Introduction
Small, relatively advanced rodents related to modern North American cricetids radiated through much of the western and central United States during the late Miocene and early Pliocene. Because they apparently did not descend from earlier, less-derived Oligocene or early Miocene North American cricetids (Martin, Reference Martin, Smith and Friedland1975; Lindsay, Reference Lindsay, Janis, Gunnell and Uhen2008), they are considered to be Asian immigrants. Originally referred to either Copemys Wilson, Reference Wilson1937, or Peromyscus Gloger, Reference Gloger1841 (Wilson, Reference Wilson1937; Shotwell, Reference Shotwell1967; Lindsay, Reference Lindsay1972), later additions and evaluations have shown late Miocene and early Pliocene cricetids to represent considerable diversity, likely including ancestors to extant North and South American cricetid clades (Jacobs, Reference Jacobs1977; Baskin, Reference Baskin1979; Lindsay and Jacobs, Reference Lindsay and Jacobs1984; Czaplewski, Reference Czaplewski1987; Lindsay, Reference Lindsay, Janis, Gunnell and Uhen2008; Korth and De Blieux, Reference Korth and De Blieux2010; Korth, Reference Korth2011; Martin and Zakrzewski, Reference Martin and Zakrzewski2019; Kelly et al., Reference Kelly, Martin and Ronez2020; Martin et al., Reference Martin, Peláez-Campomanes, Ronez, Barbière, Kelly, Lindsay and Baskin2020; Ronez et al., Reference Ronez, Martin and Pardiñas2020).
Based on small samples of cricetid dental specimens from the late Miocene Quiburis Formation of Arizona, Jacobs (Reference Jacobs1977) erected two new genera: Paronychomys, with two species (P. lemredfieldi, which is the type species, and P. tuttlei) and Galushamys, with one species (G. redingtonensis). Because of a similarity in the alternation pattern of the molar cusps, Jacobs (Reference Jacobs1977) proposed that Paronychomys was a cricetine cricetid related to the extant Onychomys Baird, Reference Baird1857, but noted that Paronychomys differed from Onychomys by having more hypsodont cheek teeth and unreduced third molars. Subsequently, four additional species of Paronychomys were described: P. alticuspis Baskin, Reference Baskin1979; P. woodburnei Martin, Reference Martin2008; P. shotwelli Korth, Reference Korth2011; and P. jacobsi Kelly, Reference Kelly2013. Kelly (Reference Kelly2013) also provisionally transferred Kellogg's (Reference Kellogg1910) Peromyscus antiquus to Paronychomys as ?Paronychomys antiquus. No further mention was made of a possible phylogenetic connection with Onychomys in any of the papers describing the latter species. Indeed, Baskin (Reference Baskin1979, p. 706) suggested that the dentition of Paronychomys “…was convergent with primitive microtines and with the South American granivore Phyllotis,” and Lindsay (Reference Lindsay, Janis, Gunnell and Uhen2008, p. 466, fig. 27.2) regarded Paronychomys as closely related to Repomys May, Reference May1981, and Galushamys.
Another mesodont cricetid, Basirepomys, was named by Korth and De Blieux (Reference Korth and De Blieux2010), with Peromyscus pliocenicus Wilson, Reference Wilson1937, as the type species. Korth and De Blieux (Reference Korth and De Blieux2010, p. 231) also suggested that Basirepomys included “…additional specimens referred to P. cf. pliocenicus elsewhere,” referring to material described by Shotwell (Reference Shotwell1967) and discussed by May (Reference May1981) from the Miocene of Oregon. However, they did not formally include the Oregon material in their synonymy. Korth and De Blieux (Reference Korth and De Blieux2010) also recognized a second species, B. robertsi, from the Hemphillian Sevier River Formation, Utah. Korth and De Blieux (Reference Korth and De Blieux2010, p. 231) noted that Basirepomys molars were higher crowned than those of Peromyscus and lower crowned than those of Repomys, and in the etymology section they indicated that Basirepomys was “…a closely related genus,” to Repomys, but no further comparisons were made with Repomys. Korth (Reference Korth2011) later emended the diagnosis of Basirepomys and transferred specimens that Jacobs and Lindsay (Reference Jacobs and Lindsay1984) previously referred to Peromyscus pliocenicus from Rome, Oregon, to the new species Basirepomys romensis Korth, Reference Korth2011. Korth (Reference Korth2011) also reallocated specimens from Pinole Junction, California, that May (Reference May1981) had previously referred to as Peromyscus cf. pliocenicus, to the new genus and species Miotomodon mayi Korth, Reference Korth2011, further proposing that “Miotomodon is derived from Paronychomys rather than Peromyscus or Basirepomys” (Korth, Reference Korth2011, p. 145).
In a paper proposing a phylogenetic model for the origin of cricetids related to modern woodrats, Martin and Zakrzewski (Reference Martin and Zakrzewski2019) established two subtribes for the Neotomini: the Neotomina (woodrats) and a redefined Galushamyina Lindsay, Reference Lindsay, Janis, Gunnell and Uhen2008 (common name: reprats). Neotomina included the extinct Tsaphanomys shotwelli (= Shotwell's [Reference Shotwell1967] Peromyscus cf. pliocenicus from Juniper Creek, Oregon = Korth's [Reference Korth2011] Paronychomys shotwelli) and extant Neotoma Say and Ord, Reference Say and Ord1825, Hodomys Merriam, Reference Merriam1894, and Xenomys Merriam, 1892 (Merriam, Reference Merriam1892a). The Galushamyina included the extinct Protorepomys, Miotomodon, Repomys, and Galushamys, plus extant Nelsonia. Protorepomys was composed of P. mckayensis Martin and Zakrzewski, Reference Martin and Zakrzewski2019, and P. bartlettensis Martin and Zakrzewski, Reference Martin and Zakrzewski2019, representing additional Miocene cricetid samples that Shotwell (Reference Shotwell1967) had previously referred to Peromyscus. At that time, Martin and Zakrzewski (Reference Martin and Zakrzewski2019) considered Paronychomys lemredfieldi to be an unusual, high-crowned relative of Onychomys Baird, Reference Baird1857, as intended by Jacobs (Reference Jacobs1977), but transferred Paronychomys shotwelli to Tsaphanomys. They also suggested that the remaining Basirepomys species were neotomines unrelated to Repomys. This study provides a more detailed phylogenetic comparison of extinct and modern cricetids that conceivably could be related to either the woodrats or reprats, with special consideration of the problematic taxonomy of Paronychomys and Basirepomys.
Materials and methods
Dental locus designations follow standard usage with upper teeth designated by capital letters and lower teeth by lowercase letters. Dental nomenclature (Fig. 1) follows Martin et al. (Reference Martin, Peláez-Campomanes, Ronez, Barbière, Kelly, Lindsay and Baskin2020), with modifications by Kelly et al. (Reference Kelly, Martin and Ronez2020) and this paper. Following Martin and Zakrzewski (Reference Martin and Zakrzewski2019, p. 1567), “enamel rings with a hollow center [on the occlusal surface] are termed ‘atolls,’ equal to ‘fossettes,’ ‘islands,’ or ‘pits’ of other authors.” North American Land Mammal ages (e.g., Clarendonian, Hemphillian, and Blancan) follow Lindsay et al. (Reference Lindsay, Mou, Downs, Pederson, Kelly, Henry and Trexler2002), Tedford et al. (Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004), and Martin et al. (Reference Martin, Peláez-Campomanes, Honey, Fox, Zakrzewski, Albright, Lindsay, Opdyke and Goodwin2008).

