Introduction
Leishmaniasis, a neglected vector-borne disease caused by the protozoan parasite Leishmania has a diverse range of clinical manifestations, ranging from a mild cutaneous variant to the life threatening visceral form (Torres-Guerrero et al., Reference Torres-Guerrero, Quintanilla-Cedillo, Ruiz-Esmenjaud and Arenas2017). The current armamentarium of anti-leishmanial drugs is far from satisfactory as they have unacceptable toxic side-effects (Sundar and Singh, Reference Sundar and Singh2016 and references therein), along with resistance to antimonials emphasizing the need to identify new compounds (Croft et al., Reference Croft, Sundar and Fairlamb2006; Ponte-Sucre et al., Reference Ponte-Sucre, Gamarro, Dujardin, Barrett, López-Vélez, García-Hernández, Pountain, Mwenechanya and Papadopoulou2017). In the ongoing search for better leishmanicidal compounds, plant-derived products have been gaining ground which includes luteolin, quassin, Aloe vera, Piper betle, berberine chloride and artemisinin among others (Sen and Chatterjee, Reference Sen and Chatterjee2011 and references therein), a common modality being their ability to mediate oxidative stress.
Leishmania do not express catalase or classical selenocysteine-containing glutathione peroxidase, the two major H2O2-metabolizing enzymes usually present in eukaryotes, which renders the parasites more vulnerable to free-radical toxicity (Krauth-Siegel and Comini, Reference Krauth-Siegel and Comini2008). Indeed, as the oxidant defense in trypanosomatids appears to depend mostly on trypanothione, enzymes synthesizing and utilizing this redox metabolite have been proposed as putative targets (Flohe et al., Reference Flohé, Hecht and Steinert1999; van Assche et al., Reference van Assche, Deschacht, da Luz, Maes and Cos2011). Additionally, due to the presence of a sole mitochondrion as the ‘powerhouse’ of Trypanosomatids like Leishmania (Konig and Fairlamb, Reference König and Fairlamb2007; van Assche et al., Reference van Assche, Deschacht, da Luz, Maes and Cos2011), enhanced generation of free radicals appears a logical target. This has been endorsed in conventional and plant-derived antileishmanials such as sodium antimony gluconate (Mookherjee et al., Reference Mookerjee Basu, Mookerjee, Sen, Bhaumik, Sen, Banerjee, Naskar, Choudhuri, Saha, Raha and Roy2006), miltefosine (Verma et al., Reference Verma, Singh and Dey2007) and amphotericin B (Shadab et al., Reference Shadab, Jha, Asad, Deepthi, Kamran and Ali2017), for which there are evidences that mitochondrion is an effective chemotherapeutic target, via modulation of the mitochondrial trans-membrane potential and/or inhibition of the mitochondrial respiratory complexes (Sen and Majumdar, Reference Sen and Majumder2008; Monzote and Gille, Reference Monzote and Gille2010; Fidalgo and Gille, Reference Fidalgo and Gille2011; Monzote et al., Reference Monzote, Lackova, Staniek, Cuesta-Rubio and Gille2015).
Artemisinin, a sesquiterpene lactone endoperoxide, isolated from the Chinese medicinal plant Artemisia annua L. (qinghaosu) is used worldwide for the treatment of malaria (Avery et al., Reference Avery, Muraleedharan, Desai, Bandyopadhyaya, Furtado and Tekwani2003; WHO). Experimental studies have established its effectiveness as an antileishmanial compound (Yang and Liew, Reference Yang and Liew1993; Sen et al., Reference Sen, Bandyopadhyay, Dutta, Mandal, Ganguly, Saha and Chatterjee2007, Reference Sen, Ganguly, Saha and Chatterjee2010a, Reference Sen, Saha, Sarkar, Ganguly and Chatterjee2010Reference Sen, Saha, Sarkar, Ganguly and Chatterjeeb; Chollet et al., Reference Chollet, Crousse, Bories, Bonnet-Delpon and Loiseau2008; Want et al., Reference Want, Islammudin, Chouhan, Ozbak, Hemeg, Chattopadhyay and Afrin2017). The anti-malarial activity of artemisinin is mediated by generation of reactive oxygen species (ROS) secondary to cleavage of its endoperoxide bridge and subsequent depolarization of the mitochondrial membrane (Mercer et al., Reference Mercer, Maggs, Sun, Cohen, Chadwick, O'Neill and Park2007; Wang et al., Reference Wang, Huang, Li, Fan, Long, Li and Zhou2010; Antoine et al., Reference Antoine, Fisher, Amewu, O'Neill, Ward and Biagini2014). Similarly, in Leishmania promastigotes, artemisinin mediated its cytotoxicity by inducing a redox imbalance following enhanced generation of ROS and concomitant depletion of non-protein thiols that culminated in a caspase-independent apoptotic-like death (Sen et al., Reference Sen, Bandyopadhyay, Dutta, Mandal, Ganguly, Saha and Chatterjee2007, Reference Sen, Saha, Sarkar, Ganguly and Chatterjee2010b). In view of artemisinin inducing an apoptotic-like death following alteration of the mitochondrial membrane potential (MMP), this study aims to delineate the contribution of the parasite mitochondrial electron transport chain (ETC) and related events.
Materials and methods
Reagents
All chemicals were of analytical grade and obtained from Sigma Aldrich Chemicals (St Louis, MO, USA), except for the adenosine triphosphatase (ATP) determination kit and MitoSOX™ red [3,8-phenanthridinediamine,5-(6-triphenylphosphoniumhexyl)-5,6-dihydro-6-phenyl] from Molecular Probes (Carlsbad, CA, USA), protein assay dye (Bio-Rad, Haryana, India), fetal bovine serum (FBS; Gibco, Thermo Fischer Scientific, Waltham, MA, USA). A stock solution of artemisinin (100 mM in DMSO) was stored at −20 °C until use.
Parasite culture
Leishmania promastigotes (MHOM/IN/1978/UR6, Mukhopadhyay et al., Reference Mukhopadhyay, Bhattacharyya, Majhi, De, Naskar, Majumdar and Roy2000) were maintained at 24 °C in blood agar slants, containing brain heart infusion agar (3.25%) supplemented with glucose (1.3%) and rabbit blood (2%) along with penicillin G (50 IU mL−1), streptomycin (50 µg mL−1) or in medium 199 (M199) supplemented with 10% FBS, penicillin G (50 IU mL−1), streptomycin (50 µg mL−1) and hemin (3.25 mg L−1). Leishmania tarentolae (LtP) promastigotes were maintained at 24 °C in brain heart infusion medium (37 g L−1) supplemented with hemin (5 mg L−1), penicillin (50 IU mL−1) and streptomycin (50 µg mL−1) in 50 mL Saarstedt tubes with gas-permeable caps with agitation in a tube shaker (0.05 s−1). For flow cytometry experiments, both parasites were cultured at 24 °C in M199 supplemented with 10% FBS, penicillin G (50 IU mL−1), streptomycin (50 µg mL−1) and hemin (3.25 mg L−1); cells were sub-cultured every 48–72 h, inoculum being 1 × 106 mL−1.
Measurement of ROS and mitochondrial superoxide in Leishmania promastigotes
In parasites, apoptosis appears to be a prominent form of cell death in response to diverse stimuli, one of them being oxidants. To determine the effect of artemisinin on generation of intracellular ROS, a live-cell permeable dye 2,7-dichlorodihydrofluorescein diacetate (H2DCFDA) was used. Briefly, UR6 or LtP promastigotes (2 × 104 in 500 µL 0.02 M phosphate buffered saline, PBS, pH 7.2 or in M199 medium) were treated with artemisinin (0–0.5 mM, 24 h, 24 °C), and after incubating with H2DCFDA (100 µM, 30 min, 37 °C), baseline ROS was measured in a flow cytometer using forward vs side scatter to initially gate the parasite population followed by a FL1 histogram to quantify the fluorescence of DCF in viable parasites (Sen et al., Reference Sen, Bandyopadhyay, Dutta, Mandal, Ganguly, Saha and Chatterjee2007). The effect of artemisinin (0.25 mM) was similarly examined from 3–24 h. Treatment with miltefosine (HePC, 1 µM, 3 h, 37 °C) served as the positive control.
