Introduction
Tympanosclerosis, which mainly affects the tympanic membrane and middle-ear mucosa, is an irreversible disease in humans. Although the aetiology and pathogenesis of tympanosclerosis are still not well known, it is widely believed that tympanosclerosis commonly develops secondary to acute or chronic inflammation of the middle ear,Reference Wielinga and Kerr 1 and as a result of other factors, such as myringotomy, ventilation tube insertion, physical trauma, various chemical agent exposure, a genetic tendency, immunity and following local metabolic changes.Reference Asiri, Hasham, al Anazy, Zakzouk and Banjar 2 – Reference Koc and Uneri 7
The typical pathological trait of tympanosclerosis is the formation of calcified plaques via the following three consecutive phases. First, there is injury of collagen fibrils by inflammation that destroys the connective tissue layer.Reference Wielinga and Kerr 1 , Reference Ferlito 8 , Reference Makishima, Toriya, Inoue, Nakashima and Igarashi 9 The reparative stage ensues, with fibroblastic invasion that results in excessive collagenisation and hyalinisation.Reference Wielinga and Kerr 1 , Reference Tos and Stangerup 10 The final irreversible stage is characterised by calcifications and occasional ossifications.Reference Olsson, Dalsgaard, Haegerstrand, Rosenqvist, Ryden and Nilsson 11 , Reference Mann, Beck and Schaefer 12
In terms of both morphology and histology, the final pathology change of tympanosclerosis is similar to several bone disorders, such as ankylosing spondylitis, osteoporosis and rheumatoid arthritis, the pathogenesis of which is regulated by bone modelling and remodelling.Reference Haynes, Pettit, Duan, Tseng, Glant and Brown 13 – Reference Walsh and Gravallese 15 Therefore, we speculate that pathological bone remodelling may be associated with the formation of local calcifications in tympanosclerosis, as in the abovementioned diseases. Interestingly, a single study showed that the osteoprotegerin/receptor activator of nuclear factor kappa B/receptor activator of nuclear factor kappa B ligand (OPG/RANK/RANKL) system may have a regulatory role in tympanosclerosis pathogenesis by affecting bone modelling and remodelling.Reference Huang, Wu, Cui, Ge and Zhang 16 , Reference Boyce and Xing 17 Therefore, more data were needed to further investigate the exact relationship between bone remodelling and tympanosclerosis.
The process of bone remodelling is characterised by coupling of resorption of the bone matrix by osteoclasts and its reformation by osteoblasts.Reference Belibasakis and Bostanci 18 If the balance is disturbed, a bone metabolic disorder will occur and may lead to a series of metabolic bone diseases. Recent research has demonstrated that the Wnt/β-catenin signalling pathway is also involved in bone metabolism, except for the OPG/RANK/RANKL system,Reference Boyce and Xing 17 , Reference Glass and Karsenty 19 – Reference Baron and Kneissel 22 and its activation increased bone mass, while its inhibition decreased bone mass.Reference Baron and Rawadi 21 , Reference Baron and Kneissel 22
Dickkopf-1 (DKK1) is the physiological antagonist of the canonical Wnt pathway, and it inhibits Wnt signalling mainly through binding to LRP5/6.Reference Fedi, Bafico, Nieto Soria, Burgess, Miki and Bottaro 23 , Reference Kawano and Kypta 24 Its inactivation can stimulate bone formation and increase bone density.Reference Glass and Karsenty 19 , Reference Glass, Bialek, Ahn, Starbuck, Patel and Clevers 20 However, to date, no previously published studies have determined the actual role of DKK1 in the development of tympanosclerosis. Therefore, the present study was designed to establish an animal model of tympanosclerosis and investigate the role of DKK1 in the pathogenesis of tympanosclerosis.