Figure 1. Dental nomenclature for cricetid teeth follows Martin et al. (Reference Martin, Peláez-Campomanes, Ronez, Barbière, Kelly, Lindsay and Baskin2020), with modifications by Kelly et al. (Reference Kelly, Martin and Ronez2020) and this paper. The anterior structures of M2 and m2 are labeled differently because they lack a procingulum and procingulid, respectively. All terms used for M2 and m2 can be applied to M3 and m3, respectively. Not all structures are present in the taxa included in the analysis.
As noted above, some investigators have suggested that Paronychomys may be related to Onychomys (i.e., Jacobs, Reference Jacobs1977; Martin and Zakrzewski, Reference Martin and Zakrzewski2019) because they both exhibit a similar degree of alternation of the primary cusps. However, other studies have not recognized this putative relationship (e.g., Lindsay, Reference Lindsay, Janis, Gunnell and Uhen2008; Korth, Reference Korth2011). In fact, Onychomys and members of Peromyscini Cockerell et al., Reference Cockerell, Miller and Printz1914 (which are Peromyscus, Habromys Hooper and Musser, Reference Hooper and Musser1964, Megadontomys Merriam, 1898 (Merriam, 1898 (Merriam, Reference Merriam1898a)), Neotomodon Merriam, 1898 (Merriam, Reference Merriam1898b), Osgoodomys Hooper and Musser, Reference Hooper and Musser1964, and Podomys Osgood, Reference Osgood1909) have very similar cusp alternation on the upper and lower molars. Lindsay (Reference Lindsay, Janis, Gunnell and Uhen2008) allied Paronychomys with Repomys and Galushamys in a separate tribe (Galushamyini) from the tribe including Onychomys, Peromyscus, and Reithrodontomys (Democricetodontini). During our study we made extensive morphological comparisons of Paronychomys and Onychomys. There are numerous significant differences between them, indicating they are not closely related (Table 1). Moreover, genetic analyses have consistently allied Onychomys as the closest sister taxon to Peromyscini (e.g., Reeder and Bradley, Reference Reeder and Bradley2004; Reeder et al., Reference Reeder, Carroll, Edwards, Kilpatrick and Bradley2006; Miller and Engstrom, Reference Miller and Engstrom2008; Keith, Reference Keith2015; Steppan and Schenk, Reference Steppan and Schenk2017).
Table 1. List of significant character differences between Paronychomys and Onychomys that indicate they are not closely related. Abbreviations: ap = greatest anteroposterior length; ht = height.

In order to determine the phylogenetic relationships of Paronychomys and Basirepomys to Neotomini and further investigate the synapomorphies supporting the neotomine subtribes, we performed a cladistic analysis based on 40 dental and mandibular characters with representative species of each genus (Fig. 2; Table 2; our list of morphological characters and character states, and our taxon/character state matrix are provided in Appendices 1, 2). We selected the type species as representatives for extinct genera and subgenera, but during our examination we discovered a number of differences in the dental morphology of species of Basirepomys and Protorepomys. In order to determine if these differences might be phylogenetically significant, we included all species of these genera in the analysis. We also included a second species of Paronychomys, P. jacobsi, because it is much better characterized than the type species. Copemys loxodon (Cope, Reference Cope1874) was used for the outgroup. Copemys, as currently recognized, is composed of a complex of nine middle to late Miocene species, probably not all congeneric (Martin and Zakrzewski, Reference Martin and Zakrzewski2019; Kelly et al., Reference Kelly, Martin and Ronez2020; Ronez et al., Reference Ronez, Martin, Kelly, Barbière and Pardiñas2021). Most later North American Miocene Neotominae were likely derived from various members of the Copemys species complex (Lindsay, Reference Lindsay, Janis, Gunnell and Uhen2008; Martin et al., Reference Martin, Peláez-Campomanes, Ronez, Barbière, Kelly, Lindsay and Baskin2020; Ronez et al., Reference Ronez, Martin and Pardiñas2020, Reference Ronez, Martin, Kelly, Barbière and Pardiñas2021). Jacobs and Lindsay (Reference Jacobs and Lindsay1984) named a new tribe, Copemyini, for Copemys, which Ronez et al. (Reference Ronez, Martin and Pardiñas2020) considered as likely valid, but a more in-depth analysis is still needed to confirm its recognition. It should be noted that in Galushamyina and Neotomina, some character transformations occur in parallel between the earliest members through the more-derived members. These include progressive increases in lophodonty and a development of flat occlusal planes during initial to early wear. These transformations also occur in a wide variety of other rodent lineages and were not included in the analysis because of convergence.

Figure 2. M1 (upper left in each quadruplet), M3 (lower left), m1 (upper right), and m3 (lower right) of species in cladistic analyses. Question mark indicates tooth position unknown. All occlusal views. (1) Copemys loxodon, LM1 (UCMP 317394, reversed), LM3 (UCMP 317400, reversed), Lm1 (UCMP 317625, reversed), Rm3 (UCMP 317546, reversed), photos by C. Ronez; (2) Basirepomys pliocenicus 1, RM1 (LACM [CIT] 1968), Rm1 (LACM [CIT] 1966, holotype), from Wilson (Reference Wilson1937); (3) Basirepomys romensis, LM1 (USNM 23564, reversed), Rm1, (USNM 23567, reversed), Lm3 (UMNH 23566), photos of E. Lindsay casts; (4) Basirepomys robertsi 2, LM1 (UMNH VP 18749, reversed), Rm1 (UMNH VP 18751, holotype, reversed), Rm3 (UMNH VP 18752), from Korth and De Blieux (Reference Korth and De Blieux2010); (5) Paronychomys lemredfieldi, RM1 and RM3 (AMNH FAM 3249), Rm1 and Rm3 (AMNH FAM 3430, reversed), photos of E. Lindsay casts; (6) Paronychomys jacobsi, RM1 (LACM 156279), LM3 (LACM 156277, reversed), Lm1 (LACM 156289), Lm3 (LACM 156287), photos by T. Kelly; (7) Protorepomys bartlettensis, LM1 (UO 24957, reversed), LM3 (UO 25078, reversed), Lm1 (UO 25591); photos by K. Tate-Jones, Lm3 (UO 21720), from Shotwell (Reference Shotwell1967); (8) Protorepomys mckayensis, RM1 (UO 26942), Lm1 (UO 24603), Rm3 (UO 21720), photos by K. Tate-Jones; (9) Miotomodon mayi 2, LM1 (UCMP 82669, reversed), LM3 (UCMP 83672, reversed), Lm1 (UCMP 82671), from Korth (Reference Korth2011); (10) Tsaphanomys shotwelli, LM1 (UO 21716, reversed), photo by K. Tate-Jones; Lm1 (UO 21719), Lm3 (UO 21270), from Korth2 (2011); (11) Lindsaymys takeuchii Kelly and Whistler, Reference Kelly and Whistler2014, RM1 (LACM 126050, holotype), RM3 (LACM 156397), Rm1 (LACM 156394, reversed), Lm3 (LACM 156521), photos by T. Kelly; (12) Neotoma (Paraneotoma) quadriplicata Hibbard, Reference Hibbard1941, LM1 (UMMP 41198, reversed); photo by A. Rountrey; LM3 (UMMP 41196, reversed), Rm1 (FHSM 14330, reversed), Rm3 (UMMP 41196), photos by R. Zakrzewski; (13) Neotoma cinerea occidentalis Baird, Reference Baird1855, RM1 and RM3 (MVZ 69441), Lm1 and Lm3 (MVZ 69441), photos by Jessica Blois, UC Merced; (14) Neotoma lepida lepida 3, LM1 and LM3 (MVZ 10438, reversed), Rm1 and Rm3 (MVZ 10438, reversed), photos by C. Conroy; (15) Hodomys alleni, LM1 and LM3 (IBUNAM:CNMA 8912, reversed), from Silva and Cuenca (Reference Silva and Cuenca2020a), Rm1 and Rm3 (IBUNAM:CNMA 46970, reversed), from Cervantes et al. (Reference Cervantes, Castañón, Sánchez, Vargas and Hortelano2016a); (16) Xenomys nelsoni Merriam, 1898 (Merriam, Reference Merriam1898a), LM1 and LM3 (IBUNAM:CNMA 5829, reversed), from Silva and Cuenca (Reference Silva and Cuenca2020b); Rm1 and Rm3 (IBUNAM:CNMA 42968, reversed), from Cervantes et al. (Reference Cervantes, Castañón, Sánchez, Vargas and Hortelano2016b); (17) Galushamys redingtonensis, RM1 (UALP 6013), RM3 (UALP 6020), Rm1 (UALP 6013, reversed), photos by T. Kelly; (18) Repomys gustelyi 2 May, Reference May1981, LM1 (UCR 20147= UCMP 320147, reversed), RM3 (UCR 20156 = UCMP 320156), Lm1 (UCR 20158 = UCMP 320158), Rm3 (UCR 20178 =UCMP 320178, reversed), from May (Reference May1981); (19) Nelsonia goldmani Merriam, Reference Merriam1903, RM1 (USNM 125816), LM3 (USNM 90893, reversed), Lm1 (USNM 125816), Lm3 (USNM 125602), photos by C. Ronez. 1With permission of the Carnegie Institution for Science; 2with permission from Taylor & Francis; 3with permission of the Museum of Vertebrate Zoology Archives, University of California, Berkeley. Not to scale, all specimens adjusted to equal size for comparison.
