Introduction
Patients with schizophrenia spectrum disorders display cognitive biases. They present a decreased competence to control their own cognition (‘thinking about one's thinking’). Typically, impairments affect the abilities to appraise and weigh information effectively, to select appropriate responses including decisions based on perceptions, to cope with cognitive limitations and to build up mental states (Lysaker et al. Reference Lysaker, Warman, Dimaggio, Procacci, LaRocco, Clark, Dike and Nicolo2008, Reference Lysaker, Gumley, Luedtke, Buck, Ringer, Olesek, Kukla, Leonhardt, Popolo and Dimaggio2013). One major cognitive bias pertains to decision-making, the executive part of meta-cognition comprising meta-cognitive monitoring and self-regulation (Flavell et al. Reference Flavell, Green and Flavell1993). In marked contrast to self-report data indicating that schizophrenia patients consider themselves as rather hesitant and insecure (Freeman et al. Reference Freeman, Garety, Kuipers, Colbert, Jolley, Fowler, Dunn and Bebbington2006), they display an objective ‘Jumping to Conclusions’ bias (JTC) – a tendency to hasty decision-making during probabilistic reasoning. The JTC bias in schizophrenia patients was initially described by Hemsley & Garety (Reference Hemsley and Garety1986). Patients display a premature acceptance of beliefs as true, even when there is limited supporting evidence (Garety & Freeman, Reference Garety and Freeman1999; Fine et al. Reference Fine, Gardner, Craigie and Gold2007; Ziegler et al. Reference Ziegler, Rief, Werner, Mehl and Lincoln2008; Lincoln et al. Reference Lincoln, Ziegler, Mehl and Rief2010). JTC is commonly assessed by the ‘beads task’ (BT; Huq et al. Reference Huq, Garety and Hemsley1988) or slightly modified versions using other stimuli (e.g. fish; Moritz et al. Reference Moritz, Veckenstedt, Bohn, Hottenrott, Scheu, Randjbar, Aghotor, Kother, Woodward, Treszl, Andreou, Pfueller and Roesch-Ely2013). The task requests a decision after a variable amount of stimuli. Incorporating only one (or two) stimuli to come to a decision is defined as JTC (Garety et al. Reference Garety, Hemsley and Wessely1991; Moritz & Woodward, Reference Moritz and Woodward2005; Van Dael et al. Reference Van Dael, Versmissen, Janssen, Myin-Germeys, van Os and Krabbendam2006; Speechley et al. Reference Speechley, Whitman and Woodward2010b ). Furthermore, in their review that included more than 200 studies Garety and Freeman found JTC to be confirmed as a characteristic of individuals with delusions (Garety & Freeman, Reference Garety and Freeman2013).
Currently, several concepts try to link the JTC bias with general cognitive theories in schizophrenia. For example, JTC and altered salience attribution are supposed to be based on a common cognitive bias (Kapur, Reference Kapur2003; Rubio et al. Reference Rubio, Ruiz-Veguilla, Hernandez, Barrigon, Salcedo, Moreno, Gomez, Moritz and Ferrin2011; Esslinger et al. Reference Esslinger, Braun, Schirmbeck, Santos, Meyer-Lindenberg, Zink and Kirsch2013): A complex dopaminergic dysfunction (Fusar-Poli & Meyer-Lindenberg, Reference Fusar-Poli and Meyer-Lindenberg2012a , Reference Fusar-Poli and Meyer-Lindenberg b ; Howes et al. Reference Howes, Kambeitz, Kim, Stahl, Slifstein, bi-Dargham and Kapur2012) is considered to lead to the attribution of aberrant salience to stimuli, which could explain why patients are prone to assuming their hypothesis as confirmed by current evidence and making hasty decisions (Hemsley, Reference Hemsley2005; Speechley et al. Reference Speechley, Whitman and Woodward2010b ). Other authors suggest JTC to be linked to disturbed reward anticipation and learning (Heinz & Schlagenhauf, Reference Heinz and Schlagenhauf2010; Murray, Reference Murray2011; Juckel et al. Reference Juckel, Friedel, Koslowski, Witthaus, Özgürdal, Gudlowski, Knutson, Wrase, Brüne, Heinz and Schlagenhauf2012) as well as to altered prediction error signalling assuming a disturbed error-dependent updating of inferences and beliefs about the world (Hemsley & Garety, Reference Hemsley and Garety1986; Bentall et al. Reference Bentall, Rowse, Shryane, Kinderman, Howard, Blackwood, Moore and Corcoran2009; Fletcher & Frith, Reference Fletcher and Frith2009; Speechley et al. Reference Speechley, Whitman and Woodward2010b ; Murray, Reference Murray2011; So et al. Reference So, Freeman, Dunn, Kapur, Kuipers, Bebbington, Fowler and Garety2012).