Materials and methods
Materials
This study was approved by the Animal Care Committee of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Forty adult male Sprague Dawley rats (weighing between 200 and 250 g) were provided by the Animal Centre of Hubei University, and the ears of the rats were examined bilaterally by otomicroscope to ensure that there was no external or middle-ear infection.
The primary antibodies were as follows: rabbit anti-DKK1 monoclonal antibody (Abcam, Cambridge, UK) and rabbit anti-β-actin monoclonal antibody (Wuhan Goodbio Technology, Wuhan, China). The secondary antibody was horseradish peroxidase conjugated goat anti-rabbit immunoglobulin G (IgG) antibody (Wuhan Goodbio Technology). The bicinchoninic acid protein assay kit and enhanced chemiluminescence kit for Western blot analysis were obtained from Wuhan Goodbio Technology.
Design and procedure
Forty adult Sprague Dawley rats with intact external auditory canals and tympanic membranes were included in the study. The rats were randomly divided into two groups, a control group (n = 20) and an experimental group (n = 20). The control group did not receive any treatment. The left tympanic membrane and middle-ear mucosa in the experimental group were slightly incised, while the right ears were untreated. The rats were intraperitoneally anaesthetised with 10 per cent chloral hydrate; then, an incision was performed on the left tympanic membrane and middle-ear mucosa using a sterile pick while viewed with an otomicroscope. The entire process was performed under aseptic conditions. Fifteen days after the incision, the development of myringosclerosis was observed by otomicroscope, and all animals were sacrificed.
Tissue preparation
After the observation by otomicroscope, the rats were decapitated and the tympanic bullae of the rats were removed by microdissection. Half of the middle-ear mucosa and tympanic membrane samples were microscopically separated and quickly frozen at −80 °C for Western blot analysis. The remaining bulla specimens were used for histological evaluation after fixing in 4 per cent formaldehyde solution overnight and decalcification with ethylenediamine tetra-acetic acid solution. After decalcification, the bulla specimens were dehydrated with graded alcohol baths. Then, the specimens embedded in paraffin were sectioned to a thickness of 4 mm, and stained with haematoxylin and eosin and immunohistochemical staining.
Histopathological evaluation
Two pathologists, who were unaware of the study groups, were asked to assess the thickness of the tympanic membrane, intensity of inflammation, and degree of fibrosis and hyaline degeneration in the lamina propria under a light microscope. In addition, the condition of the extracellular matrix and level of vascularisation were evaluated.
Immunohistochemical analysis
For immunohistochemistry, all specimen slides were deparaffinised and rehydrated; then, endogenous peroxidase activity was blocked by hydrogen peroxide. After antigen retrieval, DKK1 (1:50 dilution) immunoreactivity was detected using the EnVision™+ system. The slides were then developed with 3,3′-diaminobenzidine substrate and counterstained with haematoxylin. Phosphate-buffered saline, in place of primary antibodies, was used as the negative control.
Western blot analysis
Total proteins were extracted using radioimmunoprecipitation assay buffer according to the manufacturer's instructions. The protein content of the samples was measured using a bicinchoninic acid protein assay kit. Then, the protein extracts were separated by 10 per cent sodium dodecyl sulphate-polyacrylamide gel electrophoresis, which was followed by transfer onto nitrocellulose membranes. The membranes were incubated with rabbit anti-DKK1 monoclonal antibodies (1:1000 dilution; Abcam) overnight at 4 °C; then, the membranes were washed and incubated with horseradish peroxidase conjugated goat anti-rabbit IgG antibody (1:3000 dilution; Wuhan Goodbio Technology). Finally, the signals were detected using an enhanced chemiluminescence kit and visualised after exposure to X-ray film. The analysis of data was conducted with AlphaEase FC™ software by comparison to β-actin.
Statistical analysis
The statistical analyses were performed with GraphPad Prism® and SPSS® version 19.0 software. One-way analysis of variance was applied to analyse the data, and p < 0.05 was considered statistically significant.