Table 2. Representative species of genera included in cladistic analysis (†, extinct).
Morphological cladistic analysis using parsimony was performed with the TNT program of the Willi Hennig Society (Goloboff and Catalano, Reference Goloboff and Catalano2016) with implicit enumeration, which guarantees the shortest (optimal) tree or trees will be found. The analysis was repeated using a standard Wagner tree search including SPR (subtree-pruning-regrafting) or TBR (tree bisection reconnection) swapping algorithms, and a new technology search with the number of trees to find with minimum length set at 1,000, all of which produced the same single most parsimonious tree as implicit enumeration. Many of the extinct cricetids in the analysis are chronologically isolated and represented by a meager fossil record, often lacking knowledge of certain tooth positions. Thus, a relatively continuous series of temporal samples that would allow determination of qualitative or quantitative morphoclines to support any sequential ordering of character state transformations is lacking. In order to avoid any biases that might be introduced based on subjective ordering of character state transformations, all character states were treated as unordered (non-additive). Branch support was determined using bootstrap resampling (10,000 replicates with a 50% cutoff). Branch support values <50% are likely due to missing data for taxa that are poorly represented with some tooth positions unknown (Calede and Hopkins, Reference Calede and Hopkins2012).
Abbreviations are: ap = greatest anteroposterior length; CI = consistency index; ht = height; Ma = megannum (one million years in the radioisotopic time scale); RI = retention index; tr = transverse width.
Repositories and institutional abbreviations
AMNH FAM, American Museum of Natural History, Frick Collection, New York; IBUNAM:CNMA, Colección Nacional de Mamíferos, Departamento de Zoología, Instituto de Biología, Universidad Nacional Autónoma de México, México City; FHSM, Fort Hays Sternberg Museum, Hays; LACM, Natural History Museum of Los Angeles County, Los Angeles; LACM (CIT), California Institute of Technology specimens transferred to LACM; UALP, University of Arizona Laboratory of Paleontology, Tucson; UCMP, University of California, Museum of Paleontology, Berkeley; UCR, University of California, Riverside, transferred to UCMP (when transferred, UCR specimen numbers were modified by adding a 3 in front of the number); UMMP, University of Michigan Museum of Paleontology, Ann Arbor; UO, University of Oregon, Eugene; USNM, Smithsonian Institution National Museum of Natural History, Washington D.C.
Results
The cladistic analysis resulted in a single most parsimonious tree of 84 steps with a consistency index of 0.917 and a retention index of 0.972 (Fig. 3). Two distinct monophyletic branches are recognized in the analysis. The first branch includes Lindsaymys, Tsaphanomys, Neotoma (Paraneotoma) as successive sister taxa to an extant Neotoma-Hodomys-Xenomys clade, respectively, with the latter divided into two clades: a Hodomys-Xenomys clade and an extant Neotoma clade. The placement of the extinct subgenus Paraneotoma outside of the extant Neotoma-Hodomys-Xenomys clade suggests that the genus is not monophyletic as currently recognized. The second branch includes Protorepomys mckayensis and Miotomodon as successive sister taxa to a Galushamys-Repomys-Nelsonia clade, respectively, with the latter divided into two clades: a Galushamys clade and a Repomys-Nelsonia clade. Basirepomys, Paronychomys, and Protorepomys bartlettensis did not nest within either of the two monophyletic Neotomini clades, but are placed as successive sister taxa, respectively. The three species of Basirepomys do not form a monophyletic clade, but instead B. romensis, B. robertsi, and B. pliocenicus are placed as successive sister taxa to Paronychomys, respectively. The two species of Protorepomys also do not form a monophyletic clade.
