Introduction
There has been significant progress with our knowledge of Harpacticoida and Canuelloida (Copepoda, Crustacea) assemblages on seamounts and islands in the North-eastern Atlantic Ocean and eastern Mediterranean Sea (George & Schminke, Reference George and Schminke2002; George, Reference George2004a, Reference George2004b; Plum & George, Reference Plum and George2009; Büntzow, Reference Büntzow2011; Koller & George, Reference Koller and George2011; Packmor et al., Reference Packmor, Müller and George2015; Pointner, Reference Pointner2015, Reference Pointner2017; Packmor & George, Reference Packmor and George2016; Packmor & Riedl, Reference Packmor and Riedl2016; George et al., Reference George, Pointner and Packmor2018; Pointner, Reference Pointnerin press). As a result of this work, it has been hypothesized that seamounts and islands play an important role in meiofaunal species distribution and may even act as stepping stones or staging posts (Packmor et al., Reference Packmor, Müller and George2015) for Harpacticoida and Canuelloida. The role of seamounts in species distribution was first discussed by Hubbs (Reference Hubbs1959) for marine organisms in general and was later adopted for meiobenthos (George & Schminke, Reference George and Schminke2002; Gad & Schminke, Reference Gad and Schminke2004). This group of benthic animals typically lacks planktonic life-cycle stages (Giere, Reference Giere2009), yet several shallow-water species appear to have cosmopolitan distributions (George, Reference George2005; Song et al., Reference Song, Rho and Kim2007). Hence, if these species are dispersed in the water column or in surface waters, seamounts and islands may provide suitable habitats on which they may cross the deep sea to other shallow-water coastal habitats.
To evaluate this hypothesis, the benthic Copepoda of as many seamounts as possible need to be inventoried. This work has already begun in the North-east Atlantic Ocean, though mostly on seamounts and islands in close proximity to each other. The Great Meteor Seamount (GMS; 30°N 28°30′W; Grevemeyer, Reference Grevemeyer1994) is of special interest because it is geographically isolated from other investigated elevations (George et al., Reference George, Pointner and Packmor2018) and continental mainland (the African west coast is 800 nautical miles away; Ulrich, Reference Ulrich1971). It is ‘guyot’-shaped, the summit has a surface area of 1465 km2 (Fischer, Reference Fischer2005). On the plateau two mesoscale pinnacles rise 100 m above the middle region, one in the north and one in the south (Ulrich, Reference Ulrich1971; Mohn & Beckmann, Reference Mohn and Beckmann2002). The entire seamount rises from 4200 m depth up to 270 m below sea level (Hinz, Reference Hinz1969). Due to its geomorphology, it is surrounded by a complex current system (Beckmann & Mohn, Reference Beckmann and Mohn2002; Mohn & Beckmann, Reference Mohn and Beckmann2002). It consists of an upper thermocline layer, containing several vortexes, and a seamount summit layer with an anticyclonic flow around the summit, which are connected by upwelling above the slopes and downwelling above the centre of the plateau (Beckmann & Mohn, Reference Beckmann and Mohn2002; Mohn & Beckmann, Reference Mohn and Beckmann2002; Mohn, Reference Mohn2010). Consequently, the plateau becomes relatively isolated for passive particles retained near the seamount surface (Beckmann & Mohn, Reference Beckmann and Mohn2002). Nonetheless, tidal and internal tidal motions (Mouriño et al., Reference Mouriño, Fernández, Serret, Harbour, Sinha and Pingree2001; van Haren, Reference van Haren2005) and strong weather events on GMS may result in the resuspension of particles into the water column (Beckmann & Mohn, Reference Beckmann and Mohn2002). The two sub-mesoscale pinnacles on the plateau may influence particle movement (Mohn & Beckmann, Reference Mohn and Beckmann2002).
The GMS is one of the best studied seamounts with regards to meiofauna (George, Reference George2013 and references therein), and different aspects based on qualitative meiofaunal material have already been analysed: The plateau-fauna is considered to be related to the deep-sea fauna, due to close similarities between the plateau-species of Argestidae Por, 1986 and species of the surrounding deep sea (George, Reference George2004a). Differences in species composition across the plateau were observed in nematode Draconematidae Filipjev, 1918 (Gad, Reference Gad2009) and invertebrate megabenthos (Piepenburg & Müller, Reference Piepenburg and Müller2004). Furthermore, the meiofaunal community of only a small number of seamounts and islands has been analysed so far (George, Reference George2013). Even fewer studies have been conducted at species level of Harpacticoida and Canuelloida, but these investigations show that the same species can occur on more than one elevation (Büntzow, Reference Büntzow2011; Packmor & Riedl, Reference Packmor and Riedl2016; Packmor et al., Reference Packmor, Müller and George2015; Packmor & George, Reference Packmor and George2016; George et al., Reference George, Pointner and Packmor2018).
However, this present study is the first using quantitative material, collected in a grid-like pattern across the whole plateau using a single device. Thus, it was possible to analyse the Harpacticoida and Canuelloida (= benthic Copepoda) assemblages on the plateau as part of the inventory of Atlantic elevations. Therefore, the present contribution addresses four questions:
(1) Does the community structure of Harpacticoida and Canuelloida assemblages differ across the GMS plateau?
(2) Are the benthic copepod species found on the GMS plateau closely related to deep-sea species?
(3) According to the taxonomic diversity index, is the benthic copepod fauna of the GMS plateau distinct, with a high number of (probably endemic and) scientifically unknown species?
(4) Does the copepod community structure (composition and diversity) on the GMS plateau differ from those on other seamount summits and islands?
Materials and methods
Sampling and treatment of samples
During the RV ‘Poseidon’ cruise POS397 (‘GroMet’ expedition, March 2010, see George, Reference George2010), 21 locations equally distributed over the plateau of the GMS (Figure 1, Table 1) were sampled with a Van Veen grab (surface area: 0.1 m2). At each station three replicates were taken (exceptions: two replicates at stations #14 and #20, four replicates at station #18), adding up to 62 meiofauna samples in total. Due to the differences in the community structure found in Nematoda (Gad, Reference Gad2009) across the plateau, these locations were divided into the three regions, north (N; stations #1–#6), middle (M; stations #7–#14) and south (S; stations #15–#21). The samples were preserved with 96% undenatured ethanol on board and later in the laboratory centrifuged with a mixture of colloidal silica polymer and kaolin to separate meiofaunal organisms from the remaining sediment (for detailed information see Pointner et al., Reference Pointner, Kihara, Glatzel and Veit-Köhler2013).

