Published online by Cambridge University Press: 03 March 2004
Experimental intraperitoneal Taenia crassiceps cysticercosis in mice exhibits distinct genetical, immunological and endocrinological features possibly resulting from the complex interactive network of their physiological systems. Very notable is the tendency of parasites to grow faster in hosts of the female sex. It is also remarkable in the feminization process that the infection induces in chronically infected male mice, characterized by their estrogenization, deandrogenization and loss of sexual and aggressive patterns of behaviour. The proto-oncogene c-fos is a sex steroid-regulated transcription factor gene, expressed basally and upon stimulation by many organisms. In the CNS of rodents, c-fos is found expressed in association to sexual stimulation and to various immunological and stressful events. Hence, we suspected that changes in c-fos expression in the brain could be involved in the feminization of the infected male mice. Indeed, it was found that c-fos expression increased at different times during infection in the hypothalamus, hippocampus, less so in the preoptic area and cortex, and not in several other organs. The significant and distinctive regional changes of c-fos in the CNS of infected mice indicate that the brain of the host senses intraperitoneal cysticercosis and may also announce its active participation in the regulation of the host–parasite relationship. Possibly, the host's CNS activity is involved in the network that regulates the estrogenization and deandrogenization observed in the chronically infected male mice, as well as in the behavioural and immunological peculiarities observed in this parasitic infection.
Murine intraperitoneal (ip) cysticercosis caused by Taenia crassiceps (Freeman, 1962; Smith, Dorais & Kuhn, 1972) is an experimental infection which allows exploration of the role of biological factors (i.e. immunity, MHC genes, gender) involved in host susceptibility to cysticercosis (Sciutto et al. 1991; Larralde et al. 1995; Morales et al. 1996; Morales-Montor et al. 2001), and has also proved useful in the immunodiagnosis of human cestode diseases (Gottstein, Tsang & Schantz, 1986; Schantz, Tsang & Maddison, 1988; Larralde et al. 1990) and in testing candidate vaccines against porcine Taenia solium cysticercosis (Toledo et al. 2001).
Experimental murine T. crassiceps ip cysticercosis has progressively revealed the complexities of the interactive network between the immunological and endocrinological systems of the host and of the parasite in regulating infection (Morales-Montor et al. 2001, 2002). Briefly, a remarkable sex-associated susceptibility to T. crassiceps ip cysticercosis occurs in mice: the females of various strains bear larger parasite loads than males during early infections (Sciutto et al. 1991). After 4 weeks of infection, the parasite loads of males increase progressively and approach the massive levels of females in a few months (Larralde et al. 1995). Concomitantly, a feminization process ensues in the chronically infected male mice: serum 17β-oestradiol (E2) levels increase to reach those of females, while testosterone (T) drops to 10–15% its normal levels (Larralde et al. 1995; Morales et al. 1996; Morales-Montor et al. 2001). Infected male mice progressively lose their normal mating behaviour (Morales et al. 1996) as well as their aggressive behaviour patterns (Gourbal et al. 2001). The high E2 levels in chronically infected mice are mainly produced by the hosts gonads (Larralde et al. 1995; Morales-Montor et al. 2002), albeit the parasite is itself capable of some sex steroid synthesis (Gómez et al. 2000). High E2 levels correlate with an increase in AP-1 transcription factor (c-fos and c-jun) mRNA expression in spleen, thymus and testes (Morales-Montor et al. 1998). The incorporation of the AP-1 transcription factor genes family in the host's response to infection indicates that disease triggers crucial, interconnected physiological systems. These AP-1 genes, which include the c-fos proto-oncogene (Hyder et al. 1992; Hyder, Shipley & Stancel, 1995), are sensitive to sex steroids and have been shown to regulate the expression of many other genes in a variety of tissues and cell types. AP-1 by itself could play a major role in the outcome of infection in cysticercotic mice by a number of connected physiological circuits and its activity would suffice to explain the disease's strong association with sex steroids and modulate the hosts immune response to the parasite (Bojalil et al. 1993; Terrazas et al. 1994; Morales-Montor et al. 2001). Nonetheless, the immuno-endocrinological and AP-1 gene family changes in cysticercotic mice could also involve the hosts CNS, which has been found to express c-fos when sensing a variety of immuno-endocrinological and stressful situations (Pacheco-López et al. 2002) and is extensively interconnected with other physiological systems in the organism (Zhang et al. 2002). The possible incorporation of the host's CNS in the physiological network closely regulating parasitism was enticing.