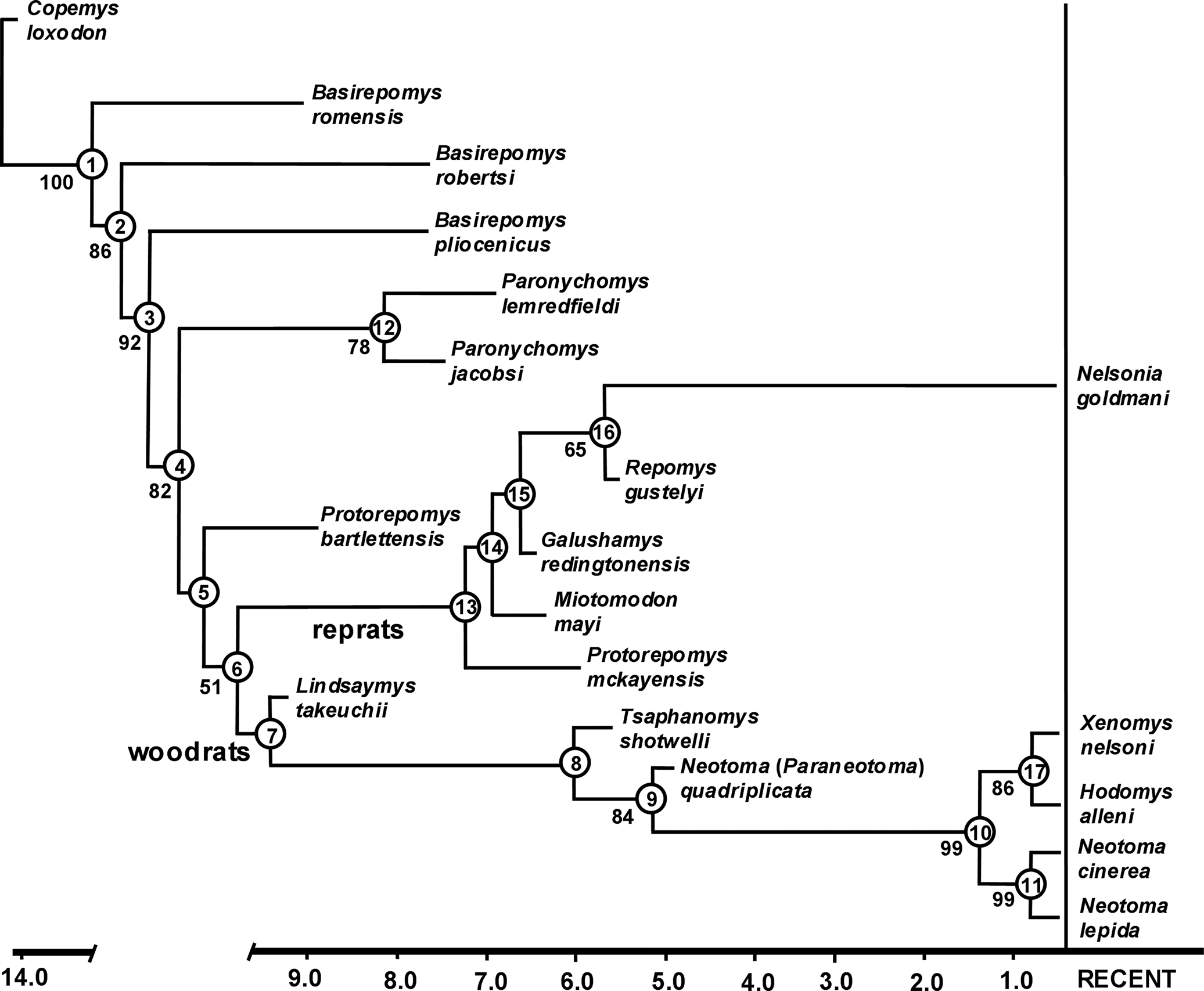
Figure 3. Single most parsimonious tree of 84 steps, CI = 0.917, RI = 0.972, using implicit enumeration. Values below branches are bootstrap support when >50%. The cladogram is supported by the following list of hypothesized ancestral synapomorphies (number to left of period denotes character number and number to right of period denotes character state). Node 1—1.2, 11.1, 17.1, 22.1, 26.2, 30.1, 33.1. Node 2—2.1, 18.4. Node 3—3.4, 4.1, 11.4. Node 4—5.1, 26.1. Node 5—34.1. Node 6—16.1, 36.1. Node 7—13.2. Node 8—26.3. Node 9—28.1, 37.1. Node 10—22.2, 23.2, 24.1, 26.5, 30.3, 34.3. Node 11—9.1, 29.1, 32.3, 35.1, 39.1. Node 12—8.1, 10.1, 13.2, 15.3, 25.1, 27.1, 30.2. Node 13—11.2, 18.3, 26.4. Node 14—3.2. Node 15—13.1, 14.1, 15.2, 16.2. Node 16—12.2. Node 17—38.3, 39.1, 40.1. Apomorphies for terminal taxa are: Basirepomys romensis 6.1, 21.1; Basirepomys robertsi 21.3; Basirepomys pliocenicus 21.2; Paronychomys lemredfieldi 21.1; Protorepomys bartlettensis 1.1, 3.1, 18.1, 23.1; Miotomodon mayi 26.3; Galushamys redingtonensis 3.3, 7.1, 11.3; Nelsonia goldmani 13.2; Neotoma lepida 7.2, 26.6; Neotoma cinerea 20.1.
Discussion
The analysis recognized the following seven ancestral synapomorphies for all ingroup taxa relative to Copemys: (1) M1 anteroloph absent; (2) M2 mesoloph short and labially directed; (3) m1 procingulid centrally positioned; (4) m1 metaconid and procingulid closely positioned lingually and fused, with wear forming a metaflexid atoll; (5) m1 protoflexid extends to base of protoconid and is unpocketed; (6) m3 mesolophid absent; and (7) mesodont to hypsodont due to coronal increase in crown height (Fig. 3, node 1). Of these, numbers 4 and 7 also distinguish all ingroup taxa in the analysis from members of Neotominae Merriam, Reference Merriam1894, and tribes Baiomyini Musser and Carleton, Reference Musser, Carleton, Wilson and Reeder2005, Reithrodontomyini Vorontsov, Reference Vorontsov1959, and Peromyscini Cockerell et al., Reference Cockerell, Miller and Printz1914.
Based on 10 dental characters, Martin and Zakrzewski's (Reference Martin and Zakrzewski2019) cladistic analysis recognized two subtribes within the tribe Neotomini: the Galushamyina and Neotomina. Galushamyina was restricted to Tsaphanomys, Neotoma, Hodomys, and Xenomys, whereas Neotomina was restricted to Protorepomys, Miotomodon, Galushamys, Repomys, and Nelsonia. They also suggested that Lindsaymys might represent a basal neotominan, but their limited cladistic analysis did not support this allocation and instead placed Lindsaymys as the closest sister taxon to Galushamyina plus Neotomini.
Our cladistic analysis with 40 characters robustly supports the monophyletic statuses and original compositions of the Galushamyina and Neotomina, except for P. bartlettensis (see below) and the addition of Lindsaymys as a basal neotominan. The latter is supported by the shared ancestral synapomorphy of the absence of an M3 protoflexus. Ancestral synapomorphies recognized for the Neotomini are enlargement of M3 talon, being only slightly smaller than the trigon (secondarily reduced in galushamyinans), and the absence of an m3 posteroflexid (Fig. 3, node 7). A previously unrecognized synapomorphy for extant Neotomini is the relative position of the choanae of the posterior nares to the tooth row, wherein the anteriormost borders of the choanae extend anteriorly well forward of the posterior margins of the M3s (Fig. 4). The pleisomorphic state for this character appears to be one in which the anterior margins of the choanae are even with the M3 posterior margins, as seen in Oligocene cricetids and one extinct species in our analysis, P. jacobsi. This character is only known for one species of Copemys, C. russelli James, Reference James1963, which also has the pleisomorphic state. In the Neotominae subtribes Baiomyini, Reithrodontomyini, and Peromyscini, the anteriormost borders of the choanae are even to well posterior of the posterior margins of the M3s. In Ochrotomyini Musser and Carleton, Reference Musser, Carleton, Wilson and Reeder2005, they are slightly anterior of the posterior margins of the M3s, but less so than extant Neotomini. This character was not included in the cladistic analysis because its state is undetermined for most of the extinct taxa.