Fig. 1. Map of sampling localities (#1 – #21) on the Great Meteor Seamount plateau during the expedition POS397 GroMet of the RV ‘Poseidon’ in 2010.
Table 1. List of stations sampled during the RV ‘Poseidon’ expedition POS397 GroMet to the Great Meteor Seamount plateau in 2010

Region (N: north, M: middle, S: south), station, number of replicates, sampling date, coordinates and depth (m) are given.
The extracted meiofaunal organisms from all samples were determined to major taxon level under a Leica MZ 12.5 stereomicroscope and enumerated. Copepoda were separated from the samples. Copepodids were counted but not identified, as they cannot be unequivocally identified to species level (George, Reference George1999). Adult specimens of Harpacticoida and Canuelloida were identified to family and then species level using a Leica DMR microscope and with reference to Huys et al. (Reference Huys, Gee, Moore and Hamond1996), Wells (Reference Wells2007) and original species descriptions. Due to the large amount of adult specimens, 18 families were haphazardly selected for species identification.
To analyse possible dispersion methods such as crawling and drifting of Harpacticoida and Canuelloida on the plateau, all identified species were divided into active emergent (drifting as possible dispersion method) and non-emergent (crawling as possible dispersion method) Copepoda following the morphological characters given by Thistle & Sedlacek (Reference Thistle and Sedlacek2004): the endopods of P2–P4 of active emergers are three-segmented and bear more than four setae at each distal segment, whereas the ones of non-emergers are at most two-segmented and bear at most four setae at the distal segments.
Statistical analysis
Stations on the plateau were compared with each other to analyse the community structure of Harpacticoida and Canuelloida. Due to the low number of replicates, the calculation of the arithmetic mean was not possible, and the median was not appropriate due to the heterogeneous structure of the species matrix. Additionally, at stations M-#14 and S-#20 only two, but at station S-#18 four, replicates were sampled. To include these stations of different sample sizes in the statistical analyses, the total abundances of the taxa identified across the plateau were standardized to the largest shared area of 0.2 m2. Nauplii are listed separately, as it is not possible to assign them clearly to Copepoda or another taxon of Crustacea.
A similarity analysis was conducted on both family- and species level data for Harpacticoida and Canuelloida, Cosine-Similarity (Pfeifer et al., Reference Pfeifer, Bäumer, Dekker and Schleier1998) was applied because it accounts for both taxa composition and abundances and can be visualized as a non-metric multidimensional scaling (nMDS) ordination.
To give a rough assessment of the number of taxa potentially overlooked (i.e. not sampled), the extrapolation procedure Jackknife1 was calculated (Heltshe & Forrester, Reference Heltshe and Forrester1983; Palmer, Reference Palmer1990, Reference Palmer1991; Colwell & Coddington, Reference Colwell and Coddington1994).
The Shannon diversity index (H’) and Pielou's Evenness (J) were calculated to analyse diversity at species level. H’ combines abundance and evenness of all species (Shannon & Weaver, Reference Shannon and Weaver1963), but changes with the number of species and/or evenness (Gray, Reference Gray1984). Hence, J was calculated to describe the distribution of specimens between species.
Average taxonomic diversity (Δ+) was calculated to assess the taxonomic relatedness of species (Clarke & Warwick, Reference Clarke and Warwick1998). Its output values range from 0–100; smaller values indicate more closely related species, and therefore fewer families/genera present in an assemblage. Linear regression analyses were conducted for these indices to identify changes across the plateau.
Additionally, a Rarefaction analysis (Achtziger et al., Reference Achtziger, Nigmann and Zwölfer1992) was applied to compare faunal diversity across the stations. This method employs interpolation to calculate the expected number of species for a consistently rising number of specimens (Gray, Reference Gray1984; George, Reference George1999) and thus enables comparison between samples of different sizes (Achtziger et al., Reference Achtziger, Nigmann and Zwölfer1992).
All analyses were conducted with R (R Core Team, 2017) and the packages ‘lsa’ (Wild, Reference Wild2015) and ‘vegan’ (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson, Solymos, Stevens, Szoecs and Wagner2017).
Results
General results
On the plateau, 263,648.5 meiofaunal organisms per 0.2 m2 were identified (387,767 ind. counted in total), belonging to 26 different major taxa. The most abundant were Nematoda (72.48%), followed by Copepoda (11.39%) and Annelida (6.55%, incl. fragments). The remaining 22 taxa each represented <1% of the total density (Figure 2; see also electronic Supplementary Table S1). Additionally, 7.25% of the identified meiofaunal organisms were nauplii. In total, 43,969 Copepoda were identified (30,013.7 ind. per 0.2 m2), of which 36,864 belong to benthic Copepoda (adults: 18,876 in total (12,891.7 ind. per 0.2 m2); copepodids: 17,988 in total (12,346 ind. per 0.2 m2)).
