INTRODUCTION
Recent changes in the global distribution of haemosporidians, such as avian malaria parasites Plasmodium sp., their arthropod vectors and their avian hosts have been associated with human settlement patterns, global trade and climate change (Garamszegi, Reference Garamszegi2011). Some parasites have become global cosmopolitans that can utilize multiple vectors and hosts, and these are often more successful or widespread (Valkiunas, Reference Valkiunas, Zehtindjiev, Dimitrov, Krizanauskiene, Iezhova and Bensch2008a). On the other hand, ‘ecological transmission barriers’, for example, cold temperatures in high latitude and/or altitude climates, can cause differentiation and isolation of parasite lineages between different host groups (Hellgren et al. Reference Hellgren, Bensch and Malmqvist2008). Parasite lineages with wide host and geographical distributions are considered to be generalist parasites, whereas lineages restricted to particular regions or endemic hosts are considered to be specialist lineages (Ishtiaq et al. Reference Ishtiaq, Clegg, Phillimore, Black and Owens2010). In Vanuatu and New Caledonia, for example, close associations between potentially specialist parasite lineages and endemic mosquito species have been described (Ishtiaq et al. Reference Ishtiaq, Guillaumot, Clegg, Phillimore, Black, Owens, Mundy and Sheldon2008). If the geographical distribution of the parasite is restricted compared to that of the host, this ‘spatial mismatch’ might be explained by climatic and ecological limiting factors associated with the invertebrate vector (Valkiunas, Reference Valkiunas2005). Islands generally have lower parasite diversity owing to isolation and small landmasses that proffer a biogeographical history distinct from that of continents, though species richness will increase with the area of the island (MacArthur and Wilson, Reference MacArthur and Wilson1967; see also Ishtiaq et al. Reference Ishtiaq, Clegg, Phillimore, Black and Owens2010). Consequently, islands such as New Zealand provide a good opportunity to discern malaria endemism and the co-evolutionary relationships between blood parasites and their hosts.
The New Zealand bellbird is an abundant and highly vagile passerine endemic to and ubiquitous throughout New Zealand (Robertson et al. Reference Robertson, Hyvonen, Fraser and Pickard2007). These characteristics make the bellbird an ideal candidate for studying parasite distribution, ecological barriers to vectors and host-parasite specificity. In New Zealand, Tompkins and Gleeson (Reference Tompkins and Gleeson2006) provided evidence for a latitudinal (north to south) decrease in abundance of one lineage of P. relictum infecting introduced European bird species. That clinal trend in malaria infections correlated positively with latitudinal trends in its putative mosquito vector, the exotic Culex quinquefasciatus (Tompkins and Gleeson, Reference Tompkins and Gleeson2006). Recently, a large public avian malaria database (MalAvi; http://mbio-serv4.mbioekol.lu.se/avianmalaria/index.html; see Bensch et al. Reference Bensch, Hellgren and Perez-Tris2009) has been constructed that uses codes for genotypes of parasites allowing researchers to deposit, query and download information on haemosporidians from around the world. Data depositions made to this international database (Ruth Brown, unpublished data; Bensch et al. Reference Bensch, Hellgren and Perez-Tris2009) show that at least 4 exotic e.g., house sparrow (Passer domesticus), European thrushes (Turdus spp.), and 6 endemic e.g., South Island robin (Petroica australis), saddleback (Philesturnus carunculatus), hihi (Notiomystis cincta), bird species have been infected by a variety of malarial parasites, including such well known exotic cosmopolitan parasites as SGS1 (P. elongatum) and GRW4 (P. relictum) in New Zealand. Not much is known about virulence of these parasites in New Zealand, however, but recent outbreaks of avian malaria causing mortality of endemic species have been recorded in New Zealand zoos e.g., mohua (Mohoua ochrocephala, Alley et al. Reference Alley, Fairley, Martin, Howe and Atkinson2008). The potential vectors for avian malaria in New Zealand are 12 species of endemic and/or native mosquito (Derraik, 2004). One of these species is continuously distributed throughout the country, Culex pervigilans; less is known about the other species (Derraik, 2004). At least 27 exotic species of mosquito have been identified from 171 interceptions recorded since 1929 by the Ministry of Agriculture and Fisheries (MAF) at ports of entry into New Zealand (Derraik, 2004). Only 4 species (Culex quinquefasciatus, Ochlerotatus notoscriptus, Ochlerotatus australis, Ochlerotatus camptorhynchus) have established successfully, however, and their distribution throughout New Zealand still appears to be patchy (Derraik, 2004; Derraik et al. Reference Derraik, Tompkins, Alley, Holder and Atkinson2008).
