Central-line–associated bloodstream infections (CLABSIs) are one of the most important healthcare-associated infections (HAIs)Reference Hodge and Puntis 1 , Reference Timsit 2 due to their morbidity, mortality, and cost. The neonatal intensive care unit (NICU) population is prone to CLABSIs because of frequent central venous catheter (CVC) use, need for medications, and/or total parental nutrition (TPN), which involve daily manipulations.Reference Vincent, Bihari and Suter 3 In addition, premature infants have both an immature immune system and an epidermal barrier that is often punctured for blood sampling or other invasive procedures. 4 – Reference Goldmann, Durbin and Freeman 7 Low birth weight (BW),Reference Geffers, Gastmeier, Schwab, Groneberg, Ruden and Gastmeier 8 – Reference de Brito, de Brito, Abdallah and Gontijo Filho 11 low gestational age (GA),Reference Auriti, Maccallini, Di Liso, Di Ciommo, Ronchetti and Orzalesi 9 , Reference de Brito, de Brito, Abdallah and Gontijo Filho 11 , Reference Marschall, Mermel and Classen 12 young chronological age,Reference Smith 13 , Reference Mahieu, De Muynck, Ieven, De Dooy, Goossens and Van Reempts 14 and presence of underlying metabolic conditionsReference Advani, Reich, Sengupta, Gosey and Milstone 15 have all been identified as CLABSI risk factors in infants. Furthermore, bloodstream infections (BSIs) and sepsis in neonates are associated with long-term neurodevelopmental effects and increased mortality.Reference Adams-Chapman and Stoll 16 – Reference Shah, Doyle and Anderson 18 Despite CLABSI surveillance, incidence rates have remained higher in NICUs than in other ICUs in Quebec and Canada. 19 , 20 Therefore, understanding risks factors for CLABSI in the NICU is a priority in the quest to eliminate CLABSIs.Reference Advani, Reich, Sengupta, Gosey and Milstone 15
Previous studies from various ICU settings, including the NICU, have shown an increased risk of CLABSI in patients with gastrointestinal conditions.Reference Niedner, Huskins and Colantuoni 21 – Reference Coffin, Klieger and Duggan 23 Elements that affect bowel wall integrity could contribute to the translocation of gastrointestinal microbiota into the bloodstream, leading to BSIs.Reference Graham, Begg, Larson, Della-Latta, Allen and Saiman 24 Bacterial translocation has been associated with gastrointestinal bacterial overgrowth, decreased intestinal blood flow, impaired immune system,Reference Wiest and Rath 25 , Reference Sherman 26 and gastrointestinal dysmotility,Reference Niedner, Huskins and Colantuoni 21 but it has also been described in patients with conditions such as abdominal surgery, parenteral nutrition, malnutrition, and bowel obstruction, and following ischemia-reperfusion injury shock.Reference Gatt, Reddy and MacFie 27 , Reference MacFie, Reddy, Gatt, Jain, Sowdi and Mitchell 28 Malnutrition, common in infants with gastrointestinal diagnoses, was also identified as an independent risk factor for HAI.Reference Al-Rawajfah, Stetzer and Hewitt 29 Most NICUs aim to eliminate CLABSIs, using bundles of measures for CVC insertion and maintenance. However, if CLABSIs in specific populations are secondary to gastrointestinal bacterial translocation, these infections are less likely to be prevented via regular preventive measures that aim to decrease infection due to microorganisms present on the skin.Reference Quach, Milstone, Perpête, Bonenfant, Moore and Perreault 30 A better understanding of the contribution of intra-abdominal pathologies to the overall risk of CLABSI is needed in the NICU population to better improve our approach to prevention. The objective of this study was to determine whether intra-abdominal conditions are an independent risk factor for CLABSI.
METHODS
Study Setting
This study was performed in 2 acute-care hospitals in Canada, part of the Pediatric Investigators Collaborating Network on Infections in Canada (PICNIC) network. All cases of CLABSI meeting the NHSN definition, as identified through infection control surveillance databases between April 2009 and March 2014, were eligible for this study.
The Montreal Children’s Hospital (MCH) NICU is a level III NICU with 24 beds and an average of 404 admissions per year; the full range of pediatric and surgical subspecialties is offered there. All patients admitted to the MCH NICU are born elsewhere, with referrals coming from McGill University hospitals and from other hospitals from the Greater Montreal Area, Northern Quebec, and Abitibi-Temiscamingue. The Royal Alexandra Hospital (RAH) NICU is a level II–III NICU with 69 beds and an average of 1,200 admissions per year. Patients admitted to the RAH NICU are both born at RAH and elsewhere, with referrals coming from Edmonton, Central Alberta, and Northern Alberta (population ~2.3 million in 2014), as well as the Northwest Territories and some parts of northern British Columbia. These 2 hospitals had similar CLABSI rates over the study period: 2.32–7.52 cases per 1,000 catheter days for the MCH and 0–6.1 cases per 1,000 catheter days for the RAH.