So far, several functional magnetic resonance imaging (fMRI) studies have defined neural correlates underlying probabilistic reasoning in healthy volunteers and schizophrenia patients, predominantly involving a fronto-striatal-thalamic network (Blackwood et al. Reference Blackwood, Fytche, Simmons, Bentall, Murray and Howard2004; Grinband et al. Reference Grinband, Hirsch and Ferrera2006; Weickert et al. Reference Weickert, Goldberg, Callicott, Chen, Apud, Das, Zoltick, Egan, Meeter, Myers, Gluck, Weinberger and Mattay2009; Furl & Averbeck, Reference Furl and Averbeck2011; Koch et al. Reference Koch, Wagner, Schachtzabel, Schultz, Guellmar, Reichenbach, Sauer and Schloesser2011; Bach & Dolan, Reference Bach and Dolan2012; Morris et al. Reference Morris, Vercammen, Lenroot, Moore, Langton, Short, Kulkarni, Curtis, O'Donnell, Weickert and Weickert2012).
Recently, a hypo-activation in the ventral tegmental area (VTA) and the right ventral striatum (VS) during the point of decision-making was found in schizophrenia patients compared with healthy controls, while a broad cortical activation pattern became apparent during the entire process of probabilistic reasoning (Rausch et al. Reference Rausch, Mier, Eifler, Esslinger, Schilling, Schirmbeck, Englisch, Meyer-Lindenberg, Kirsch and Zink2014). A comparably reduced activation pattern within the right VS was also revealed in patients with an ‘at-risk mental state’ (ARMS; Rausch et al. Reference Rausch, Mier, Eifler, Fenske, Schirmbeck, Englisch, Schilling, Meyer-Lindenberg, Kirsch and Zink2015), which illustrates that underlying neurobiological alterations are already present in the ARMS population. Pathogenetic studies in schizophrenia are limited by illness- and treatment-related confounds, which is why comprehensive investigations of ARMS patients are an extraordinarily useful tool to gain insight into the development of pathology over time.
ARMS patients are commonly identified using cognitive basic symptoms (BS) or ‘ultra-high-risk’ (UHR) criteria. Patients fulfilling UHR criteria present with attenuated psychotic symptoms (APS) and/or brief limited intermittent psychotic symptoms (BLIPS) or display a genetic risk and a deterioration syndrome (Fusar-Poli et al. Reference Fusar-Poli, Borgwardt, Bechdolf, Addington, Riecher-Roessler, Schultze-Lutter, Keshavan, Wood, Ruhrmann, Seidman, Valmaggia, Cannon, Velthorst, de Haan, Cornblatt, Bonoldi, Birchwood, McGlashan, Carpenter, McGorry, Klosterkoetter, McGuire and Yung2013).On average, about 22% of ARMS patients convert to psychosis later on (McGorry et al. Reference McGorry, Nelson, Amminger, Bechdolf, Francey, Berger, Riecher-Rössler, Klosterkötter, Ruhrmann, Schultze-Lutter, Nordentoft, Hickie, McGuire, Berk, Chen, Keshavan and Yung2009; Ruhrmann et al. Reference Ruhrmann, Schultze-Lutter, Salokangas, Heinimaa, Linszen, Dingemans, Birchwood, Patterson, Juckel, Heinz, Morrison, Lewis, von Reventlow and Klosterkötter2010; Fusar-Poli et al. Reference Fusar-Poli, Bonoldi, Yung, Borgwardt, Kempton, Valmaggia, Barale, Caverzasi and McGuire2012).
