Introduction
Smooth newts Lissotriton vulgaris inhabit humid and aquatic areas in most of Europe, from sea level to 2150 m above sea level (asl) in the Austrian Alps. A considerable range of studies on newt populations covering most of their geographical range yielded a well-established overview on their helminth endoparasites (Barus & Groschaft, Reference Barus and Groschaft1962; Vojtková, Reference Vojtková1963a, Reference Vojtkováb; Avery, Reference Avery1971; Vojtek, Reference Vojtek1972; Scholz & Moravec, Reference Scholz and Moravec1990; Caffara et al., Reference Caffara, Bruni, Paoletti, Gustinelli and Fioravanti2014). Local diversity rarely exceeds five parasite species within a single host population, whereas a total of 31 different helminth species have been reported to use L. vulgaris as host in general, i.e. two acanthocephalans: Acanthocephalus anthuris, A. ranae; two cestodes: Batrachotaenia carpathica and Bothriocephalus claviceps; 14 trematodes (metacercariae or adults): Alaria alata (a foodborne human pathogen), Astiotrema monticelli, A. trituri, Clinostomum complanatum, Diplodiscus subclavatus, Halipegus kessleri, Leptophallus nigrovenosus, Opistioglyphe ranae, Paralepoderma cloacicola, Parastrigea robusta, Pleurogenes claviger, Pleurogenoides medians, Strigea sphaerula and S. strigis; and 13 nematodes: Agamospirura sp. larva, Amphibiocapillaria filiformis, A. tritonispunctati, Anguillicola crassus, C. longicauda, C. ornata, Hedruris androphora, Megalobatrachonema terdentatum, Oswaldocruzia duboisi, O. goezei, O. filiformis, O. molgeta and Oxysomatium brevicaudatum (Lewis, Reference Lewis1928; Grabda, Reference Grabda1959; Barus & Groschaft, Reference Barus and Groschaft1962; Vojtková, Reference Vojtková1963a, Reference Vojtkováb; Avery, Reference Avery1971; Vojtková & Vojtek, Reference Vojtková and Vojtek1972; Moravec & Vojtková, Reference Moravec and Vojtková1975; Bertman & Okulewicz, Reference Bertman and Okulewicz1987; Chumak, Reference Chumak1989; Scholz & Moravec, Reference Scholz and Moravec1990; Ben Slimane et al., Reference Ben Slimane, Durette-Desset and Chabaud1993; Iskova et al., Reference Iskova, Sharpilo, Sharpilo and Tkach1995; Moravec & Skoríková, Reference Moravec and Skoríková1998, Shimalov et al., Reference Shimalov, Shimalov and Shimalov2001; Shimalov, Reference Shimalov2002; Caffara et al., Reference Caffara, Bruni, Paoletti, Gustinelli and Fioravanti2014). Yet, helminth parasite diversity of smooth newts could turn out to be significantly lower because the list comprises taxa within the nematode genera Amphibiocapillaria and Oswaldocruzia which are morphologically indistinguishable (Moravec & Vojtková, Reference Moravec and Vojtková1975; Yildirimhan et al., Reference Yildirimhan, Bursey and Goldberg2005). Reliable taxonomic identification of newt helminths is hampered by the fact that many parasite species have a broad host spectrum (some infest a wide range of anuran, urodele and reptile species) and that subsequent morphological variation among conspecific parasites collected from distinct hosts may lead to erroneous taxonomic decisions (e.g. Moravec & Vojtková, Reference Moravec and Vojtková1975). Moreover, early developmental stages of nematodes and encysted metacercariae in amphibian hosts often do not offer reliable morphological features to distinguish sufficiently among species (Odening, Reference Odening1967; Petter & Chabaud, Reference Petter and Chabaud1971; Locke et al., Reference Locke, McLaughlin, Lapierre, Johnson and Marcogliese2011; Patrelle et al., Reference Patrelle, Portier, Jouet, Delorme and Ferté2015).
