INTRODUCTION
The ciliate genus Euplotes Ehrenberg, 1830 has often been reported in taxonomic–morphological, ecological, biochemical and toxicological studies as being an important group of benthic communities in various habitats (e.g. Curds, Reference Curds1975; Valbonesi et al., Reference Valbonesi, Apone and Luporini1997; Xu et al., Reference Xu, Song and Warren2004; Lobban et al., Reference Lobban, Modeo, Verni and Rosati2005; Schwarz et al., Reference Schwarz, Zuendorf and Stoeck2007; Trielli et al., Reference Trielli, Cervia, Giuseppi, Ristori, Kruppel, Burlando, Guella, Viarengo, Bagnoli, Corrado and Dini2008; Wilbert & Song, Reference Wilbert and Song2008; Yi et al., Reference Yi, Song, Clamp, Chen, Gao and Zhang2009; Jiang et al., unpublished). Some investigations have been made on the morphogenesis of Euplotes in the last decades (e.g. Hammond & Kofoid, Reference Hammond and Kofoid1936; Wise, Reference Wise1965; Washburn & Borror, Reference Washburn and Borror1972; Ruffolo, Reference Ruffolo1976), which demonstrated that the pattern of cell development in Euplotes is considerably stable except for some variation in the formation of frontoventral cirri (Voss, Reference Voss1989; Wang & Song, Reference Wang and Song1995; Foissner, Reference Foissner, Hausmann and Bradbury1996). The present species was first reported by Müller (Reference Müller1786) as Kerona vannus, transferred to Euplotes by Diesing (Reference Diesing1850), and re-described in detail by Tuffrau (Reference Tuffrau1960), Song & Packroff (Reference Song and Packroff1997) and Kwon et al. (Reference Kwon, Kang and Shin2007). In terms of morphogenesis, however, it has not been completely studied yet. As a contribution, its divisional morphogenesis has been investigated here emphasizing the development of the dorsal ciliature.
MATERIALS AND METHODS
Two populations of Euplotes vannus were isolated from different occasions in the Jiaozhou Bay, Qingdao (Tsingtao, 120°18′E 36°04′N), China (water temperature about 22°C, salinity ~30‰) in September, 2003 and May, 2006. Uniprotistan and clonal cultures were used, to which some rice grains were added to enrich bacterial food. Protargol staining (Wilbert, Reference Wilbert1975) was used to reveal the infraciliature. Drawings were made with the help of camera lucida and photomicrographs at a magnification of 1250×. To illustrate the changes during morphogenesis, parental cirri are depicted by contour whereas new ones are shaded black (Hu, Reference Hu2008).
Terminology is mainly according to Song et al. (Reference Song, Shao, Yi, Li, Warren, Al-Rasheid and Yang2009). Notably, a primary mode of dorsal kinety formation (or morphogenesis) is that the dorsal kinety anlagen of both proter and opisthe develop from a single primordium per parental kinety. Subsequently, the primordium divides to form the anlagen for both dividers, while the secondary mode is from two primordia formed separately per parental kinety (Li et al., Reference Li, Shao, Yi, Song, Warren, Al-Rasheid, Al-Farrah, Al-Qursisht, Zhang, Hh, Zhu and Ma2008; Shao et al., Reference Shao, Song, Li, Warren, Al-Rasheid, Al-Quraishy, Al-Farraj and Lin2008).
RESULTS
Infraciliature of non-dividers
Euplotes vannus has been reinvestigated in detail by Song & Packroff (Reference Song and Packroff1997) hence no further description is necessary. The infraciliature of population I is as shown in Figure 1A, B: adoral zone sharply curved posteriorly, composed of about 60 membranelles; 10 frontoventral, 2 marginal, 2–3 caudal and 5 transverse cirri; 9–10 dorsal kineties, with ~18 dikinetids in the middle row (N > 30). Macronucleus C-shaped. Silverline system of single-vannus type. Population II differs from population I only in the number of caudal cirri (3–4 versus 2–3; N > 30).
