Introduction
One in six couples experience fertility problems and in about a third of these cases the primary cause is of male origin. Oligoasthenoteratozoospermia (OAT) is defined by a combined low count < 20 × 106 sperm/ml, poor motility < 50 % forward progression or < 25 % rapid linear progression and abnormal morphology (5–8 % normal using Kruger strict criteria). Approximately 25 to 30 % of men who are investigated for severe male factor infertility have OAT of unknown cause. A large number of OAT patients have karyotype anomalies that interfere with spermatogenesis and can lead to the production of unbalanced gametes; others with a normal karyotype, however, may be at either low or high risk of having germ cell aneuploidy (Calogero et al., Reference Calogero, De Palma, Grazioso, Barone, Burrello, Palermo, Gulisano, Pafumi and D'Agata2001a,Reference Calogero, De Palma, Grazioso, Barone, Romeo, Rappazzo and D’Agatab; Griffin & Finch, Reference Griffin and Finch2005). The results of sperm aneuploidy studies in OAT patients are controversial. Some reports highlight elevated frequencies of diploidy, autosomal and sex chromosome disomy and nullisomy, while other studies have shown no significant difference in the sperm aneuploidy rate between fertile and infertile patients (In't Veld et al., Reference In't Veld, Broekmans, de France, Pearson, Pieters and van Kooij1997; Pang et al., Reference Pang, Hoegerman, Cuticchia, Moon, Doncel, Acosta and Kearns1999; Pfeffer et al., Reference Pfeffer, Pang, Hoegerman, Osgood, Stacey, Mayer, Oehninger and Kearns1999; Calogero et al., 2001a,b; Tempest & Griffin, Reference Tempest and Griffin2004).
While extensive work has been performed on sperm samples of OAT males (reviewed in Tempest & Griffin, Reference Tempest and Griffin2004), very little has been performed on the cytogenetic constitution of the resulting embryos that arise following ICSI form these men. Here we report an interesting case of the cytogenetic constitution of three embryos surplus to IVF requirements from a couple that was referred for ICSI because of OAT. Collectively, these embryos shed light on possible mechanisms that occur in human preimplantation development, particularly of tetraploid embryos.
Materials and methods
Source of human embryos
This study was performed on three surplus human embryos donated from a couple undergoing ICSI treatment because of OAT in the Assisted Conception Unit (ACU) of the Leeds General Infirmary (LGI), with their written consent and under a licence by the Human Fertilization and Embryology Authority (HFEA).
Ovarian stimulation
A standard protocol was used to induce superovulation and control timing of egg retrieval. On day 1 of the cycle the oral contraceptive pill was prescribed for 2 weeks. Pituitary downregulation was achieved by the administration of Nafarelin nasal spray (Synarel), at 200 μg three times a day for the first 10–14 days, followed by ovarian stimulation with between 150–225 IU/day of human menopausal gonadotrophin (hMG) (Menogon) for 12–14 days during which time the dose of Nafarelin was reduced to twice daily at 200 µg. The patient was monitored by ultrasound regularly starting on day 6 of gonadotrophin administration and between days 12–14 when adequate follicular development had been demonstrated (≥3 follicles of 17 mm in diameter), 10 000 IU of human chorionic gonadotrophin (hCG) (Profasi; Serono) was administered to trigger ovulation. At 36 h after the hCG administration, transvaginal ultrasound guided egg retrieval was performed.
Embryo culture
Six MII oocytes were retrieved from the 31-year-old female into Medicult flushing medium (Medicult) incubated in 5 % CO2 in air at 37 °C and subsequently fertilized by ICSI and cultured until the time of embryo transfer in Medicult universal M2 medium. On day 1, assessment of fertilization was performed by scoring the number of pronuclei and polar bodies, which revealed that five out of six injected oocytes, were normally fertilized (2PN). Those normally fertilized zygotes were transferred to fresh microdrops of the same medium and after the two best embryos were selected for transfer on day 2, the remaining ‘spare’ embryos were processed for FISH on day 4 postinsemination.
Embryo spreading
The embryos were spread in 0.01 N HCl (BDH), 0.1 % Tween-20 (Sigma-Aldrich) on poly-l-lysine slides (BDH) as described previously (Chatzimeletiou et al., Reference Chatzimeletiou, Morrison, Panagiotidis, Prapas, Prapas, Rutherford, Grudzinskas and Handyside2005a). The spreading solution was removed and replaced until all the zona and cytoplasm were dissolved. The slides were left to air dry, washed in PBS (Sigma-Aldrich) and dehydrated through ethanol series (BDH).