Molecular taxonomy aims to use DNA sequence diversity of one or more uniform target genes as a standardized tool, and has been proposed to solve the shortcomings of the morphological approach in the affiliation of parasites with a lack of morphological diagnostic characters in adults and larval stages that have not yet been characterized (e.g. Besansky et al., Reference Besansky, Severson and Ferdig2003; Vanhove et al., Reference Vanhove, Tessens, Schoelinck, Jondelius, Littlewood, Artois and Huyse2013). Internal transcribed spacer 2 (ITS2) sequences proved useful for the assignment of Trematoda metacercariae to the family level, but identification at species level failed, due to the absence of sequences obtained from adult specimens, which had been identified morphologically (Chontananarth et al., Reference Chontananarth, Tejangkura, Wetchasart and Chimburut2017). Cytochrome c oxidase 1 (CO1) sequences distinguished 52 species among 2000 diplostomid metacercariae in North America, Europe and Africa, but only 12 were assignable to described species (Locke et al., Reference Locke, Al-Nasiri and Caffara2015). In cyathostomin nematodes, reliable morphological species differentiation is limited to adults (Bredtmann et al., Reference Bredtmann, Krücken, Murugaiyan, Kuzmina and von Samson-Himmelstjerna2017). These study results imply that morphological and molecular datasets are widely unlinked, resulting in a low number of reliably identified helminth species with corresponding sequence data deposited in GenBank. Thus, the real diversity of helminth taxa is still in question (Luque et al., Reference Luque, Pereira, Alves, Oliva and Timi2017).
DNA sequences of morphologically identified endoparasitic helminths of the smooth newt in Germany are presented in this paper. So far, GenBank provides sequences (mainly the 28S rRNA gene) for only 12 (1 cestode, 8 trematodes, 3 nematodes) out of 31 species known to use L. vulgaris as host. At least two of them are predominately fish parasites (A. crassus, C. complanatum) being atypically found in newts. The five parasitic helminth taxa infesting L. vulgaris in western Germany are presented morphologically, together with their corresponding DNA sequences for future molecular identification.
Materials and methods
Collection and examination of newts
Adult L. vulgaris were collected from two localities at the former military training area Schmidtenhöhe near Koblenz (Rhineland-Palatinate, Germany) (Sinsch & Breuer, Reference Sinsch and Breuer2018): (1) 44 males and 5 females in a permanent eutrophic pond (50.347°N, 7.674°E, 333 m asl; 10 June 2016, 26 April, 31 May and 21 June 2017) and (2) 20 males in a temporarily water-filled ditch (50.346°N, 7.644°E, 279 m asl; 16 June 2016). All specimens were sacrificed immediately after collection and the body cavity, digestive tract, lungs, kidneys and bladder were subsequently examined macroscopically and microscopically for the presence of helminth parasites. Carcasses of newts were fixed in 10% buffered formalin, and transferred to 70% ethanol for long-term storage. One to three helminth specimens (encysted metacercariae or adult nematodes) of each infected newt were isolated in 1.5-ml reaction tubes with 96% ethanol for DNA extraction, while the remaining helminths were stored in 4% buffered formalin for morphological species identification and documentation. Finally, the newts examined and voucher specimens of helminths were deposited in the collection of the Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany (ZFMK).
Morphological helminth identification
Only encysted trematode metacercariae and larval and adult nematodes were detected. Ellipsoidal cysts were photographed in life under slight coverslip pressure, and axes were measured to the nearest micrometre. Metacercariae were identified based on the keys of Dubois (Reference Dubois1968) and Niewiadomska (Reference Niewiadomska, Gibson, Jones and Bray2002). Features of tentatively identified taxa were compared with those given in specific references on these taxa (e.g. Odening, Reference Odening1965, Reference Odening1967). Nematodes were photographed in life and formalin-preserved. Each individual sample was cleared in a drop of lactophenol on a microscope slide, a cover slip was added and it was examined under a compound microscope. The nematodes were identified following the keys of Anderson et al. (Reference Anderson, Chabaud and Willmott2009) and Gibbons (Reference Gibbons2010).