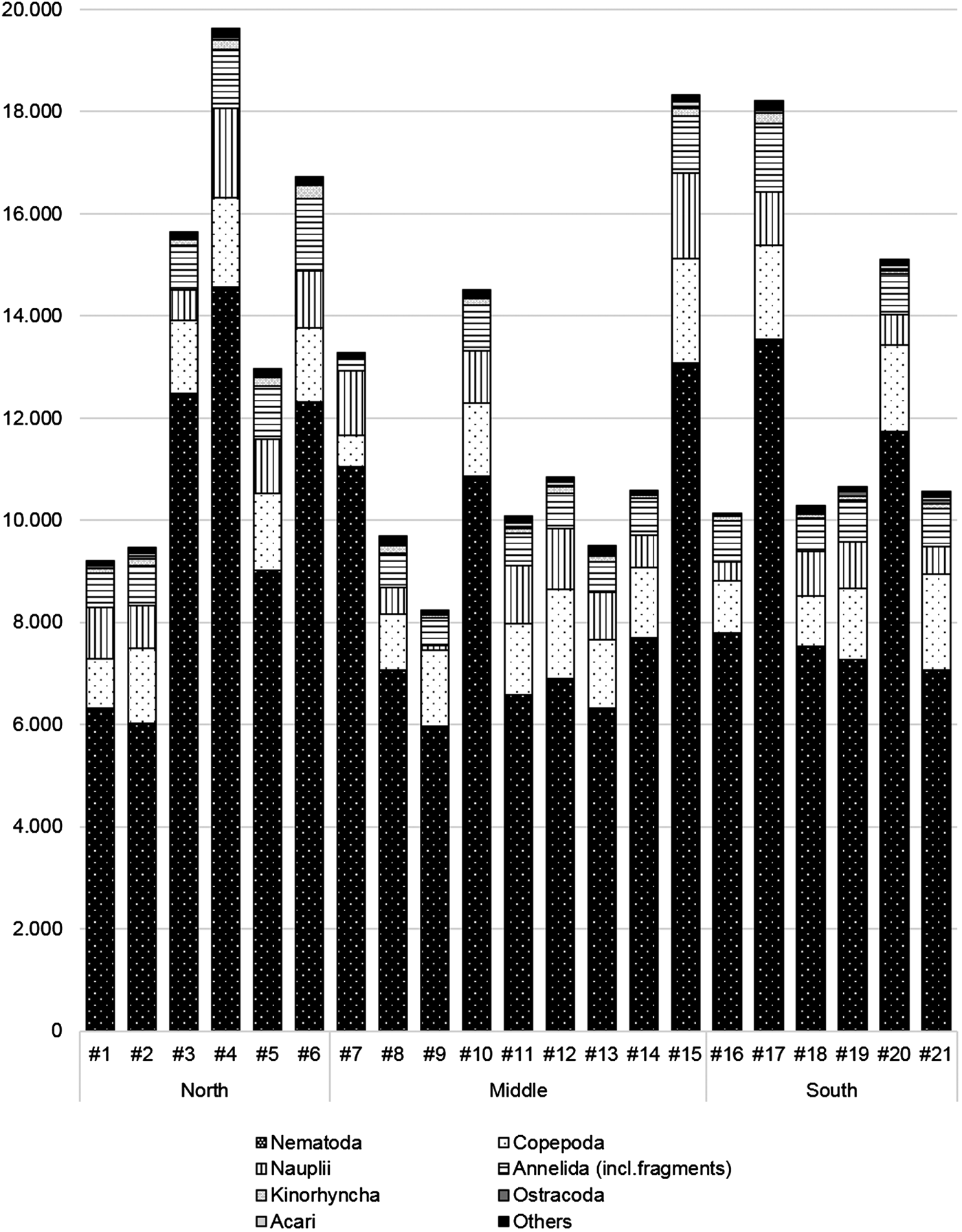
Fig. 2. Densities (ind. per 0.2 m2) of meiofaunal taxa at each station (#1 – #21) on the Great Meteor Seamount plateau. Others = Brachiopoda, Chaetognatha, Coelenterata, Cumacea, Gastropoda, Holothuria, Pantopoda, Priapulida, Rotifera, Sipunculida, Tantulocarida.
The Harpacticoida and Canuelloida community structure of the GMS plateau
Analysis of similarity
The investigated material contained 12,891.7 ind. per 0.2 m2 adult Harpacticoida and Canuelloida (18,863 ind. in total), belonging to 24 different families (Table 2). Within these, the most common families were Paramesochridae Lang, 1944 (26.36%), Miraciidae Dana, 1846 (17.34%), Zosimeidae Seifried, 2003 (16.46%), Ameiridae Boeck, 1865 (10.87%) and Ectinosomatidae Sars, 1903 (10.77%). All other families were represented by less than 4% of the total individual number. Within all benthic Copepoda, 117 individuals (0.68%) could not be identified to family and are listed as ‘Harpacticoida incertae sedis’.
Table 2. Densities (ind. per 0.2 m2) of all adult copepods belonging to Harpacticoida and Canuelloida families at each station (#1 – #21) on the Great Meteor Seamount plateau, empty cell: absent

The total densities (SUM) for each station in the last row, and for each taxon in the penultimate column. Relative abundances (%) on the plateau for each taxon in the last column.
All families (except Harpacticidae Dana, 1846 and Idyanthidae Lang, 1944) were present in each region of the plateau, but not in equal numbers (Table 2). Zosimeidae (19.65%) and Canuellidae Lang, 1944 (3.62%) were most abundant in the northern region, Miraciidae (20.14%), Cylindropsyllidae Sars, 1909 (3.18%) and Leptastacidae Lang, 1948 (2.92%) in the middle region, and Ectinosomatidae (12.99%) and Ameiridae (12.05%) in the southern region. Harpacticidae and Idyanthidae were recorded in the middle and southern regions and were absent from the northern region. Nonetheless, the analysis of similarity at the family level revealed no differences between the three regions of the plateau (Figure 3A).

Fig. 3. Two-dimensional non-metric multidimensional scaling (nMDS) ordination based on the Cosine-Similarity of density data of Harpacticoida and Canuelloida communities on the GMS plateau. (A) all families identified (stress: 0.11) and (B) identified species from the investigated families (stress: 0.15). Symbols indicate the three geographic regions of the stations: ○ = north, x = middle, Δ = south.
Specimens from 18 families, i.e. 6403 individuals (accounting for 33.95% of all adult Harpacticoida and Canuelloida), were identified to species level. This resulted in 106 species, 94 of which are scientifically unknown ‘working species’ (electronic Supplementary Table S2). The number of species recorded for each family are as follows: Aegisthidae Giesbrecht, 1893 (3 species), Argestidae Por, 1986 (13 species), Canthocamptidae Brady, 1880 (15 species), Canuellidae (1 species), Cletodidae T. Scott, 1904 (2 species), Cletopsyllidae Huys & Willems, 1989 (1 species), Cylindropsyllidae (6 species), Dactylopusiidae Lang, 1936 (2 species), Harpacticidae (3 species), Idyanthidae (3 species), Laophontidae T. Scott, 1904 (7 species), Leptastacidae (2 species), Leptopontiidae Lang, 1948 (5 species), Nannopodidae Brady, 1880 (4 species), Neobradyidae Olofsson, 1917 (6 species), Pseudotachidiidae Lang, 1936 (14 species), Tetragonicipitidae Lang, 1944 (7 species) and Zosimeidae (12 species). The extrapolation procedure Jackknife1 estimated a maximum of 133.69 ± 11.61 species within the investigated families, indicating that 72.95–86.83% of the species were sampled and recognized during this investigation.
Analysis of similarity on species level data again showed no differences among the three regions (Figure 3B); in fact, several stations from different regions were more similar to each other than to stations from the same region (e.g. S-#18, N-#3 and M-#12). Several species were heterogeneously distributed across the plateau: They were not present at every station of a region (S2), but they were found in several stations from different regions (e.g. Dactylopodopsis sp.1, Mesocletodes sp.1, Metahuntemannia sp.4, Zosime sp.4).