Promastigotes were stained with MitoSOX™ to measure the generation of superoxide in parasite mitochondrion as previously described (Shadab et al., Reference Shadab, Jha, Asad, Deepthi, Kamran and Ali2017). Briefly, promastigotes UR6 or LtP (2 × 104 in 250 µL PBS) were pre-stained with MitoSOX™ (2.5 µM, 1 h, 37 °C). Cells were then washed twice, pellets resuspended in 500 µL PBS or in M199 medium and treated with antimycin A (0.01 mM, 1 h, 37 °C) or artemisinin (0–0.5 mM, 24 h, 37 °C). Fluorescence was acquired in the FL3 channel of a flow cytometer. A time-dependent effect of artemisinin (0.25 mM, 3–24 h) on promastigotes was similarly measured.
Isolation of mitochondria
Mitochondria from log phase promastigotes were isolated following hypotonic lysis and differential centrifugation as previously described (Roy et al., Reference Roy, Ganguly, Bose Dasgupta, Das, Pal, Jaisankar and Majumder2008) with some modifications. Briefly, Leishmania (UR6, 1–2 × 109) were washed twice with PBS and the cell pellet was resuspended in Tris-HCl (5 mM, pH 7.4, 25 °C for 10 min) to osmotically lyse the cells. The suspension was then passed through a needle (26 gauge) followed by homogenization using a pre-chilled Dounce homogenizer (~10 cycles, each cycle for 5 min), and observed microscopically for optimum cell lysis. This was followed by immediate addition of a one-third volume of mitochondria stabilization buffer (1 m sucrose, 35 mM EDTA, 50 mM Tris). The stabilized homogenate was then centrifuged (1000 g, 10 min, 4 °C), followed by further centrifugation of the supernatant (13 000g, 20 min, 4 °C). The resultant pellet containing mitochondria was resuspended in phosphate buffer (50 mM, pH 7.4) containing protease inhibitor cocktail and protein concentrations were measured. The isolated mitochondria were stored in aliquots at −20 °C until use.
Nicotinamide adenine dinucleotide (NADH)-ferricyanide reductase assay
The NADH:ferricyanide reductase activity of artemisinin-treated mitochondria (0–0.5 mM, 25 °C) was estimated using ferricyanide as an electron acceptor as previously described (Chen et al., Reference Chen, Zhai, Christensen, Theander and Kharazmi2001) with minor modifications. The reaction was initiated by addition of the mitochondrial lysate (30–50 µg protein in a final volume of 200 µL phosphate buffer, 50 mM, pH 7.4) containing NADH (0.2 mM) and ferricyanide (0.5 mM). The rate of oxidation of NADH was immediately monitored by measuring the decrease in absorbance at 340 nm every 15 s for 5 min and enzyme activity was expressed as nmoles of NADH oxidized min−1 mg−1 protein, molar extinction coefficient of NADH being 6.22 mM−1 cm−1. Rotenone (250 µM), a complex I inhibitor, served as the positive control.
Complex II (succinate dehydrogenase) activity
In mitochondria isolated from promastigotes (30–50 µg protein in 200 µL), the complex II activity of artemisinin-treated (0–0.5 mM, 25 °C) was assayed in phosphate buffer (100 mM, pH 7.4) containing EDTA (100 µM), potassium succinate (25 mM), rotenone (250 µM), antimycin A (10 µM) and sodium azide (2.5 mM). Following addition of 2,6-dichlorophenol-indophenol (DCIP, 0.05 mM) and phenazine methosulphate (0.5 mM), the decrease in absorbance of DCIP was monitored every 15 s for 5 min at 600 nm (Spectramax M2e, Molecular Devices, San Jose, USA, Chen et al., Reference Chen, Zhai, Christensen, Theander and Kharazmi2001). Results were expressed as nanomoles of DCIP reduced min−1 mg−1 protein, molar extinction coefficient of DCIP being 20.5 mM−1 cm−1.
Complexes I–III [NADH cytochrome c (Cyt c) reductase, NCC] coupled assay
The activity of complexes I–III (NCC) was measured in mitochondria isolated from UR6 promastigotes by the NADH-supported reduction of ferricytochrome c to ferrocytochrome c, absorbances being measured at 550 nm (Roy et al., Reference Roy, Ganguly, Bose Dasgupta, Das, Pal, Jaisankar and Majumder2008). Briefly, mitochondria (30–50 µg protein in a final volume of 200 µL of phosphate buffer, 50 mM, pH 7.4) containing NADH (250 µM), Cyt c (1.2 mg mL−1), sodium cyanide (2 mM) and EDTA (0.01 mM) were incubated with artemisinin (0–0.5 mM, 25 °C); the reference cuvette contained all these reagents, except the sample. The increase in absorbance was immediately measured at 550 nm every 15 s for 5 min in a quartz cuvette (Spectramax M2e, Molecular Devices, San Jose, USA), with rotenone (inhibitor of complex I, 0.25 mM) serving as the positive control. The enzyme activity was calculated in terms of nanomoles of Cyt c reduced min−1 mg−1 protein, the molar extinction coefficient of Cyt c being 29.5 mM−1 cm−1.
Complexes II–III (succinate Cyt c reductase, SCC) coupled assay
The activity of complexes II–III (SCC) in parasite mitochondria (30–50 µg protein) treated with artemisinin (0–0.5 mM, 25 °C) was assayed by monitoring the succinate supported reduction of ferricytochrome c to ferrocytochrome c at 550 nm (Roy et al., Reference Roy, Ganguly, Bose Dasgupta, Das, Pal, Jaisankar and Majumder2008). Briefly, mitochondria (30–50 µg) was incubated in 200 µL of phosphate buffer (100 mM, pH 7.4) containing succinate (2 mM), sodium cyanide (1.0 mM), EDTA (0.3 mM) and Cyt c (1.2 mg mL−1); the reference cuvette contained all the reagents, except the sample and increase in absorbance at 550 nm was monitored every 15 s for 5 min (Spectramax M2e, Molecular Devices, San Jose, USA). Thenoyltrifluoroacetone (TTFA, 0.4 mM) an inhibitor of complex II confirmed the assay specificity and enzyme activity was calculated in terms of nanomoles of Cyt c reduced min−1 mg−1 protein, molar extinction coefficient of Cyt c being 29.5 mM−1 cm−1.
Measurement of leishmanial oxygen consumption
OxoPlates (OP96U PreSens, Regensburg, Germany) with integrated fluorescence oxygen sensors were used to measure the cellular oxygen concentration in a multimode plate reader (EnSpire, PerkinElmer, MA, USA) using excitation wavelength 540 nm and two emission wavelengths (reference dye 590 nm, I Ref, O2-sensing dye 650 nm, I Ind). The fluorescence intensity ratio I R = I Ind/I Ref was used to calculate the oxygen concentration (in terms of μM O2) according to the manufacturer's instructions:
OxoPlates were calibrated with air-saturated buffer (I R = k 100) and sodium dithionite-treated buffer (1% w/v) (I R = k 0). After calibration, plates were loaded either with 200 µL of medium (control for drift corrections) or 50 µL medium (untreated) or 50 µL medium containing test compounds CCCP (0.001 mM) or artemisinin (0.2 mM). Immediately before measurement, 150 µL of well-aerated LtP promastigotes (1–1.7 × 108 mL−1) were added to respective wells. Finally on the top of each well, 50 µL paraffin oil was layered, and fluorescence measured from the bottom at 27 °C at 590 and 650 nm simultaneously at 5 min intervals (Monzote et al., Reference Monzote, Lackova, Staniek, Steinbauer, Pichler, Jäger and Gille2017). In all the cases, Schneider's insect medium supplemented with hemin (5 mg L−1), penicillin (50 IU mL−1 penicillin) and streptomycin (50 µg mL) was used. From the linear part of the O2 decay, the slopes were calculated and corrected for the medium drift for further statistical evaluation. Each concentration was assayed in quadruplicates and the results were expressed as percentage of oxygen consumption in comparison with the untreated control or in μM O2 min−1. The highest concentration of the vehicle control (1% DMSO) caused only 2% inhibition.