Nucleic acid extractions and polymerase chain reactions
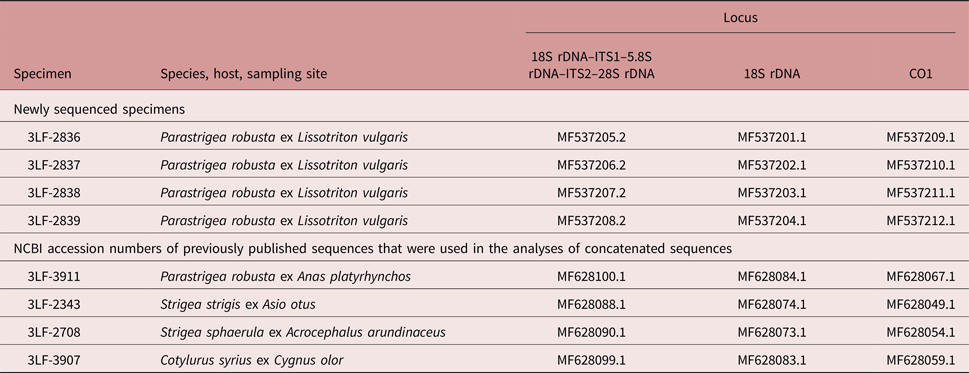
Metacercariae (n = 4) were washed twice for 15 min using 1 ml of buffer containing 10 mm Tris–HCl (pH 7.5) and 5 mm EDTA. Subsequently, the samples were processed as described previously (Sitko et al., Reference Sitko, Bizos and Heneberg2017). The primers targeted nuclear 18S rDNA and ITS1–5.8S–ITS2 loci, and the mitochondrial CO1 locus (table 1). The resulting consensus DNA sequences were submitted to GenBank under accession numbers MF537201–MF537212 (see table 2).
Table 1. Primers used for the amplification and sequencing of nuclear and mitochondrial DNA loci in the helminths examined.
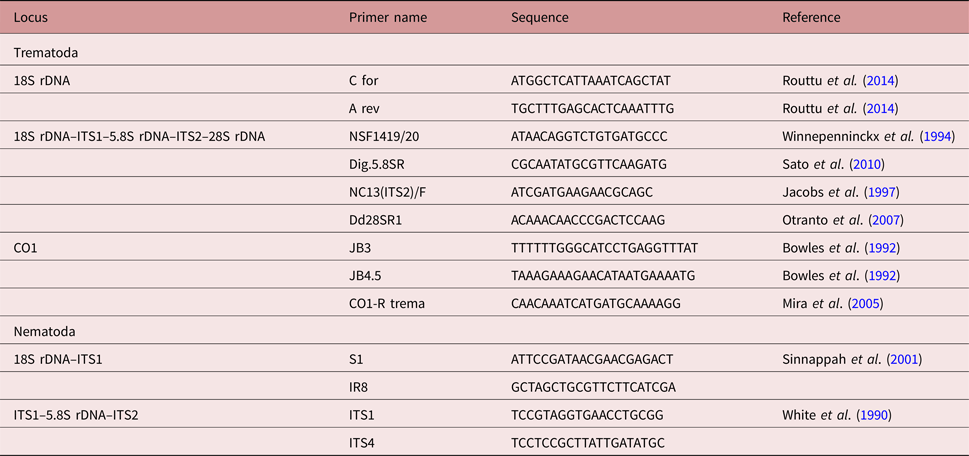
Table 2. Sequences of helminth parasites isolated from L. vulgaris in the course of the present study. New sequences of Parastrigea robusta and the previously published sequences of Strigeidae adults (Heneberg et al., submitted), which were used in the comparative phylogenetic analyses of concatenated sequences in order to allow molecular identification of the new material. The National Centre for Biotechnology Information (NCBI) GenBank accession numbers are indicated.