Thus, in search of a sign of CNS involvement in the host's complex immuno-endocrine reaction to murine ip cysticercosis, we decided to study the effects of T. crassiceps ip infection upon c-fos expression in the brain of infected male mice.
Chemical reagents were purchased from Sigma (St Louis, MO, USA) and Gibco-BRL (Gaithersburg, MD, USA). Taq polymerase was purchased from Tecnologías Universitarias (Facultad de Veterinaria, U.N.A.M., Mexico City).
Male Balb/c AnN (H2-d) inbred mice were used in all experiments. They were fed Purina Diet 5015 (Purina, St Louis, MO) and water ad libitum. The fast-growing ORF strain of T. crassiceps (Dorais & Esch, 1969) was used for infection in all experiments. Larvae were obtained from 3 to 6-month-old infected female donor mice. Ten non-budding T. crassiceps larvae (approximately 2 mm in diameter) were suspended in 0·3 ml of phosphate-buffered saline (PBS: 0·15 M NaCl, 0·01 M sodium phosphate buffer, pH 7·2) and injected intraperitoneally into each of ten 42-day-old male mice using a 0·25 gauge needle. Ten uninfected mice were used as age-matched controls. Mice were sacrificed by cervical dislocation after ether anaesthesia at 4, 8 and 16 weeks of infection. Peritoneal cysticerci were collected and counted after rinsing the peritoneal cavity with PBS. Animal care and experimental practices at the Universidad Nacional Autonoma de Mexico are validated by the University's Animal Care and Use Committee in order to ensure compliance with established international regulations and guidelines.
Blood for steroid determination was collected by cardiac puncture of anaesthetized mice. After incubation for 5 h at room temperature and 18 h at 4 °C, the blood was centrifuged and serum obtained. E2 and T serum levels were determined using an enzyme immunoassay kit from ALPCO (Windham, NH, USA) according to the manufacturer's instructions.
The brain, kidney, heart, lungs and intestines of infected and normal mice were excised immediately after their humane sacrifice (cervical dislocation while under pentobarbital anaesthesia) and processed as described below.
Mice brains were rapidly removed and chilled in cold 0·9% saline solution. Hypothalamus, hippocampus, pre-optic area and frontal cortex were immediately excised according to the ‘Atlas of Developing and the Adult Brain’ (Paxinos, Ashwell & Tork, 1994) and the ‘Atlas of the developing rat nervous system’ (Paxinos & Watson, 1998). Briefly, the pre-optic area was dissected by making a razor cut just rostral to the optic chiasm to the anterior commisure whose antero-ventral limit was the nucleus of the diagonal band of the lateral Broca. The hypothalamus was dissected by one razor cut just behind the optic chiasm to the caudal portion of the mamillary bodies that laterally limit with hypothalamic commisures. The hippocampus was obtained by making a razor cut underneath the frontal cortex, while the frontal cortex was excised directly from the frontal lobes. In all cases, CNS samples from 5 animals were pooled because of the microgram amount of tissue collected from each region of the brain of each mouse. All experiments were performed in triplicate.
Total RNA was isolated from the hypothalamus, hippocampus, pre-optic area and frontal cortex, as well as from kidneys, heart, intestines, and lungs of control and 4, 8 and 16 week parasitized males, by the single-step extraction method using TRIzol reagent (Gibco-BRL, NY, USA). Briefly, each tissue was removed and immediately disrupted in TRIzol reagent (1 ml/0·1 g tissue), and 0·2 ml of chloroform were added per ml of TRIzol. The aqueous phase was recovered after 10 min centrifugation at 14000 g. RNA was precipitated with isopropyl alcohol, washed with 75% ethanol, and redissolved in RNAase-free water. RNA concentration was determined by absorbance at 260 nm and its purity was verified after electrophoresis in 1·0% denaturing agarose gel in the presence of 2·2 M formaldehyde. Total RNA from all tissues extracted was reverse transcribed followed by specific PCR amplification of c-fos and β-actin genes sequences.