Figure 4. Ventral views of posterior palates showing synapomorphic character state of anterior position of anteriormost choanae borders of posterior nares (thick black lines) relative to posterior margins of M3s (thick gray lines) in representatives of extant Neotomini versus pleisomorphic state seen in Paronychomys, Copemys russelli, and primitive Oligocene cricetids where anteriormost choanae borders are even with posterior borders of M3s. (1) Paronychomys jacobsi 1, LACM 156274 (holotype, choanae anterior borders outlined by thin black lines because posterior nares filled with rock matrix; thick black line overlaps thick gray line with both equal in position); (2) Neotoma cinerea occidentaltis 2 (MVZ 11152); (3) Neotoma lepida lepida 3 (MVZ 10438); (4) Hodomys alleni elatturus 4 Osgood, 1938 (IBUNAM:CNMA 8912); (5) Xenomys nelsoni 5, (IBUNAM:CNMA 5829); (6, 7) Nelsonia goldmani 6 (USNM 91965 and USNM 90893, respectively). 1Photo by T. Kelly; 2 photo by J. Blois; 3with the permission of The Museum of Vertebrate Zoology Archives, University of California, Berkeley; 4, 5from Silva and Cuenca (Reference Silva and Cuenca2020a, Reference Silva and Cuenca2020b); 6photos by C. Ronez. Not to scale, all adjusted to equal M1–3 lengths.
A common ancestral node uniting the three species of Basirepomys was not recognized in the analysis; instead the species are placed as successive sister taxa of successive common ancestors to Paronychomys, Protorepomys bartlettensis plus Galushamyina and Neotomina. Although B. romensis possesses several pleisomorphic states relative to B. robertsi and B. pliocenicus, the following apomorphies were recognized: (1) a strongly bilobed M1 procingulum, and (2) a slightly bilobed m1 procingulid during very early wear. Basirepomys robertsi is more derived than B. romensis by having the following ancestral synapomorphies also shared with the common ancestor of B. pliocenicus plus all remaining taxa analyzed: (1) an M1 parastyle absent, and (2) an m1 mesolophid absent (Fig. 2, node 2). Basirepomys pliocenicus is more derived than B. robertsi in the following ancestral synapomorphies also shared with the common ancestor of Paronychomys plus all remaining taxa analyzed: (1) M1 mesolophid absent, (2) M1 mesostyle absent, and (3) M2 mesoloph absent (Fig. 2, node 3). Our analysis does not support any species of Basirepomys as closely related to Repomys or any other member of Galushamyina. The acquisition of successive derived character states in the species of Basirepomys suggests that the genus as currently recognized is paraphyletic and should be evaluated further.
Paronychomys is placed as the closest sister taxon to P. bartlettensis plus Galushamyina and Neotomina (Fig. 2, node 4). The monophyly of Paronychomys is well supported by the following synapomorphies (Fig. 3, node 12): (1) well-developed, shelf-like M1–2 labial flexi and hypoflexus; (2) well-developed, shelf-like m1–2 lingual flexids and hypoflexid; (3) M3 hypoflexus absent to vestigial; (4) M3 hypocone weakly developed; and (5) m1 procingulid anterolabial cingulid well developed, extending posteriorly and connecting to base of protoconid, forming a distinct pocketed protoflexid. May (Reference May1981) and Korth (Reference Korth2011) proposed that Repomys may have been derived from Miotomodon. In our analysis, Miotomodon is placed as the closest sister taxon to a Galushamys-Repomys-Nelsonia clade, supported by two ancestral synapomorphies (Fig. 2, node 14): (1) M2 mesolophid significantly reduced to a mesolophule; and (2) a shallow m1 metaflexid, with the metaconid and procingulid connected during initial wear. Korth (Reference Korth2011) also proposed that Miotomodon was derived from Paronychomys rather than Basirepomys, which our analysis does not support.
Kellogg (Reference Kellogg1910) named Peromyscus antiquus based on a partial dentary with m1–3 from the early Hemphillian Thousand Creek Beds of northern Nevada (Fig. 5), and Wilson (Reference Wilson1937) referred a partial dentary with m1–3 (LACM[CIT] 1812) to the species from the early Hemphillian Coal Valley Formation of Smith Valley, Nevada. Wilson (Reference Wilson1937) and Hoffmeister (Reference Hoffmeister1945) noted that P. antiquus may represent a genus distinct from Peromyscus because of its larger size and higher crowned teeth. Peromyscus antiquus is at least 1.5 Ma older or more than the oldest species of Paronychomys sensu stricto, P. jacobsi (Kelly, Reference Kelly2013). Kelly (Reference Kelly2013) provided a detailed comparison of P. antiquus to Paronychomys and proposed that it was likely ancestral to later species of Paronychomys and questionably referred it to the genus as ?Paronychomys antiquus. It exhibits the following shared characters with other Paronychomys species (Kelly, Reference Kelly2013; this paper): (1) mesodont; (2) m1–2 primary cusps alternate and slant slightly inwards towards the centerlines of the teeth; (3) m1 procingulid subcircular and centrally positioned; (4) m1 metaconid positioned close lingually to the procingulid, which would form a shallow metaflexid atoll with further wear; (5) m1 protoflexid pocketed; (6) m1–3 accessory molar stylids and lophids absent; (7) well-developed and shelf-like labial and lingual cingulids; (8) relatively long, procumbent diastema; (9) ascending ramus rises between m2–3; and (10) masseteric scar relatively narrow, terminating anteriorly below m1 protoconid. It differs from later species of Paronychomys by the following (Kelly, Reference Kelly2013; this paper): (1) slightly lower crowned (mean ratio of m1 protoconid ht/ap = 0.49, versus mean range for other species = 0.53–0.65); (2) m2–3 anterior cingulids better developed; (3) m1–3 occlusal surfaces with slightly less tendency to form flat occlusal planes in early wear; (4) m1 metaconid and procingulid joined lingually at slightly later wear stage; (5) m3 entoconid slightly less developed; and (6) m3 slightly less reduced relative to m2 (ratio of m3 ap/m2 ap = 0.89, versus mean range for other species = 0.77–0.82). These differences indicate that later species of Paronychomys are more derived. We did not include P. antiquus in the analysis because so many of its character states are unknown. However based on the above shared character states, we provisionally assign P. antiquus to Paronychomys sensu lato, recognizing that additional specimens, including upper molars, are needed to confirm this assignment.

Figure 5. Paronychomys antiquus, holotype left dentary with m1–3 (UCMP 12571): (1) occlusal view; (2) labial view; (3) lingual view. Scale bars for (1) and (2, 3) 1 mm. Photos by P. Holroyd, UCMP.
A common ancestral node uniting the two species of Protorepomys was not recognized in the analysis; instead P. bartlettensis is placed as the closest sister taxon to Neotomini and P. mckayensis is recognized as a basal galushamyinan and the sister taxon to all other galushamyinans (Fig. 3, nodes 5, 13). Protorepomys mckayensis is more derived than P. bartlettensis by the following: (1) M1 anteroloph absent; (2) M1 mesoloph absent; (3) m1 metaflexid very shallow, lacking an atoll between the procingulum and metaconid; (4) m1 mesolophid reduced to a mesolophulid; and (5) m3 posteroflexid absent. Protorepomys bartlettensis also possesses the following apomorphies: (1) M1 anteroloph fused labially to procingulum forming an atoll; (2) extremely deep m1 protoflexid extending to near the lingual enamel margin between the metaconid and procingulid forming a shallow atoll at its termination; and (3) very well-developed, long m1 procingulid anterolabial cingulid. The position of P. bartlettensis relative to that of P. mckayensis in the analysis implies that Protorepomys is paraphyletic. Even though P. bartlettensis possesses a number of pleisomorphic states relative to galushamyinans and neotominans as recognized in the analysis, several characters appear transitional. The m1 mesolophid is distinct, but reduced, slightly anterolingually directed and somewhat fused to the entolophid. This appears transitional between the pleisomorphic state of a more labially directed mesolophid with its origin clearly from the protolophid 2 and the mesolophulid seen in galushamyinans. The molars of P. bartlettensis wear relatively flat after moderate wear and exhibit some lophodonty, which also is seen in P. mckayensis. Protorepomys bartlettensis is ca. 3 Ma older than P. mckayensis (Shotwell, Reference Shotwell1967; McClellan and Smith, Reference McClellan and Smith2020), so it is not surprising that it possesses a number of pleisomorphic characters. Despite our analysis, the possibility that P. mckayensis was derived from P. bartlettensis or an unknown species closely related to it cannot be ruled out. Unfortunately further comparisons are difficult because both species are known from a very limited number of teeth, with some tooth positions unknown for each species. Although P. bartlettensis possesses a number of characters that differ from P. mckayensis and our analysis suggests that Protorepomys is paraphyletic, until additional more-complete material is available for further study to confirm this, we tentatively retain it in Protorepomys.