Each ethanol-fixed nematode specimen was incubated, with open caps, at 40°C in a Thermomixer (Eppendorf, Wesseling-Berzdorf, Germany) until the ethanol was completely evaporated. Samples were mixed with 180 μl ATL buffer, 20 μl proteinase K and incubated at 56°C until specimens were completely lysed, and processed following the protocol for DNA purification from tissues of the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). The extracted DNA was used to amplify parts of 18S rRNA and ITS1 locus using primers S1 and IR8, or partial ITS1, 5.8S rDNA and partial ITS2 locus using primers ITS1 and ITS4, respectively (White et al., Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990; Sinnappah et al., Reference Sinnappah, Lim, Rohde, Tinsley, Combes and Verneau2001). Polymerase chain reaction (PCR) using Taq PCR core kit (Qiagen) was conducted as recommended by the manufacturer. The PCR protocol consisted of an initial cycle at 94°C for 3 min; 40 cycles at 94°C for 1 min, 50°C for 1 min and 72°C for 3 min; with a last extension step at 72°C for 7 min. The amplicons were separated on a 1% agarose gel, stained with ethidium bromide and visualized on a UV transilluminator. PCR products were purified using QIAquick PCR Purification Kit (Qiagen).
Sequencing and phylogenetic analysis
PCR amplicons were bidirectionally sequenced using the same primers as for PCR. Sequences were assembled by DNA baser (Heracle BioSoft, http://www.dnabaser.com/) and deposited in GenBank (tables 2 and 3). Sequences obtained from metacercariae and sequences of adult Strigeidae that were published previously (Heneberg et al., submitted) were aligned using ClustalW (http://www.genome.jp/tools-bin/clustalw) (gap opening penalty 7, gap extension penalty 2 for both pairwise and multiple alignments, DNA weight matrix IUB, transition weight 0.1). Cotylurus syrius served as an outgroup. The alignments were corrected manually for any inconsistencies and the aligned sequences were trimmed to the length covered by all the sequences included. The sequences were trimmed to ITS2 (267 bp, trimmed to nucleotides 1131–1397 of MF537205.2), 18S rDNA (695 bp, corresponds to the full-length MF537201.1) and CO1 (295 bp, trimmed to nucleotides 35–329 of MF537209.1). The trimmed aligned sequences were concatenated and only these concatenated sequences were used for further analyses. Closest matches of nematode sequences were identified by a BLAST search against GenBank entries (Altschul et al., Reference Altschul, Gish, Miller, Myers and Lipman1990).
Table 3. Sequences of helminth parasites isolated from L. vulgaris in the course of the present study. New sequences of nematode parasites. NCBI GenBank accession numbers are indicated.

Phylogenetic trees were constructed from the alignments using the Maximum Likelihood algorithm with MEGA 5.2 (Tamura et al., Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011). Branch support was calculated based on 1000 bootstraps. The nearest-neighbour interchange served as the maximum likelihood heuristic method of choice to determine the tree inference when the initial tree was formed using a neighbour-joining algorithm. The best model according to the Bayesian information criterion, corrected Akaike information criterion and maximum likelihood values was Hasegawa–Kishino–Yano, with the non-uniformity of evolutionary rates among sites modelled using a discrete Gamma distribution with five rate categories, and by assuming that a certain fraction of sites are evolutionarily invariable (+I). The maximum likelihood method was used to estimate inter- and intraspecific evolutionary divergence in the analysed sequences. The number of base differences per site was calculated by averaging over all sequence pairs between groups (distance) ± SE and by employing a bootstrap procedure at 1000 replicates. The model that was used to estimate inter- and intrasite evolutionary divergence was identical to that used to construct the respective maximum likelihood tree.
Results
The endoparasitic helminth diversity detected in smooth newts collected from the two study sites included one trematode and four nematode species (table 4, fig. 1). 13 (19%) out of the 69 newts inspected were free of helminth parasites. Most newts (60%) were infested by only one parasite species, 21% by the trematode and one nematode species, while a simultaneous infection of two distinct nematode species was not observed. Trematode cysts were attached exclusively to the wall of the body cavity, mostly at the dorsal part. All adult nematodes were found within the digestive tract of the newt, whereas larval stages also moved through the body cavity. Lungs, liver, kidneys, bladder and reproductive system were found to be not infected.