So far, the associations between JTC and neurocognitive properties have been studied in patients with schizophrenia spectrum disorder (Bentall et al. Reference Bentall, Rowse, Shryane, Kinderman, Howard, Blackwood, Moore and Corcoran2009; Garety et al. Reference Garety, Waller, Emsley, Jolley, Kuipers, Bebbington, Dunn, Fowler, Hardy and Freeman2014), in patients with first-episode psychosis (Falcone et al. Reference Falcone, Murray, Wiffen, O'Connor, Russo, Kolliakou, Stilo, Taylor, Gardner-Sood, Paparelli, Jichi, Di, David, Freeman and Jolley2014), in patients with current and remitted delusions (Dudley et al. Reference Dudley, John, Young and Over1997; Colbert et al. Reference Colbert, Peters and Garety2010), in delusion-prone individuals (Colbert & Peters, Reference Colbert and Peters2002; White & Mansell, Reference White and Mansell2009) and in ARMS patients (Broome et al. Reference Broome, Johns, Valli, Wooley, Tabraham, Brett, Valmaggia, Peteres, Garety and McGuire2007). As meta-cognitive impairments are associated with the development of delusions (Fletcher & Frith, Reference Fletcher and Frith2009; Speechley et al. Reference Speechley, Murray, McKay, Munz and Ngan2010a ; Moritz et al. Reference Moritz, Andreou, Schneider, Wittekind, Menon, Balzan and Woodward2014; Ross et al. Reference Ross, McKay, Coltheart and Langdon2015), it seems reasonable that the majority of studies assessed JTC in ARMS samples with upcoming psychotic positive symptoms according to the UHR criteria. However, the ARMS sample can be comprehensively characterized if cognitive BS as well as UHR criteria are assessed using sensitive instruments, such as the Early Recognition Inventory based on IRAOS (ERIraos; Häfner et al. Reference Häfner, Bechdolf, Klosterkötter and Maurer2012; Rausch et al. Reference Rausch, Eifler, Esser, Esslinger, Schirmbeck, Meyer-Lindenberg and Zink2013; Maurer et al. 2015), or the Schizophrenia Proneness Instrument – Adult Version (SPI-A; Schultze-Lutter et al. Reference Schultze-Lutter, Addington, Ruhrmann and Klosterkötter2007) combined with the Structured Interview for Prodromal Symptoms (SIPS; Miller et al. Reference Miller, McGlashan, Rosen, Cadenhead, Ventura, McFarlane, Perkins, Pearlson and Woods2003), or the Comprehensive Assessment of At-Risk Mental States (CAARMS; Yung et al. Reference Yung, Yuen, McGorry, Phillips, Kelly, Dell'Olio, Francey, Cosgrave, Killackey, Stanford, Godfrey and Buckby2005). So far, no data are available on JTC in ARMS stages presenting with cognitive BS only and potential differences compared to ARMS stages fulfilling UHR criteria.
Therefore, we evaluated the baseline data of the secondary prevention of schizophrenia study (PREVENT; Bechdolf et al. Reference Bechdolf, Müller, Stutzer, Wagner, Maier, Lautenschlager, Heinz, Janssen, Gaebel, Michel, Schneider, Lambert, Naber, Brüne, Krüger-Özgürdal, Wobrock, Riedel and Klosterkötter2011). Within this study, several ARMS subgroups were comprehensively characterized for cognitive BS and UHR criteria, and the JTC bias was assessed. We hypothesized we would find a more pronounced JTC bias in ARMS patients fulfilling UHR criteria (ARMS-UHR) in contrast to ARMS patients only presenting with cognitive BS (ARMS-BS). Secondary endpoints were the comparison of the ARMS group and a group of healthy control participants that were assessed in a parallel study, as well as correlations of the JTC severity with psychometric data.
Method and materials
The protocol of the clinical multi-centre PREVENT study (registry identifier: ISRCTN: 02658871) was approved by the respective institutional ethical committees of the trial sites. All participants were provided with detailed information about the study, and written informed consent was obtained prior to study entry. Detailed descriptions of design and setting have been published separately (Bechdolf et al. Reference Bechdolf, Müller, Stutzer, Wagner, Maier, Lautenschlager, Heinz, Janssen, Gaebel, Michel, Schneider, Lambert, Naber, Brüne, Krüger-Özgürdal, Wobrock, Riedel and Klosterkötter2011), but the most important characteristics are summarized below.
Setting and subjects
The ongoing interventional trial PREVENT was conducted at 12 German Early Intervention Centres (Aachen, Berlin, Bochum, Bonn, Cologne, Dresden, Düsseldorf, Göttingen, Hamburg, Heidelberg, Mannheim, Munich). First, subjects were screened by an Inclusion Criteria Checklist (ICC). For the detailed assessment of UHR criteria, the SIPS including the Scale of Prodromal Symptoms (SOPS) was applied (Miller et al. Reference Miller, McGlashan, Rosen, Cadenhead, Ventura, McFarlane, Perkins, Pearlson and Woods2003). Additionally, cognitive BS were assessed by the SPI-A (Schultze-Lutter et al. Reference Schultze-Lutter, Addington, Ruhrmann and Klosterkötter2007).
Inclusion criteria for the ARMS group
Age between 18 and 49 years and attribution to one of the following groups: (A) attenuated positive symptoms – presence of at least one of the following symptoms (SOPS scores 3–5): unusual thought content/delusional ideas, suspiciousness/persecutory ideas, grandiosity, perceptual abnormalities/hallucinations, disorganized communication. (B) Brief limited intermittent psychotic symptoms – presence of at least one of the following symptoms: ⩽7 days resolving spontaneously (SOPS score = 6): hallucinations, delusions, formal thought disorder. (C) Predictive basic symptoms – presence of at least two of the following nine symptoms (SPI-A ⩾3) at least three times a week during the last 3 months: inability to divide attention, thought interferences, thought pressure, thought blockages, disturbance of receptive speech, disturbance of expressive speech, disturbance in abstract thinking, unstable ideas of reference, captivation of attention by details of the visual field. (D) Family risk plus reduced functioning: any DSM-IV psychotic disorder in first-degree relatives or DSM-IV schizotypal personality disorder of the index person plus impaired global functioning [a 30% drop in the Global Assessment of Functioning Scale (GAF) compared to the premorbid level or a score of ⩽50 during the last 12 months].