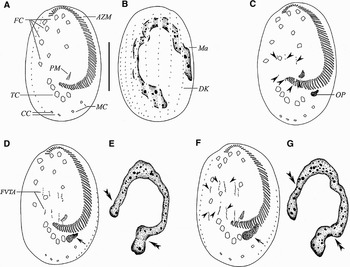
Fig. 1. Early stages of morphogenesis in Euplotes vannus (after protargol impregnation). (A, B) Ventral and dorsal views of the same specimen, to demonstrate the general pattern of the infraciliature; (C) ventral view of an early stage to show the oral primordium, arrowheads show the FVT-anlagen; (D, E) ventral view and nuclear apparatus of the same specimen at an early stage to show that two sets of four streaks are formed to the right of buccal cavity. Note the developing oral primordium (arrow) and macronuclear replication bands (double arrowheads); (F, G) slightly later stage, ventral view and nuclear apparatus of the same specimen, indicating 5 FVT-cirral streaks (arrowheads) and oral primordium within which membranelles are forming (arrow); double arrowheads point to the replication bands. AZM, adoral zone membranelles; CC, caudal cirri; DK, dorsal kinety; FC, frontoventral cirri; FVTA, frontoventral-transversal anlagen, Ma, macronucleus; MC, marginal cirri; OP, oral primordium; PM, paroral membrane; TC, transverse cirri. Scale bar = 40 µm.
Morphogenesis in cell division
Unless otherwise indicated, the description and figures below are based on the population I with 2–3 caudal cirri.
STOMATOGENESIS
Cortical morphogenesis in E. vannus starts with the formation of a small patch of kinetosomes (the opisthe's oral primordium) within a pouch beneath the cortex, ahead of the marginal cirri (Figures 1C & 4A, arrowhead). As the pouch enlarges, kinetosomes begin to align into the new membranelles from the anterior end towards the posterior (Figures 1D, F, arrows & 2A, C).
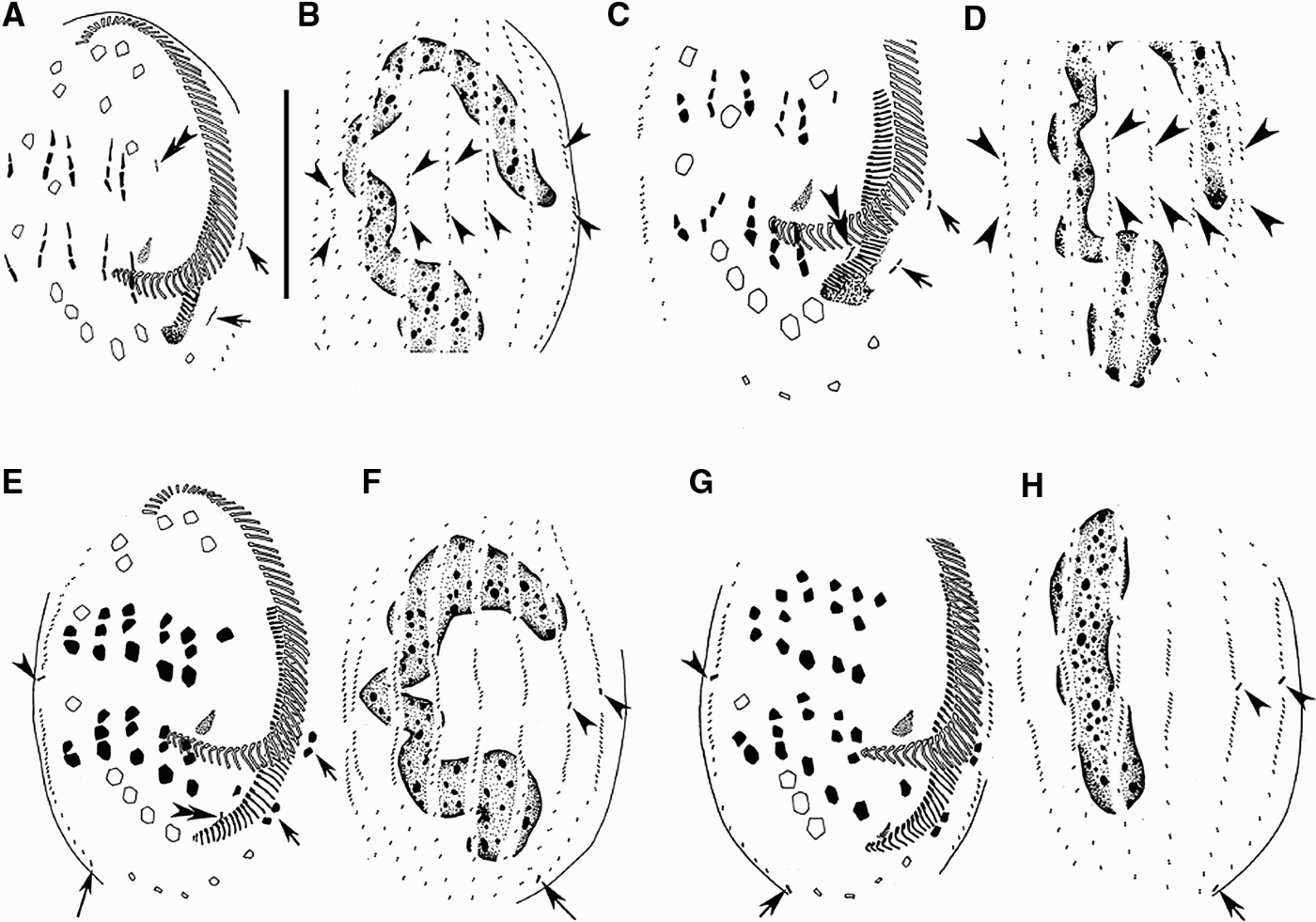