Sequential fluorescent in situ hybridization (FISH)
FISH was performed in three rounds according to Chatzimeletiou et al., (Reference Chatzimeletiou, Morrison, Panagiotidis, Prapas, Prapas, Rutherford, Grudzinskas and Handyside2005a). The probes used in each round were: (1) AneuVysion probe set; CEPX (Spectrum Green) CEPY (Spectrum Orange) CEP18 (Spectrum Aqua) (Abbott Molecular Diagnostics); (2) UroVysion probe set; CEP3 (Spectrum Red) CEP7 (Spectrum Green) CEP9 (Spectrum Gold) CEP17 (Spectrum Aqua) (Abbott Molecular Diagnostics); and (3) AneuVysion probe set; LSI13 (Spectrum Green) LSI21 (Spectrum Red) (Abbott Molecular Diagnostics).
Results
The embryos were scored for morphology and cell number on days 2 and 4 postfertilization (Table 1). After spreading, 12 nuclei (four from each embryo) were visualized on slides and analysed by FISH in three successive hybridizations. The results for each chromosome in each nucleus are summarized in Fig 1. In general terms, all embryos were female and showed evidence of polyploidy, but each one had quite a different pattern of chromosome segregation.

Figure 1 The chromosomal constitution of the three embryos as documented by the patterns of probe hybridization. (A) Embryo 1. (B) Embryo 2. (C) Embryo 3. Each hybridization signal is represented by a ‘spot’ and corresponds to a chromosome in a matrix in which each chromosome is allocated a row. The chromosomes appear in the order they were hybridized. Each column represents an individual nucleus. On the right of each spotogram there is a diagrammatic representation of the embryo and the chromosomal constitution of each nucleus.
Table 1 Embryo scoring data on days 2 and 4 postfertilization
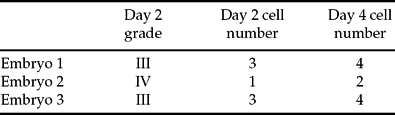
Grade I, excellent quality; Grade IV, poor quality.
Embryo 1 was uniformly tetraploid for all chromosomes tested. That is, for all chromosomes analysed, each blastomere had four copies.
Embryo 2 showed evidence of karyokinesis but not cytokinesis, i.e. there were four nuclei but only two cells (two nuclei in each). In this case chromosome numbers were unequal in each cell but they were, on the whole, multiples of 8. That is, for chromosome X, eight chromosomes appeared in two nuclei but none in the others. For chromosomes 18 and 13 there were five chromosomes in two nuclei and three in the others (a 5:3 segregation pattern); for chromosomes 3, 17 and 21, nuclei had 7:1, 7:1 and 6:2 segregation patterns respectively. Only chromosomes 7 and 9 deviated from this pattern with a 6:1 arrangement for both set of nuclei with respect to chromosome 7 and, for chromosome 9, nuclei with 9, 1, 5 and 1 chromosomes respectively.
While one-sided divisions were prominent in all three rounds of hybridization in embryo 2, embryo 3 was undergoing unbalanced divisions in a more chaotic form and the chromosomes were incorporated into the derivative blastomeres in a more random way than in embryo 2. Embryo 3 also showed some evidence of the ‘multiples of 8’ segregation pattern seen in embryo 2, i.e. chromosomes X, 18, 13 and 21 had 5:3 patterns. However there was also evidence of significant chromosome loss with chromosomes 3, 7, 9, 17 having fewer chromosomes than would be expected for a tetraploid embryo (1, 3, 5 and 4 for chromosome 3; 2, 1, 3 and 3 for chromosome 7, 2, 2, 3 and 3 for chromosome 9 and 5, 1 3 and 6 for chromosome 17).
Discussion
Molecular cytogenetic analysis of nine chromosomes revealed that all three embryos were polyploid (mostly tetraploid), suggesting that this couple may be particularly prone to tetraploidy. Given that fertilization of triploid sperm is rare, tetraploidy in these embryos most likely arose through failure in cytokinesis in the presence or absence of karyokinesis.