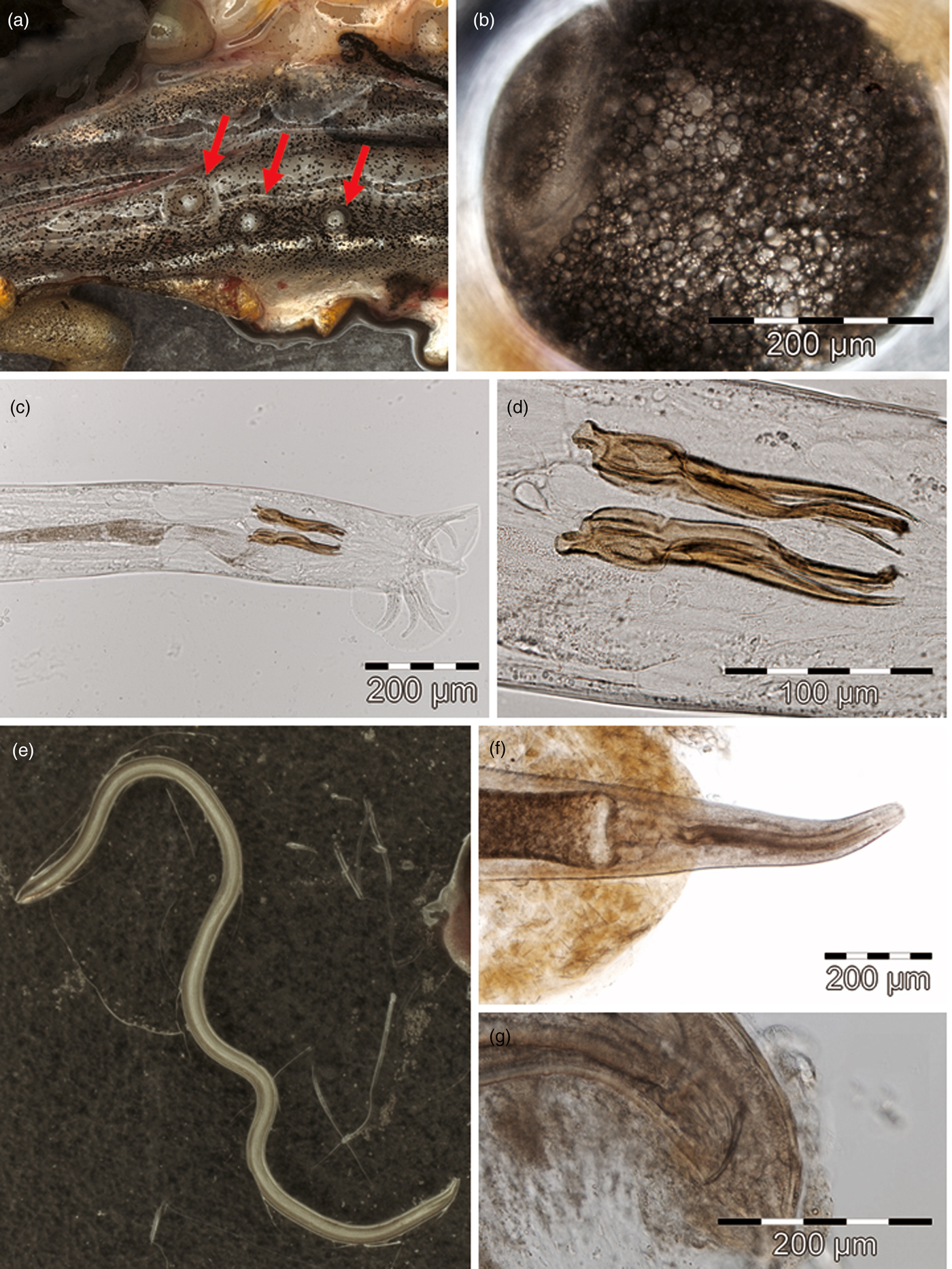
Fig. 1. Morphological features of smooth newt parasites. (a) Dorsal body cavity wall with three encysted metacercariae of P. robusta (indicated by red arrows), (b) metacercaria within the hyaline inner cyst wall, showing lipid globes within the paranephridial plexus. (c) Posterior extremity of a male O. filiformis (= O. molgeta) with spicules and bursa, (d) spicules. (e) Female M. terdentatum of length 22.7 mm. (f) Anterior extremity with oesophagus and bulbus of a male C. longicauda, (g) posterior extremity with speculum.