Exclusion criteria for the ARMS group
(A) Prior or present antipsychotic treatment for >1 week, (B) prior psychotic episode for >1 week, (C) present suicidality or self-harming behaviour, (D) alcohol or substance dependence, (E) presence of an organic brain disease, (F) intelligence quotient (IQ) <70, (G) contemporary or planned pregnancy, breastfeeding or missing reliable method of contraception in case of sexual activity.
Healthy control participants
Thirty healthy control participants were recruited in parallel in the Mannheim study centre. Control subjects were matched for age, gender, level of education and premorbid verbal intelligence. Prior to study entry, all of the participants were comprehensively evaluated to exclude any positive family history of schizophrenia, bipolar disorder or suicide in first-degree relatives, any previous or current psychiatric disorders according to the Mini-International Neuropsychiatric Interview (M.I.N.I.) and any former or present psychopharmacological treatment.
Methods of cross-sectional assessments
Socio-demographic parameters such as age, gender, educational level and estimated level of premorbid verbal intelligence [Multiple-Choice Vocabulary Intelligence Test, version B (MWT-B)] were assessed. For psychometric ratings, the SIPS, the SPI-A, the Positive and Negative Syndrome Scale (PANSS), the Montgomery–Asberg Depression Scale (MADRS), the Social and Occupational Functioning Assessment Scale (SOFAS), and the Clinical Global Impression Scale (CGI) were applied. Furthermore, to assess lifetime diagnoses of co-morbid disorders, the Structured Clinical Interviews for DSM-IV, SCID-I and SCID-II were performed at baseline. Additionally, several neurocognitive and meta-cognitive tests including a JTC task to assess a tendency towards hasty decision-making during probabilistic reasoning were applied. The JTC task was conducted on a PC screen and requested a probabilistic decision after a variable amount of stimuli. Participants successively viewed a total of ten fish in two different colours being fished out of a lake and had to decide which of two possible lakes they were coming from. Colour ratios in the lakes were 80/20% or 20/80%, respectively. The coloured fish were presented in a pre-defined fashion (1–1–1–2–1–1–1–1–2–1). After each fish, subjects were asked to estimate the probability of the fish being taken from lake A or lake B. Afterwards they were asked if this probability was already sufficient for them to decide for one of the two lakes. This task was not repeated, but consisted of a single run (Moritz et al. Reference Moritz, Veckenstedt, Bohn, Hottenrott, Scheu, Randjbar, Aghotor, Kother, Woodward, Treszl, Andreou, Pfueller and Roesch-Ely2013). It has been developed based on the classical BT (Huq et al. Reference Huq, Garety and Hemsley1988), where subjects viewed beads of two colours being drawn out of a jar and had to decide which of two jars they were drawn from. Further instructions are identical. The ARMS patients underwent the fish task within the PREVENT study whereas the healthy control participants who allowed secondary analyses completed the classical BT and preponderantly also a modified JTC fish task within fMRI scanning, which was part of another study (also see limitations section). Both tasks are detailed described elsewhere (Esslinger et al. Reference Esslinger, Braun, Schirmbeck, Santos, Meyer-Lindenberg, Zink and Kirsch2013; Rausch et al. Reference Rausch, Mier, Eifler, Fenske, Schirmbeck, Englisch, Schilling, Meyer-Lindenberg, Kirsch and Zink2015).
Primary outcome
The primary endpoint of this cross-sectional investigation was the comparison of the ‘draws to decision’ (DTD; number of fish needed for a decision) between the different ARMS subgroups either presenting cognitive BS or fulfilling UHR criteria. The UHR group contains a subgroup presenting with attenuated psychotic symptoms (ARMS-APS) and a subgroup presenting with brief limited intermittent psychotic symptoms (ARMS-BLIPS) (solely or additionally to APS). We hypothesized we would find significantly less DTD in the UHR group. Additionally, we compared the number of subjects showing JTC (defined as one or two draws) expecting to find more JTC in the UHR group.
Within the PREVENT study, patients with genetic risk for psychosis in parallel to reduced functioning were classified as ‘vulnerability group’ and separated from the ARMS subgroups either presenting cognitive BS or fulfilling UHR criteria. We therefore excluded them from our primary analysis, but not from secondary evaluations.
