgeon et al., Reference Dudgeon, Davison and Vadas1990; Pearson & Davison, Reference Pearson and Davison1993). Similar studies have not been undertaken on MPB but comparable results would be likely as these macroalgae were located in intertidal zones and are likely to have been exposed to freezing temperatures during exposure at low tide.
The results of the present study for temperate MPB show that freezing does not have a large impact on photosynthesis, indicating that MPB are tolerant to freezing and they are able to resume photosynthesis immediately following periods of exposure to freezing. As with intertidal macroalgae, MPB is mostly confined to the intertidal zone, where it can be exposed to freezing temperatures during periods of exposure in winter. In this field study freezing temperatures occurred for a much shorter period, i.e. just for a few hours around sunrise, and this occurred relatively infrequently. Under these conditions it is likely that the cells would have been light-limited and photosynthetic activity would have been low. Therefore, the length of time the MPB was exposed to freezing would not have had a significant impact on the photosynthetic responses of the MPB.
Several factors need to be considered in determining the reason for the freezing tolerance demonstrated by the MPB in this study. Firstly, photoinhibition and/or cell bleaching might only occur after repeated freezing events over a relatively long period of time (i.e. several weeks) (Dudgeon et al., Reference Dudgeon, Davison and Vadas1990). Dudgeon et al. (Reference Dudgeon, Davison and Vadas1990) suggested that cumulative damage to membrane integrity from frequent freezing leading to permanent damage might require longer recovery periods. In the present study, the MPB did not experience prolonged periods of freezing due to the low number of frosty nights (~10 per annum) with minimum temperatures always greater than −5°C and mean daytime temperatures above 10°C, so even though damage to the plasmalemma may have occurred, freezing temperatures were not sustained and so did not prevent full recovery. Furthermore, it is not likely that high irradiance impacted MPB photosynthesis in winter because freezing temperatures only occurred around dawn when irradiance was low. Consequently, MPB are unlikely to experience photochemical stress caused by freezing temperatures. However, the relatively infrequent occurrence of freezing temperatures during winter might have had some negative impact because the MPB are probably not well adapted to an environment where freezing of sediment occurs.
Although the sediment temperature increased with depth, temperature alone cannot be considered as the reason for the changes in photosynthetic parameters, as other factors, such as dissolved oxygen and light penetration, are also strongly correlated with sediment depth (Boudreau & Jorgensen, Reference Boudreau and Jorhensen2001). There was a significant negative relationship between F V /F M and rETRmax and depth, with higher values at the surface in both the ambient conditions and in vitro studies. This was unexpected, as it was hypothesized that the freezing at the surface would most likely inhibit MPB and produce a downward migration of cells. This result was possibly an artefact of the sampling method as depth resolution was 2 mm and all migration may have occurred within this top interval (Jordan et al., Reference Jordan, McMinn and Wotherspoon2008). The photosynthetic parameter α is usually considered to be temperature independent (Falkowski & Raven, Reference Falkowsi and Raven1997). Lower values of this parameter at the surface therefore may provide evidence that MPB migrated away from the freezing sediment surface. However, similar trends in F V /F M and rETRmax were not observed and so it was not possible to positively determine if the MPB had migrated to avoid freezing. Furthermore, Morris & Kromkamp (Reference Morris and Kromkamp2003) showed that the α of the benthic diatom, Cylindrotheca closterium, decreased at extreme low temperature, but this was not seen here.
Results of the current study show that only F V /F M values were affected by freezing, and impacts on the other photosynthetic parameters were not apparent. Although it was expected that MPB would undergo acclimation under low temperature conditions, results from this study show that they were freezing tolerant rather than acclimating to the freezing conditions. This freezing tolerance may have been associated with the resistance of light-harvesting reaction centres to freezing, recovery of the plasmalemma integrity and cryoprotection.
ACKNOWLEDGEMENTS
The authors would like to thank Simon Wotherspoon, for his advice and assistance in the statistical analysis and Peter McGoldrick, for guiding and supervising the grain size analysis. The authors would also like to thank Shi Hui Ng, Chia Min Tan and Yit Ning Beh for assistance in the field.