The main objectives of this study are to assess (1) the diversity of Plasmodium and Haemoproteus parasites infecting a highly mobile passerine endemic to New Zealand, (2) the distribution of these parasites relative to that of their host and (3) the worldwide distribution and phylogenetic relationships of bellbird parasite lineages. This study can be useful for comparison with future studies on changes in host-specificity and parasite biogeography associated with environmental change.
MATERIALS AND METHODS
Blood sampling
To assess the diversity and distribution of avian malaria parasites, we collected 693 blood samples from individual bellbirds at 9 sites over a large latitudinal range in New Zealand between 2007 and 2010 (Fig. 1). These sites extend from the northern locations of Poor Knights Islands (Aorangi) and Hauraki Gulf (Hauturu, Tiritiri Matangi and Tawharanui), through to the southern North Island of New Zealand (Tongariro Forest and Kapiti Island), the South Island (Kaikoura and Dunedin), and finally, to the Sub-Antarctic Islands (Adams Island) (Fig. 1). Under permission of the Department of Conservation (DoC) and Massey University Animal Ethics Committee (MUAEC), bellbirds were captured via mist nets, individuals were marked with DOC Banding Office stainless-steel and colour leg bands and blood samples were extracted by venipuncture of brachial vein and samples were stored in either lysis buffer or 95% ethanol. Blood smears were air-dried on microscope slides, preserved with 100% methanol and then stained using Giemsa stain.

Fig. 1. Location map showing our nine host population sampling locations in New Zealand. The numbers in percentages and parentheses represent Plasmodium infection prevalence (number of individuals infected/individuals screened) using PCR. The pie charts show relative proportion of positive infections represented by each Plasmodium lineage at each location. The approximate New Zealand bellbird (Anthornis melanura) distribution range is shown in light grey shading.
PCR detection of the parasite
DNA was extracted from blood samples using a DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA). Samples were screened for Plasmodium and Haemoproteus infections using a nested polymerase chain reaction (PCR) method that amplifies a 478 base pair (bp) fragment of mitochondrial DNA (mtDNA) cytochrome b gene (Hellgren et al. Reference Hellgren, Waldenstrom and Bensch2004). This nested PCR comprises 2 rounds of PCR reactions performed in 15 μl volumes using positive and negative controls. The forward and reverse primers used in the first round of reactions were HaemNFI (5′- CATATATTAAGAGAAITATGGAG-3′) and HaemNR3 (5′-ATAGAAAGATAAGAAATACCATTC-3′), respectively. Each 15 μl reaction included 2 μl of genomic DNA, 0 75 mm of each dNTP, 0 6 μ m of each primer, 1 5 mm MgCl2 and 0 3 units of Taq DNA polymerase (Invitrogen). The thermal profile consisted of a 3-min, 94°C activation step, followed by 20 cycles of 94°C for 30 sec, 50°C for 30 sec and 72°C for 45 sec, ending with an elongation step of 72°C for 10 min. In the second round of PCR reactions, primers HaemF (5′-TGGTGCTTTCGATATGCATG-3′) and HaemR2 were used (5′-GCATTATCTGGATGTGATAATGGT-3′). The protocol for the second round of PCR was the same as the first, except that 2 μl of PCR product from the first reaction was used as template instead of genomic DNA and the number of cycles in the thermal profile was increased to 35 cycles. Three μl of PCR products were run on 1 5% agarose then stained with ethidium bromide and viewed under UV. PCR products containing bands around 500-bp in size were considered malaria positive and purified using SureClean (Bioline Inc.), then sequenced using the forward primer HaemF on an ABI 3730 DNA Analyzer (Applied Biosystems, Inc.). Sequences edited and aligned in mega 5 (Tamura et al. Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011).