Secondary outcomes
In order to examine the occurrence of the JTC bias in our ARMS sample per se we compared the total ARMS group with a group of healthy control participants who had been investigated in a parallel project in the Mannheim study centre regarding DTD, as a secondary outcome (Rausch et al. Reference Rausch, Mier, Eifler, Fenske, Schirmbeck, Englisch, Schilling, Meyer-Lindenberg, Kirsch and Zink2015). Again, we evaluated the number of subjects showing JTC in the different groups.
For exploratory reasons, we further stratified the ARMS group for vulnerability, cognitive BS, APS and BLIPS to evaluate group differences. Moreover, we evaluated possible correlations of DTD with clinical characteristics.
Statistics
Statistical analyses were performed using the SPSS software (IBM SPSS v. 21.0, IBM Corp., USA). Socio-demographic characteristics as well as the primary endpoint were assessed using Student's t tests. Secondary endpoints were evaluated using analysis of variance (ANOVA), two-sided Student's t test and Fisher's exact test to investigate group-specific differences. Correlations were expressed by Pearson's correlation coefficient.
Results
In the framework of the PREVENT study, 234 ARMS patients were recruited and 188 subjects could be included in the final data analysis. In parallel, a sample of 30 healthy control participants was characterized and could be included in the final data analysis. ARMS patients were identified using SIPS and SPI-A. The mean SIPS sum score was 29.2, the mean SPI-A sum score was 60.54. A total of 42 patients were allocated to ARMS-BS and 132 patients were attributed to ARMS-UHR, presenting APS and/or BLIPS (Table 1). Furthermore, 14 subjects were attributed to the vulnerability group (see Method section, inclusion criteria, group D). There were no significant differences regarding age, gender, education and premorbid verbal intelligence between ARMS-BS and ARMS-UHR (Table 1).
Table 1. Socio-demographic and psychopathological characteristics of ARMS-BS and ARMS-UHR subgroups
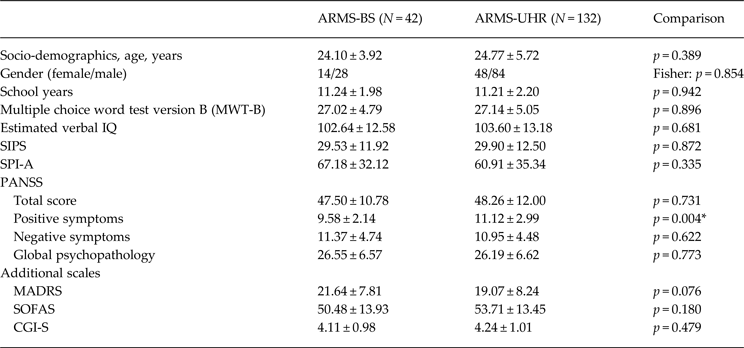
Data is reported as mean ± standard deviation (s.d.).
ARMS, At-risk mental state; BS, basic symptoms; CGI-S, Clinical Global Impression – Severity subscore; MADRS, Montgomery–Asberg Depression Scale; PANSS, Positive and Negative Syndrome Scale; SIPS, Structured Interview for Prodromal Symptoms; SOFAS, Social and Occupational Functioning Assessment Scale; SPI-A, Schizophrenia Proneness Instrument – Adult Version; UHR, ultra-high risk;
* Significant.
Comparison of the ARMS subgroups (ARMS-BS v. ARMS-UHR)
As related to the primary endpoint of this study, the DTD significantly differed between ARMS-BS (3.66 ± 2.35) and ARMS-UHR (2.62 ± 2.25) as UHR patients needed fewer fish to make a decision (T = 2.30, df = 172, p = 0.023, d = 0.41; see Fig. 1). The mean level of certainty (%) at the point of decision did not differ between the ARMS subgroups (ARMS-BS: 84.77 ± 16.62; ARMS-UHR: 84.10 ± 13.39; T = 0.26, df = 152, p = 0.799, d = 0.05).

Fig. 1. Displays the mean DTD in the ARMS subgroups. ARMS, At-risk mental state; BS, basic symptoms; DTD, draws to decision; UHR, ultra-high risk.
Furthermore, ARMS-UHR tended to fulfil behavioural criteria for JTC (43.2%) more often than ARMS-BS (26.2%) (Fisher's exact test, p = 0.069, d = 0.28).
Comparison with healthy control participants
The PREVENT sample of 188 ARMS patients was compared within a secondary analysis with healthy control subjects (N = 30) who did not differ significantly regarding age (p = 0.429), gender (p = 0.313), education as measured by the number of school years (p = 0.232) and estimated premorbid verbal intelligence (MWT-B; p = 0.336). A between-group comparison regarding DTD revealed significant differences, as ARMS patients (2.90 ± 2.32) needed less stimuli than controls (4.20 ± 2.44) to make a decision (T = 2.83, df = 216, p = 0.005, d = 0.56; see Fig. 2). The mean level of certainty (%) at the point of decision differed significantly between groups, as controls were more secure with their decision (ARMS: 84.64 ± 13.90; controls: 89.67 ± 8.09; T = 2.76, df = 64.5, p = 0.008, d = 0.43).