In addition, LtP (108 mL−1) were pre-incubated with artemisinin (0.04 mM) for 0, 6, and 24 h at 25 °C in Schneider's medium supplemented with 5 mg L−1 hemin. The respective vehicle controls were treated with 1% DMSO under otherwise identical conditions. Oxygen consumption rates of LtP (1–2.4 × 108 mL−1) were measured with a Clark-type oxygen electrode (Hansatech, Germany) and analysed using software MCREC (Monzote et al., Reference Monzote, Lackova, Staniek, Steinbauer, Pichler, Jäger and Gille2017). The uncoupling effect of artemisinin was determined by adding oligomycin (5 µM, ATP synthase inhibitor) to the respiring LtP followed by addition of an uncoupler CCCP (0.5 µM). As an indirect parameter of intracellular mitochondrial coupling the respiratory control ratios (RCRs) of vehicle- and artemisinin-pretreated LtP were calculated as the ratio of oxygen consumption rates in the presence of CCCP to oligomycin-inhibited LtP. Each experiment was performed in quadruplicates.
Measurement of MMP
In order to assess the mitochondrial transmembrane electrochemical gradient, a cell permeable, cationic, lipophilic dye, JC-1 was used which aggregates within mitochondria to form dimers and fluoresces red at higher transmembrane potential. However, at lower transmembrane potential, JC-1 remains in the cytosol as monomers, emitting a green fluorescence. Accordingly, the ratio of red to green fluorescence of JC-1 is an efficient indicator of the cellular mitochondrial transmembrane potential and the impact of artemisinin (0–0.25 mM, 3 h, 37 °C) was measured in UR6 promastigotes as previously described (Sen et al., Reference Sen, Bandyopadhyay, Dutta, Mandal, Ganguly, Saha and Chatterjee2007). Briefly, parasites (5 × 105 mL−1) were stained with JC-1 (20 µM, 24 °C, 10 min), and fluorescence was measured in a flow cytometer. To distinguish between monomers and J-aggregates, gates P2 and P3 respectively were set using hydrogen peroxide (H2O2, 40 mM, 30 min, 37 °C).
Measurement of ATP
ATP was measured using an ATP determination kit, wherein the luminescence generated is proportional to the amount of ATP present, based on the utilization of ATP by luciferase (Emmax 560 nm, pH 7.8) (Roy et al., Reference Roy, Ganguly, Bose Dasgupta, Das, Pal, Jaisankar and Majumder2008). Promastigotes (UR6, 1 × 106) treated with artemisinin (0–0.5 mM, 3 h, 37 °C) were lysed using Triton X-100 (0.25%), and the lysed cells (10 µL) were added to a reaction mix of 190 µL containing luciferin (0.5 mM), luciferase (1.25 µg mL) and reaction buffer in H2O. The amount of intracellular ATP was measured in a total volume of 200 µL in a 96 well black, clear bottom microtest plate (Optilux, BD Falcon, CA, USA) in terms of chemiluminescence (Spectramax M2e, Molecular Devices, San Jose, USA). The amount of ATP was calculated from a standard curve of ATP (0–1000 nm) and expressed as % of control; 2-deoxy-d-glucose (2-DG, 2.5 mM) served as the glycolytic inhibitor.
Pyruvate kinase/lactate dehydrogenase-coupled F0F1-ATPase/ATP synthase activity
The F 0F 1-ATPase/ATP synthase activity was measured as previously described (Roy et al., Reference Roy, Ganguly, Bose Dasgupta, Das, Pal, Jaisankar and Majumder2008). Briefly, mitochondria from UR6 promastigotes were initially permeabilized by incubating for 30 min at 4 °C in a digitonin (1%) containing solubilizing buffer comprising Tris-HCl (50 mM, pH 7.4), NaCl (120 mM), KCl (5 mM), MgSO4 (1 mM), CaCl2 (1 mM) and glycerol (10%). This ATPase activity of solubilized mitochondria was measured in terms of the oxidation of NADH via pyruvate kinase and lactate dehydrogenase, molar extinction coefficient of NADH being 6.22 mM−1 cm−1 (Roy et al., Reference Roy, Ganguly, Bose Dasgupta, Das, Pal, Jaisankar and Majumder2008). Accordingly, the reaction mixture (200 µL) contained mitochondrial protein (30–50 µg) in Tris-HCl (50 mM, pH 8.0) along with ATP (1.0 mM), MgCl2 (1.0 mM), KCl (25 mM), EDTA (0.10 mM), NADH (0.10 mM), phosphoenolpyruvate (0.5 mM), pyruvate kinase (2.5 U), lactate dehydrogenase (4 U), cyanide (5 µM), rotenone (5 µM) and antimycin A (5 µM). These assay conditions minimized the contribution of other transport ATPases, such as Na+ and K+-ATPase. Artemisinin (0–0.5 mM, 25 °C) or oligomycin (0.01 mM, 25 °C), a complex V inhibitor were added and decrease in absorbance was measured at 340 nm every 15 s for 5 min (Genesys 10S, UV-VIS, Thermo Fisher Scientific, MA, USA). Data were expressed as F 0F 1-ATP synthase activity in terms of % of control.
Flow cytometry
Promastigotes (2 × 104 per 500 µL) from different experimental groups were monitored for their intracellular fluorescence on a flow cytometer (FACS Verse, Becton Dickinson, CA, USA). The parasites were gated based on their forward and side scatter and fluorescence was then measured in the log mode using BD FACS Suite™ software (BD Biosciences, CA, USA). Acquisition was performed on 8000 gated events and data expressed as geometric mean fluorescence channel (GMFC) i.e. average or central tendency of fluorescence of analysed particles. Data were analysed using BD FACS Suite™ (BD Biosciences, CA, USA).
Statistical analysis
Each experiment was performed at least thrice and results expressed as mean ± standard error of the mean (s.e.m.). Statistical analysis wherever applicable was evaluated by Kruskal–Wallis multiple comparison test followed by Dunnett's multiple comparison test for non-parametric data using GraphPad Prism software, version 5 (La Jolla, CA, USA); P < 0.05 was considered as statistically significant.
Results
Artemisinin induced generation of ROS in Leishmania promastigotes
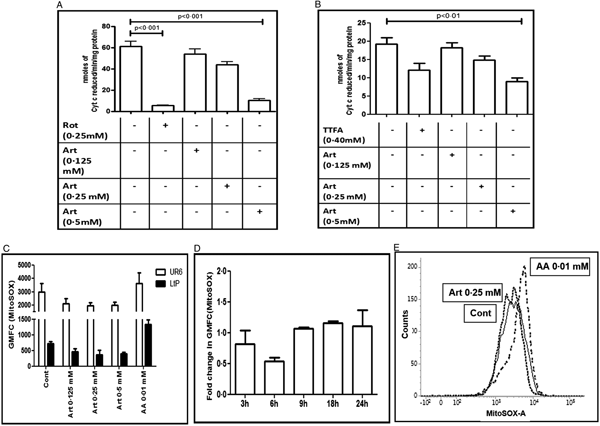
To evaluate the ability of artemisinin to generate free radicals in Leishmania promastigotes, H2DCFDA was used based on its ability to be oxidized by ROS and produce a fluorescent compound DCF (Wan et al., Reference Wan, Myung and Lau1993), the resultant fluorescence being indicative of the amount of ROS generated. As the IC50 and IC90 of artemisinin in Leishmania donovani promastigotes was 0.16 and 0.5 mM (Sen et al., Reference Sen, Bandyopadhyay, Dutta, Mandal, Ganguly, Saha and Chatterjee2007), the dose range selected in this study was 0.125–0.5 mM. In UR6, artemisinin (0–0.5 mM, 24 h) increased the GMFC to 1724.00 ± 110.00 (0.125 mM), 2221.00 ± 196.70 (0.25 mM, P < 0.01) and 1993.00 ± 163.30 (0.5 mM, P < 0.05), the baseline fluorescence being 1305.00 ± 131.20; this translated into a fold increase of 1.32, 1.70 and 1.52, respectively (Fig. 1A). In LtP promastigotes, the baseline fluorescence was lower being 254.80 ± 16.01. However, the fold increase in DCF fluorescence by artemisinin was similar as the GMFC increased to 313.30 ± 30.06 (0.125 mM), 420.80 ± 14.78, P < 0.01 (0.25 mM) and 339.30 ± 14.19, P < 0.05 (0.5 mM), translating into a fold increase of 1.23, 1.65 and 1.33, respectively (Fig. 1A). A time-dependent effect of artemisinin (0.25 mM) was studied at 3, 6, 9, 18 and 24 h, wherein the increase in GMFC levels was expressed as fold change with respect to control (Fig. 1B). As the fluorescence increase was maximal from 0.25 mM onwards, a time-dependent kinetics was also studied wherein the fold increase was maximal at 24 h, being 1.79 ± 0.13 (Fig. 1B and C). Miltefosine, a prominent anti-leishmanial drug known to generate ROS in Leishmania, served as the positive control and a 31.5-fold increase was observed (8026.00 ± 108.00, Fig. 1C).