Phylogenetic analysis of the haemosporidian parasite
The GTR+I+G model of sequence evolution best fitted our 4 cytochrome b mitochondrial DNA haplotype dataset (N=93 individuals) as determined using maximum likelihood analyses and the Akaike Information Criterion (AIC) in jmodeltest 0.1.1 (Guindon and Gascuel, Reference Guindon and Gascuel2003; Posada, Reference Posada2008). This GTR+I+G model assumes base frequencies A=0 2994, C=0 1352, G=0 1310, T=0 4345) with proportion of invariable sites (I) <0 001 and a substitution rate matrix A-C=2 6951, A-G=1 2005, A-T=5 8779, C-G=1 2931, C-T=20 3541 and G-T=1 0. Using the GTR+I+G model, we estimated the phylogeny of haplotypes using 5000 bootstrap replicates of the maximum likelihood (ML) analysis in mega. The phylogenetic relationships among parasite lineages were estimated using cytochrome b sequences ⩾400-bp. We used all avian malaria sequences in the MalAvi database and some additional southern hemisphere Meliphagidae parasite sequences from GenBank (Beadell et al. Reference Beadell, Gering, Austin, Dumbacher, Peirce, Pratt, Atkinson and Fleischer2004; Bensch et al. Reference Bensch, Hellgren and Perez-Tris2009) to aid in the identification and biogeographical relationships of haemosporidian lineages found in bellbirds. A mammalian Plasmodium outgroup sequence was used to root the tree (see Wood et al. Reference Wood, Cosgrove, Wilkin, Knowles, Day and Sheldon2007). Pair-wise LogDet sequence divergence estimates (Lockhart et al. Reference Lockhart, Steel, Hendy and Penny1994) were calculated in mega.
RESULTS
Phylogenetic identification and prevalence of avian malaria lineages
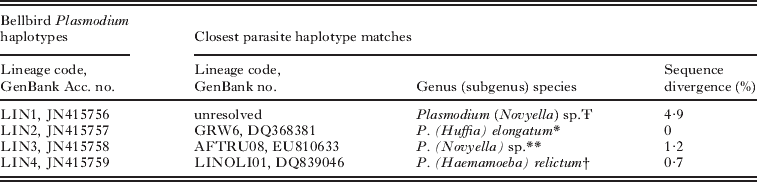
Phylogenetic analysis on cytochrome b sequences identified 4 distinct lineages (referred to as lineage LIN1, LIN2, LIN3 and LIN4 for the remainder of the text) of a single genus, Plasmodium (Table 1; Fig. S1, online version only). There is strong bootstrap support (91%) that LIN1 falls within a known clade of unresolved Plasmodium (subgenus Novyella) sp. sampled from Australian and Papua New Guinean Meliphagidae species (Beadell et al. Reference Beadell, Gering, Austin, Dumbacher, Peirce, Pratt, Atkinson and Fleischer2004) (Fig. 2). LogDet sequence divergence between LIN1 and its 2 closest phylogenetic matches, MELANA01 and MELNOT01, is 4 9% (22BASE-PAIR CHANGES/454COMMON SITES) and 5 1% (23BASE-PAIR CHANGES/454COMMON SITES), respectively (Table 1; see Fig. S2, online version only for LogDet pair-wise sequence divergence estimates). LIN1 parasites comprise 80% (N=74/93) of positive malaria infections in bellbirds and our country-wide prevalence estimate is 11% (N=74/693) (Fig. 1). We identified LIN2 to be the prolific cosmopolitan P. (Huffia) elongatum GRW06 (Perez-Tris et al. Reference Perez-Tris, Hellgren, Krizanauskiene, Waldenstrom, Secondi, Bonneaud, Fjeldsa, Hasselquist and Bensch2007; see Valkiunas et al. Reference Valkiunas, Zehtindjiev, Dimitrov, Krizanauskiene, Iezhova and Bensch2008a). The LIN2 and GRW06 cytochrome b sequences are an exact