Nucleotide sequences of the primers used for amplification flanked the mouse c-fos cDNA sequence from +258 (sense primer 5′-TGTACGATCA CTGAACTG CA-3′) to +505 (anti-sense primer 5′-AGTCAGTTATATCCTGGC-3′), and for β-actin primers the sequences were: sense primer 5′-CTACAATGAGCTGCGTGTGG-3′ and anti-sense primer 5′-AGGAAGGCTGA AGAGTGC-3′. Briefly, 1 μg of total RNA from each tissue was incubated at 37 °C for 1 h with 400 units of M-MLV reverse transcriptase (Applied Biosystems, Boston, MA) in 20 μl of reaction volume containing 50 mM of each dNTP and 0·05 μg oligo (dt) primer (Gibco, NY). Ten μl of the cDNA reaction were subjected to PCR in order to amplify specific sequences of the specified genes. The 50 μl PCR reaction included 10 μl of previously synthesized cDNA, 25 μl of 10× PCR-buffer (Biotecnologias Universitarias, Mexico) 1 mM MgCl2, 0·2 mM of each dNTP, 0·05 μM of each primer, and 2·5 units of Taq DNA polymerase (Biotecnologias Universitarias, Mexico). After an initial denaturation step at 94 °C for 4 min, temperature cycling was initiated as follows: 94 °C for 55 sec, 50 °C for 5 sec and 72 °C for 45 sec during 30 cycles for c-fos and β-actin. Then 25 μl of the total PCR reaction products of each sample were electrophoresed on a 2% agarose gel. PCR products were visualized by staining with ethidium bromide. A single band was detected in all cases, and the size of each specific amplification product was 274 nt (c-fos) and 321 nt (β-actin) as expected. In order to determine that c-fos as well as β-actin both were in the exponential phase of amplification, and to be assured that changes in c-fos are not an artefact (such as β-actin being in the stationary phase), we performed RNA cycling and temperature curves for each region of the brain analysed, that proved that the 30 cycles were in the exponential phase of amplification (data not shown).
In order to verify that PCR products were indeed from c-fos and β-actin genes, PCR products were purified in a QIAquick PCR purification kit (Qiagen, Chatsworth, CA). Pure DNA was eluted in 20 μl of molecular biology grade H2O. The DNA sample was directly sequenced using a Thermo Sequenase Radiolabelled Terminator Cycle Sequencing kit, using 33P ddNTP terminators (Amersham Pharmacia Biotech, Buckinghamshire, UK), under conditions specified by the manufacturer. Four μl of the total sequencing reaction of each sample were electrophoresed on 6% denaturing polyacrylamide gels, at 1300 V during 3 h. The gel was dried and exposed for autoradiography during 24 h at room temperature. Comparisons of homology of mouse c-fos and β-actin sequences were done with the Entrez Gene Pub Med data bank program (NIH).
The experimental design is a three factorial experiment. Independent variables are (1) brain region under study (4 levels: hypothalamus, hippocampus, pre-optic area and frontal cortex); (2) time of infection at which samples were taken (3 levels: 4, 8, 16 weeks); (3) infection (2 levels: Yes, No). The dependent variable is the expression of c-fos in the tissue sample as measured by the optical density (OD) of the corresponding gel divided by the OD density of β-actin in the same tissue sample in the same gel, used as control gene for amplification technology. The complete design was repeated 3 times and the tissues used in each experiment at each time of infection were those pooled from 5 normal or infected mice. A total of 120 mice were used in the study. Statistical analysis of Variance Components was performed with the software Statistica (StatSoft, Inc., v2001–2002). When performed, post hoc individual contrasts of group means by t-tests used the sum of Residual and Three Factor Interactions variance to test for significant differences.