Fig. 2. Displays the mean DTD of ARMS patients and healthy control participants. ARMS, At-risk mental state; DTD, draws to decision.
Moreover, the ARMS group tended to show more JTC (38.8%) compared to the control group (20.0%) (Fisher's exact test, p = 0.064, d = 0.25).
Exploratory analysis
The between-group comparison of the entire model including the vulnerability group, ARMS-BS, ARMS-UHR-APS, ARMS-UHR-BLIPS and healthy controls using a one-way ANOVA revealed a significant difference (F = 3.64, df = 4, p = 0.007, d = 0.56). Fig. 3 displays the group means of DTD for all subgroups.

Fig. 3. Displays the mean DTD in the different symptom groups and the healthy control participants. APS, Attenuated psychotic symptoms; ARMS, at-risk mental state; BLIPS, brief limited intermittent psychotic symptoms; BS, basic symptoms; DTD, draws to decision.
Additionally, a prognostic score allowing for an individualized estimation of the transition risk was recently proposed by Ruhrmann et al. (Reference Ruhrmann, Schultze-Lutter, Salokangas, Heinimaa, Linszen, Dingemans, Birchwood, Patterson, Juckel, Heinz, Morrison, Lewis, von Reventlow and Klosterkötter2010) and Müller et al. (unpublished data). The prognostic score was calculated as (1.571 × SIPS-positive score > 16) + (0.865 × SCID-II score for schizotypal personality disorder = 3) + (0.793 × bizarre thinking score > 2) + (1.037 × sleep disturbance score > 2) + [((100 – highest SOFAS in the past year) – 34.64) × 0.033] + [(years of education recoded – 12.52) × 0.250]. Stratifying the ARMS group for the corresponding risk classes revealed a decrease of mean DTDs in parallel to an assumed increase of the risk for transition, with DTD = 3.15 in risk class 1, 2.79 in risk class 2, 2.73 in risk class 3 and 2.00 in risk class 4. The between-group comparison including the four risk classes and the healthy control group using a one-way ANOVA revealed a significant difference (F = 2.59, df = 4, p = 0.038, d = 0.57).
Furthermore, we evaluated possible associations of DTD with the early recognition scales SIPS and SPI-A and their subscales, but no significant correlations became apparent. However, in the entire ARMS group the correlation of DTD with clinical characteristics revealed significant correlations with the PANSS total score (r = −0.248, p = 0.001) and all scores of the PANSS five-factor model (Van der Gaag et al. Reference Van Dael, Versmissen, Janssen, Myin-Germeys, van Os and Krabbendam2006) except Emotional Distress that displayed a trend (Positive: r = −0.228, p = 0.003; Negative: r = −0.178, p = 0.023; Disorganization: r = −0.223, p = 0.004; Excitement: r = −0.199, p = 0.011; Emotional Distress: r = −0.147, p = 0.061; all two-sided). Stratifying the ARMS group for ARMS-UHR and ARMS-BS, similar correlations became apparent in ARMS-UHR, but in ARMS-BS no significant correlations between DTD and PANSS scores were revealed. Regarding MADRS, SOFAS and CGI, no correlations became evident.
Furthermore, in patients the mean level of certainty (%) at the point of decision significantly correlated with DTD (r = 0.360, p ⩽ 0.001), as subjects were more secure when they evaluated more stimuli before making a decision. However, no correlations with clinical characteristics were observed.
As DTD might be affected by the premorbid verbal intelligence (MWT-B) we tested for possible associations in the total sample, but no significant correlations became apparent (r = 0.021, p = 0.763).
Due to the exploratory character of the correlation study, the levels of statistical significance were reported without correction for multiple testing.
Discussion
Our findings indicate that ARMS patients fulfilling UHR criteria, in contrast to patients only presenting with cognitive BS, display an increased behavioural propensity to hasty decision-making. Furthermore, the comparison of ARMS patients and healthy control participants revealed that ARMS patients needed fewer stimuli than controls to reach a decision, confirming previous findings (Broome et al. Reference Broome, Johns, Valli, Wooley, Tabraham, Brett, Valmaggia, Peteres, Garety and McGuire2007; Rausch et al. Reference Rausch, Mier, Eifler, Fenske, Schirmbeck, Englisch, Schilling, Meyer-Lindenberg, Kirsch and Zink2015).