match (0BASE-PAIR CHANGES/454COMMON SITES) (Table 1). LIN2 is the most geographically widespread infection throughout New Zealand and occurs in 14% (N=13/93) of positive infections, but country-wide prevalence is low (2%, N=13/693) (Fig. 1). LIN3 is phylogenetically similar to infections previously found in African and Seychelles passerines, AFTRU08 Plasmodium (Novyella) sp. (Beadell et al. Reference Beadell, Covas, Gebhard, Ishtiaq, Melo, Schmidt, Perkins, Graves and Fleischer2009) (Fig 1). There is only a 0 7% LogDet sequence difference between LIN3 and AFTRU08 (Table 1). Finally, we found that LIN4 only has a 0 7% (3BASE-PAIR CHANGES/478COMMON SITES) sequence difference from the P. relictum LINOLI01 (Beadell et al. Reference Beadell, Ishtiaq, Covas, Melo, Warren, Atkinson, Bensch, Graves, Jhala, Peirce, Rahmani, Fonseca and Fleischer2006) (see Table 1). This lineage is an exact match with both P. relictum FOUSEY01 and CINCQ01 sampled in Seychelles passerines (Beadell et al. Reference Beadell, Ishtiaq, Covas, Melo, Warren, Atkinson, Bensch, Graves, Jhala, Peirce, Rahmani, Fonseca and Fleischer2006) (Fig. 2). We detected LIN3 and LIN4 in 0 7% (N=5/693) and 0 1% (N=1/693) of individuals screened throughout New Zealand, respectively (Fig. 1).

Fig. 2. The maximum likelihood (ML) tree shows the estimated phylogenetic relationships among parasite lineages using cytochrome b sequences (length 478-bp). Leucocytozoon and a mammalian Plasmodium were used as outgroups to root the tree. The numbers at the branches represent ML bootstrap support ⩾70% (5000 replicates). Previously documented sequences have their Latin, MalAvi database and GenBank names at the end of each branch.
Table 1. Sequence divergence estimates of the bellbird Plasmodium parasites detected in this study and the closest sequence matches documented in the MalAvi database and GenBank
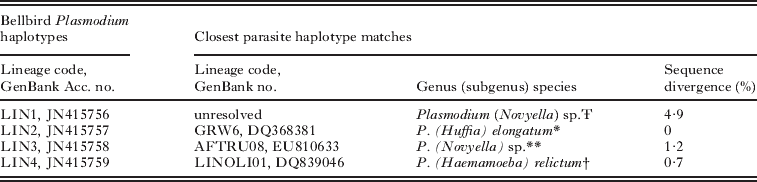
Ŧ This sequence could not be resolved to a known malaria lineage, but belongs to the subgenus Novyella and a clade of Plasmodium found only in Australopacific honeyeaters (Beadell et al. Reference Beadell, Gering, Austin, Dumbacher, Peirce, Pratt, Atkinson and Fleischer2004).
* Mitochondrial cytochrome b lineages found in bellbirds that were identical to a well-known previously defined cosmopolitan species (Perez-Tris et al. Reference Perez-Tris, Hellgren, Krizanauskiene, Waldenstrom, Secondi, Bonneaud, Fjeldsa, Hasselquist and Bensch2007; Valkiunas et al. 2008a).
** This sequence matches most closely to AFTRU08 found in a thrush (Turdus pelios) host in Cameroon, western Africa (Beadell et al. Reference Beadell, Covas, Gebhard, Ishtiaq, Melo, Schmidt, Perkins, Graves and Fleischer2009).
† This sequence matches most closely to LINOLI01 (a P. relictum lineage very similar to the cosmopolitan SGS01) collected from a Seychelles sunbird (Cinnyris dussumieri) host off the coast of eastern Africa (Beadell et al. Reference Beadell, Ishtiaq, Covas, Melo, Warren, Atkinson, Bensch, Graves, Jhala, Peirce, Rahmani, Fonseca and Fleischer2006).