Fig. 2. Morphogenesis of Euplotes vannus after protargol impregnation. (A, B) Ventral and dorsal views of the same specimen to show the anlagen of marginal cirri (arrows), cirrus I/1 for the proter (double-arrowheads) and dorsal kineties (arrowheads); (C, D) ventral and dorsal views of a slightly later stage (the same specimen) to indicate the fragmentation of the cirri anlagen, the formation of cirrus I/1 anlage for the opisthe (double-arrowheads), the development of the anlagen of marginal cirri (arrows) and dorsal kineties (arrowheads); (E, F) ventral and dorsal views of the same specimen at a middle stage to show the completion of the cirral evolution, the differentiation of caudal cirri (arrowheads for the proter's, long tail arrows for the opisthe's), the appearance of the UM-anlage for the opisthe (double-arrowheads) and the development of the new marginal cirri (arrows); (G, H) ventral and dorsal views of a later middle stage (the same specimen) showing the development of caudal cirri (arrowheads for for the proter's, long tail arrows for the opisthe's) and the nodule-shaped macronucleus (H). Scale bar = 40 µm.
An additional anlage, the primordium for the paroral membrane, appears within the subcortical pouch, which is located close to the posterior end of the oral primordium (Figures 2E & 5I, J, double-arrowheads).
In the following periods, the UM-anlage begins to lengthen and then becomes broader (Figures 2G & 3A, C). The parental adoral zone remains nearly intact throughout the entire morphogenetic process.
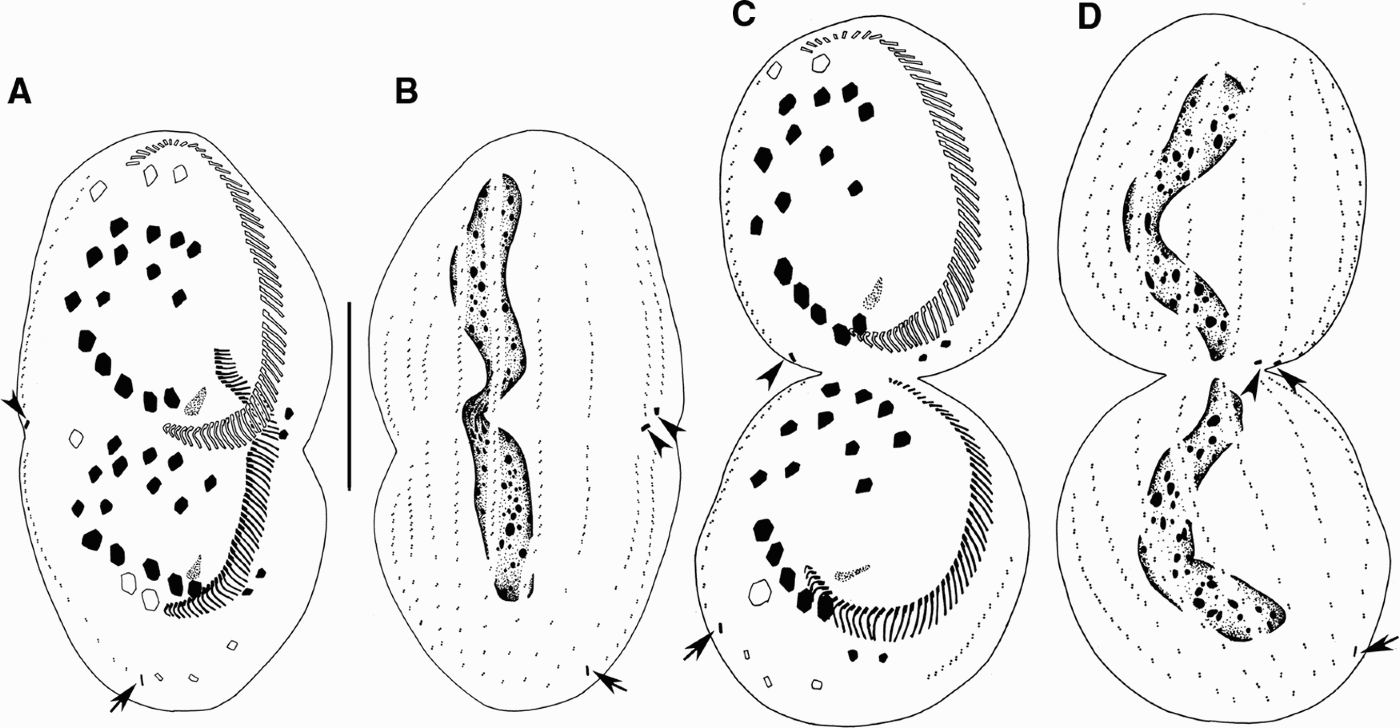