Figure 1 shows the increment in the number of parasites recovered from the peritoneal cavity of infected male mice with time of infection, reaching 1450±321 per mouse at 16 weeks of infection. No parasites were found outside the peritoneal cavity. The progressive oestrogenization and deandrogenization effects in infected male mice are also shown in Fig. 1. At 4 weeks of infection, serum oestradiol levels increased in male mice to 30% (P<0·01) above normal levels, reaching a maximum of 150-fold increase at 16 weeks of infection (P<0·01). Serum levels of testosterone in infected mice began to decrease 35% at 4 weeks of infection, reaching a 75% and 85% decrement by 8 and 16 weeks after infection, respectively (P<0·01). Levels of testosterone and oestradiol in normal age-matched male mice were stable throughout the study.

Fig. 1. Number of Taenia crassiceps cysticerci and serum oestradiol and testosterone levels throughout the infection course in male mice. Data are represented as the mean±S.D. of 3 experiments (n=5), each serum sample was determined in duplicate. E2=estradiol, T4=Testosterone. *P<0·05, **P<0·01, both compared to the control group.
The c-fos expression was assessed by RT-PCR in brain, lungs, kidney, heart, intestines, testes, seminal vesicles and epididymus of normal and infected mice at 4, 8 and 16 weeks of infection. The brain was sampled by regions: hypothalamus, hippocampus, pre-optic area and frontal cortex. The expression of β-actin was used as internal control at all times and in all organs and brain regions studied. In all samples, a single product of 224 nt, corresponding to the amplification fragment expected for c-fos, and another of 357 nt corresponding to β-actin were obtained. Identity of the amplified genes was verified by sequencing, and found to correspond to that of mouse c-fos and β-actin (100% identity, data not shown).
Figure 2 shows a representative PCR image of the expression of c-fos and β-actin and their quantitation in optical density (OD) for c-fos and β-actin expression in the mouse brain regions is shown in Fig. 3. Table 1 includes all quantitative PCR expression data obtained for the complete experimental design in the study of brain regions. Visual inspection of data in Table 1 and Figs 2 and 3 indicates the variability in expression of both individual genes but it is clear that levels in c-fos expression with respect to β-actin vary significantly in relation to infection and with time of infection in the different brain regions. The OD quotient for each gene (c-fos/β-actin) in each brain region was calculated at each time of infection to control background variation in PCR amplifications at different brain regions, times of infection and replications of experiments. The graphs in Fig. 3 strongly suggest that the quotient c-fos/β-actin is higher in infected mice in all brain regions studied and at all times of infection, with the exception of the hypothalamus at 16 weeks of infection, in which c-fos expression was similar in infected and non-infected mice. Three-Way Analysis of Variance strengthened the visual impressions, since it revealed statistically significant differences (P<0·001) between infected and normal mice at all times in all brain regions with the exception of the hypothalamus at 16 weeks. Comparisons between brain regions indicate that hypothalamus and hippocampus expression of infected mice nearly doubled those of normal mice, whereas pre-optic area and frontal cortex were also expressed more intensely in infected mice, albeit to a lesser extent. Analysis of variance showed a significant interaction between time of infection and brain region studied, mainly originating from c-fos expression peaking at 8 weeks of infection in the hypothalamus and less so in the hippocampus, pre-optic area and frontal cortex. In response to a sharp observation of a referee and because the levels of c-fos expression in the control mice varied at the different times of infection (possibly reflecting changes with age or in environmental conditions in the animal house or differences in the handling of the mice) the kinetics of gene expression in the infected mice was corrected to the ratio (infected OD c-fos-β-actin)/(control OD c-fos-β-actin) (Fig. 3, square symbols). The resulting kinetics varied in form, especially in the hypothalamus, which was the earliest area to show the highest differences compared to controls. These increases then progressively decayed to similar levels at 16 weeks of infection. The pre-optic area and frontal cortex response peaked at 8 weeks and then tended to decay with time of infection. The hippocampus also reacted rapidly to double that of controls at 2 weeks and continue to increase steadily at 8 and 16 weeks of infection, where it triples that of controls. The main result that levels of expression in the infected mice are significantly higher than in controls remained statistically significant (P<0·001). The expression of c-fos was not altered in other examined tissues of infected mice (lung, kidney, intestine and heart), excepting testes and seminal vesicles, which did show some changes that are still under verification. Histological examination of the sampled brain regions of infected and normal mice did not reveal abnormalities (data not shown).