DISCUSSION
Diversity and distribution of bellbird Plasmodium lineages within New Zealand
We detected 4 lineages of Plasmodium and no Haemoproteus parasites from a widely distributed endemic passerine host throughout the island archipelago of New Zealand. Our parasite diversity estimates are low compared to studies on continental locations; for example, typically in Europe and North America a host population can have up to 12–15 lineages of malaria within a small geographical area (see Fallon et al. Reference Fallon, Ricklefs, Latta and Bermingham2004; Kimura et al. Reference Kimura, Dhondt and Lovette2006; Bensch et al. Reference Bensch, Waldenstrom, Jonzen, Westerdahl, Hansson, Sejberg and Hasselquist2007; Wood et al. Reference Wood, Cosgrove, Wilkin, Knowles, Day and Sheldon2007; Cosgrove et al. Reference Cosgrove, Wood, Day and Sheldon2008; Norte et al. Reference Norte, Araujo, Sampaio, Sousa and Ramos2009). The low malaria diversity and prevalence (⩽20%) in the New Zealand bellbird is congruent, however, with findings from other endemic Australopacific island host bird populations (Jarvi et al. Reference Jarvi, Farias, Baker, Freifeld, Baker, Van Gelder, Massey and Atkinson2003; Beadell et al. Reference Beadell, Gering, Austin, Dumbacher, Peirce, Pratt, Atkinson and Fleischer2004).
The distribution of Plasmodium parasite, LIN1, was geographically restricted compared to its bellbird host in 2 key ways. First, the most numerous avian malaria parasite, LIN1, was restricted geographically to the Hauraki Gulf region and, second, LIN2 (P. elongatum GRW06) was the only lineage that reflected the bellbird distribution and was ubiquitous throughout most of New Zealand. As with LIN1, parasite lineages LIN3 and LIN4 were only detected in northern New Zealand. The lack of any Plasmodium detected at Tongariro may be due to low parasitaemia and/or high altitude (∼1120 m above sea level), where all other sites were at sea level (Knowles et al. Reference Knowles, Wood, Alves, Wilkin, Bensch and Sheldon2011). In the Sub-Antarctic Auckland Islands a complete absence of mosquito vectors precludes transmission at that location. The availability of competent vectors or suitable environmental and climatic variables in addition to the geographical distribution of the host likely play an important role in the biogeographical distribution of avian malaria parasites (Hellgren et al. Reference Hellgren, Waldenstrom, Perez-Tris, Szollosi, Hasselquist, Krizanauskiene, Ottosson and Bensch2007; Ishtiaq et al. Reference Ishtiaq, Clegg, Phillimore, Black and Owens2010).
Biogeographical origins of bellbird Plasmodium parasites
Our detailed phylogenetic analysis using the complete MalAvi database (Bensch et al. Reference Bensch, Hellgren and Perez-Tris2009) and GenBank provides no mitochondrial DNA sequence match close enough to resolve the LIN1 lineage to the species level, let alone a particular lineage for LIN1. However, we found that the LIN1 lineage belongs to a clade of parasites known to infect only Australopacific meliphagids: the yellow-spotted honeyeater (Meliphaga notata) that inhabits Australia; Lewin's honeyeater (Meliphaga lewinii) that inhabits northeastern Australia and Papua New Guinea (Beadell et al. Reference Beadell, Gering, Austin, Dumbacher, Peirce, Pratt, Atkinson and Fleischer2004). In a recent study, Ricklefs and Outlaw (Reference Ricklefs and Outlaw2010) demonstrated that the rate of molecular evolution of haemosporidian parasites is ∼1 2% sequence divergence per million years. Bellbirds are thought to have evolved ∼2 9 million years ago (see Beadell et al. Reference Beadell, Gering, Austin, Dumbacher, Peirce, Pratt, Atkinson and Fleischer2004; Driskell and Christidis, Reference Driskell and Christidis2004). Thus our results here of a 4 9% sequence divergence between Australian and New Zealand Meliphagid Plasmodium spp. date the origin of the LIN1 parasite to the historical Meliphagidae radiation from Australia to New Zealand. A convincing argument would be that the ancestral bellbird carried its parasites from Australia and that the bellbird and LIN1 have been locked in an ancient evolutionary arms race (Dawkins and Krebs, Reference Dawkins and Krebs1979). However, Ricklefs and Outlaw (Reference Ricklefs and Outlaw2010) concluded that a new disease might emerge in a host, through host switching, soon after its origin and at any time thereafter, thus it is still plausible that the modern-day bellbird Plasmodium LIN1 lineage may have originated in New Zealand earlier than 3 million years ago, independently of the evolutionary history of the bellbird.