The similar stations M-#7 and M-#14 as well as station S-#20 plotted separately, owing to high dominance of species that were otherwise found in low abundances across the plateau (e.g. Argestidae gen.1 sp.1 at station M-#7, Zosime sp.2 at station M-#14 and Sextonis sp.2 at station S-#20), combined with a rarity or absence of species (e.g. Boreopontia heipi Willems, Reference Willems1981 at station M-#7 and M-#14, Sextonis sp.1 at station S-#20, Zosime anneae Koller & George, Reference Koller and George2011 at M-#7, M-#14 and S-#20), being present in high abundances elsewhere (S2).
While the assemblages of Harpacticoida and Canuelloida of the GMS are relatively homogenous on family level, their species composition is heterogeneous.
Analysis of diversity
Diversity analysis of species-level data found a relatively uniform diversity across the plateau (Table 3, Figure 4), with H’ slightly higher in the southern region (linear regression: coefficient r = 0.4971; stability index r 2 = 0.2471). This increased diversity reflects a higher number of species: J is more consistent across the plateau and does not correlate with the geographic region (r = 0.2773; r 2 = 0.0769; Figure 4B). Additionally, Δ+ fluctuates from 74.46 (M-#13) to 77.91 (S-#21) and is slightly higher in the southern region (Δ+: r = 0.2946; r 2 = 0.0868).

Fig. 4. Diversity indices for each station in the northern, middle and southern regions. (A) number of species (S), (B) Pielou's Evenness (J), (C) rarefaction values 160 individuals (E(S 160)).
Table 3. Diversity indices for each region (North, Middle, South; merged) and each station

N, number of individuals (ind. per 0.2 m2); S, number of species; H’, Shannon diversity index; J, Pielou's Evenness; Δ+, average taxonomic diversity; E(Sn), rarefaction values for 30, 160, 500 and 1800 individuals.
The slightly higher average diversity in the southern region is further highlighted by Rarefaction analysis (Figure 5A): With lower individual numbers (N < 30 specimens) the curves indicate similar evenness, but as the number of specimens rises the curves diverge. The gradient of the species accumulation curves increases from the northern region to the middle and then the southern region. From the northern region 56 species were sampled, 73 from the middle region and 90 from the southern region. This compares with 55, 68 and 86 estimated species by rarefaction, E(S1,800) (Figure 5A, Table 3). Additionally, two species were exclusively recorded from the northern region, compared with nine from the middle region and 26 unique species reported from the southern region.

Fig. 5. Rarefaction curves for the number of expected Copepoda species (E(S n)) on the Great Meteor Seamount plateau, based on the number of specimens (N). (A) Merged curves for each region (north, middle, south); and estimated diversity curves for (B) the northern stations, (C) the middle stations and (D) the southern stations.
However, differences noted between regions were confirmed when single station data were examined (Figure 5B–C, Table 3). Estimated diversity for some northern stations (N-#3, N-#4) was higher than for some middle region stations (M-#13, M-#14) and even more so than for southern stations (S-#16, S-#18). Several stations in the middle region (M-#11, M-#12) also had a greater estimated diversity than southern stations (e.g. S-#19, S-#20). Nevertheless, all northern stations had a similar estimated species richness (Figure 4C; lowest E(S160): 21 species at station N-#1; highest E(S160): 26 species at station N-#3). However, it was more variable in the middle and the southern regions, ranging from E(S160) 22 (M-#13) to 33 (M-#7) species in the middle region, and 27 (S-#18) to 37 (S-#17) species in the southern region. The maximum diversity was expected for different numbers of species and with different numbers of sampled specimens in each region (Figure 4A): 35 species were expected within 377 specimens in the northern region (N-#4), 37 species within 374 specimens in the middle region (M-#12) and 50 expected specimens within 353 specimens in the southern region (S-#17). Hence, the southern region is expected to have the highest species diversity.
Analysis of emergence of Harpacticoida and Canuelloida
According to the distinctive characters given by Thistle & Sedlacek (Reference Thistle and Sedlacek2004), 56.60% of the identified plateau-species are non-emergent and 37.74% are actively emergent Copepoda, 5.66% could not be clearly determined (S2).
The origin of the plateau fauna
Of the 106 species identified, 40 (37.74%) belonged to genera solely recorded in shallow water (0–200 m), a further 27 (25.47%) to genera which exclusively inhabit the deep sea and the remaining 39 species (36.79%) belong to eurybathic genera. So far, nine species recorded on the GMS plateau have a recorded eurybathic distribution.
Analysis of isolation on the plateau
On the plateau of the GMS 106 species were identified, but only 16 (15.09%) were already known to science (S2), 10 of which are known exclusively from the GMS (Bodinia meteorensis George, 2004, B. peterrummi George, 2004, Cylindropsyllus flexibilis Pointner, in press, Cylindropsyllus valentini Pointner, Reference Pointnerin press, Meteorina magnifica George, 2004, Microcanuella secunda Pointner, Reference Pointner2015, Monsmeteoris reductus Pointner, Reference Pointnerin press, Monsmeteoris wiesheuorum Pointner, in press, Zosime carsteni Pointner, Reference Pointner2017 and Zosime eliasi Pointner, Reference Pointner2017; George, Reference George2004a, Reference George2004b; Pointner, Reference Pointner2015, Reference Pointner2017, Reference Pointnerin press). The six (5.66%) remaining known species have a geographically wide distribution (Table 4): Asellopsis intermedia (T. Scott, Reference Scott1895) has been recorded from the Arctic Ocean to the Mediterranean Sea; Boreopontia heipi from the North Sea and the Atlantic Ocean, Huntemannia jadensis Poppe, Reference Poppe1884 from the Barents Sea and White Sea to the English Channel and Pacific Ocean; Selenopsyllus dahmsi Moura & Pottek, Reference Moura and Pottek1998 from the South-east Atlantic Ocean and the Antarctic Ocean, and Zosime bergensis Drzycimski, 1968 from the Norwegian Sea and the North-east Atlantic Ocean; whilst Zosime anneae has been previously recorded only on the Seine Seamount, and is the sole species shared between GMS and Seine seamount.