Results
Otomicroscopic findings
The histological structure of the tympanic membranes was normal, and no inflammatory exudates of the bilateral middle ear were observed, in the control group (Figure 1a). Similarly, no obvious abnormalities were observed in the right untreated ears of the rats in the experimental group. Conversely, the incisions of the left tympanic membranes in the experimental group healed completely at day 15, and sclerotic lesions were observed in 14 of 20 experimental ears (Figure 1b). The total calcification ratio was 70 per cent.

Fig. 1 Otomicroscopic views of tympanic membranes at day 15. (a) The tympanic membrane was normal and no inflammatory exudates of the middle ear were observed in the control group. (b) The tympanic membrane incision had healed and the sclerotic lesions (arrows) on the tympanic membranes were confluent, forming a horseshoe-shaped pattern in the experimental group.
Histopathological findings
The haematoxylin and eosin staining showed that the morphological structure of the tympanic membrane and middle-ear mucosa was normal in the control group (Figure 2a,b). Similarly, no obvious changes were observed in the right untreated ears of the experimental group. Compared to the tympanic membrane of the control group, the thickness of the membrane, inflammatory infiltration and fibroblast proliferation were significant in the left tympanic membrane of the experimental group (Figure 2c). In addition to the abovementioned changes, a dense network of collagen fibres, vascularisation and a thickened extracellular matrix were seen in the middle-ear mucosa of the experimental group (Figure 2d).
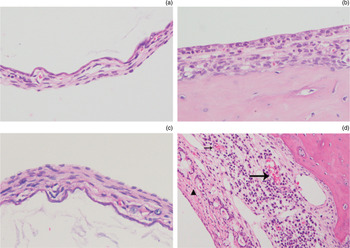
Fig. 2 In the control group, the tympanic membrane (a) and middle-ear mucosa (b) had a normal morphological structure. In the experimental group, significant thickness, inflammatory cell infiltration and fibroblast proliferation were seen in the tympanic membrane (c), and inflammatory infiltration, fibroblast proliferation, a dense collagen fibre (arrowhead) network, vascularisation (arrow) and increased extracellular matrix thickness were seen in middle-ear mucosa (d). (H&E; ×200)
Expression of DKK1 on immunohistochemistry
Immunohistochemical staining revealed DKK1-positive cells in all tympanic membrane and middle-ear mucosa samples in the present study. As shown in Figure 3, the DKK1-positive cells distributed in the control and experimental groups' tympanic membranes were mainly located in the cytoplasm of epithelial cells and fibroblasts. In addition, the DKK1-positive cells distributed in the control and experimental groups' middle-ear mucosa were mainly located in the cytoplasm of epithelial cells.

Fig. 3 In the control group tympanic membranes (a) and experimental group tympanic membranes (c), DKK1-positive cells were observed in the epithelial cells and fibroblasts, and they were mainly located in the cytoplasm of epithelial cells and fibroblasts. In the control group middle-ear mucosa (b) and experimental group middle-ear mucosa (d), DKK1-positive cells were observed in the epithelial cells, and they were mainly located in the cytoplasm of epithelial cells. Arrows indicate DKK1-positive cells. (Immunohistochemical staining; ×400)
Expression of DKK1 on Western blot
In this study, we used an immunoblotting technique to investigate the protein expression of DKK1 in the middle-ear mucosa. The ratios for the grey scale of DKK1 protein to internal reference protein are shown in Table I. As seen in Figure 4, the grey scale value of DKK1 protein in the experimental (tympanosclerosis) group was lower than that in control group. Statistical analyses revealed that the expression level of DKK1 was significantly down-regulated in the experimental group compared with the control group (p < 0.05).