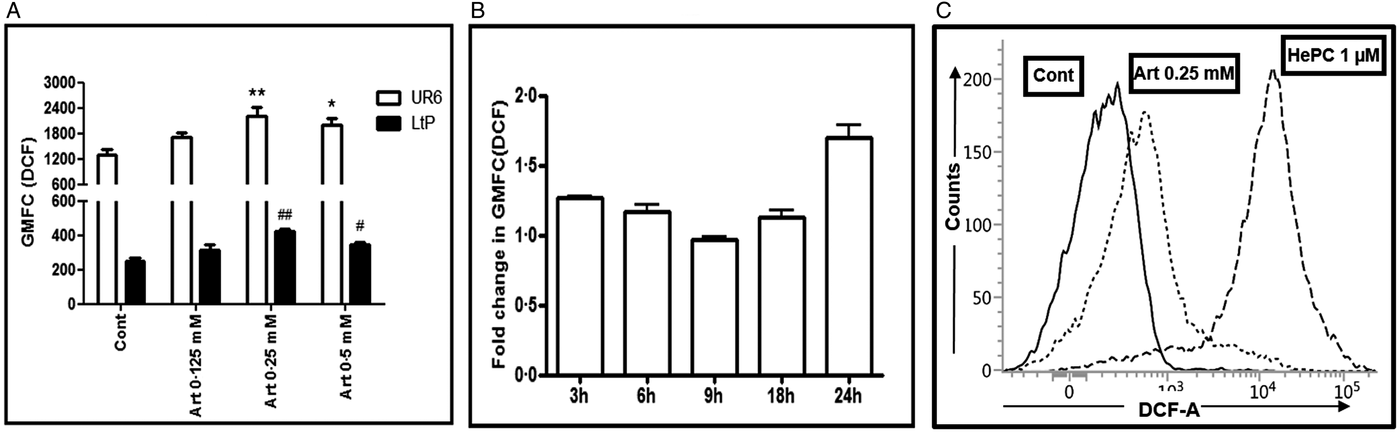
Fig. 1. Effect of artemisinin on generation of ROS in Leishmania promastigotes. (A) Log phase promastigotes UR6 (□) or Leishmania tarentolae (LtP, ■), 2 × 104 per 500 µL were incubated with artemisinin (Art, 0.125–0.5 mM, 24 h) and after labelling with H2DCFDA (100 µM at 37 °C), fluorescence was acquired and analysed as described in the Materials and methods section. Data are expressed as the mean ± s.e.m. of GMFC of at least three experiments in duplicate. *P < 0.05, **P < 0.001 as compared with respective controls; #P < 0.05 and ##P < 0.001 as compared with respective controls. (B) Time kinetics of the generation of ROS in log phase UR6 promastigotes (2 × 104 per 500 µL) following incubation with artemisinin (0.25 mM) and labelling with H2DCFDA (100 µM, 37 °C). The fluorescence is expressed in terms of fold change as described in the Materials and methods section. (C) Representative histogram profiles of UR6 promastigotes (2 × 104 per 500 µL, Cont, —) treated with artemisinin (Art, 0.25 mM, ⋯) or miltefosine (HePC, 1 µM, - - -) stained with H2DCFDA (100 µM, 37 °C) as described in the Materials and methods section.
Effect of artemisinin on the ETC of Leishmania promastigotes
Inhibition of the mitochondrial ETC is a potent source of ROS, especially superoxide (Staniek et al., Reference Staniek, Gille and Kozlov2002; Chen et al., Reference Chen, Vazquez, Moghaddas, Hoppel and Lesnefsky2003; Nohl et al., Reference Nohl, Gille and Kozlov2003). To explore whether the enhanced generation of ROS by artemisinin in Leishmania promastigotes (Fig. 1) is secondary to modulation of the mitochondrial functions, inhibition of the respiratory chain was examined. In UR6 promastigotes, artemisinin (0.125–0.5 mM) did not strongly inhibit the NADH ferricyanide reductase activity or complex II activity (Table 1), but inhibited complexes I–III NCC activity in a dose dependent manner, the fold decrease being 1.13 (0.125 mM), 1.39 (0.25 mM) and 6.05 (0.5 mM) (Fig. 2A, Table 1). Likewise, artemisinin inhibited complexes II–III SCC activity, the fold decrease with 0.25 and 0.5 mM being 1.3 and 2.13 fold, respectively (Fig. 2B, Table 1). The positive controls for NCC activity (rotenone, 0.25 mM, Fig. 2A) and SCC activity (TTFA, 0.4 mM, Fig. 2B) demonstrated significant inhibition (Table 1).
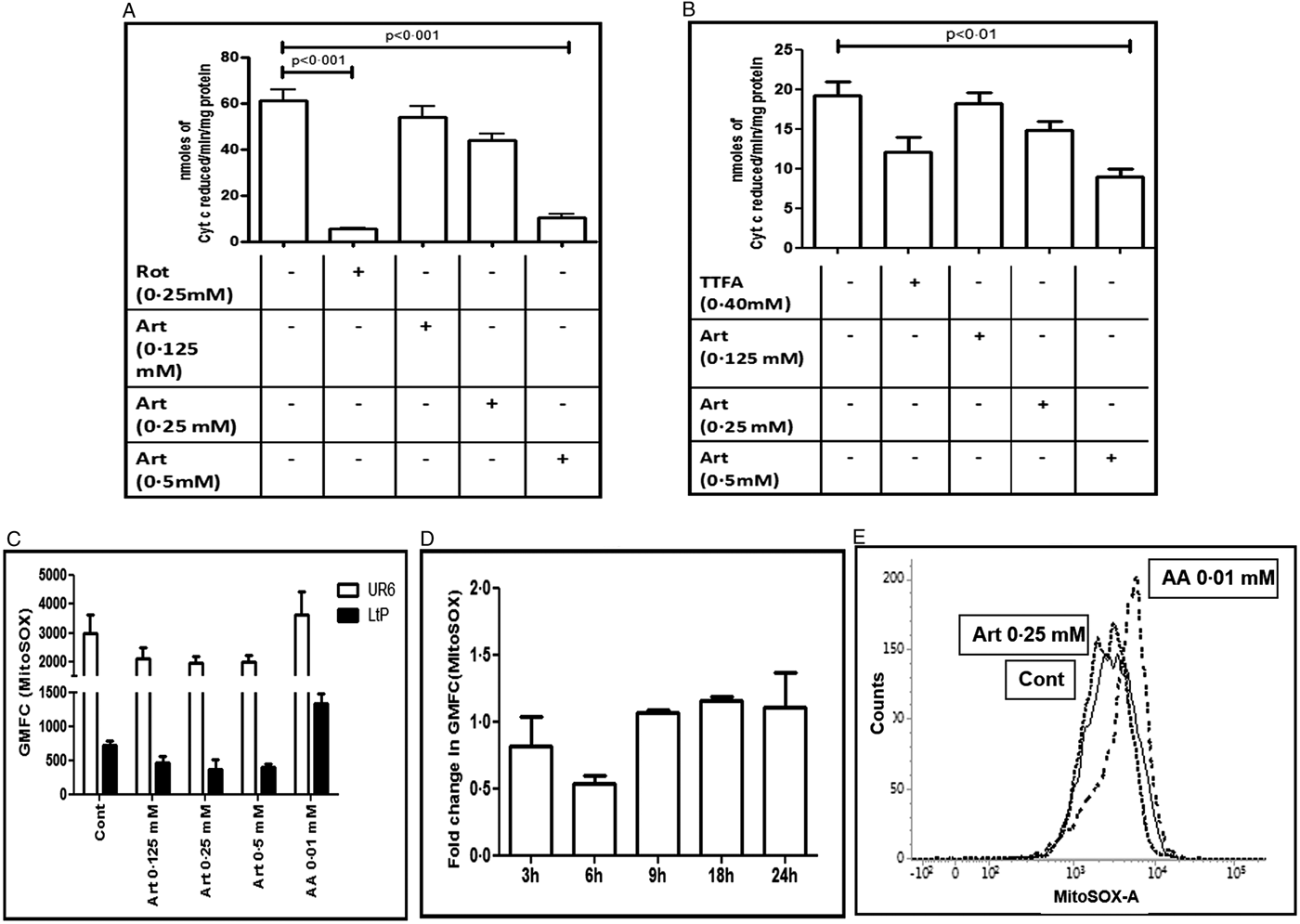
Fig. 2. Effect of artemisinin on the Leishmania mitochondrial respiratory chain. (A and B) Crude mitochondria sourced from UR6 promastigotes were incubated with artemisinin (Art, 0.125–0.5 mM) and complexes I–III (A) and complexes II–III (B) assays were performed as described in the Materials and methods section. Data are expressed as mean ± s.e.m. of nmoles of Cyt c reduced min−1 mg−1 protein of at least three experiments in duplicate. (C) Log phase UR6 (□) or L. tarentolae (LtP, ■) promastigotes (2 × 104 per 500 µL, Cont) were incubated with artemisinin (Art, 0.125–0.5 mM) and after labelling with MitoSOX™ (2.5 µM, 37 °C), fluorescence was acquired and analysed as described in the Materials and methods section. Data are expressed as the mean ± s.e.m. of GMFC of at least three experiments in duplicate. (D) Time kinetics of generation of MitoSOX™ in log phase UR6 promastigotes (2 × 104 per 500 µL) that were treated with artemisinin (0.25 mM) and labelled with MitoSOX™ (2.5 µM, 37 °C). Fluorescence was acquired and analysed as described in the Materials and methods section; data are expressed as the fold change (mean ± s.e.m.) in GMFC of at least three experiments in duplicate. (E) Representative histogram profiles of UR6 promastigotes (2 × 104 per 500 µL, —, Cont) treated with artemisinin (Art, 0.25 mM, ⋯) or antimycin A (AA, 0.01 mM, - - -) that were stained with MitoSOX™ (2.5 µM, 37 °C) and fluorescence was analysed and acquired as described in the Materials and methods section.