Table 4. Worldwide distribution of Asellopsis intermedia, Boreopontia heipi, Huntemannia jadensis, Selenopsyllus dahmsi and Zosime bergensis

Comparison of Harpacticoida and Canuelloida assemblages on GMS and other seamount summits and islands
On the GMS plateau, average Copepoda density was 7.15 ind. per 10 cm2 (1429.2 ind. per 0.2 m2), ranging from 3.01 ind. per 10 cm2 (602.7 ind. per 0.2 m2) at station M-#7 to 10.20 ind. per 10 cm2 (2039.3 ind. per 0.2 m2) at station M-#15 (Table S1). In comparison, average Copepoda density was 36.72 ind. per 10 cm2 on Seine Seamount (Büntzow, Reference Büntzow2011), and 14.41 ind. per 10 cm2 on Anaximenes Seamount (ranging from 5.33 ind. per 10 cm2 on the north-western slope to 63.90 ind. per 10 cm2 on the north-eastern slope: George et al., Reference George, Pointner and Packmor2018). No density values are available for Copepoda on the Sedlo Seamount, but adult Harpacticoida density ranged from 1.0 to 159 ind. per 10 cm2 on Madeira Island (Packmor & George, Reference Packmor and George2016) and 56.7 to 155.0 ind. per 10 cm2 on Porto Santo Island (Packmor & George, Reference Packmor and George2016). Thus, Copepoda density on the GMS plateau is the lowest recorded so far.
The occurrence of Copepoda families across the studied seamount summits and islands and their diversity identifies a number of differences (Table 5). On GMS plateau 26 families of Harpacticoida and Canuelloida have been recorded (Table 5), compared with 24 on Seine Seamount (Büntzow, Reference Büntzow2011), 19 on Sedlo Seamount (Büntzow, Reference Büntzow2011), 27 on Madeira Island and seven on Porto Santo Island (Packmor & George, Reference Packmor and George2016) in the Atlantic, as well as 32 families recorded on Anaximenes Seamount (George et al., Reference George, Pointner and Packmor2018) in the eastern Mediterranean. Despite this, only three families (Ameiridae, Ectinosomatidae, Paramesochridae) have been recorded on all elevations (Table 5). The GMS shares seven families with the above elevations, with the exception of Porto Santo Island, seven further families are shared with Madeira Island, two with each of Seine Seamount and Porto Santo Island, and one each with Sedlo and Anaximenes seamounts. Two families are exclusively known from the GMS: Cletopsyllidae, detected on the GMS plateau for the first time by George & Schminke (Reference George and Schminke2002) as well as during the present study, and Rometidae Seifried & Schminke, (Reference Seifried and Schminke2003). The latter was recorded by Seifried & Schminke (Reference Seifried and Schminke2003) but not during the present study. Two families are solely reported from the Seine, four from Madeira Island and 10 from Anaximenes, although the species Harpacticoida fam.1 – fam.9 recorded at this latter seamount require further study (George et al., Reference George, Pointner and Packmor2018).
Table 5. Comparison of all families identified on the following elevations (abbreviation; reference): Great Meteor Seamount (GMS; George & Schminke, Reference George and Schminke2002; Seifried & Schminke, Reference Seifried and Schminke2003; present contribution), Seine Seamount (SeiS; Büntzow, Reference Büntzow2011), Sedlo Seamount (SedS; Büntzow, Reference Büntzow2011), Anaximenes Seamount (AS; George et al., Reference George, Pointner and Packmor2018), Madeira Island (M; Packmor & George, Reference Packmor and George2016) and Porto Santo Island (PS; Packmor & George, Reference Packmor and George2016). Bold: investigated families on the GMS plateau in the present contribution; x: present, empty cell: absent

At the species level a very different picture is revealed. For this analysis, only the 18 families identified to species level on the GMS plateau were compared. Within these families, 106 species were identified on the GMS, 41 species on Seine seamount, 30 on Sedlo seamount, 115 on Anaximenes seamount, 46 on Madeira Island and only one on Porto Santo Island. However, only one species was found on both GMS and another elevation (Zosime anneae, also recorded on Seine) whilst Seine and Sedlo shared six species (Büntzow, Reference Büntzow2011), the islands Madeira and Porto Santo shared one species (Packmor & George, Reference Packmor and George2016), Madeira Island and Anaximenes had one species in common (George et al., Reference George, Pointner and Packmor2018). One species (Stylicletodes longicaudatus (Brady, 1880)) has been found on four elevations (Seine, Sedlo, Anaximenes, Madeira; George et al., Reference George, Pointner and Packmor2018). Thus, it seems that the fauna of GMS plateau differs from those of the other studied elevations and supports a distinct fauna.
Discussion
The community structure of the benthic plateau fauna
The Great Meteor Seamount is one of the most intensively studied seamounts with regards to benthos (e.g. Brenke, Reference Brenke2002; Heinz et al., Reference Heinz, Ruepp and Hemleben2004; Martin & Nellen, Reference Martin and Nellen2004; Piepenburg & Müller, Reference Piepenburg and Müller2004; George, Reference George2013 and references therein). Previous studies have identified distinct regions on the GMS plateau (megabenthos: Piepenburg & Müller, Reference Piepenburg and Müller2004; meiofaunal nematodes Draconematidae: Gad, Reference Gad2009), but these regions are based only on qualitative data and therefore may be an artefact of sampling. Indeed, the geomorphological features of the GMS plateau (Ulrich, Reference Ulrich1971; Beckmann & Mohn, Reference Beckmann and Mohn2002; Mohn & Beckmann, Reference Mohn and Beckmann2002; Fischer, Reference Fischer2005) may suggest different habitats on the plateau and thus also different communities. Hence, it was hypothesized that the community structure of Harpacticoida and Canuelloida assemblages differs across the plateau (H1). However, the present study, the first to present quantitative data, revealed a uniform community with evenly distributed Copepoda families and a heterogeneous species composition. Therefore, H1 must be rejected.
All families were present in nearly all stations in every region, as were the studied species. Thus, it seems that the hydrodynamic and geomorphological features of the plateau do not influence Harpacticoida and Canuelloida distribution patterns, as already assumed for Zosimeidae (Pointner, Reference Pointner2017).
Perhaps owing to the complex hydrodynamic regime, the GMS plateau is homogenously covered by coralline, biogenic carbonate sediment (grain size: 92%: 125–2000 µm; 8%: <125 µm; Hesemann, Reference Hesemann2013), an ideal habitat for interstitial benthic Copepoda (Hicks & Coull, Reference Hicks and Coull1983). The homogenous sediment type, combined with the eurybathic species composition (indicating that the differences in the plateau depth are without influence on these species) as well as with the more or less similar abundance values, suggest that the entire GMS plateau, including its pinnacles, provides a potentially continuous habitat. Indeed 65.09% of all identified species were found across the plateau. Furthermore, Δ+ indicates closely related species across the plateau and a more taxonomic diverse community in the southern region, as it is slightly higher in this region.