Table 4. Helminth parasites of smooth newts L. vulgaris at two localities in the study area. Local infection rates of newts as well as developmental stages of the detected parasites are given.

Encysted metacercariae, identified as Parastrigea robusta Szidat, 1928
Morphological diagnosis
The detected cysts (fig. 1a, b) showed the typical features of strigeid tetracotyle metacercariae, i.e. the inner hyaline cyst wall is of parasite origin, the outer walls of host origin, and reserve bladder forms network filling entire body, with free excretory bodies in canals (Dubois, Reference Dubois1968; Niewiadomska, Reference Niewiadomska, Gibson, Jones and Bray2002). Average life size of seven ellipsoid cysts under slight coverslip pressure was 356 μm (286–407) × 301 μm (241–336). They were compared to strigeid trematodes that are known to form metacercaria cysts in smooth newts or other central European amphibians, i.e. P. robusta (264–388 μm × 228–308 μm; Odening, Reference Odening1965), Strigea falconis (440–607 μm × 220–404 μm; Odening, Reference Odening1967), S. sphaerula (440–734 μm × 301–499 μm; Odening, Reference Odening1967) and S. strigis (573–1049 μm × 360–433 μm; Odening, Reference Odening1967). Consequently, the measured cyst size range matched exclusively with data on P. robusta in the literature, providing a tentative narrowing among candidates.
Molecular diagnosis
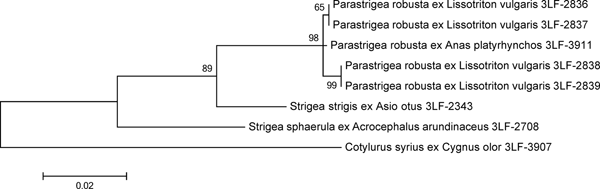
The DNA isolated from the cysts was variable in the CO1 locus (eight informative sites within the 368-bp-long sequence), whereas all the analysed nuclear loci (two partial 18S rDNA sequences, complete ITS1, 5.8S rDNA, ITS2 and partial 28S rDNA) were invariant. The 18S rDNA, ITS2 and CO1 sequences of an adult P. robusta obtained previously from its definitive host Anas platyrhynchos were identical with those of the cysts. ITS1, 5.8S rDNA and partial 28S rDNA were not available for the adult specimen. Based on the maximum likelihood analysis (fig. 2), DNA from all the four cysts was identified as P. robusta. The analysis of concatenated DNA sequences of ITS2, 18S rDNA and CO1 showed that the four sequences were 99.8–99.9% identical with the control sequence of an adult P. robusta. Conspecifity with S. sphaerula or S. strigis could therefore be excluded.