Protorepomys mckayensis plus all other galushamyinans (Galushamyina) are united by the following the ancestral synapomorphies (Fig. 3, node 13): (1) M2 mesolophule present; (2) m1 mesolophulid present; and (3) a shallow m1 metaflexid, with the metaconid and procingulid connected at initial wear. Miotomodon is recognized as the closest sister taxon of a Repomys-Nelsonia clade plus Galushamys, united by the ancestral synapomorphy of an M1 mesolophule present (Fig 3, node 14). Galushamys and the Repomys-Nelsonia clade are united by the following ancestral synapomorphies (Fig. 3, node 15): (1) a shallow M3 protoflexus; (2) a shallow, weakly developed M3 hypoflexus; (3) a weakly developed M3 hypocone; and (4) M3 talon significantly reduced relative to trigon (talon tr/trigon tr ratio ≤0.50).
Galushamys is distinguished from other galushamyinans by having the following autapomorphies: (1) the M1–2 mesoloph is significantly reduced, fused anteromedially to the paraloph and connected to the metacone by early wear, resulting in an atoll between the paracone; and metacone; and (2) the M1 protoflexus is usually very shallow and slightly provergent.
The Nelsonia-Repomys clade is supported by a single synapomorphy (Fig.3, node 16): the M3 occlusal outline wears to a distinctive F-shaped pattern due to a deep paraflexus and reduced metaflexus that commonly forms an atoll at initial to very early wear (Fig. 2). When considering only extant species, our analysis placed Nelsonia as the closest sister taxon to a Neotoma-Hodomys-Xenomys clade. This relationship also has been recognized in recent molecular studies (e.g., Steppan and Schenck, Reference Steppan and Schenk2017; León-Tapia and Cervantes, Reference León-Tapia and Cervantes2021).
Martin and Zakrzewski (Reference Martin and Zakrzewski2019) concluded that the extinct Neotoma (Paraneotoma) and extant Neotoma formed a clade that was the sister clade to a Hodomys-Xenomys clade. However no hypothesized ancestral synapomorphies were identified in support of the common ancestors of either of these clades. Our analysis places Neotoma (Paraneotoma) as the closest sister clade to an extant Neotoma clade plus a Hodomys-Xenomys clade. This is supported by the following ancestral synapomorphies shared by extant Neotoma, Hodomys and Xenomys (Fig. 3, node 10) and lacking in Neotoma (Paraneotoma): (1) m1 procingulid labially positioned; (2) m1 protoflexid nearly perpendicular to long axis of tooth; (3) m1 entoflexid deep and nearly perpendicular to long axis of tooth; (4) m1 metaflexid deep and wide, with the metaconid and procingulid well separated throughout wear; (5) m1 procingulid anterolabial cingulid weakly developed and widely separated from protoconid; and (6) m3 entoflexid deep, relatively straight, and widely open lingually through wear never forming an atoll. These synapomorphies imply that extant Neotoma, Hodomys, and Xenomys share a common ancestor that split off from that of their common ancestor with Neotoma (Paraneotoma). This further implies that if Neotoma (Paraneotoma) and extant Neotoma are included in the same genus, Neotoma becomes paraphyletic. Hibbard (Reference Hibbard1967, p. 130) proposed that Neotoma (Paraneotoma) “…is closely related to or is the stock that gave rise to Neotoma [= Hodomys] alleni [Merriam, 1892] [Merriam, Reference Merriam1892b].” Here we tentatively retain Paraneotoma as a subgenus of Neotoma, but recognize that if future detailed analyses support this phylogenetic scenario, Paraneotoma would deserve elevation to generic rank.
The Xenomys-Hodomys clade is supported by the following ancestral synapomorphies: (1) a reduced, small mental foramen; (2) a reduced incisor capsular process; and (3) the masseteric scar is positioned low on the horizontal ramus (Fig. 3, node 17). Although not included in the analysis because the character states for extinct species are unknown, an additional soft-tissue character allying Xenomys and Hodomys is the morphology of the glans penis (Hooper, Reference Hooper1960; Genoways and Birney, Reference Genoways and Birney1974; Carleton, Reference Carleton1980). They both share the following characters (Hooper, Reference Hooper1960, pl. 8): (1) relatively short, length/width ratio = 1.3–1.9; (2) mid-dorsal and mid-ventral troughs; (3) two urethral processes and dorsal papillae present; and (4) terminal crater nearly filled, with a bulbous mass covering tip of baculum that is free of the crater. Neotoma cinerea Ord, Reference Ord and Guthrie1815, and N. lepida Thomas, Reference Thomas1893, differ from Xenomys and Hodomys by the following (Hooper, Reference Hooper1960, pls. 6, 7): (1) greater length/width ratio (4.5–9.2); (2) simpler form (lack bulbous mass); (3), single, undivided urethral process; and (4) troughs lacking. Although the glans penis of certain other extant Neotoma are relatively short with length/width ratios varying from 1.26–2.2 (e.g., N. albigula Hartley, Reference Hartley1894), all lack troughs and the bulbous mass in the terminal crater (Hooper, Reference Hooper1960).
Further supporting evidence that Xenomys and Hodomys are closely related to extant Neotoma include molecular analyses that place Xenomys and Hodomys as either the closest successive sister taxa to extant Neotoma, respectively (Reeder and Bradley, Reference Reeder and Bradley2004; Matocq et al., Reference Matocq, Shurtliff and Feldman2007) or within a Hodomys-Xenomys clade that is the closest sister clade to extant Neotoma (Steppan and Schenk, Reference Steppan and Schenk2017; León-Tapia and Cervantes, Reference León-Tapia and Cervantes2021).
Conclusions
The division of the Neotomini subtribes, Galushamyina (reprats) and Neotomina (woodrats), is well supported by our analysis. Galushamyina is comprised of Protorepomys mckayensis, Miotomodon, Galushamys, Repomys, and extant Nelsonia. Neotomina is composed of Lindsaymys, Neotoma (Paraneotoma), Hodomys, Xenomys, and extant Neotoma. An additional synapomorphy uniting extant Neotomini is the anterior position of the choanae of the posterior nares relative to the posterior margins of the M3s. Neotoma (Paraneotoma) is the closest sister taxon to an extant Neotoma-Hodomys-Xenomys clade, the latter divided into an extant Neotoma clade and a Hodomys-Xenomys clade. This suggests that Neotoma (Paraneotoma) and extant Neotoma are paraphyletic at the generic level, but further study will be necessary to make this determination. An additional synapomorphy uniting Hodomys and Xenomys, not included in the analysis because it is unknown for extinct taxa, is the distinctive suite of shared characters seen in the glans penis.