LIN2 was an exact match with the P. elongatum GRW06 lineage originally described in the great reed warbler (Acrocephalus arundinaceus; Perez-Tris et al. Reference Perez-Tris, Hellgren, Krizanauskiene, Waldenstrom, Secondi, Bonneaud, Fjeldsa, Hasselquist and Bensch2007; see also Valkiunas et al. Reference Valkiunas, Zehtindjiev, Dimitrov, Krizanauskiene, Iezhova and Bensch2008a) and is a global cosmopolitan species found in many species of avian hosts throughout Europe, Africa, Australia and North America (Table 2). Despite its relatively low prevalence in bellbirds, the geographical distribution of LIN2 is ubiquitous throughout New Zealand (this study) and this same parasite has been sampled from several endemic and exotic avian species throughout New Zealand (Ruth Brown, unpublished data). Furthermore, it is unlikely that the more rare lineages LIN2-4 maintain their population solely within the bellbird (Bensch et al. Reference Bensch, Waldenstrom, Jonzen, Westerdahl, Hansson, Sejberg and Hasselquist2007). The most probable reservoir for LIN2-4 parasite lineages are the European and Asian passerines, and indeed Passer domesticus is the most common host listed in the MalAvi database for LIN2 infections. Taken together with the lack of nucleotide base pair substitutions, we suggest that GRW06 (LIN2) has arrived in New Zealand relatively recently.
Table 2. Worldwide biogeographical distribution of Plasmodium lineages most closely matching each parasite sequence found in New Zealand bellbirds (Anthornis melanura) in this study (information only for sequences with <2% LogDet sequence divergence from LIN1-4)

The 2 remaining lineages grouped within 2 Plasmodium clades representing species reported from African birds only: LIN3, Plasmodium (Novyella) sp. AFTRU08 (Beadell et al. Reference Beadell, Ishtiaq, Covas, Melo, Warren, Atkinson, Bensch, Graves, Jhala, Peirce, Rahmani, Fonseca and Fleischer2006); and LIN4, P. (Haemamoeba) relictum LINOLI01. A single bellbird screened positive for Plasmodium relictum, LIN4, at Hauturu and it is unknown to date whether this exact lineage has been detected in other New Zealand birds. The P. relictum lineages documented by Tompkins and Gleeson (Reference Tompkins and Gleeson2006) cannot be compared directly to our results because the cytochrome b markers they used differed from those in our study and those generally used in the MalAvi database. The P. relictum lineage that we detected in bellbirds may be a distant variant of the well-known GRW04 P. relictum lineage (LIN4 vs GRW04: 1 7% sequence divergence; LIN4 vs LINOLI01: 0 7% sequence divergence). GRW04 is well known to be responsible for the decimation of Hawaiian avifauna (Perkins and Schall, Reference Perkins and Schall2002). That tragedy has not occurred in New Zealand likely because mosquitos are endemic, unlike in Hawaii (Derraik, Reference Derraik2004). Thus, endemic New Zealand birds should have some acquired resistance to exotic strains of malaria as well as endemic strains (Jarvi et al. Reference Jarvi, Schultz and Atkinson2002).