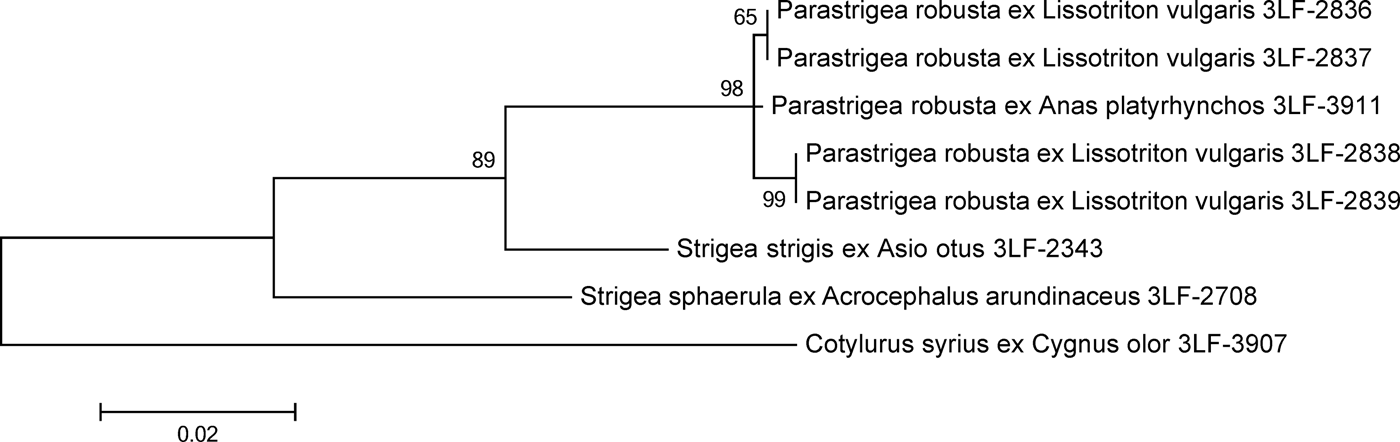
Fig. 2. Maximum likelihood analysis of concatenated nuclear and mitochondrial DNA loci from P. robusta and related species of Strigeidae. The scale bar indicates the number of substitutions per one nucleotide. Support values of maximum likelihood bootstrap analysis (1000 replicates) are indicated.
Nematode species 1, identified as Oswaldocruzia filiformis (Goeze, 1782)
Morphological diagnosis
The absence of a buccal ring and extradorsal rays indicated the taxonomic assignment of this male trichostrongylid nematode to the family Molineidae (Anderson et al., Reference Anderson, Chabaud and Willmott2009). Generic allocation to Oswaldocruzia was supported by the shape of the bursal dorsal ray, multiple processes of the spicules (fig. 1c, d) and the absence of a gubernaculum (Travassos, Reference Travassos1937). Morphological features of the specimen corresponded to those of O. molgeta (Lewis, Reference Lewis1928; Barus & Groschaft, Reference Barus and Groschaft1962), but also fell into the range of variability found in O. duboisi, O. filiformis and O. goezei (Moravec & Vojtková, Reference Moravec and Vojtková1975; Ben Slimane et al., Reference Ben Slimane, Durette-Desset and Chabaud1993). As morphological diagnostic features have not yet been proposed to distinguish among these taxa, assignment of the examined specimen to any taxon remained unreliable.
Molecular diagnosis
Using primers targeting partial 18S rDNA and ITS1–5.8S rDNA–ITS2 loci, DNA sequences for both loci in one specimen and for 18S rDNA in another were obtained (table 3). Specimen ZX3-1701 could be confirmed to be conspecific with O. filiformis obtained from Pelophylax [Rana] ridibunda by 99% (sequence similarity 99% at the ITS1 locus; 635/638 identities without gaps) (fig. 3). At the 18S rDNA locus, specimens examined showed a sequence similarity of 100% with O. filiformis (since the sequence of specimen ZX3-1701 has only a 437-nucleotide overlap with the respective O. filiformis database entry, this locus was not chosen for phylogeny) and of 90% with Spiculopteragia houdemeri (Trichostrongylidae: Ostertagiinae) as the closest match in GenBank (803/893 identities and 47 gaps in the alignment).

Fig. 3. Maximum likelihood analysis of ITS1–5.8S rDNA–ITS2 loci of an O. molgeta specimen isolated from the small intestine of the smooth newt L. vulgaris (ZX3-1701) and related nematode species. Support values of maximum likelihood bootstrap analysis (1000 replicates) are given. Scale bar = substitutions per site.