Table 1. Effect of artemisinin upon the mitochondrial ETC of Leishmania promastigotes

The effects of artemisinin upon the activities of NADH ferricyanide reductase and complexes II, I–III and II–III were evaluated as described in the Materials and methods section. For NADH ferricyanide reductase and complex II activities (with rotenone and TTFA respectively being the inhibitors), data are expressed as mean ± s.e.m. of nmoles of NADH oxidized min−1 mg−1 protein and nmoles of DCIP reduced min−1 mg−1 protein respectively, while for the NCC and SCC activities (with rotenone and TTFA respectively being the inhibitors), data are expressed as mean ± s.e.m. of nmoles of Cyt c reduced min−1 mg−1 protein. The % inhibition with respect to control is indicated in the parenthesis.
Artemisinin failed to generate mitochondrial superoxide in Leishmania promastigotes
In mammalian mitochondria, inhibition of the ETC leads to enhanced formation of superoxide radical. Accordingly, the generation of mitochondrial superoxide was studied in promastigotes using MitoSOX™, a fluorogenic dye that permeates live cells and targets mitochondria. The entry of the probe is dependent on the membrane potential and once inside the mitochondria, it is rapidly oxidized by superoxide, and importantly, not by any other free radical (Polster et al., Reference Polster, Nicholls, Ge and Roelofs2014). Antimycin A, a known trigger for mitochondrial superoxide production was examined along with artemisinin (Fig. 2C and E). Artemisinin failed to impact on the generation of mitochondrial superoxide in UR6 or LtP promastigotes (Fig. 2C–E), which was corroborated by a time kinetic study (Fig. 2D and E).
Artemisinin marginally impaired oxygen consumption in Leishmania parasites
In view of artemisinin significantly decreasing complexes I–III and II–III activity, it was expected to translate into reduced consumption of oxygen. Artemisinin is sufficiently lipophilic to be immediately taken up by Leishmania and access the mitochondria within minutes. Accordingly, oxygen consumption was measured in artemisinin-treated LtP in parallel with antimycin A, a direct inhibitor of the mitochondrial ETC (Fig. 3). Antimycin A strongly inhibited mitochondrial oxygen consumption causing a 98.30% decrease in oxygen consumption (0.11 ± 0.01 vs 6.47 ± 0.15 µM min−1, P < 0.001, Fig. 3A) whereas artemisinin (0.2 mM) failed to inhibit oxygen consumption, as a negligible 3.10% decrease was demonstrated (6.27 ± 0.32 µM min−1, Fig. 3A).

Fig. 3. Effect of artemisinin upon mitochondrial oxygen consumption. (A) Oxygen consumption was measured in log phase L. tarentolae (LtP) promastigotes (1 × 108 mL−1) treated with antimycin A (AA, 0.01 mM) or artemisinin (Art, 0.2 mM) as described in the Materials and methods section. Data are expressed as mean ± s.e.m. of oxygen consumed (μM min−1) of at least three experiments. (B) Oxygen consumption was measured in log phase LtP promastigotes (1 × 108 mL−1) treated with oligomycin (Oligo, 0.01 mM) or artemisinin (Art, 0.04 and 0.20 mM) in the presence of an uncoupler, CCCP (0.001 mM) as described in the Materials and methods section. The oxygen consumption rate is expressed as % control (mean ± s.e.m.) of at least three experiments. (C) Uncoupling effects were monitored in well-aerated cell suspensions of LtP promastigotes (1–2.4 × 108 mL−1) that were incubated for 0, 6, and 24 h with artemisinin (Art, 0.04 mM) or 1% DMSO (vehicle cont). The RCR was measured in the presence of oligomycin followed by CCCP with a Clark-type oxygen electrode as described in the Materials and methods section. Data are the mean ± s.e.m. of four experiments.
A major mitochondrial function is generation of ATP, which can be impaired by (i) inhibition of the ETC and/or (ii) uncoupling of ETC from ATP synthase following collapse of the MMP (Monzote et al., Reference Monzote, Lackova, Staniek, Steinbauer, Pichler, Jäger and Gille2017). Upon addition of oligomycin (a classical inhibitor of ATP synthase), oxygen consumption decreased by 27.86% (6.06 ± 0.09 vs 8.40 ± 0.35 µM min−1, Fig. 3B). Following inclusion of the uncoupler CCCP, the maximally uncoupled respiration was obtained, which translated into a 22.51% increase with respect to the decrease caused by oligomycin (7.82 ± 0.42 µM min−1, P < 0.05, Fig. 3B). Artemisinin (0.04 and 0.2 mM) in the presence of oligomycin, reverted inhibition of oxygen consumption marginally by 10.09 and 12.55%, respectively (6.74 ± 0.32 and 6.93 ± 0.001 µM min−1, respectively, Fig. 3B).
The minimal uncoupling effect of artemisinin (0.04 mM) was further confirmed using the Clark-type electrode method wherein the CCCP/oligo ratio representative of respiratory control was evaluated in a time-dependent manner. This RCR is an indication of the cells ability to respond to increased ATP demands and is linked to the ‘spare respiratory capacity’ of the mitochondria. Therefore, a high RCR is indicative of a healthy cell and a decreased RCR reflects an impaired stress response. The basal RCR of LtP promastigotes was 1.64 ± 0.12 which was unaltered by artemisinin (0.04 mM) at 0 time point (1.69 ± 0.04, Fig. 3C), and even at later time points (6 and 24 h), remained comparable with vehicle-treated cells (Fig. 3C).
Artemisinin induced mitochondrial transmembrane depolarization
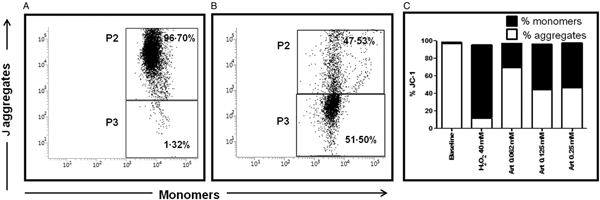
In L. donovani promastigotes, artemisinin induced a loss of MMP (Sen et al., Reference Sen, Bandyopadhyay, Dutta, Mandal, Ganguly, Saha and Chatterjee2007, Reference Sen, Saha, Sarkar, Ganguly and Chatterjee2010b) and was confirmed in UR6 promastigotes, wherein an increase in green monomers was evident (Fig. 4A). In healthy cells, % JC-1 monomers was only 1.49 ± 0.12, which with the addition of artemisinin increased progressively to 27.55 ± 2.63 (0.062 mM) and 51.52 ± 2.99 (0.125 mM, Fig. 4B), but remained unchanged at 0.25 mM (50.89 ± 11.96%), Fig. 4C. This translated into a decline in the red/green fluorescence ratio from 65.59 ± 4.92 at baseline to 2.60 ± 0.44, 0.87 ± 0.14 and 1.16 ± 0.54 with 0.062, 0.125 and 0.25 mM artemisinin, respectively. H2O2 (40 mM, 30 min, 37 °C) served as the positive control, wherein the red/green fluorescence ratio decreased to 0.16 ± 0.06.