Fig. 2. c-fos and β-actin gene expression in different regions of the brain of normal (N) and infected (I) male mice during intraperitoneal infection with Taenia crassiceps cysticerci. A representative RT-PCR image is shown with total RNA from hypothalamus, hippocampus, pre-optic area and frontal cortex (see Materials and Methods section).

Fig. 3. c-fos relative expression reported as densitometric data of the autoradiographic signal. The relative expression was obtained by correcting the expression of c-fos with respect to that of β-actin. Data represent pools of 5 mice, and each experiment was done in triplicate. Values are expressed as the mean of the triplicate experiments in infected ([utrif ]) and uninfected ([bull ]) age-matched mice. ([squf ]) Represents the kinetics of c-fos expression as the quotient of infected OD c-fos/β-actin/control OD c-fos-β-actin.
Table 1. c-fos/β-actin expression in the brain regions of normal and intraperitoneally Taenia crassiceps- infected male mice at different weeks after infection (The upper panel shows the c-fos/β-actin OD quotients of the three factorial design. The lower panel shows the variance components of the results as obtained from three-way analysis of variance which supports the satistically significant (P<0·001) contribution to variance of the three factors plus a significant (P<0·001) two factor interaction between time of infection and brain region (i.e. c-fos expression of regions differ at the different times of infection), the variance component possibly arising from the peaking of the response variable at 8 weeks after infection in the hypothalamus in contrast to the more stable dynamics of the hippocampus, pre-optic area and frontal cortex.)

This study reports changes in c-fos expression in the brain of male mice experimentally infected in the peritoneal cavity with T. crassiceps cysticerci and well into their feminization process. The OD quotient c-fos/β-actin showed considerable variations. However, the experimental design allowed statistical analysis of variance. ANOVA was employed to study the sources of variation involved in our experiments and to test the null hypotheses of no main effects of the experimental variables (infection, time, brain regions) upon gene expression. The residual variation was significantly smaller than those from the variables. The significant interaction between time and brain regions indicates that the differences in gene expression in the brain regions differ at the different times of infection. Thus, the null hypotheses were rejected. The residual variation may come from biological and technical factors. The brain's exquisite sensitivity and responsiveness to many external and internal stimuli (i.e. temperature cycles, stress signals) may have contributed to the variation but to a significantly lesser extent than the experimental variables under study. Reasons other than the statistically improbable artefactual source of our findings would be (i) the lack of response in other organs and tissues and the careful technical optimization of the RT-PCR employed (the amount of RNA employed, (ii) the number of cycles and the temperature used in amplification were those found optimal (data not shown)), and (iii) the constitutively expressed gene (β-actin) used as positive control of expression in each sampled RNA, and its incorporation into the response variable (c-fos/β-actin in experiment)/(c-fos/β-actin in control) at each time of infection to control for differences in time of infection coming from other stimuli associated with time (i.e. ageing, environmental changes).
Thus, there are indications of increased expression of c-fos in the hypothalamus and hippocampus of infected mice with respect to normal mice and to lesser extents in the pre-optic area and frontal cortex. Other organs simultaneously assessed did not show such changes. The expression of c-fos in the different brain regions differed with time of infection. The pre-optic area and frontal cortex peaked at 8 weeks of infection, whilst the hippocampus steadily increased throughout the 16 week interval and was the highest at 4 weeks and then decreased steadily to reach normal values at 16 weeks of infection.