Given that embryo 1 was uniformly tetraploid for all chromosomes tested, it is possible that this embryo was normal diploid up to the day it became 4-cell, but then lack of karyokinesis and cytokinesis during the third cleavage divisions and after complete DNA replication in interphase, led the blastomeres to become tetraploid. Alternatively tetraploidy may have arisen prior to the 4-cell stage, at the 2-cell (second cleavage division) or indeed at the 1-cell zygote stage through failure in karyokinesis and cytokinesis or through failure of the centrosome to duplicate during the first mitotic cycle or failure of the duplicated centrosome to move to opposite poles, leading to a monopolar spindle that in turn led to a tetraploid complement (Fig. 2B). Thereafter the centrosome was able to duplicate and move to opposite poles giving rize to exact tetraploid copies. The latter scenario, however, would be compatible only with an embryo that showed retarded development on day 2 and there was no such evidence for embryo 1, which had reached the 3-cell stage on day 2. Therefore either failure in karyokinesis and cytokinesis at the 4-cell stage or at the 2-cell stage followed by faithful 4:4 chromosome segregation may be the most likely explanation.

Figure 2 Possible scenarios for the formation of tetraploid and complex polyploid nuclei.
In a similar way, based on the observation that embryo 2 was still 1-cell on day 2, it seems likely that this embryo might have been the result of a normal fertilization, but an inability of the 1-cell zygote to divide, leading to a tetraploid 1-cell zygote, either through a monopolar spindle or failure of karyokinesis and cytokinesis (Fig. 2B). Once the first tetraploid blastomere was created, it then perhaps tried, unsuccessfully, to divide equally. A possible explanation for embryo 2 not being able to divide equally might be the fact that the tetraploid blastomere produced by failure of karyokinesis and cytokinesis had two centrosomes that, when duplicated, would produce four centrosomes. If there is unequal allocation of these centrosomes on the spindle poles (i.e. 3:1), then inevitably unequal numbers of spindle fibres will be present and one-sided divisions would ensue (Fig. 2B). Therefore, in the case of embryo 2, it is possible that the tetraploid nucleus divided into two nuclei, but due to the 3:1 centrosome allocation, most of the chromosomes attached and moved towards the spindle pole with the greatest number of centrosomes.
Figures 3–5 show a schematic representation of the hypothesized events that were responsible for the final arrangement of chromosomes in embryo 2. In brief, all four chromosomes X, one chromosome 18 and 13 pairs of chromosomes 7 and 21 and three chromosomes 3, 9 and 17 were subject to non-disjunction. During anaphase, surprisingly enough, all these chromosomes moved towards the same pole, while each of the chromatids of the normally segregated chromosomes was pulled to opposite poles with the only exemption of a single chromatid 7, which was excluded by anaphase lag. As a consequence the two blastomeres had a combination of different chromosome complements ranging from octasomic for chromosome X, heptasomic for chromosomes 3 and 17, hexasomic for chromosomes 7 and 21, pentasomic for chromosomes 13 and 18 (nucleus B), to nullisomic for chromosome X, trisomic for chromosomes 13 and 18, disomic for chromosome 21 and monosomic for chromosomes 3, 7, 9 and 17 (nucleus A). These two nuclei further divided into the FISH analysed nuclei that are shown in Figs 3–5 but, although karyokinesis occurred, cytokinesis did not occur causing these two blastomeres to become binucleate. Nucleus A faithfully copied itself, giving rize to A1 and A2, but nucleus B failed to make an exact copy and divided into two uneven nuclei for chromosome 9. This was due to non-disjunction of two chromosomes 9 that were incorporated into nucleus B1, causing it to become eniasomic and leaving the other nucleus B2 carrying five copies of chromosome 9. The rest of the chromosomes normally divided into opposite poles.

Figure 3 The chromosomal constitution of embryo 2 as revealed by analysis of chromosomes X and 18. (A1–B2) FISH photomicrographs of the four nuclei in embryo 2 following hybridization with probes X (Green) and 18 (aqua). Note that B1 and B2 are octasomic for X/pentasomic for 18, while A1–A2 are nulisomic for X/trisomic for 18. (I–III) Diagrammatic representation of the events that most likely led to the final arrangement of chromosomes X and 18 in embryo 2. (I) The embryo started as a normal diploid 1-cell zygote. However, lack of karyokinesis and cytokinesis led to the formation of a tetraploid nucleus, which now has two centrosomes. During the next cell division each centrosome duplicates but there is uneven allocation of these centrosomes on the spindle poles (3:1). As a result most of the chromosomes become attached to the spindle fibres extending from the pole with the greater number of centrosomes. Non-disjunction of all four chromosomes Xs and one chromosome 18 leads to the production of two nuclei possessing 0 × X, 3 × 18 (A; precursor of A1 + A2) and 8 × X, 5 × 18 (B; precursor of B1 + B2). Nucleus A makes an exact copy of itself (A1 and A2) shown in (II) and nucleus B also faithfully divides giving rise to B1 and B2 (III).