Study Design
We performed a retrospective, matched case–control study. A case was considered an occurrence of CLABSI during the study period. If a patient had >1 CLABSI, only the first was considered; a CLABSI case was excluded if no matched controls could be identified. All controls were patients who had the potential to develop a CLABSI case; some controls may have developed a CLABSI after their matched date or outside our study period. However, each patient was enrolled in the study only once. Each patient with a CLABSI case was matched to a maximum of 3 control patients from the same center based on 3 criteria: (1) National Healthcare Safety Network (NHSN) BW category, (2) CVC dwell time (number of days between CVC insertion and removal, ±10%), and (3) patient chronological age at the time of CVC insertion (±7 days if <90 days old, or ±14 days if 90 days old or more). The date of onset (using the infant’s chronological age) for each CLABSI was defined as T0. Charts of case patients and control patients were reviewed using a standardized case report form collecting demographic information, details about insertion and removal of CVC, CLABSI duration (time between first positive and first negative blood culture), pathogens and treatment, possible risk factors (ie, heel punctures, other invasive devices, TPN, blood products, and intravenous locks), prior and current underlying medical conditions, prior surgeries, and mortality. In patients without arterial lines, numbers of heel punctures were tracked retrospectively by the number of blood gases obtained for each patient.
Definitions
Using the NHSN definition, 31 a CLABSI was defined as a positive blood culture not related to another site of infection in a symptomatic patient with a CVC in place at the time of or removed within 2 days prior to the onset of infection. 4 , Reference Wenzel, Thompson and Landry 32
Ethics approval
The research ethics boards of both hospitals approved this study.
Statistical Analyses
Case patients and control patients were characterized by potential risk factors for BSIs including primary diagnosis, comorbidities, invasive device use, surgery, and heel punctures. In our primary analysis, we performed univariate and multivariate conditional logistic regression analyses to test covariates that were potential risk factors for CLABSI in neonates. We calculated odds ratios (OR) and 95% confidence intervals (95% CI) to evaluate the strength of each association. Final model selection was performed using Bayesian information criteria, and results were confirmed using likelihood ratio testing. For all calculations, a 2-tailed P<.05 was considered significant.
Our secondary analysis was a subanalysis comparison of CLABSI cases with and without a mucosal barrier injury (MBI) pathogen. 31 We performed a χ2 test on risk factors, a deviance test for BW categories, univariate logistic regression, and a multivariate analysis. We also repeated the matched case–control analyses using only MBI CLABSI cases. All analyses were performed using STATA v.14.0 (StataCorp, College Station, Texas).
RESULTS
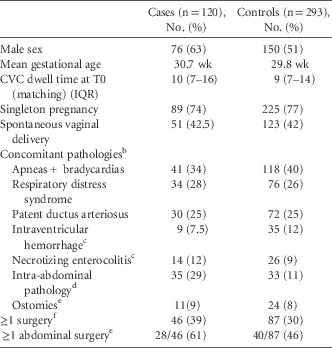
From April 2009 to March 2014, 120 cases were identified and matched to 293 controls. A single case from RAH was excluded because no control could be identified, given the long CVC dwell time. Overall, 51 of 120 case patients (43%) were <28 weeks gestational age (GA; median, 29 weeks; IQR, 26–37); 24 of 120 (20%) had a BW of <750 g (median, 1,105 g; IQR, 813–1,748), 89 of 120 (74%) were singleton pregnancies, and 76 of 120 (63%) were male. Infants from RAH were slightly younger (median GA, 28 weeks; IQR, 26–37) and smaller (median BW, 1,105 g; IQR, 880–1,370) than infants from MCH (median GA, 29 weeks; IQR, 26–36; median BW, 1,405 g; IQR, 770–2,865). Case patients and control patients, matched on center, birth weight, age at time of line insertion, and CVC dwell time, were comparable in the distribution of baseline characteristics, with the exception of male sex and intra-abdominal pathologies (Table 1). Case patients and control patients were also comparable in their concomitant pathologies, as recorded on their discharge summaries. The main primary diagnosis was prematurity, which occurred in 47 of 120 case patients (39%) and 159 of 293 control patients (54%). The second most common primary diagnosis among case patients was intra-abdominal pathology, which occurred in 27 of 120 case patients (23%); among control patients, patent ductus arteriosus was the second most common diagnosis, occurring in 21 of 293 control patients (7%).