Thus, our data indicate that the changes in c-fos expression in the brain of the infected mice point to the host's CNS sensing which may react to the presence of the parasites, even if they are located in the peritoneal cavity. The extensive connectivity of the CNS with other organs and physiological systems, including the immune and endocrine systems, is probably involved in this CNS sensing (Besedovsky & Del Rey, 2002). The precise mechanism remains to be elucidated, as does the interpretation of the distinct kinetic differences between brain areas. Present and past experimental evidence suggests that mutually regulating roles of interleukins, hormones and neural activity are induced in the host by parasitism, and that they interact with the parasites' mechanisms of survival and reproduction. Indeed, c-fos expression by the CNS would appear as a stereotyped form of response of the brain to a variety of stimuli (Svarnik et al. 2003; Fraley et al. 2003; Xu et al. 2003). Oestrogens and androgens and their receptors have been shown to induce or mediate c-fos expression in various regions of the CNS in a number of instances related with sexual stimulation and reproduction (Bigsby & Li, 1994; Kumar, Dudley & Moss, 1999; Wersinger & Rissman, 2000). c-fos changes in CNS, unrelated with levels of sex steroids, have been reported in other infections, including helminthiasis (Palmer, Wong-Riley & Sharkey, 1998; Lee et al. 2000), in non-infection related immune responses (Pacheco-López et al. 2002) and in stressful situations not involving sex steroids (Kumei et al. 2000). So, in the case of T. crassiceps ip cysticercosis, both hormonal (sex steroids and stress) mediation and infection-related intermediaries (i.e. interleukins, antigens, immune-complexes) could be inducing the c-fos expression changes in the brain of the murine host. However, the regional distribution of distinct levels of c-fos expression indicates that other factors are involved besides high levels of sex steroids (like the turning off/on of cellular receptors, a distinct affinity distribution of receptors in different cells and organs, or variation in the states of their connecting intracellular signal transduction reactions). The possibility that the CNS directly senses the peritoneum through its nervous endings cannot be excluded.
Regardless of how they arise, the c-fos expression changes in the brain of the feminized infected male mice may be something more than just a sign of it sensing the parasite: it may also announce CNS reaction and involvement in the host--parasite relationship via its ample repertoire to access and influence the acquired and innate immune responses, as well as other physiological systems and behaviour.
The incorporation of the CNS in the already complex immuno-endocrine network underlying ip T. crassiceps cysticercosis brings to mind the structure of scale-free networks. These networks have strong stability to random perturbations but are weak to directed (strategic) attack, their cause-effect relationships distribute over the whole network instead of in single sectors, and other emergent properties, result from their numerous and heterogeneous actors, extensive connectivity and non-normal distribution of the number of connections in the nodes (Ravasz et al. 2002; Oltvai & Barabasi, 2002). Recalling that ip murine cysticercosis is accompanied by a number of unique events (i.e. a bias towards the female environment, a feminization of male hosts, a permissive TH2 inclined TH1/TH2 balance in the immune response), that this infection does not kill the host (in spite of the huge parasite loads), and that the host's immune response does not totally reject the parasite, one is led to suspect that such host--parasite conviviality rests upon unusually effective mechanisms of achieving physiological stability for survival of both host and parasite. Perhaps the restraint of belligerence in the confrontation between host and parasite and/or its possible pathogenic consequences requires a neuro-immuno-endocrine scale-free network in the decision-making process and resulting events. A sketch of such a convivial network possibly in operation in murine ip cysticercosis is shown in Fig. 4, with its principal actors, connections and outcomes.

Fig. 4. Simplified chart of the network involved in the host--parasite relationship between Taenia crassiceps and BALBc/AnN male mice, showing the protagonist role of the c-fos gene at times of infection when the host is feminized and in apparently good health, despite the hundreds of cysticerci in the peritoneal cavity. The chart shows the principal nodes or clusters of nodes (in squares) of the parasite (reproductive system, other physiological systems) and of the host (immune system, gonads, adrenals and brain) or of both host and parasite (stars) (c-fos gene). Bidirectional arrows correspond to the most prominent connections between clusters, tips indicating to the directional flux of the signals. Connections belong to the parasite (antigens (Ags), toxins (t), other molecules (OP)) or to the host (hormones (Ho), antibodies (Abs), interleukins (IL's), immunocytes (c), chemical mediators of inflammation (i), and others (OH)). The network may adopt different states of activation and may result in quite different events, possibly varying with each pathogen. In the case illustrated here the network's major outputs are hormonal feminization of the host, with behavioural consequences, and a change in the host's endocrinological and immunological environment that is permissive of parasite reproduction. The high levels of oestrogen and low levels of DHT in the host, acting through c-fos expression, among many other possible nodes, would favour parasite proliferation, change the host's sexual and aggressive behaviour patterns and, also, tame its immune response towards the parasite by up-regulating the TH2 response with consequent down regulation of TH1 and perhaps interfering with inflammation and innate immune responses that could potentially be harmful to the host.