Fig. 4. Effect of artemisinin on MMP in Leishmania promastigotes. (A and B) Representative profiles of JC-1 mediated red (P2): green (P3) fluorescence in UR6 promastigotes (5 × 105 mL−1), (A) treated with artemisinin (0.125 mM), (B) as described in the Materials and methods section. (C) Log phase UR6 promastigotes (5 × 105 mL−1) incubated with artemisinin (0.06–0.25 mM, 3 h) were stained with JC-1 and analysed as described in the Materials and methods section. Data are expressed as mean % JC-1 green monomers (■)/red aggregates (□) of at least three experiments in duplicate.
Artemisinin decreased ATP levels in Leishmania promastigotes
As oxidative stress and mitochondrial dysfunction can directly or indirectly increase the energy demand of Leishmania parasites, the effect of artemisinin upon levels of ATP were evaluated. The assay specificity was confirmed using oligomycin A, an established inhibitor of the F 0F 1 ATPase complex which decreased ATP levels by 75.89 vs 100.00% (P < 0.001, Fig. 5A). Similarly, artemisinin depleted ATP levels in a dose dependent manner by 35.93 (0.125 mM), 68.80 (P < 0.001, 0.25 mM) and 84.27% (P < 0.001, 0.5 mM), respectively (Fig. 5A). As ATP can be generated from mitochondria and glycolysis, 2DG (an established inhibitor of the glycolytic pathway, 2.5 mM) was added to pinpoint the target of artemisinin mediated depletion of ATP. As 2DG decreased the ATP levels marginally by 18.13% (Fig. 5A), it endorsed that in Leishmania, glycolysis is not the major source of ATP (Blum, Reference Blum1994).
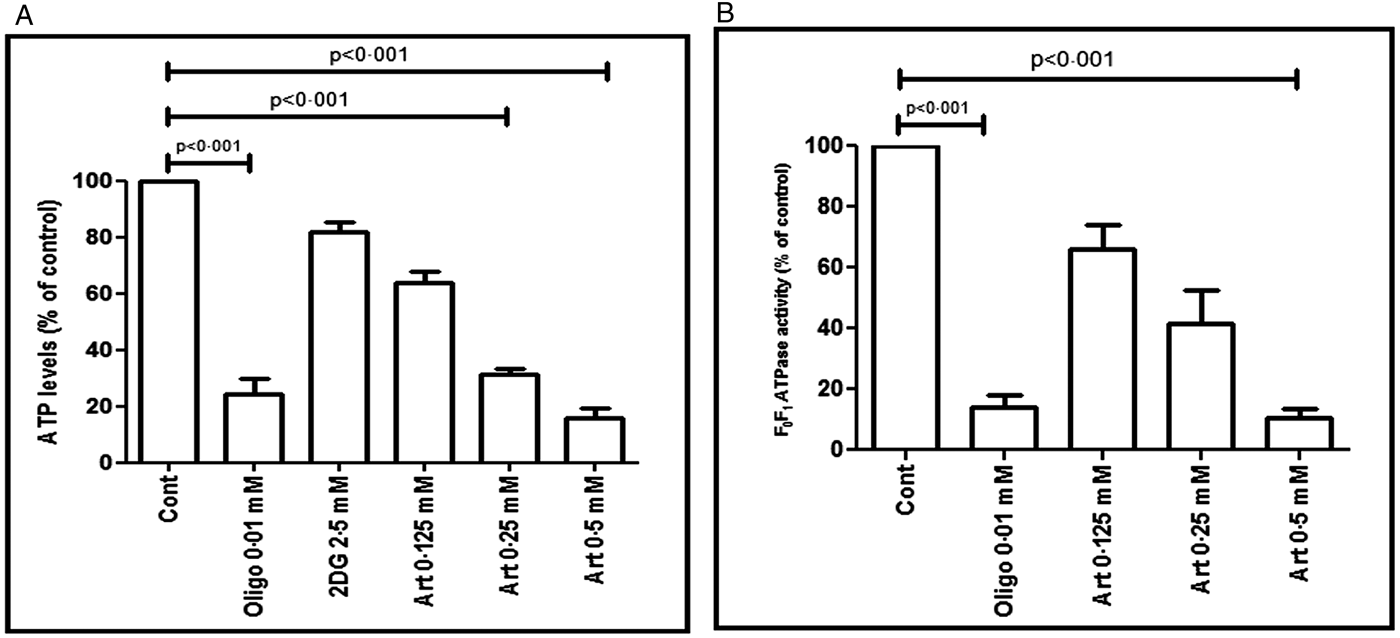
Fig. 5. Effect of artemisinin on ATP levels and F 0F 1-ATPase activity in Leishmania promastigotes. (A) The levels of ATP were determined in UR6 promastigotes (1 × 106 mL−1) following treatment with artemisinin (Art, 0.125–0.5 mM), oligomycin (Oligo, 0.01 mM) or 2DG (2.5 mM). The ATP content was determined by the luciferin/luciferase reaction as described in the Materials and methods section. The results are expressed as the mean ± s.e.m. of ATP levels relative to control (which was considered as 100%) of at least three experiments in duplicate. (B) Mitochondria from UR6 promastigotes treated with artemisinin (Art, 0.125–0.5 mM) were assayed for the F 0F 1-ATPase activity. The pyruvate kinase/lactate dehydrogenase-coupled assay was performed as described in the Materials and methods section, with oligomycin (Oligo, 0.01 mM) as the positive control. Values represent the mean ± s.e.m. (% of control) of at least three experiments in duplicate.
Artemisinin inhibited ATP hydrolysis in UR6 promastigotes
The F 0F 1-ATP synthase (complex V) is responsible for the last step of mitochondrial oxidative phosphorylation. It catalyses ATP synthesis, and an essential requirement for this activity is the presence of a proton gradient. However, if the proton gradient is lost or absent, as for example when there is a collapse of the MMP, only ATP hydrolysis would occur (Faccenda and Campanella, Reference Faccenda and Campanella2012). Oligomycin, a potent inhibitor of complex V, inhibited F 0F 1-ATPase by 84.68% (18.97 ± 5.14 vs 123.90 ± 27.83 nmoles of NADH oxidized min−1 mg−1 protein, P < 0.001, Fig. 5B). Similarly, artemisinin caused a dose dependent inhibition by 23.01, 57.19 and 91.14% as the nmoles of NADH oxidized min−1 mg−1 protein was 95.38 ± 12.38 (0.125 mM), 53.05 ± 1.54 (0.25 mM) and 10.98 ± 4.48 (0.5 mM), respectively (Fig. 5B).