Fig. 3. Morphogenesis of Euplotes vannus after protargol impregnation. (A, B) Ventral and dorsal views, showing the migration of cirri and the development of dorsal kineties and caudal cirri; (C, D) ventral and dorsal sides of the same divider at late divisional stage showing infraciliature and nuclear apparatus. Arrowheads indicate the caudal cirri in the proter and arrows point to those in the opisthe. Scale bar = 40 µm.
DEVELOPMENT OF FRONTAL–VENTRAL–TRANSVERSE CIRRI (FVT)-ANLAGEN
At the same time as the formation of the oral primordium commences, two sets of FVT-anlagen originate de novo to the right of the buccal cavity as multiple basal bodies (Figures 1C & 4B, arrowheads). With further proliferation of basal bodies, the FVT-anlagen develop to two sets of four streaks first (Figure 1D), and then five (Figures 1F & 4C, arrowheads). Each cirral streak extends to both directions with proliferation of basal bodies. Clearly, no parental ciliary organelles are involved in the formation of these new primordia. Subsequently, the cirral anlagen streaks broaden, break apart as 3:3:3:3:2 pattern and then migrate developing as distinct cirri to daughter cells (Figures 2A, C, E, G; 3A, C; 4D, G & 5D, K).
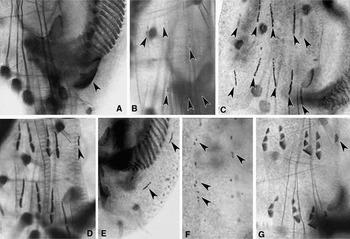
Fig. 4. Photomicrographs of morphogenesis in Euplotes vannus (protargol impregnated specimens). (A, B) Ventral views of a very early divider to show the appearance of oral primordium (arrowhead in A) and FVT-anlagen (arrowheads in B); (C) ventral view of an early divider demonstrating two sets of FVT-anlagen streaks (arrowheads); (D–F) ventral views of the same early divider showing the fragmentation of cirri-anlagen, appearance of the anlage of frontal cirrus I/1 in the proter (arrowhead in D), marginal cirri (arrowheads in E) and dorsal kineties (arrowheads in F); (G) ventral view of a later divider indicating frontal cirrus I/1 in the proter (arrowhead).