The southern region of the GMS plateau exhibits slightly greater species diversity than the other regions, possibly indicating the presence of microhabitats. Similar findings were made for Cylindropsyllidae (Pointner, Reference Pointnerin press) and nematodes (Draconematidae: Gad, Reference Gad2009) and, with the hydrological data, might support the identification of stronger internal tidal flow at the southern slope (Mohn & Beckmann, Reference Mohn and Beckmann2002).
In contrast to previously conducted qualitative studies on the GMS (e.g. George & Schminke, Reference George and Schminke2002; Plum & George, Reference Plum and George2009), the present study is based on quantitative material of an extensive sampled area. But even for this study, sampling artefacts may remain a possible factor in regional differences in recorded diversity, with Jackknife1 analysis estimating that, at most, 86.83% of species were sampled. Also, those species with a restricted distribution were only recorded in very low abundances, and therefore, their recorded absence from other stations/regions may simply reflect undersampling. Consequently, sampling more intensively in a smaller area might reveal species currently considered rare (i.e. only reported in the southern region in the present contribution). Abiotic and biotic factors must also be sampled across the whole plateau in order to identify potential microhabitats or possible environmental heterogeneity and therefore advance our understanding of the community structure of benthic copepods on the plateau.
Considering the isolated nature of the GMS plateau, it is surprising that meiofaunal benthic copepods occur in such a high diversity. Their widespread distribution across the plateau might result from a number of dispersal mechanisms, including (1) crawling, (2) drifting and (3) rafting (Gerlach, Reference Gerlach1977; Hicks, Reference Hicks1988; Giere, Reference Giere2009):
(1) Historically GMS breached the sea surface as an island (Hinz, Reference Hinz1969) providing habitat for shallow-water species. Having reached the island, they would then have been able to colonize GMS by crawling in/on the sediment.
(2) Representatives of both non-emergent (e.g. Cerviniopsis sp.1, Cylindropsyllus valentini, Malacopsyllus sp.2, Laophonte sp.1) and emergent (e.g. Bodinia peterrummi, Pseudomesochra sp.3, Zosime eliasi) Copepoda were widely distributed on the plateau, suggesting that both active and passive dispersal by drifting may occur. When fauna enter the water at the sediment–water interface, either actively or passively, they are caught by currents moving above the surface sediment. These drifting meiofaunal organisms are reported to have a range of 10 km (Hagerman & Rieger, Reference Hagerman and Rieger1981) to >50 km per day (Palmer & Gust, Reference Palmer and Gust1985). Hence the GMS plateau, measuring 54 km × 31 km (Ulrich, Reference Ulrich1971), is within the drifting range of meiofaunal organisms. Furthermore, the current system around GMS is changeable, influenced by a number of factors including flow velocity and strength of winds, which affect dispersal distance and direction, respectively. Consequently, species may drift over different routes on different days/times of year (Mouriño et al., Reference Mouriño, Fernández, Serret, Harbour, Sinha and Pingree2001; Fischer, Reference Fischer2005).
(3) Meiofauna are known to raft on macroalgae (Houle, Reference Houle1999) and bacterial mats which grow at the sediment/water interface (Giere, Reference Giere2009). To date, bacterial mats have only been observed in shallow water (Faust & Gulledge, Reference Faust and Gulledge1996), however, they may potentially occur on the GMS plateau owing to its shallowness (Mouriño et al., Reference Mouriño, Fernández, Serret, Harbour, Sinha and Pingree2001) and its easily suspended coralline sediment.
It is the homogenous nature of the plateau sediment that makes such dispersal successful: after drifting or rafting species must find sediment with both suitable abiotic and biotic characteristics to colonize on descent. It is possible that such dispersal also takes fauna from the seamount to the deep sea; future studies in the surrounding deep sea will identify if there are more eurybathic species on GMS than currently known, which would support colonization scenario 2 (for details see section ‘Colonization of the plateau fauna’).
Colonization of the plateau fauna
Qualitative investigations of the harpacticoid fauna of the GMS (George & Schminke, Reference George and Schminke2002; Seifried & Schminke, Reference Seifried and Schminke2003; George, Reference George2004a, Reference George2006), including the summit region (plateau and upper slope) and the surrounding deep sea, have revealed a clear distinction between these areas (George & Schminke, Reference George and Schminke2002). Moreover, three different scenarios for the colonization of the plateau have been suggested (George, Reference George2004a): Scenario 1, geographic immigration via drifting in the water column; scenario 2, bathymetric immigration from the deep sea; and scenario 3, species elevation alongside the geological evolution of the seamount, the scenario considered most viable by the author. Additionally, a close relation between species of Argestidae on the plateau with species of the surrounding deep sea was suggested (George, Reference George2004a). Hence, it was hypothesized that the benthic copepod species found on the GMS plateau were closely related to deep-sea species (H2).
In the present investigation, nearly the same ‘deep-sea taxa’ were identified as in former studies. Among the 24 families, Aegisthidae (3 genera, 3 species), Argestidae (6 genera, 13 species), Neobradyidae (2 genera, 6 species) and Zosimeidae (1 genus, 12 species) were recorded (though no Ancorabolidae).This finding supports colonization scenario 3 (George, Reference George2004a); possibly they colonized the seamount as it formed. The morphology of all these species suggests they prefer muddy sediments (Hicks & Coull, Reference Hicks and Coull1983), as is generally found in the deep sea. This is surprising since the GMS summit region is covered with biogenic carbonate sediment, which is more suitable for interstitial fauna (George & Schminke, Reference George and Schminke2002).
Based on the same qualitative material as in George & Schminke (Reference George and Schminke2002), further studies on the taxa Paramesochridae and Zosimeidae (Plum & George, Reference Plum and George2009; Koller & George, Reference Koller and George2011) revealed eight more species which occur in the summit region as well as at the seamount base, and two which were already known from geographically and bathymetrically different areas. Hence, the composition of copepods in these two regions becomes increasingly similar. The present contribution concentrates on the material sampled only on the plateau, so no conclusion on bathymetrical exchange can be drawn. Nevertheless, it was possible to compare the species with those recorded in the qualitative survey, and more species were found to be eurybathic, for example for Zosimeidae (Pointner, Reference Pointner2017) and Cylindropsyllidae (Pointner, Reference Pointnerin press). On the plateau itself, a large number of species were assigned to genera which occur in shallow and deeper waters and are therefore seen as eurybathic. Nearly the same number of species was assigned to shallow-water genera, the fewest species to genera inhabiting exclusively the deep sea. Hence, H2 has to be rejected. However, the true bathymetric distribution of the newly recorded species cannot be known, unless they are recorded elsewhere in the future. Despite this, the colonization scenario 2 suggested by George (Reference George2004a) seems likely owing to the number of eurybathic species across the increasing number of investigated families.