Discussion
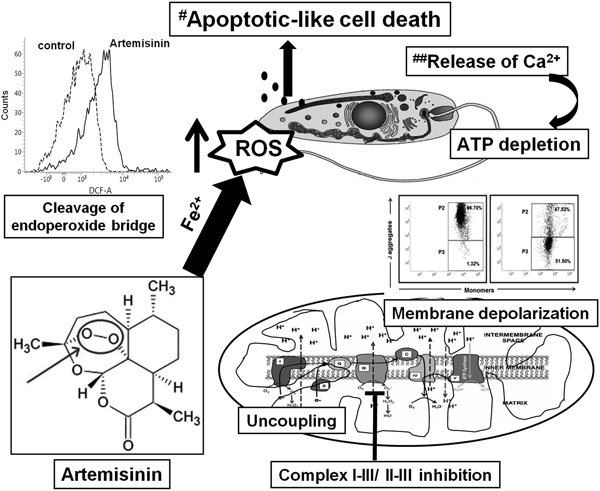
Artemisinin, a sesquiterpene lactone contains an unusual peroxide bridge which is generally unstable in alkaline or acidic conditions as also in the presence of reducing agents such as Fe2+, haem and Cu2+ (Geroldinger et al., Reference Geroldinger, Tonner, Hettegger, Bacher, Monzote, Walter, Staniek, Rosenau and Gille2017). The endoperoxide moiety present in artemisinin is considered crucial for its antiparasitic and anticancer properties (Mercer et al., Reference Mercer, Maggs, Sun, Cohen, Chadwick, O'Neill and Park2007). Similarly, in L. tarentolae, the presence of low molecular iron facilitates cleavage of the endoperoxide bridge leading to generation of carbon and oxygen centred free radicals (Dong and Vennerstorm, Reference Dong and Vennerstrom2003; Geroldinger et al., Reference Geroldinger, Tonner, Hettegger, Bacher, Monzote, Walter, Staniek, Rosenau and Gille2017). This critical involvement of Fe2+ in mediating the leishmanicidal activity of artemisinin was endorsed by Sen et al. (Reference Sen, Saha, Sarkar, Ganguly and Chatterjee2010b). Additionally, the radical generation by artemisinin in Leishmania promastigotes is associated with depolarization of the MMP along with release of Ca2+ that culminates in an apoptotic-like death as evidenced by an increased proportion of the sub-G0 population (Sen et al., Reference Sen, Bandyopadhyay, Dutta, Mandal, Ganguly, Saha and Chatterjee2007). This alteration of MMP can occur secondary to (i) inhibition of the ETC, (ii) stimulation of uncoupling proteins and/or (iii) permeabilization of the inner membrane (Fidalgo and Gille, Reference Fidalgo and Gille2011). Accordingly, this study aims to delineate whether the leishmanicidal activity of artemisinin is secondary to disruption of mitochondrial functions and/or cleavage of the endoperoxide bridges.
As studies with mitochondria require high cell densities, this was achievable in non-pathogenic strains of Leishmania (UR6 and L. tarentolae) promastigotes. However, both strains showed comparable IC50 (around 160 µM), and more importantly, was similar to IC50 obtained in pathogenic strains (Sen et al., Reference Sen, Bandyopadhyay, Dutta, Mandal, Ganguly, Saha and Chatterjee2007). In L. tarentolae, it has been reported that artemisinin acts as a pro-oxidant and thereby mediates its cytotoxicity (Geroldinger et al., Reference Geroldinger, Tonner, Hettegger, Bacher, Monzote, Walter, Staniek, Rosenau and Gille2017). Consequently, the enhanced generation of free radicals by artemisinin was confirmed in UR6 and LtP promastigotes (Fig. 1).
In Leishmania, the single mitochondrion is considered as the major source of ATP, and it is logical to extrapolate that its dysfunction/inhibition would be a potent chemotherapeutic target. Indeed, the parasiticidal activity of conventional anti-leishmanial drugs like amphotericin B, miltefosine and pentamidine has been attributed to enhanced membrane permeability and collapse of the MMP (Lee et al., Reference Lee, Bertholet, Debrabant, Muller, Duncan and Nakhasi2002). The scenario is similar with regard to several plant-derived compounds namely chalcones, phenylphenalenones and aurones (Chen et al., Reference Chen, Zhai, Christensen, Theander and Kharazmi2001; Luque-Ortega and Rivas, Reference Luque-Ortega and Rivas2007 and references therein). Externalization of the phosphatidylserine present in the inner leaflet of the plasma membrane is considered as a marker of apoptosis and endoperoxides like artemisinin induce phosphatidylserine externalization in Leishmania promastigotes, secondary to membrane depolarization (Sen et al., Reference Sen, Bandyopadhyay, Dutta, Mandal, Ganguly, Saha and Chatterjee2007).
The maintenance of the MMP is essential for cell survival as it drives the synthesis of ATP and maintains oxidative phosphorylation (Gottlieb, Reference Gottlieb2001). In view of artemisinin being reported to cause collapse of the MMP in malaria parasites and Leishmania (Sen et al., Reference Sen, Bandyopadhyay, Dutta, Mandal, Ganguly, Saha and Chatterjee2007; Antoine et al., Reference Antoine, Fisher, Amewu, O'Neill, Ward and Biagini2014), it was corroborated in UR6 promastigotes (Fig. 4) and considered logical to examine the impact of artemisinin on the ETC of mitochondria sourced from UR6 and/or LtP. In mammalian mitochondria, complex III has been reported to be the major site for generation of ROS (St-Pierre et al., Reference St-Pierre, Buckingham, Roebuck and Brand2002). Plant-derived compounds like xanthohumol exert their anti-leishmanial activity, secondary to inhibition of the mitochondrial electron transfer complexes II–III (Monzote et al., Reference Monzote, Lackova, Staniek, Steinbauer, Pichler, Jäger and Gille2017). Endoperoxides like ascaridole exhibit their leishmanicidal activity by disrupting the MMP (Monzote et al., Reference Monzote, García, Pastor, Gil, Scull, Maes, Cos and Gille2014). The immediate inhibition by artemisinin of NCC and SCC activity (Fig. 2A and B), representative of complexes I–III and II–III activity, respectively, in Leishmania suggested impairment of the mitochondrial ETC, and would translate into enhanced generation of superoxide. However, artemisinin at higher concentrations and prolonged incubation failed to generate mitochondrial superoxide in Leishmania promastigotes (UR6 and LtP, Fig. 2C and D), implying that although complexes I–III and II–III were inhibited by artemisinin, it did not enhance the formation of ROS via mitochondria, at least under the experimental conditions maintained this study. This was corroborated by the inability of artemisinin to impact on oxygen consumption within Leishmania mitochondria (Fig. 3).
The survival of cells hinges on its ability to meet its energy requirements. During normal/unstressed conditions, mammalian cells utilize a fraction of their mitochondrial bioenergetic capacity. However, when energy demand exceeds supply, the difference between the maximal respiratory capacity and basal respiratory capacity, referred to as the ‘spare or reserve respiratory capacity’ ensures a greater availability of ATP. This spare capacity is generally measured in terms of the maximum uncoupler-stimulated mitochondrial respiration subtracted from the coupled mitochondrial respiration. In a mammalian J774 macrophage cell line, the uncoupler-accelerated respiration is more than 200% of mitochondrial respiration whereas in LtP, the maximal uncoupled mitochondrial respiration is barely above normal mitochondrial respiration (Monzote et al., Reference Monzote, Lackova, Staniek, Steinbauer, Pichler, Jäger and Gille2017, Fig. 3B). This inherent low coupling efficiency of Leishmania confirmed the parasites limited ‘spare respiratory capacity’ and accounted for the limited increase in O2 consumption following inclusion of artemisinin with oligomycin (Fig. 3B and C).
The depletion of ATP by artemisinin (Fig. 5A) can principally result from multiple sources that include (i) inhibition of the ETC, (ii) inhibition of complex V (F 0F 1-ATP synthase), (iii) disruption of the MMP and/or (iv) increased ATP hydrolysis. The F 0F 1-ATPase in Leishmania spp. consists of two oligomeric components F 0 and F 1, the former being an integral membrane protein that contains the proton channel, whereas the latter is a peripheral membrane protein (Sen et al., Reference Sen, Das, Ganguly, Mukherjee, Bandyopadhyay and Majumder2004; Roy et al., Reference Roy, Ganguly, Bose Dasgupta, Das, Pal, Jaisankar and Majumder2008). Importantly, the synthesis of ATP requires involvement of both the F 0 and F 1 components and a chemiosmotic gradient (Monzote and Gille, Reference Monzote and Gille2010). It can therefore be proposed that loss of MMP by artemisinin (Sen et al., Reference Sen, Bandyopadhyay, Dutta, Mandal, Ganguly, Saha and Chatterjee2007) can have a deleterious impact on the F 0 − F 1 ATPase and cause ATP depletion. However, this was not the case with artemisinin as it failed to impact on O2 consumption (Fig. 3). Plant-derived anti-leishmanial compounds like camptothecin and 3,3′-diindolylmethane have been demonstrated to cause depolarization of MMP and inhibition of complex V, F 0F 1-ATP synthase leading to decreased levels of ATP (Sen et al., Reference Sen, Das, Ganguly, Mukherjee, Bandyopadhyay and Majumder2004; Roy et al., Reference Roy, Ganguly, Bose Dasgupta, Das, Pal, Jaisankar and Majumder2008). In view of artemisinin and oligomycin significantly inhibiting the F 0F 1-ATPase activity (Fig. 5B), it suggested that both compounds decreased ATP hydrolysis. However, unlike artemisinin, oligomycin strongly inhibited oxygen consumption (Fig. 3B) and thus displayed inhibition of ATP synthase and ATP hydrolysis (Grover et al., Reference Grover, Atwal, Sleph, Wang, Monshizadegan, Monticello and Green2004), the former being stronger and accounted for the depletion of ATP (Fig. 5B).