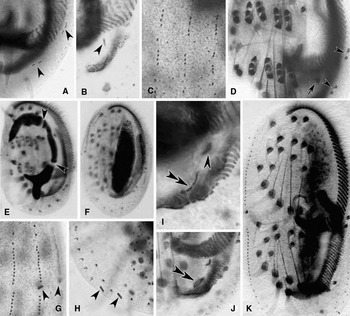
Fig. 5. Photomicrographs of morphogenesis in Euplotes vannus (protargol impregnated specimens). (A–C) Ventral and dorsal views, to show the break of marginal-cirri-anlagen (arrowheads in A), the anlage for cirrus I/1 in the opisthe (arrowhead in B) and the development of the anlagen of dorsal kineties; (D) ventral view, arrow indicates the frontal cirrus I/1 in the opisthe, arrowheads point to the marginal cirri in the proter and the opisthe; (E, F) to show the macronuclear apparatus of cells in earlier and middle stages, arrowheads show the replication bands; (G, H) to show the caudal cirri in the proter (arrowheads in G) and the opisthe (arrowheads in H); (I, J) ventral views of the opisthe to show the development of the PM-anlage (double-arrowheads) and the cirrus I/1 (arrowhead); (K) ventral view of a middle divider showing the migration of cirri and the split of macronucleus.
As soon as the FVT-anlagen breaks apart, a small patch of basal bodies appears to the left of streaks forming the anlage of the left-most frontal cirrus for the proter (Figures 2A, double arrowhead & 4D, arrowhead). Also, the opisthe's one appears close to the posterior of the new adoral zone (Figures 2C, double arrowheads & 5B, arrowhead). These two anlagen are formed de novo, i.e. apparently no old cirrus or paroral membrane joins in the formation. And then, these two anlagen develop by rapid proliferation of kinetosomes and migrate to their final positions (Figures 2E, G; 3A, C & 5I, arrowhead).
DEVELOPMENT OF MARGINAL CIRRI
One anlage for the marginal cirri forms de novo as a short row of kinetosomes near the parental adoral zone in the proter and the other near the newly formed adoral zone in the opisthe (Figures 2A, arrows & 4E, arrowheads). Each anlage subsequently breaks into two (Figures 2C, arrows & 5A, arrowheads) to form the two marginal cirri (Figures 2E, arrows & 5D, arrowheads).
NUCLEAR DIVISION
A replication band is recognizable in early stage at each end of the macronucleus (Figure 1E, double arrowheads). The macronucleus changes the shape as the replication bands progress (Figures 1G; 2B, D, F & 5E, arrowheads), which then becomes a short strand after the process of replication of the nuclear apparatus is completed (Figures 2H & 5F). As the daughter cells separate, the nodular macronucleus divides to form the C-shaped nucleus in each daughter cell (Figures 3B, D & 5K). Micronucleus not observed.
DEVELOPMENT OF DORSAL CILIATURE
On the dorsal side, the proliferation of new basal bodies occurs in the middle of each parental dorsal kinety in a non-typical primary mode, i.e. no dorsal kinety anlagen composed of densely arranged basal bodies are formed (Figures 2B, arrowheads & 5C). With further proliferation, these loosely arranged anlagen then move anteriorly and posteriorly and eventually replace the old structures (Figures 2D, F).
In the proter of population I, one caudal cirrus is formed at the posterior end of the right-most three dorsal kinety anlagen each (Figures 2E–H; 3A–D, arrowheads & 5G, arrowheads). Meanwhile, in the opisthe, one caudal cirrus forms at end of the right-most two old dorsal kinety rows each (Figures 2E, F, long tail arrows; 2G, H, arrows; 3A–D, arrows & 5H, arrowheads). So 50% of the specimens have 3 and 50% have 2 caudal cirri (Table 1).
Table 1. Distribution of caudal cirri in two clones of Euplotes vannus. CC, caudal cirri; Pop, population.

By contrast, in the population II, the morphogenetic process is the same as in the population I except for the number of caudal cirri during cell division and interphase stage. One caudal cirrus is formed at the end of the 4 rightmost dorsal kinety anlagen (proter) and 3 rightmost old dorsal kineties (opisthe) each. As a result, 50% of the specimens have 4 and 50% have 3 caudal cirri (Table 1).