In contrast to bathymetric exchange, geographic exchange seems less plausible: The large number of unknown species indicates an isolated plateau (George & Schminke, Reference George and Schminke2002; Plum & George, Reference Plum and George2009), as it was hypothesized that the benthic copepod fauna of the GMS plateau is distinct, with a high number of scientifically unknown species (H3). As 88.68% of identified species were unknown to science, the isolation of the plateau is strongly supported by the present contribution, and H3 was accepted. This isolated character of the GMS plateau and its geographic isolation (closest islands: Azores, about 450 nautical miles away; closest mainland coast: Africa, about 800 nautical miles distant; Ulrich, Reference Ulrich1971) suggests consistent dispersion via drifting (Giere, Reference Giere2009) is very unlikely (George & Schminke, Reference George and Schminke2002; George, Reference George2004a; Pointner, Reference Pointner2015).
However, the remarkable diversity of identified interstitial Copepoda on the plateau (Cylindropsyllidae: 4 genera, 6 species; Leptastacidae: 1 genus, 2 species; Leptopontiidae: 2 genera, 5 species; Tetragonicipitidae: 5 genera, 7 species; Paramesochridae: present on the plateau with several species but not further investigated) cannot be explained by the accidental arrival of species. It is more likely that the seamount was once connected to other islands/seamounts, acting as a ‘stepping stone’ (sporadic arrival of species by chance) or even as a ‘staging post’ (continuous species exchange within critical dispersal distances; Packmor et al., Reference Packmor, Müller and George2015 and references therein). The seamount is of volcanic origin, but it is unclear whether it is part of a tectonic hotspot track or whether it developed by lithospheric fracturing (Wendt et al., Reference Wendt, Kreuzer, Müller, von Rad and Raschka1976; Morgan, Reference Morgan1983; Duncan, Reference Duncan1984; Müller et al., Reference Müller, Royer and Lawver1993; Grevemeyer, Reference Grevemeyer1994; Heaman & Kjarsgaard, Reference Heaman and Kjarsgaard2000). However, it seems to have been generally accepted (Hinz, Reference Hinz1969; Ulrich, Reference Ulrich1971; Wendt et al., Reference Wendt, Kreuzer, Müller, von Rad and Raschka1976; Grevemeyer, Reference Grevemeyer1994) that the GMS developed from the Oligocene to the Miocene (55 to 10 my ago; Hinz, Reference Hinz1969; Wendt et al., Reference Wendt, Kreuzer, Müller, von Rad and Raschka1976). It is assumed that the GMS seamount was once an island, as suggested by its typical ‘guyot’-shape, and that the northern Atlantic Ocean used to be narrower (Scotese, Reference Scotese1991). Therefore, the continental coasts and oceanic elevations (e.g. Canary Islands Seamount Province (age: 142 to 0.2 my; van den Bogaard, Reference van den Bogaard2013) and the Madeira Archipelago (age: 67 to 5 my; Geldmacher et al., Reference Geldmacher, Hoernle, Hanan, Blichert-Toft, Hauff, Gill and Schminke2011)) were geographically closer. Concerning this, a geographic distribution by drifting in the water column (scenario 1; George, Reference George2004a) is also a possible source of Copepoda colonization. Over time, as the Atlantic Ocean widened, the plateau and the fauna living on it would have become isolated and, due to the low subsidence rate of the GMS (10 mm per 1000 years; Hinz, Reference Hinz1969), an adaptation of the ‘shallow-water species’ to the changing conditions (amount of light, pressure, temperature, etc.) might have been possible.
Today, the GMS plateau is below the commonly accepted 200 m barrier between the littoral and the bathyal (Tardent, Reference Tardent2005), but it may still be considered a shallow-water region (Mouriño et al., Reference Mouriño, Fernández, Serret, Harbour, Sinha and Pingree2001): abiotic and biotic factors are similar to other shallow-water habitats (Mouriño et al., Reference Mouriño, Fernández, Serret, Harbour, Sinha and Pingree2001) and the sandy sediment on the plateau is comparable to other shallow-water regions all over the world (e.g. North Sea: Sylt (Kuhnert et al., Reference Kuhnert, Veit-Köhler, Büntzow and Volkenborn2010); Irish Sea: e.g. Isle of Man (Oh et al., Reference Oh, Hartnoll and Nash2001); Mediterranean Sea: Algier (Bakalem et al., Reference Bakalem, Ruellet and Dauvin2009); Atlantic Ocean: coast of Madeira (Packmor & George, Reference Packmor and George2016), Porcupine Seabight (Gheerardyn et al., Reference Gheerardyn, De Troch, Vincx and Vanreusel2009), Bermuda (Pratt, Reference Pratt1963); Pacific Ocean: Pudget Sound (Wieser, Reference Wieser1959)). Hence, the plateau is a suitable habitat for ‘shallow-water species’ and if species are able to settle, they should be able to colonize and even radiate across the plateau (George, Reference George2004a). However, if the GMS plateau was still connected to another shallow-water region (e.g. Azores, Madeira), a larger number of known species would be expected. Nevertheless, several species known to have wider distribution patterns were present on GMS (George & Schminke, Reference George and Schminke2002; Plum & George, Reference Plum and George2009; Packmor et al., Reference Packmor, Müller and George2015). Additionally, Zosime bergensis and Zosime anneae, both identified by Koller & George (Reference Koller and George2011), were identified in the present survey, and Asellopsis intermedia, Boreopontia heipi, Huntemannia jadensis and Selenopsyllus dahmsi were recorded for the first time on the plateau. These findings lead to two considerations.
Firstly, as shown above, the benthic copepods of the plateau appear to have been isolated for a long time. Additionally, due to the large number of scientifically unknown, mainly closely related species, it seems likely that radiation occurs on the plateau (George, Reference George2004a; Pointner, Reference Pointner2017, Reference Pointnerin press). Conversely, the presence of the coastal Asellopsis intermedia and Huntemannia jadensis on the plateau is surprising, but at the very least their bathymetric range has to be expanded towards 289 m depth. These species have no closely related species on the plateau and both are present in only low abundances in a single location (Asellopsis intermedia, eight individuals and Huntemannia jadensis, one individual), suggesting that they recently arrived on the plateau by accident. Hence, it is questionable if these individuals are able to colonize the plateau. Similarly, George & Schminke (Reference George and Schminke2002) identified 12 individuals of Retrocalcar brattstroemi but this species was not recovered in the present contribution despite extensive sampling.