The mobilization of Ca2+ is an essential requirement for programmed cell death, as most endonucleases utilize Ca2+ to cleave DNA strands (Lee et al., Reference Lee, Bertholet, Debrabant, Muller, Duncan and Nakhasi2002). Therefore, it can be proposed that the observed apoptosis by artemisinin enhanced the release of Ca2+ (Sen et al., Reference Sen, Saha, Sarkar, Ganguly and Chatterjee2010b), which translated into an upregulation of ATP dependent Ca2+ efflux pumps. This resultant demand for ATP (Jiang et al., Reference Jiang, Mousawi, Yang and Roger2017) perhaps outnumbered the parasites ability to ATP depletion (Fig. 5A).
The enhanced presence of ROS (Fig. 1) can also be attributed to artemisinin manipulating the relatively weak anti-oxidant arsenal of Leishmania parasites. The anti-oxidant defense of Leishmania includes enzymatic (e.g. superoxide dismutase and ascorbate peroxidases) along with non-enzymatic defenses (tryparedoxin and related molecules) (Hermans et al., Reference Hermans, Cos, Maes, De Bruyne, Vanden Berghe, Vlietinck and Pieters2007; van Assche et al., Reference van Assche, Deschacht, da Luz, Maes and Cos2011; Das et al., Reference Das, Aich and Shaha2015). In antimonial resistant L. donovani field isolates, the increased levels of thiols have been proposed to confer resistance by protecting them against antimony mediated oxidative stress (Mandal et al., Reference Mandal, Wyllie, Singh, Sundar, Fairlamb and Chatterjee2007). Similarly, Sen et al. (Reference Sen, Bandyopadhyay, Dutta, Mandal, Ganguly, Saha and Chatterjee2007) demonstrated that artemisinin caused depletion of thiols, collectively indicating that the enhanced generation of ROS by artemisinin could be attributed to cleavage of the endoperoxide bridge, along with depletion of thiols. This would be secondary to modulation of ROS mediated signalling pathways which remains to be examined. Taken together, the enhanced labile iron pool in Leishmania (Geroldinger et al., Reference Geroldinger, Tonner, Hettegger, Bacher, Monzote, Walter, Staniek, Rosenau and Gille2017) facilitated cleavage of the endoperoxide bridge, rendering the drug to itself act as a radical (Fig. 6). In Leishmania promastigotes, artemisinin demonstrated a minimal inhibitory effect on the mitochondrial ETC, as also showed no immediate uncoupling effect. However, its ability to cause depolarization of the mitochondrial transmembrane potential, perhaps secondary to release of Ca2+ caused depletion of ATP which culminated in an apoptotic-like cell death (Fig. 6).
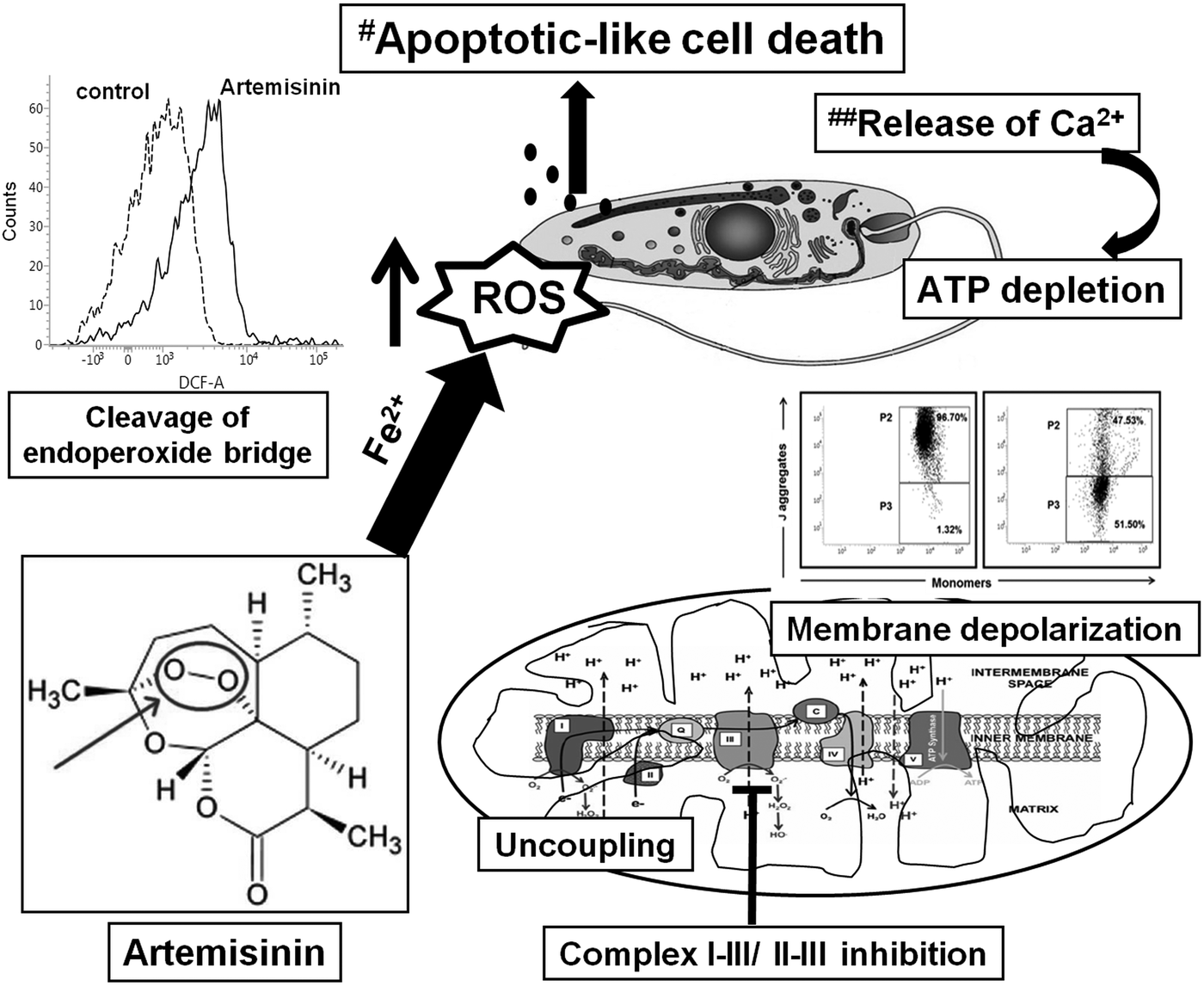
Fig. 6. A proposed model for artemisinin mediated cell death in Leishmania promastigotes. The cleavage of the endoperoxide bridge of artemisinin was the major contributor towards generation of ROS in Leishmania promastigotes, along with mitochondrial dysfunction as evidenced by depolarization of the mitochondrial membrane, marginal uncoupling and partial inhibition of the mitochondrial ETC. This translated into depletion of ATP and along with an enhanced release of Ca2+ (##Sen et al., Reference Sen, Ganguly, Saha and Chatterjee2010a, Reference Sen, Saha, Sarkar, Ganguly and Chatterjee2010b), led to an apoptotic-like death (#Sen et al., Reference Sen, Bandyopadhyay, Dutta, Mandal, Ganguly, Saha and Chatterjee2007).
Financial support
The work was supported by the International Bilateral Cooperation Division, Dept. of Science & Technology (DST), Govt. of India INT/AUSTRIA/BMWF/P-06/2017, and Austrian Exchange Office (OEAD) in the Scientific & Technological Cooperation project with India IN 04/2017, Austrian Science Fund (FWF), grant P 27814-B22, Fund for Improvement of S&T infrastructure in Universities and Higher Educational Institutions (FIST) Program, DST, Govt. of India, SR/FST/LS1-049/2010 and SR/FST/LS1-663/2016 and Dept. of Health Research, Govt. of India, ‘Establishment of Multidisciplinary Research Unit’ no. V.25011/103/2016-HR. AD is a recipient of a Junior Research Fellowship from University Grants Commission, Govt. of India.
Conflict of interest
None.
Ethical standards
Not applicable.
Author ORCIDs
Lars Gille http://orcid.org/0000-0003-1223-0201.
Mitali Chatterjee http://orcid.org/0000-0003-1203-1384.