Lack of Haemoproteus infections and underestimation of parasite prevalence
We found no Haemoproteus infections in bellbirds despite the fact that their usual hippoboscid vector (Valkiunas, Reference Valkiunas2005) was observed on most individuals captured in this study (S.M.B., unpublished data). The New Zealand bellbird host lineage originated from Australian meliphagids (Driskell et al. Reference Driskell, Christidis, Gill, Boles, Barker and Longmore2007), in which Haemoproteus infections are common (Beadell et al. Reference Beadell, Ishtiaq, Covas, Melo, Warren, Atkinson, Bensch, Graves, Jhala, Peirce, Rahmani, Fonseca and Fleischer2006). Thus, we suggest that Haemoproteus infections in bellbirds (1) might have been lost through the process of host population bottleneck, (2) are too low in numbers to detect, or (3) have never been part of the New Zealand meliphagid system. PCR detection of parasites has been experimentally shown to underestimate prevalence by 30%, especially (1) in cases of low parasitaemia characteristic of chronic infection and (2) if infections temporarily evacuate the peripheral blood stream (Jarvi et al. Reference Jarvi, Schultz and Atkinson2002; Valkiunas et al. Reference Valkiunas, Iezhova, Krizanauskiene, Palinauskas, Sehgal and Bensch2008b). This large margin of error alone may explain the absence of Haemoproteus in our study and means that our Plasmodium prevalence estimates are underestimated. Microscopy yields varying results depending on both the skill level of the observer and quality of specimens. For most non-specialized researchers microscopy techniques will underestimate prevalence by 70% (Jarvi et al. Reference Jarvi, Schultz and Atkinson2002). In this study, we found that in our initial 97 samples, 39% (16/26) of the malaria detected by nested PCR was not detected by microscopy performed by a professional haematologist experienced in malaria detection. A more sensitive malaria detection technique involves serological assay of antibodies, but current infection status and parasite lineage identification is not possible (Jarvi et al. Reference Jarvi, Schultz and Atkinson2002). The most promising blood parasite detection approach for future studies is a relatively new quantitative PCR (qPCR) that has been shown to have higher detection sensitivity than nested PCR and it can be used to estimate parasitaemia, or concentration of parasites in a given amount of blood (see Knowles et al. Reference Knowles, Wood, Alves, Wilkin, Bensch and Sheldon2011).
Conclusions
Students of biogeography have long centred their studies on islands. Because of its large almost continental sized islands, range of climatic and altitudinal variation and long distance from source areas, New Zealand is an ideal island to study replicate natural experiments that allow a focus on a wide variety of issues including ecological transmission barriers, area and distance effects, endemic versus widespread species and extinction rates. Relative to continental bird populations, we found a low diversity of haematozoan parasites in New Zealand bellbirds. Avian malaria parasite diversity and abundance was concentrated in northern New Zealand and the only malaria lineage present throughout the country was an exotic P. elongatum GRW06 (LIN2, this study). Our phylogenetic analysis provides strong evidence that the LIN2 parasite (as well as LIN3 and LIN4) has been recently introduced to New Zealand. On the other hand, sequence divergence of the LIN1 parasite within its clade indicates that LIN1 may have existed in New Zealand for several millions of years. The mismatch we reveal between host and parasite spatial distributions suggests that this putative endemic parasite is limited by vector distribution, possibly for climatic reasons. As climatic conditions change both in New Zealand and around the world, the use of the MalAvi database, as well as phylogenetics and qPCR techniques will be important tools in future studies on rates of entry of invading parasites in New Zealand, parasite virulence and ecological transmission barriers across variable climatic environments.
ACKNOWLEDGEMENTS
Gracious thanks especially to Jordi Segers, Morag Fordham and Simon Fordham, and also to Barbara Elgi, Eva Krause and Birgit Ziesemann who helped capture bellbirds in the Hauraki Gulf. Michael Anderson and Yuri Shanas were crucial to fieldwork at the Poor Knights Islands. Graeme Elliott of DoC generously collected blood samples from the Auckland Islands. Finally, we extend many thanks to Dr Peter Ritchie for permission to work in his genetics lab and discussion group at School of Biological Sciences, Victoria University of Wellington, Wellington, New Zealand. All blood collection from birds was performed under permit of New Zealand Department of Conservation (DoC), Auckland Regional Council (ARC) and Massey University Animal Ethics Committee (MUAEC). We thank two anonymous reviewers for their comments and advice, which were a valuable contribution to this paper.
FINANCIAL SUPPORT
This study was funded by a Canadian Natural Science and Engineering Research Council (NSERC) PGS scholarship to S.M.B. and the New Zealand Institute of Natural Resources (INS) Massey University.