TABLE 1 Baseline Characteristics of Neonatal CLABSI Cases and Their ControlsFootnote a
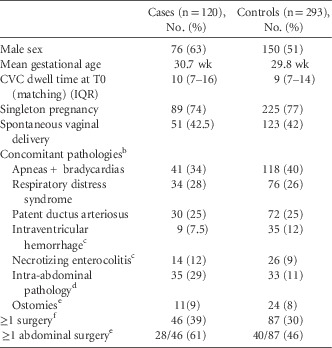
NOTE. CLABSI, central-line–associated bloodstream infection; CVC, central venous catheter; T0, the date of onset for each CLABSI.
a Matching criteria: Hospital, National Healthcare Safety Network (NHSN) birth weight categories, CVC dwell time (±10%) and patient chronological age at time of CVC insertion (±7 d if <90 d old, ±14 d if 90 d old or more).
b As found on discharge summaries.
c All grades/stages included.
d Present and unresolved 7 d prior to onset (T0).
e Includes colostomy, ileostomy, duodenostomy,gastrostomy, jejenuostomy, tracheostomy.
f Any time prior to onset (T0).
Risk Factors for CLABSI
Overall, 35 of 120 case patients (29%) had 1 or more active intra-abdominal pathology in the week preceding CLABSI onset, compared with 33 of 293 of control patients (11%). Intra-abdominal pathologies included but were not limited to necrotizing enterocolitis (NEC), as written in the chart and not meeting NHSN definition, Hirschsprung’s disease, short bowel syndrome, gastroschisis, omphalocele, gastrointestinal perforations, volvulus, among others. In infants without an arterial line, 31 of 102 of case patients (30%) and 23 of 247 control patients (9%) had ≥3 heel punctures in the 48 hours prior to CLABSI onset. Of 35 patients with an intra-abdominal pathology, 10 patients had a CLABSI associated with MBIs or Candida; 1 C. albicans, 1 C. parapsilosis, 4 E. faecalis and, 4 E. coli. Surgeries at any time prior to T0, in particular abdominal surgeries, were more prevalent in case patients (28 of 46; 61%) than in control patients (40 of 87; 46%). Surgeries mostly consisted of bowel resections/manipulations and cardiac surgeries. Surgeries among case patients consisted of various forms of bowel resections and G-tube placements. The MBI pathogens associated with CLABSIs in case patients that had abdominal surgery within the 7 days prior to onset (2 of 8) were 2 Klebsiella spp. 31 In total, 31 case patients had ≥3 heel punctures in the 48 hours preceding CLABSI, and the following pathogens were isolated: 20 coagulase-negative staphylococci, 4 S. aureus, 5 MBI pathogens (Enterococci spp., Klebsiella spp., and S. marcescens), and 1 Candida sp.
Unadjusted Analysis
The following factors were associated with an increased risk of CLABSI: presence of an active intra-abdominal pathology in the 7 days prior to CLABSI (OR, 3.39; 95% CI, 1.81–6.36), abdominal surgery in the 7 days prior to CLABSI (OR, 3.49; 95% CI, 1.12–10.90), ≥3 heel punctures in the 48 hours preceding CLABSI (OR, 4.01; 95% CI, 1.93–8.33), and male sex (OR, 1.7; 95% CI, 1.05–2.63) (Table 2). CVC insertion attempts were not associated with increased risk of CLABSI.
TABLE 2 Matched Univariate and Multivariate Analyses of Risk Factors for CLABSI in Neonates

NOTE. CLABSI, central-line–associated bloodstream infection; OR, odds ratio; CI, confidence interval; T0, the date of onset for each CLABSI; CVC, central venous catheter.
a In 7 d prior to T0.
b Any time prior to onset (T0).
c ≤48 h prior to onset (T0).
Adjusted Analysis
Adjusting for potential confounders (Table 2), 2 factors remained independently associated with the risk of CLABSI: active intra-abdominal pathology in the preceding week (OR, 5.9; 95% CI, 2.50–14.05) and ≥3 heel punctures within the 48 hours preceding CLABSI onset (OR, 5.36; 95% CI, 2.37–12.15). While being male did not remain a statistically significant risk factor (OR, 1.76; 95% CI, 0.98–3.16), it was included in the final model because it significantly confounded our main determinant. Case patients had, on average, 3 heel punctures (median, 1; IQR, 1–3) compared with control patients, who underwent an average of 1.7 heel punctures (median, 1; IQR, 0–2) in the 48 hours preceding CLABSI onset.