Figure 4 The chromosomal constitution of embryo 2 as revealed by analysis of chromosomes 3, 7, 9 and 17. A1–B2) FISH photomicrographs of the four nuclei in embryo 2 following hybridization with probes 3 (red), 7 (green), 9 (gold) and 17 (aqua). Note that B1 and B2 are eptasomic for 3, 9, 17, exasomic for 7, while A1–A2 are monosomic for 3, 7, 9,1 7. (I–III) Diagrammatic representation of the events that most likely led to the final arrangement of chromosomes 3, 7, 9, 17 in embryo 2. (I) The embryo started as a normal diploid 1-cell zygote. However, lack of karyokinesis and cytokinesis led to the formation of a tetraploid nucleus, which now has two centrosomes. During the next cell division each centrosome duplicates but there is uneven allocation of these centrosomes on the spindle poles (3:1) as described in Fig. 2. As a result most of the chromosomes become attached to the spindle fibres extending from the pole with the greater number of centrosomes. Non-disjunction of two chromosome 7s (but loss of one through anaphase lag) and one chromosome 3, 9, 17 leads to the production of two nuclei with 1 × 3, 1 × 7, 1 × 9, 1 × 17 (A, precursor of A1 + A2) and 7 × 3, 6 × 7, 7 × 9, 7 × 17 (B, precursor of B1 + B2). Nucleus A makes an exact copy of itself (A1 and A2) shown in (II), but nucleus B fails to segregate two chromosome 9s giving rise to (B1 and B2) that has 7 × 3, 6 × 7, 12 × 9, 7 × 17 (B1) and 7 × 3, 6 × 7, 5 × 9, 7 × 17 (B2) shown in (III).
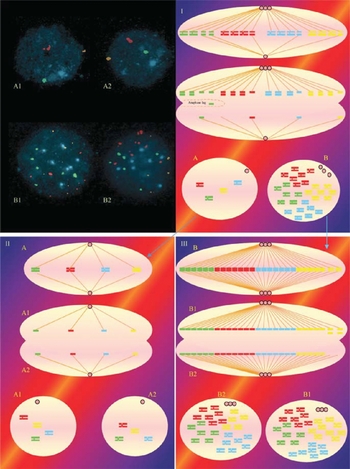
Figure 5 The chromosomal constitution of embryo 2 as revealed by analysis of chromosomes 13 and 21. (A1–B2) FISH photomicrographs of the four nuclei in embryo 2 following hybridization with probes 13 (red) and 21 (green). Note that B1 and B2 are pentasomic for 21/exasomic for 13, while A1 and A2 are trisomic for 21/disomic for 13. (I–III) Diagrammatic representation of the events that most probably led to the final arrangement of chromosomes 3, 7, 9, 17 in embryo 2. (I) The embryo started as a normal diploid 1-cell zygote. However, lack of karyokinesis and cytokinesis led to the formation of a tetraploid nucleus, which now has two centrosomes. During the next cell division each centrosome duplicates, but there is uneven allocation of these centrosomes on the spindle poles (3:1) as described in Fig. 2. As a result most of the chromosomes become attached to the spindle fibres extending from the pole with the greater number of centrosomes. Non-disjunction of two chromosomes 13s and one chromosome 21 leads to the production of two nuclei with 3 × 21, 2 × 13 (A, precursor of A1 + A2) and 6 × 13, 5 × 21 (B, precursor of B1 + B2). Nucleus A and B then make an exact copy of themselves (A1 and A2).
Abnormal segregation of chromosomes was also evident in embryo 3. It is suggested that this embryo also started as diploid, but absence of cytoplasmic division during the second cleavage division led the two blastomeres to become tetraploid. Those two tetraploid blastomeres then were unsuccessful in their attempts to divide faithfully. They both failed to segregate one chromosome X and 18 and one chromosome 13 and 21. As a consequence the derivative nuclei had either five copies of chromosomes X, 18, 13 and 21 (pentasomic) or three copies (trisomic). The postzygotic divisions for chromosomes 3, 7, 9 and 17 were more complicated. Perhaps, a centrosome defect, non-disjunction, as well as premature chromatid predivision, chromosome loss by anaphase lag and late replication of chromatids were all responsible for the abnormal complements shown.