Fig. 4 DKK1 expression level evaluated by Western blot. (a) The grey scale value of DKK1 protein in the experimental (tympanosclerosis) group was lower than that in the control group. (b) Compared to the control group, DKK1 expression was statistically significantly decreased in the experimental group (*p < 0.05). TS = tympanosclerosis
Table I Western blot data

Discussion
Previous studies have shown that incisional myringotomy increases the incidence of myringosclerosis events compared to other methods, and there is no difference in the myringosclerosis rates induced by incisional myringotomy between ears with otitis media and ears without infection.Reference Sakalli, Baylancicek, Yuksel, Erdurak and Dadas 25 – Reference Mattsson, Magnuson and Hellstrom 27 Meanwhile, myringosclerosis appeared in 66.7 per cent of the experimental ears at day 15.Reference Song, Kwon, Cho and Park 28 Therefore, we established a tympanosclerosis model via incision and observed the development of myringosclerosis at day 15.
In the present study, the incision in the left ears of Sprague Dawley rats resulted in calcification and histological alteration in most rats. Moreover, there was marked appearance of inflammatory infiltration and hyaline degeneration in the tympanic membranes and middle-ear mucosa in the experimental group. Conversely, neither calcifications nor histological changes appeared in the untreated right ears and normal ears. These findings are consistent with the typical pathological traits of tympanosclerosis,Reference NaderPour, Moghaddam, Peirovifar, Mollajavadi, Abbasi and Mohajeri 29 – Reference Li, Sarosi, Cattley, Pretorius, Asuncion and Grisanti 32 suggesting that the animal model of tympanosclerosis was successfully established by myringotomy.
DKK1, a secreted glycoprotein, is not only expressed within osteoblasts and maturing osteocytes,Reference Ueland, Otterdal, Lekva, Halvorsen, Gabrielsen and Sandberg 33 it is observed in endothelium and other tissues.Reference Yamaguchi, Passeron, Hoashi, Watabe, Rouzaud and Yasumoto 34 Our immunohistochemistry results, for the first time, showed that DKK1 was mainly located in the cytoplasm of epithelial cells, which were widely distributed in the tympanic membranes and middle-ear mucosa. Meanwhile, the Western blot results revealed that the DKK1 expression level was significantly down-regulated in the experimental group, indicating that DKK1 may be involved in the formation of local calcifications in the tympanosclerosis.
In recent decades, some studies have paid more attention to the canonical Wnt pathway, which plays a crucial role in the regulation of bone homeostasis. Activation of this pathway increases bone formation and its inhibition decreases bone formation.Reference Glass and Karsenty 19 , Reference Glass, Bialek, Ahn, Starbuck, Patel and Clevers 20 As previously mentioned, DKK1 mainly inhibits Wnt signalling through binding to LRP5/6,Reference Fedi, Bafico, Nieto Soria, Burgess, Miki and Bottaro 23 , Reference Kawano and Kypta 24 showing that there is a negative relationship between the DKK1 level and the activation of Wnt signalling. Our results, which showed that the protein expression of DKK1 significantly decreased in the middle ears in the experimental tympanosclerosis model, indicated that the down-regulated expression of DKK1 contributes to the formation of tympanosclerosis by reducing the inhibitory effect on Wnt/β-catenin signalling.
-
• An animal model of tympanosclerosis was successfully established by myringotomy
-
• DKK1 expression was significantly decreased in the tympanosclerosis group
-
• DKK1 may be involved in tympanosclerosis pathogenesis by regulating the Wnt/β-catenin signalling pathway
In conclusion, our study demonstrates, for the first time, that the level of DKK1 was down-regulated in the middle-ear mucosa of rats with tympanosclerosis, providing direct evidence that a physiological antagonist of Wnt/β-catenin signalling, DKK1, is involved in tympanosclerosis formation. However, while animal models suggest that DKK1 regulates the pathogenesis of tympanosclerosis, its role in humans remains unclear. Therefore, further research is needed to investigate the effect of Wnt/β-catenin signalling and its antagonist, DKK1, on the formation of tympanosclerosis in humans.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (grant number 81271072).