CLABSI Caused by MBI Versus Non-MBI Pathogens: Cohort Analysis
Of 120 CLABSI cases, 27 (22.5%), had they occurred in an oncology population (stem cell transplants or neutropenic), met the NHSN definition for a mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBI). 31 We performed a cohort subanalysis of neonatal MBI-LCBI versus non–MBI-LCBI cases. There were no statistically significant differences between the 2 groups in age at CLABSI onset, mean birth weight, birth weight categories, or CVC dwell time. There was no statistically significant difference in the time to CVC removal after onset of CLABSI with neonatal MBI CLABSIs (5.14 days; standard deviation [SD], 7.01) compared with coagulase-negative Staphylococcus CLABSIs (6.68 days; SD, 15.51). Concurrently, there was no difference between MBI-LCBI versus non–MBI-LCBI cases in the proportion of cases in which CVC removal occurred before or after the first negative culture. In addition, the duration of neonatal MBI cases was significantly shorter than that of non-MBI cases (Table 3). We did not find an association between the presence of an intra-abdominal pathology or surgery and a CLABSI caused by MBI pathogens (Table 4).
TABLE 3 CLABSI Cases: Comparison of Baseline Risk Factors Between Cases Caused by MBI Pathogens and Non-MBI Pathogens

NOTE. MBI, mucosal barrier injury; SD, standard deviation; T0, defined as the date of onset for each CLABSI; BW, birth weight; CLABSI, central line-associated bloodstream infection; CVC, central venous catheter.
a ≤48 h prior to onset (T0).
b Deviance test.
TABLE 4 CLABSI Cases: Univariate and Multivariate Logistic Regression Analysis of Cases Caused by MBI and Non-MBI Pathogens

NOTE. MBI, mucosal barrier injury; CLABSI, central line-associated bloodstream infection; CVC, central venous catheter; T0, the date of onset for each CLABSI.
a In 7 d prior to T0.
b ≤48 h prior to onset (T0).
c Any time prior to onset (T0).
MBI-CLABSI: Matched Case–Control Analysis
Keeping only neonatal MBI-CLABSIs and their matched controls, univariate and multivariate analyses showed that both intra-abdominal pathology and having ≥3 heel punctures in the 48 hours preceding CLABSI onset were statistically significant (Table 5).
TABLE 5 Matched Univariate and Multivariate Analysis of Risk Factors: Cases Caused by MBI Pathogens and Their Respective Controls

NOTE. MBI, mucosal barrier injury; CLABSI, central line-associated bloodstream infection; T0, the date of onset for each CLABSI.
a In 7 d prior to T0.
b Any time prior to onset (T0).
c ≤48 h prior to onset (T0).
DISCUSSION
In our case–control study performed in 2 level-III NICUs in Canada, intra-abdominal pathologies and multiple heel punctures were independently associated with an increase in the risk of CLABSI. A secondary analysis of only neonatal MBI-CLABSIs and their matched controls substantiated these findings.
Intra-abdominal pathologies have been previously reported as risk factors for CLABSI in the literature reporting adult patient cohorts.Reference Gatt, Reddy and MacFie 27 , Reference MacFie, Reddy, Gatt, Jain, Sowdi and Mitchell 28 Disruption of intestinal flora leading to BSIs was demonstrated in adult surgical patients.Reference MacFie, Reddy, Gatt, Jain, Sowdi and Mitchell 28 , Reference Taur, Xavier and Lipuma 33 Pediatric studies have reported an association between gastrointestinal conditions and Gram-negative and Candida BSIs.Reference Graham, Begg, Larson, Della-Latta, Allen and Saiman 24 , Reference Feja, Wu and Roberts 34 , Reference Saiman, Ludington and Pfaller 35 Multiple cohort studies have also reported an increased prevalence of gastrointestinal conditions in pediatric bacteremic patients.Reference Niedner, Huskins and Colantuoni 21 , Reference Blanchard, Fortin and Rocher 22 , Reference Squires, Duggan and Teitelbaum 36 This association, however, was not seen when the study population was expanded to include patients outside the ICU.Reference Advani, Reich, Sengupta, Gosey and Milstone 15
Elements that affect bowel wall integrity could contribute to translocation of Gram-negative bacteria and fungi into the bloodstream, causing bacteremia.Reference Graham, Begg, Larson, Della-Latta, Allen and Saiman 24 Of all BSIs in the NICU, a portion is caused by MBI pathogens. Of this subset, some CLABSIs associated with MBIs can originate from line contamination (ie, true CLABSIs) because enteric organisms can colonize the skin and the CVC,Reference Polin, Denson and Brady 37 especially in patients with ostomies. The other portion of MBI-associated CLABSIs can be secondary to intestinal bacterial translocation and are therefore unaffected by catheter care preventive measures. Waters et alReference Waters, Larson and Wu 38 showed that isolated BSI organisms were unique to patients. They were not found on caretakers’ hands or in neighboring neonates, which suggests that some infections do not originate from cutaneous cross contamination. How can we differentiate BSIs caused by translocation from those caused by contamination if both have MBIs as culprit pathogens?