In comparison with healthy controls, the ARMS patients were more insecure about their decision. This pattern of findings suggests that ARMS patients tend to decide after fewer stimuli and on the basis of less certainty, which corresponds to findings of Moritz and colleagues, pointing to hasty decision-making based on low subjective certainty (Moritz et al. Reference Moritz, Woodward and Hausmann2006) and suggesting a liberal acceptance bias to be responsible for decision-making biases in schizophrenia (Moritz et al. Reference Moritz, Woodward and Lambert2007, Reference Moritz, Woodward, Jelinek and Klinge2008). Moderating effects of the entire amount of information on the subjective certainty seem possible, as suggested be the observed correlation of DTD and certainty.
To our knowledge, so far there is no study comparing different ARMS subgroups (ARMS-BS and ARMS-UHR) regarding JTC. Our data correspond to the results of studies exclusively assessing UHR samples (Broome et al. Reference Broome, Johns, Valli, Wooley, Tabraham, Brett, Valmaggia, Peteres, Garety and McGuire2007). Furthermore, our findings add to data on schizophrenia patients that suggest JTC as a ‘state’ associated with psychotic symptoms as well as a maintaining factor for delusions (Jolley et al. Reference Jolley, Thompson, Hurley, Medin, Butler, Bebbington, Dunn, Freeman, Fowler, Kuipers and Garety2014). On the other hand there are findings that support the theory of JTC as a trait phenomenon (Moritz & Woodward, Reference Moritz and Woodward2005; Van Dael et al. Reference Van Dael, Versmissen, Janssen, Myin-Germeys, van Os and Krabbendam2006; Garety & Freeman, Reference Garety and Freeman2013; Falcone et al. Reference Falcone, Murray, Wiffen, O'Connor, Russo, Kolliakou, Stilo, Taylor, Gardner-Sood, Paparelli, Jichi, Di, David, Freeman and Jolley2014).
However, the JTC bias seems to be an early cognitive marker of the emerging psychotic state. In a recent fMRI investigation, ARMS patients defined according to the ERIraos (Häfner et al. Reference Häfner, Bechdolf, Klosterkötter and Maurer2012; Rausch et al. Reference Rausch, Eifler, Esser, Esslinger, Schirmbeck, Meyer-Lindenberg and Zink2013; Maurer et al. unpublished data) also presented with JTC and showed reduced activation of the right VS during probabilistic decision-making (Rausch et al. Reference Rausch, Mier, Eifler, Fenske, Schirmbeck, Englisch, Schilling, Meyer-Lindenberg, Kirsch and Zink2015). Our results support associations of JTC with general delusion development, as the ARMS-BLIPS group in average needed the fewest fish to come to a decision. This might indicate a mechanism for the development of BLIPS and disturbed cognition during prodromal states. Taking into account the correlation of DTD with Positive, Negative, Disorganization and Excitement subscores of the PANSS, the JTC bias might be a hint towards general and underlying cognitive alterations that induce the development of psychotic symptoms in general, not only positive symptoms.
Our findings further underpin the proposals of disturbances related to reward anticipation (Heinz & Schlagenhauf, Reference Heinz and Schlagenhauf2010; Murray, Reference Murray2011; Juckel et al. Reference Juckel, Friedel, Koslowski, Witthaus, Özgürdal, Gudlowski, Knutson, Wrase, Brüne, Heinz and Schlagenhauf2012) and the ability to propagate prediction errors in a hierarchical Bayesian inference framework between lower- and higher-level systems in schizophrenia patients (Lee & Mumford, Reference Lee and Mumford2003; Fletcher & Frith, Reference Fletcher and Frith2009; Friston, Reference Friston2010; Dura-Bernal et al. Reference Dura-Bernal, Wennekers and Denham2012), extending them to the ARMS.
Furthermore, since our findings point to a successive increase of JTC during the course of the ARMS, this might suppose the late ARMS stage as a progress – not only theoretically, but underpinning the patients’ increasing cognitive impairment. This fact is reflected in the primary endpoint of the PREVENT trial, defining both transitions from the early to the late ARMS and to the psychotic state. Thus, JTC might display a measure of progressing functional alterations predominantly assigned to striatal brain regions that is accessible for scientific observation.
Besides JTC, several other measures are supposed to reflect the neurobiology in early psychotic states. For instance, Bodatsch et al. (Reference Bodatsch, Ruhrmann, Wagner, Müller, Schultze-Lutter, Frommann, Brinkmeyer, Gaebel, Maier, Klosterkötter and Brockhaus-Dumke2011) found a significant reduction in the duration mismatch-negativity in those ARMS patients who later converted to psychosis, compared to those without a transition. These results might contribute to an individualized prediction of the transition risk. The study of Frommann et al. (Reference Frommann, Pukrop, Brinkmeyer, Bechdolf, Ruhrmann, Berning, Decker, Riedel, Möller, Wölwer, Gaebel, Klosterkötter, Maier and Wagner2011) revealed more pronounced neurocognitive impairments (e.g. in the domains of working memory, processing speed and memory) in an UHR sample compared to patients only presenting with cognitive BS. Similarly, Koutsouleris et al. (Reference Koutsouleris, Davatzikos, Bottlender, Patschurek-Kliche, Scheuerecker, Decker, Gaser, Möller and Meisenzahl2012b ) found executive functioning and verbal IQ deficits to be particular properties of the late ARMS stage. Moreover, they suggest MRI-based biomarkers, e.g. alterations in prefrontal perisylvian and subcortical brain structures, to depict a helpful tool for an improved estimation of the psychosis risk (Koutsouleris et al. Reference Koutsouleris, Borgwardt, Meisenzahl, Bottlender, Möller and Riecher-Rössler2012a ).