Paronychomys shares with Neotomini a closely positioned m1 metaconid and procingulid, fused either initially or during early wear, a progressive coronal increase in molar crown height, and a tendency to form a flat occlusal plane during early wear, suggesting that Paronychomys is the sister taxon to Protorepomys bartlettensis plus Neotomini. Although Basirepomys may be paraphyletic, all three species exhibit a closely positioned m1 metaconid and procingulid, but these structures do not fuse until very late wear, indicating that they are less derived than the condition seen in Paronychomys, P. bartlettensis, and Neotomini. Species of Basirepomys also exhibit a number of other pleisomorphic characters relative to Paronychomys, P. bartlettensis, and Neotomini, further indicating they are more removed from Neotomini than Paronychomys. Whether the three species of Basirepomys represent a side branch of successive sister taxa of Neotominae or the successive sister taxa to Paronychomys, P. bartlettensis, and Neotomini is uncertain at this point, but none of them is closely related to Repomys or other Galushamyina. If the paraphyly of Basirepomys as recognized in our analysis is confirmed by additional studies, then establishing two new generic ranks for B. romensis and B. robertsi to separate them from the type species, B. pliocenicus, would be warranted.
Protorepomys mckayensis nests within Galushamyina as a basal galushamyinan, but P. bartlettensis exhibits a number of pleisomorphic characters that suggest the genus is paraphyletic, with P. bartlettensis as the possible closest sister taxon to Neotomini. However, because P. bartlettensis is currently represented by only five isolated teeth representing three tooth positions (Shotwell, Reference Shotwell1967; Martin and Zakrzewski, Reference Martin and Zakrzewski2019), erecting a new genus is unjustified at this time. However, if the discovery of additional material and further study confirms this paraphyly, erecting a new genus for P. bartlettensis would be warranted.
Acknowledgments
We are grateful to P. Holroyd and C. Mejia of the UCMP, and S. McLeod of the LACM for providing access to the specimens in the collections under their supervision. Special thanks are given to E. Lindsay for providing numerous casts of cricetid taxa relevant to our study, J. Blois and the Blois Laboratory (UC Merced), C.E. Castañón, F.A. Cervantes, C. Conroy, J.V. Cuenca, P. Holroyd, Y. Hortelano, J.A. Sánchez, M.H. Silva, W. Stone, K. Tate-Jones, C. Ronez, J. Vargas, and R.J. Zakrzewski for specimen photos. We thank L.L. Jacobs and J. Saunders for their considerate assistance in procuring a loan of Galushamys specimens from the UALP. Three anonymous reviewers provided constructive comments and suggestions on the original draft of this paper, which significantly improved the final version.
Appendix 1. Characters/Character States Used in Cladistic Analyses
1. M1 anteroloph: 0, usually present (>50% of available specimens), long and separated labially from procingulum, originating from junction of procingulum and protoloph 1; 1, short, labially directed, originating from junction of procingulum and protoloph 1 and fused labially with procingulum forming an atoll; 2, absent or vestigial; 3, anteroloph reduced to anterolophule, a short posterolabially directed spur fused to the anterolingual loph at its junction with procingulum in early wear resulting in a narrowing of the paraflexus entrance.
2. M1 parastyle: 0, present; 1, absent.
3. M1 mesoloph/mesolophule: 0, mesoloph present, long, labially directed, isolated from paracone and metacone and originating from hypoloph 1; 1, mesoloph short, labially directed, isolated from paracone and metacone and originating from hypoloph 1; 2, mesoloph significantly reduced to a mesolophule, a short lophule fused anteromedially to paraloph and posterolabially directed; 3, mesoloph significantly reduced, fused anteromedially to paraloph, connecting to metacone in moderate wear; 4, mesoloph/mesolophule absent.
4. M1 mesostyle (not equivalent to the mesostyle of Mou [Reference Mou2011]): 0, usually present (>50%) or present in holotype; 1, absent or usually absent.
5. M1 low cingulum (anterior lingual cingulum, Fig. 1) extending between anterolingual base of protocone to posterolingual base of procingulum, not the anterolingual cingulum of procingulum): 0, present; 1, absent or vestigial.
6. M1 procingulum bilobed by development of anterolingual conule and well-developed anteromedian flexus: 0, absent or vestigial in very early to early wear; 1, strongly bilobed.
7. M1 protoflexus depth and orientation in occlusal view in early wear: 0, moderately deep, postvergent; 1, shallow, slightly postvergent; 2, usually absent or may be present as slight indentation during early wear.
8. M1 labial flexi in lateral view and labial cingula: 0, V- to U-shaped without shelf-like cingula; 1, U-shaped with well-developed shelf-like cingula.
9. M1 relative position of hypoflexus/paraflexus apices: 0, hypoflexus apex posterior of paraflexus apex; 1, hypoflexus apex nearly opposite of paraflexus apex throughout wear.
10. M1 hypoflexus in lateral view and lingual cingulum: 0, V- to U-shaped without shelf-like cingulum; 1, U-shaped with well-developed shelf-like lingual cingulum.
11. M2 mesoloph/mesolophule: 0, mesoloph long, labially directed, isolated from paracone and metacone and originating from hypoloph 1; 1, mesoloph short, labially directed, isolated from paracone and metacone and originating from hypoloph 1; 2, mesoloph significantly reduced to a mesolophule, a short lophule fused anteromedially to paraloph and posterolabially directed; 3, mesoloph significantly reduced, fused anteromedially to paraloph, connecting to metacone in moderate wear; 4, mesoloph/mesolophule absent.
12. M3 occlusal outline pattern with wear: 0, round to oval; 1, E-shaped; 2, F-shaped.
13. M3 protoflexus: 0, moderately developed; 1, shallow; 2, absent.
14. M3 hypoflexus: 0, moderately to well developed; 1, weakly developed, shallow; 2, absent to vestigial.
15. M3 hypocone: 0, moderately developed; 1, well developed; 2, weakly developed.
16. M3 relative size of talon to trigon (ratio of talon tr/trigon tr), talon includes hypocone, metacone, mesoloph (if present), posteroloph and their connecting lophs, trigon includes anterior cingula (if present), paracone, protocone and their connecting lophs: 0, moderately smaller (0.65-0.75); 1, slightly smaller (≥0.80); 2, significantly smaller (≤0.50).
17. m1 crown height (ratio of m1 protoconid ht/m1 ap when unworn to very early wear): 0, brachydont (<0.50); 1, mesodont to hypsodont due to coronal increase in height (≥0.50).
18. m1 mesolophid (not equal to mesolophid of Zakrzewski, Reference Zakrzewski, Martin and Barnosky1993)/mesolophule: 0, mesolophid long, lingually directed and originating from protolophid 2; 1, mesolophid short, anterolingually directed and originating from protolophid 2; 3, mesolophid significantly reduced to mesolophulid, a short, anterolingually directed projection fused anteromedially to entolophid at initial through early moderate wear; 4, mesolophid/mesolophulid absent.
19. m1 ectolophulid (short, anterolabially directed projection originating from the hypolophid 2 on the occlusal plane: 0, absent; 1, present in very early or early wear.
20. m1 anterior lateral dentine tract: 0, absent; 1, present.
21. m1 procingulid bilobed with addition of second conulid (anterolabial conulid): 0, absent; 1, vestigial to very slightly in very early wear; 2, present through moderate wear; 3, present throughout wear.