Previous studies have attempted to define this subset of BSIs. Coffin et alReference Coffin, Klieger and Duggan 23 proposed a definition for MBI-CLABSI in neonates: NHSN-BSI caused by an MBI pathogen in a patient with an eligible MBI-GI condition and TPN exposure. They subsequently conducted a retrospective cohort study on neonates with CLABSIs, and they observed a similar distribution of pathogens between cases meeting the MBI-CLABSI neonatal definition and cases that did not. This finding brings the theory of translocation into question. However, their study was not designed to differentiate cases with MBI pathogens colonization of skin and a real CLABSI versus those caused by bacterial translocation. Furthermore, a temporal microbiota study in premature infants showed that coagulase-negative Staphylococcus (CoNS), classically thought to be a skin organism, was the predominant bacteria in the gut of these infants for the first 3 weeks of life.Reference Aujoulat, Roudiere and Picaud 39 Notably, however, their aim was not to determine whether gastrointestinal conditions were a risk factor for CLABSI in the NICU.
Our study design allowed us to detect an independent and almost 6-fold increase in risk of CLABSI when intra-abdominal pathologies were present, while simultaneously studying multiple exposures. Essentially all recruited patients were on TPN at the time of or in the 7 days preceding CLABSI onset. We also observed a statistically significantly shorter duration of CLABSI caused by MBI pathogens (mean, 2.27 days vs 3.63 days) in the absence of any differences in CVC removal time; this finding suggests a different underlying pathophysiology.
Because active intra-abdominal pathologies are an independent risk factor for CLABSI, we recommend refining the CLABSI definition to include a subcategory of cases associated with bacterial translocation in the NICU, similar to the 2013 revised definition for CLABSIs in hematology–oncology patients. 31 Operationally, this definition should include neonates admitted to the NICU with a CVC, a documented BSI with an MBI pathogen, and the presence of an active intra-abdominal pathology and/or a recent intra-abdominal surgery, in the absence of a primary intra-abdominal infection according to NHSN criteria.
Our study also revealed that multiple heel punctures were independently associated with a 5-fold increase in risk of all CLABSIs. The theory of invasive procedures and skin disruption increasing the risk of CLABSI in the NICU has been shown previously,Reference Healy, Baker, Palazzi, Campbell and Edwards 40 but only 1 case report suggests heel punctures as a possible risk factor for healthcare-associated infections.Reference Onesimo, Fioretti, Pili, Monaco, Romagnoli and Fundaro 41 Because our data collection was retrospective, it was impossible to determine causality; heel punctures ordered in the 48 hours prior to T0 may have been associated with an already deteriorating infant requiring a closer follow-up (confounding by indication). Infants with MBI pathogens had a lower average number of heel punctures compared with infants with CLABSI caused by non-MBI pathogens, which is not explained by the difference in the proportion of infants with an arterial line.
Our study was retrospective and was thus limited in its ability to demonstrate causation. We were also restricted to data available in the clinical charts, which may have caused observation bias due to strong reliance on physician and nursing notes. Our study was also limited in its capacity to remove potential confounding by indication in the association between an increased number of heel punctures and the development of CLABSI. A prospective cohort study would be needed to address this limitation. Having a large cohort of >400 infants and conducting the study in 2 separate NICUs in different Canadian provinces, our study is likely generalizable to level III and IV NICUs.
Given our findings, we recommend refining the CLABSI definition to include a subcategory of cases potentially associated with gastrointestinal bacterial translocation. Future studies should validate the impact of the change in definition.
ACKNOWLEDGMENTS
We thank M. Althaide and B. Kamstra for providing useful feedback regarding the case report form and reviewing all the clinical charts at Royal Alexandra Hospital.
Financial support: This study was funded by an investigator-initiated grant from Sage. Sage played no role in the current study: Sage did not provide CHG-impregnated clothes, was not involved in the study design, and did not have any input on the manuscript. Caroline Quach received funding from GlaxoSmithKline, Pfizer, and AbbVie (all for research grant or support). The remaining authors have no financial relationships relevant to this article to disclose.
Potential conflicts of interest: The authors have no conflicts of interest relevant to this article to disclose.