However, it remains to be seen if a predictive value regarding transitions to psychosis can also be attributed to the JTC bias. Therefore, follow-up investigations within the PREVENT sample are mandatory.
Finally, our results underscore the importance of JTC and meta-cognitive deficits in general within cognitive theories of the developing psychosis as JTC is suggested as a state predominantly co-occurring with UHR stages. On the other hand, one could argue that subjects that are not liable to show JTC perhaps will not develop APS or BLIPS. Moreover, these findings highlight the need for complemented psychosis and ARMS treatments with bias modification programmes like meta-cognitive (Moritz et al. Reference Moritz, Andreou, Schneider, Wittekind, Menon, Balzan and Woodward2014) and reasoning (Ross et al. Reference Ross, Freeman, Dunn and Garety2011) training. For instance, Andreou et al. (Reference Andreou, Treszl, Roesch-Ely, Kother, Veckenstedt and Moritz2014) observed a positive association between an improvement of the JTC bias and the vocational outcome in patients with schizophrenia, also underpinning the importance of interventions regarding meta-cognitive deficits.
Limitations
This study is limited by its cross-sectional design, thus prohibiting accounts on the inherent transition risk and on differences regarding the transition risk in several ARMS subgroups. Therefore, the analysis of the longitudinal data of the PREVENT study is crucial. Furthermore, it must be re-emphasized that the healthy control participants were not recruited within PREVENT and underwent the classical BT instead of the fish task as described above. Regarding DTD, the instructions were identical, but outside this construct slight wording-differences were present. It might be discussed, whether the more salient fish paradigm might have enforced a tendency towards JTC behaviour (Dudley et al. Reference Dudley, John, Young and Over1997; Young & Bentall, Reference Young and Bentall1997). However, we were able to control for this caveat: a comparison of DTD according to the classical BT (mean 4.20 ± 2.44) revealed no significant difference to DTD extracted from a JTC fish task that was applied to a subgroup (n = 28) of the healthy control participants during fMRI scanning (mean 4.14 ± 2.29). Therefore we propose that it is feasible to draw a robust measure like DTD out of this data, especially because the mean DTD in our control group matches to scores of control groups reported in other studies (Moritz & Woodward, Reference Moritz and Woodward2005; White & Mansell, Reference White and Mansell2009; Colbert et al. Reference Colbert, Peters and Garety2010; Garety and Freeman, Reference Garety and Freeman2013; Ermakova et al. Reference Ermakova, Ramachandra, Corlett, Fletcher and Murray2014; Rausch et al. Reference Rausch, Mier, Eifler, Esslinger, Schilling, Schirmbeck, Englisch, Meyer-Lindenberg, Kirsch and Zink2014).
Conclusions
Data found in our study indicate an enhancement of hasty decision-making in patients fulfilling UHR criteria compared to patients presenting with cognitive BS only. Finally, interactions of JTC with the liability of psychotic transitions and therapeutic interventions should be unravelled during the longitudinal phase of PREVENT.
Acknowledgements
The authors acknowledge the support of this project provided by Judith Gimpel, M.Sc. Psych., Dr Dana Beck, Dipl.-Psych., Prof. Dr. Dr René Hurlemann, NN. The German Research Foundation provided funding to Prof. Dr. J. Klosterkötter (DFG, grant KL 970/7-1) and Prof. Dr. M. Zink (ZI 1253/3-1 and 3-2).
Declaration of Interest
S. Englisch has received travel expenses and consultant fees from AstraZeneca, Bristol-Myers Squibb, Eli-Lilly, Janssen Cilag, Lundbeck, Otsuka Pharma, Pfizer Pharma, Roche Pharma and Servier. M. Zink has received unrestricted scientific grants from the German Research Foundation (DFG), and Servier; further speaker and travel grants were provided from AstraZeneca, Lilly, Pfizer Pharma GmbH, Bristol–Myers Squibb Pharmaceuticals, Otsuka, Servier, Lundbeck and Trommsdorff. All other authors report no conflict of interest.