22. m1 relative position of procingulid in occlusal view to long axis of tooth: 0, slightly lingually; 1, centrally; 2, slightly labially to labially.
23. m1 protoflexid depth and orientation in occlusal view: 0, moderately deep to deep, provergent; 1, extremely deep, constricted near termination forming incipient atoll, provergent; 2, moderately deep and nearly perpendicular to long axis of tooth.
24. m1 entoflexid depth and orientation in occlusal view (or mesoflexid when well-developed, long mesolophid present dividing the reentrant): 0, very deep, provergent; 1, very deep and slightly provergent to nearly perpendicular to long axis of tooth.
25. m1 labial flexids in lateral view and labial cingulid: 0, V- to U- shaped without shelf-like cingulids; 1, U-shaped with well-developed shelf-like labial cingulids.
26. m1 metaflexid relative positions of metaconid/procingulid and atoll: 0, moderately deep, U-shaped, metaconid separated from procingulid until very late wear; 1, moderately deep, metaconid and procingulid lingual margins closely positioned, fusing in early to early moderate wear forming an atoll; 2, moderately deep, metaconid and procingulid closely positioned, but separate through moderate wear, fusing during very late wear; 3, shallow, metaconid and procingulid connected in initial to very early wear, atoll present near anterolabial margin of metaflexid; 4, shallow, metaconid and procingulid connected in initial wear, atoll sometimes present in center of procingulid; 5, deep, wide V-shaped with metaconid and procingulid well separated through very late wear; 6, usually absent, but sometimes present as small transient notch.
27. m1 hypoflexid in lateral view and labial cingulid: 0, V- to U-shaped without well-developed shelf-like cingulid; 1, U-shaped with well-developed shelf-like labial cingulid.
28. m1 posteroflexid depth and orientation in occlusal view: 0, deep, provergent; 1, deep, slightly provergent to nearly perpendicular to long axis of tooth.
29. m1 hypoflexid/posteroflexid relative positions of apices in occlusal view: 0, hypoflexid apex anterior of posteroflexid apex; 1, hypoflexid apex opposite to posteroflexid apex.
30. m1 procingulid anterolabial cingulid/protoflexid pocket (also applies to m2). Pocket is defined as deep protoflexid completely enclosed labially by a tall anterolabial cingulid that forms a pocket-like appearance in occlusal view (Fig. 2.6); 0, moderately to well developed, extending posteriorly and connected to base of protoconid, protoflexid not pocketed; 1, moderately to well developed not extending to base of protoconid, protoflexid not pocketed; 2, cingulid well developed, extending posteriorly and connected to base of protoconid, protoflexid strongly pocketed; 3, cingulid weakly developed, widely separated from protoconid, protoflexid not pocketed.
31. m2 ectolophulid (short, anterolabially directed projection originating from hypolophid 1 on occlusal plane): 0, absent; 1, present in very early or early wear.
32. m3 occlusal outline pattern: 0, S-shaped; 1, oblique keyhole-shaped (talonid flexed lingually); 2, keyhole-shaped; 3, dumbbell-shaped (bilobed).
33. m3 mesolophid (not equal to mesolophid of Zakrzewski [1993]): 0, present, long; 1, present, short; 2, absent.
34. m3 entoflexid (or mesoflexid if well-developed mesolophid present in reentrant)/atoll: 0, moderately deep, open lingually, does not close with wear, no atoll; 1, deep, initially open lingually and then closes off by early moderate to moderate wear forming an atoll; 2, shallow, entoflexid closed at initial wear with atoll present between metaconid and protoconid; 3, entoflexid very deep, straight, widely open lingually throughout wear, no atoll.
35. m3 entoflexid/hypoflexid apices: 0, entoflexid apex anterior to hypoflexid apex; 1, entoflexid apex opposite of hypoflexid apex.
36. m3 posteroflexid: 0, present; 1, absent.
37. m3 talonid size relative to trigonid size (talonid tr/trigonid tr ratio), talon includes hypoconid, entoconid, mesolophid (if present), posterolophid and their connecting lophids), trigon includes anterior cingulids (if present), metaconid, protoconid and their connecting lophids: 0, moderately smaller (0.65–0.75); 1, slightly smaller (≥0.80); 2, significantly smaller (≤0.55).
38. Dentary, mental foramen relative size and position on horizontal ramus: 0, large size, lateral and moderately high; 1, moderate size, lateral and moderately high; 2, moderate size, lateral and very high; 3, small size, lateral and moderately high.
39. Dentary, position of masseteric scar on horizontal ramus: 0, about the middle; 1, low.
40. Dentary, development of incisor capsular process: 0, well developed; 1, weakly developed.
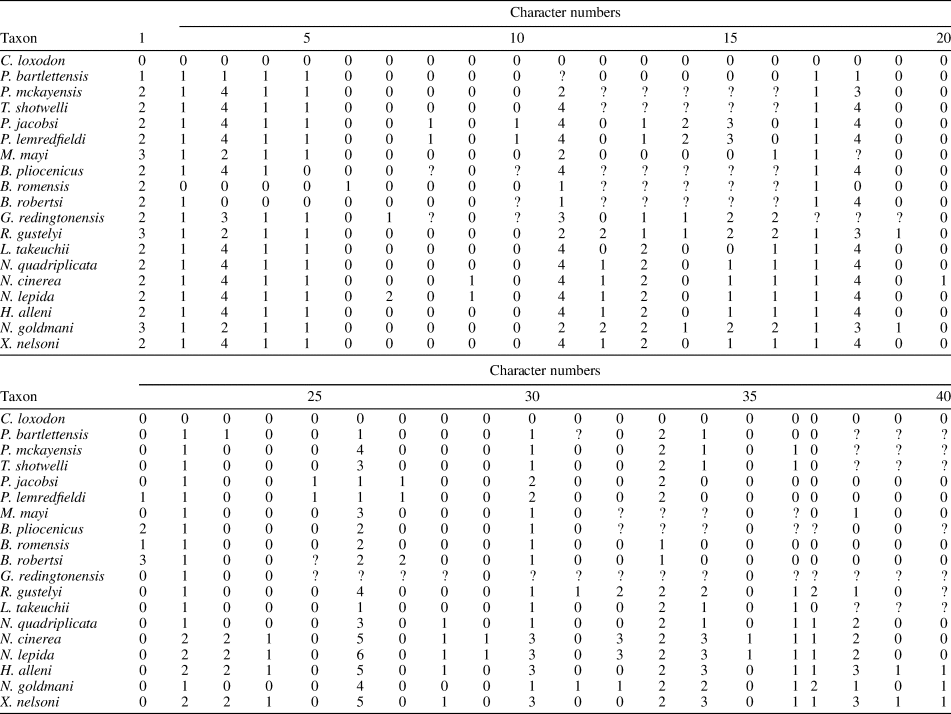
Appendix 2. Character state matrix for analysis with the following species: Copemys loxodon, Protorepomys bartlettensis, P. mckayensis, Tsaphanomys shotwelli, Paronychomys jacobsi, P. lemredfieldi, Miotomodon mayi, Basirepomys pliocenicus, B. romensis, B. robertsi, Galushamys redingtonensis, Repomys gustelyi, Lindsaymys takeuchii, Neotoma (Paraneotoma) quadriplicata, Neotoma cinerea, Neotoma lepida, Hodomys alleni, Nelsonia goldmani, and Xenomys nelsoni.