Financial support was provided by Dirección General de Asuntos del Personal Académico, UNAM, IN217401 to C.L. and J.M.M., and by Consejo Nacional de Ciencia y Tecnologia, México (CONACyT, 136430-N and 40072/A to J.M.M.). L.I. del Castillo has a research assistant scholarship from Sistema Nacional de Investigadores, CONACyT. I. Perez-Montfort corrected the English language in the original version and the text's literary structure. The authors thank Dr Gabriel Gutierrez-Ospina for his valuable criticism of the manuscript. The authors are grateful to Professor C. Arme and the several unknown colleagues (referees) of Parasitology who patiently struggled with our manuscript to make it acceptably intelligible.

Fig. 1. Number of Taenia crassiceps cysticerci and serum oestradiol and testosterone levels throughout the infection course in male mice. Data are represented as the mean±S.D. of 3 experiments (n=5), each serum sample was determined in duplicate. E2=estradiol, T4=Testosterone. *P<0·05, **P<0·01, both compared to the control group.
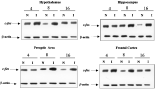
Fig. 2. c-fos and β-actin gene expression in different regions of the brain of normal (N) and infected (I) male mice during intraperitoneal infection with Taenia crassiceps cysticerci. A representative RT-PCR image is shown with total RNA from hypothalamus, hippocampus, pre-optic area and frontal cortex (see Materials and Methods section).

Fig. 3. c-fos relative expression reported as densitometric data of the autoradiographic signal. The relative expression was obtained by correcting the expression of c-fos with respect to that of β-actin. Data represent pools of 5 mice, and each experiment was done in triplicate. Values are expressed as the mean of the triplicate experiments in infected ([utrif ]) and uninfected ([bull ]) age-matched mice. ([squf ]) Represents the kinetics of c-fos expression as the quotient of infected OD c-fos/β-actin/control OD c-fos-β-actin.

Table 1. c-fos/β-actin expression in the brain regions of normal and intraperitoneally Taenia crassiceps- infected male mice at different weeks after infection
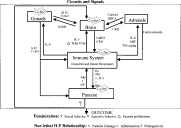
Fig. 4. Simplified chart of the network involved in the host--parasite relationship between Taenia crassiceps and BALBc/AnN male mice, showing the protagonist role of the c-fos gene at times of infection when the host is feminized and in apparently good health, despite the hundreds of cysticerci in the peritoneal cavity. The chart shows the principal nodes or clusters of nodes (in squares) of the parasite (reproductive system, other physiological systems) and of the host (immune system, gonads, adrenals and brain) or of both host and parasite (stars) (c-fos gene). Bidirectional arrows correspond to the most prominent connections between clusters, tips indicating to the directional flux of the signals. Connections belong to the parasite (antigens (Ags), toxins (t), other molecules (OP)) or to the host (hormones (Ho), antibodies (Abs), interleukins (IL's), immunocytes (c), chemical mediators of inflammation (i), and others (OH)). The network may adopt different states of activation and may result in quite different events, possibly varying with each pathogen. In the case illustrated here the network's major outputs are hormonal feminization of the host, with behavioural consequences, and a change in the host's endocrinological and immunological environment that is permissive of parasite reproduction. The high levels of oestrogen and low levels of DHT in the host, acting through c-fos expression, among many other possible nodes, would favour parasite proliferation, change the host's sexual and aggressive behaviour patterns and, also, tame its immune response towards the parasite by up-regulating the TH2 response with consequent down regulation of TH1 and perhaps interfering with inflammation and innate immune responses that could potentially be harmful to the host.