What is striking from this study, is that all embryos were arrested on day 4 showing polyploid complements due to cytokinetic failure and although no uniform aneuploidy was detected, abnormal postzygotic segregation of chromosomes was evident in two out of three embryos. Whether behind these abnormal events was a malfunctioning sperm centrosome, warrants further investigation.
The ability of human embryos to undergo normal development has been shown to be subject to strong paternal (sperm-derived) effects. Tesarik et al., Reference Tesarik, Mendoza and Greco2002 compared the quality of zygotes and cleaving embryos resulting from sibling donor oocytes fertilized by sperm from different patients. They documented that fertilizations with sperm from certain individuals repeatedly resulted in the formation of zygotes with abnormal pronuclear morphology that tended to cleave slowly and show extensive fragmentation and blastomere irregularities. These authors concluded that these paternal effects might be both of genetic origin (related to the minor gene activity of the male pronucleus) or epigenetic origin (related to the sperm-derived oocyte-activating factor or sperm centrosome). In our recent study, we provided the first direct evidence of the existence of spindle abnormalities in cleavage and blastocyst stage human embryos (Chatzimeletiou et al., Reference Chatzimeletiou, Morrison, Prapas, Prapas and Handyside2005b). We also suggested that multipolar spindles might arize following cytokinetic failure and incorporation of two centrosomes in the blastomere. One of the cleavage stage embryos with a tetrapolar spindle in that study was indeed from an OAT patient.
It is therefore possible that the abnormal divisions of the embryos in the present study were due to an inherent abnormality passed on by the sperm. If this is the case, then it is not surprising that all spare embryos arrested in cleavage and the female partner failed to become pregnant, even though initially she had a borderline HCG assay, indicating a biochemical pregnancy. If the cause of cleavage arrest in these embryos is indeed a centrosomal defect, then it is likely that the problem will persist in subsequent cycles too. It would be advisable for OAT patients, therefore, instead of undergoing several IVF attempts and having their hopes falsely raised, especially when biochemical pregnancies occur, to undergo either extended culture of embryos, which may select against those with a tendency to binucleation, cytokinetic failure and arrest or preimplantation genetic screening which can identify polyploid and complex polyploid/aneuploid complements. It would also be desirable to investigate any potential centrosomal defects and if present explore other ways to fatherhood, as the latter may not allow those patients to father their own children.
This case report demonstrates representative examples of the possible fates of tetraploid (or possibly any other) embryos at the preimplantation stages. The first is normal cell division with faithful chromosome segregation. This is represented by embryo 1. Although tetraploidy is rarely compatible with life in humans, it seems that a likely fate of this embryo, had it continued to develop, would have been spontaneous abortion. The second, if these results are indeed representative, is unequal segregation but without significant loss or gain of chromosomes. This is represented by embryo 2 where it seems likely that an unequal chromosome segregation pattern occurred in the first cleavage division, which was maintained in the second. This pattern may have been facilitated by a 3:1 centrosome arrangement which could be confirmed in future studies by staining of α-tubulin and γ-tubulin to highlight the spindle fibre and poles. Larger studies involving more embryos might also reveal whether a 3:1 arrangement of the centrosomes also predisposes the cell to increased levels of non-disjunction. If this was the case we might expect to see the majority of cells with a 4:4 pattern in embryos with two centrosomes at each pole but significant deviations from a 6:2 pattern when an unequal representation of centrosomes were present. It might be argued that preferential segregation to one pole was also observed in embryo 3 and thus that similar mechanisms were in place. In this case, however, there is evidence of significant loss of whole chromosomes and therefore a tendency towards ‘chaos’. Embryo 3 is therefore an example representing the third possible fate of a tetraploid embryo. Chaos is considered a separate class to aneuploidy in human embryos and may arise through the breakdown of mitotic checkpoint genes.
The study of human embryos derived by IVF and ICSI provides the basis through which clues about our early development as well as the safety of radical infertility treatment can be gleaned. This case report provides useful examples of how such information can be generated and interpreted.