These observations indicate that even an isolated seamount like GMS plays an important role in meiobenthic species distribution.
Comparison of the Harpacticoida and Canuelloida community of the GMS plateau with those on other seamounts and islands
Copepoda abundance is lower on the GMS plateau than on the other elevations of the North-eastern Atlantic Ocean. Even the deep-sea Mediterranean seamount, Anaximenes, has Copepoda abundance greater than the shallow GMS. The low copepod densities on GMS and Anaximenes might be a result of the location of these two seamounts: Both are situated in oligotrophic regions, with reduced sediment chlorophyll a (compared to Seine seamount and Madeira and Porto Santo islands; Mendonça et al., Reference Mendonça, Arístegui, Vilas, Montero, Ojeda, Espino and Martins2012; George et al., Reference George, Pointner and Packmor2018). Whilst oligotrophy at Anaximenes reflects its Mediterranean location, at GMS it is probably a result of the long distance from the mainland and therefore nutrient-rich coastal waters (Blain et al., Reference Blain, Guieu, Claustre, Leblanc, Moutin, Quéguiner, Ras and Sarthou2004; Tardent, Reference Tardent2005). Of course, it should be considered that the seamounts were sampled in different years and at different times of the year. There is the possibility therefore that both interannual and seasonal difference in nutrient levels have affected the recorded abundance levels (Hirch, Reference Hirch2009; Denda & Christiansen, Reference Denda and Christiansen2011).
Recent intensive inventory of Harpacticoida and Canuelloida on Atlantic and Mediterranean elevations (George & Schminke, Reference George and Schminke2002; George, Reference George2004a; Plum & George, Reference Plum and George2009; Büntzow, Reference Büntzow2011; Koller & George, Reference Koller and George2011; Packmor et al., Reference Packmor, Müller and George2015; Pointner, Reference Pointner2015, Reference Pointner2017; Packmor & Riedl, Reference Packmor and Riedl2016; George et al., Reference George, Pointner and Packmor2018; Pointner, Reference Pointnerin press) enables preliminary conclusions to be drawn on their distribution. Therefore, it was hypothesized that the copepod community structure (composition and diversity) on the GMS plateau does not differ from those of other elevations (H4). On more than three elevations 25 families were identified, and it might, therefore, be expected that they exhibit similar faunas and that faunal exchange may occur between them. However, families often comprise species with different ecologies (Hicks & Coull, Reference Hicks and Coull1983; Chertoprud et al., Reference Chertoprud, Chertoprud, Garlitskaya, Azovsky and Kondar’2007), and only species-level data can truly answer this question.
It has been shown that seamounts and islands play an important role in the distribution of Paramesochridae (Packmor et al., Reference Packmor, Müller and George2015) and Normanellidae (Packmor & Riedl, Reference Packmor and Riedl2016) at the species level. These studies examined distributions across the North-east Atlantic Ocean elevations Seine, Sedlo and GMS seamounts and Madeira and Porto Santo islands. Both found species that were present on at least two elevations, including GMS. However, previous investigations have assumed the GMS plateau to be isolated due to the large number of unknown and apparently closely related species (George & Schminke, Reference George and Schminke2002; George, Reference George2004a; Plum & George, Reference Plum and George2009). As 84.91% of the identified species are unknown to science, the isolation of the plateau is strongly supported by the present contribution. On the GMS plateau, 16 species were already described, six of which have only been documented. Nonetheless, six known species with a wider distribution range (Zosime anneae, Zosime bergensis), or even with an amphi-oceanic range (Asellopsis intermedia, Boreopontia heipi, Huntemannia jadensis, Selenopsyllus dahmsi) were recorded on the GMS and not on any other seamount/island, suggesting that it still plays an important role in the dispersal of meiobenthic organisms. Hence, H4 can be accepted on family level but has to be rejected on species level.
Future studies to catalogue the Harpacticoida and Canuelloida in the deep-sea sediments surrounding GMS and on other neighbouring seamounts (e.g. the Small Meteor Seamount, the Hyères and Irving Seamounts) are needed to clarify the role of the GMS in the distribution of species.
Conclusion
The extensive quantitative sampling on the plateau of the GMS made it possible to conduct a community analysis of the Harpacticoida and Canuelloida. Species were heterogeneously distributed across the plateau and most families were present at each station. The southern region was slightly more diverse than the other regions due to a higher number of species belonging to more genera/families. Thus, only one community was detected on the plateau, despite variable geomorphological features, and probably resulting from a number of different dispersal methods.
The comparison of the GMS plateau fauna with other seamounts and oceanic islands revealed a similar composition at family level, but a distinct assemblage at species level, suggesting an isolated plateau that might not act as a stepping stone. Additionally, most species found on GMS can be assigned to genera known from shallow-water areas, suggesting that GMS and adjacent elevations were once connected as ‘stepping stones’ or even as ‘staging posts’. Isolation is likely to have occurred following Atlantic sea-floor spreading and the subsidence of GMS. Nevertheless, the findings of species with wider distribution ranges indicate that the GMS still plays an important role in the dispersal of Harpacticoida and Canuelloida, even though the arrival of specimens might happen only by chance.
A number of additional studies are needed to clarify the role of GMS and the distribution of Harpacticoida and Canuelloida. Firstly, on the deep-sea surrounding GMS but also on more seamounts and islands, in order to examine the ‘accidental arrival’ of species on the plateau and secondly to gather more information about the distribution of shallow-water species on Atlantic elevations, to understand the role played by seamounts in meiofaunal species distribution.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315419000444
Author ORCID
Karin Richter, 0000-0002-0437-3707
Acknowledgements
The authors are thankful to the technical staff, Marco Bruhn, Annika Hellmann, Stefan Gogulla and Rebekka Schüller (Senckenberg am Meer, DZMB, Wilhelmshaven) for their support in centrifuging and sorting samples. Katja Uhlenkott (Senckenberg am Meer, DZMB, Wilhelmshaven) is thanked for her patient support in R. The authors are also grateful for the revision of the English text by Dr Natalie Barnes (Lee-on-the-Solent, Hampshire, UK). Additionally, the authors thank the three anonymous reviewers for their constructive remarks on the manuscript.
Financial support
This research was supported by the Deutsche Forschungsgemeinschaft [DFG-Gz: GE 1086/15-1].