DISCUSSION
The most important events of the morphogenetic process in our populations of E. vannus are as follows:
(1) the oral primordium in the opisthe generates de novo in a subcortical pouch;
(2) the paroral membrane anlage develops independently from the oral primordium within the same subcortical pouch;
(3) the anlagen for the frontal, ventral and transverse cirri in both dividers develop separately from the oral primordium or parental cirri, and are formed in a secondary mode;
(4) the anlagen for the cirrus I/1 and marginal cirri also are formed de novo and separately; and
(5) the dorsal kinety anlagen occur within the parental structures at mid-body, of which the ends of the right-most three/four contributes three/four caudal cirri for the proter, while only two/three caudal cirri are formed at the ends of the right most two/three old dorsal kineties for the opisthe.
The morphogenesis of Euplotes vannus has previously been investigated on several occasions by Fleury & Fryd-Versavel (Reference Fleury and Fryd-Versavel1981), Hufnagel & Torch (Reference Hufnagel and Torch1967), Tuffrau et al. (Reference Tuffrau, Tuffrau and Genermont1976) and Voss (Reference Voss1989), although all lacked some important stages. Hufnagel & Torch (Reference Hufnagel and Torch1967) mentioned the variations in the number of caudal cirri in clones of E. vannus with plausible reason but without any illustrations or photomicrographs. Since many important stages, especially the development of dorsal ciliature, have not been presented in detail, a comprehensive understanding of the processes of cortical development could not be obtained.
In Euplotes vannus, the oral primordium, FVT-anlagen, marginal cirri and macronuclear apparatus, develop in a typical euplotid mode (Fleury, Reference Fleury1991a, Reference Fleuryb; Hill, Reference Hill1980; Voss, Reference Voss1989; Washburn & Borror, Reference Washburn and Borror1972). The segmentation pattern of the FVT-anlagen in this species is 1:3:3:3:3:2, which is the same as in E. charon, E. focardii, E. harpa, E. moebiusi, E. trisulcatus, E. rariseta and E. mediterraneus (Klein, Reference Klein1936; Hill, Reference Hill1980; Fernández-Leborans & Zaldumbide, Reference Fernández-Leborans and Gastro de Zaldumbide1985; Serrano et al., Reference Serrano, Sola, Guinea, Arregui and Fernández-Galiano1992; Wang & Song, Reference Wang and Song1995; Pang et al., Reference Pang, Liu and Ren1998; Ma et al., Reference Ma, Jiang, Hu, Shao and Song2008). As a result, all of these species have 10 frontoventral cirri. This contrasts with another two patterns (1:3:3:3:2:2, 1:2:2:3:3:2) resulting in the formation of 9 and 7 frontoventral cirri respectively.
Another remarkable feature is its formation mode of caudal cirri: 1 caudal cirrus is formed at the posterior ends of the right-most 3 (or 4) dorsal kinety anlagen each in the proter; meanwhile, in the opisthe, 1 at the ends of right-most 2 (or 3) old dorsal kineties each. As a result, the proter always gets 3 or 4 caudal cirri while the opisthe acquires only 2 or 3, independent of the number of caudal cirri in the parental cell (Figures 2E–H & 3A–D). A similar phenomenon during physiological regeneration was already described by Fleury & Fryd-Versavel (Reference Fleury and Fryd-Versavel1981); by contrast, in other congeners of Euplotes, both dividers get an equal number of caudal cirri (e.g. Voss, Reference Voss1989; Foissner, Reference Foissner, Hausmann and Bradbury1996).
Conclusively, the number of caudal cirri in Euplotes vannus is unstable (2–3 or 3–4), and thus cannot be regarded as a key feature for species distinction.
ACKNOWLEDGEMENTS
We are grateful to Professor Weibo Song and Xiaozhong Hu, Laboratory of Protozoology, OUC, for their valuable comments and critical review of the manuscript. This work was supported by the Natural Science Foundation of China (Project No. 4090605) and a joint grant from the Center of Excellence in Biodiversity, King Saud University.