Nematode species 2, identified as Megalobatrachonema terdentatum (Linstow, 1890)
Morphological diagnosis
Adult specimens examined (fig. 1e) showed the typical morphological features of helminths of the genus Megalobatrachonema (Kathlaniidae) in having a cylindrical oesophageal isthmus with a glandular bulb lacking valves and poorly developed lips (Anderson et al., Reference Anderson, Chabaud and Willmott2009). The morphological features of these adult nematodes entirely matched those of M. terdentatum (Linstow, 1890) as described in Hartwich (Reference Hartwich1960) and Bertman & Okulewicz (Reference Bertman and Okulewicz1987). Larval specimens (stages III and IV) found in the body cavity lacked reliable diagnostic features (Petter & Chabaud, Reference Petter and Chabaud1971).
Molecular diagnosis
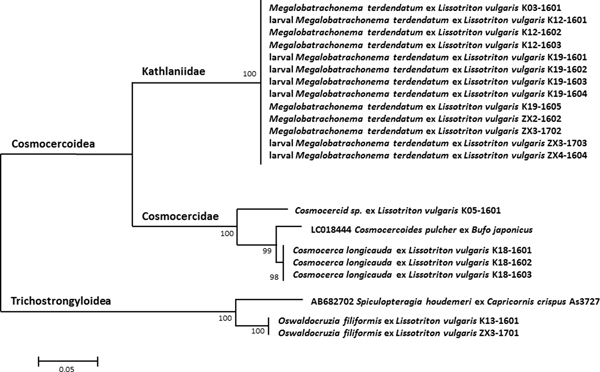
Using primers targeting 18S rDNA, sequences were obtained for a total of 13 specimens, including six morphologically diagnosed adults and seven undetermined larval stages collected moving in the body cavity (table 3). All the sequences obtained were identical. The closest match to sequences deposited in GenBank yielded a similarity of 94% with Cosmocercoides pulcher (Cosmocercidae: Cosmocercinae) (fig. 4).

Fig. 4. Maximum likelihood analysis of concatenated partial 18S rDNA and ITS1 sequences of the nematodes isolated from the small intestine of the smooth newt L. vulgaris and related species. Support values of maximum likelihood bootstrap analysis (1000 replicates) are given. Scale bar = substitutions per site.
Nematode species 3, identified as Cosmocerca longicauda (Linstow, 1885)
Morphological diagnosis
Four adult specimens of this nematode taxon resulted from a single newt individual. Three of them were inspected briefly before storing for molecular identification. The fourth specimen, examined morphologically, was C. longicauda (Cosmocercidae), exhibiting the features of a young male according to Grabda-Kazubska (Reference Grabda-Kazubska1974) (fig. 1f, g).
Unidentified nematode species 4
Morphological diagnosis
One nematode specimen, looking distinct from M. terdentatum and C. longicauda, was collected from the large intestine of a newt and identified briefly as a Cosmocercidae taxon before storing for molecular identification. To avoid any contamination of the sample, a thorough morphological examination of the specimen was avoided.
Molecular diagnosis
Using primers targeting 18S rDNA, a sequence for this individual specimen was obtained (table 3). The genetic similarity among this taxon and the identified Cosmocercoidea species M. terdentatum and C. longicauda was 70–72% and 85%, respectively. The closest match to sequences deposited in GenBank was again C. pulcher (Cosmocercidae: Cosmocercinae) with a similarity of 95% (fig. 4).
Discussion
Studying an amphibian's helminth community is a non-trivial taxonomic task because all morphological features of specimens collected should be linked to molecular data to confirm the identification, especially in areas where the avifauna is widespread and diverse (Patrelle et al., Reference Patrelle, Portier, Jouet, Delorme and Ferté2015). This study provides the DNA sequences for five parasitic helminth species infesting smooth newts, from which four can be linked to unequivocally identified specimens. Therefore the number of newt parasites with a morphology–sequence link increased from six to seven trematode species (P. robusta added) and from three to five nematode species (C. longicauda and M. terdentatum added). Moreover, morphologically indistinct digenean metacercariae and larval nematode stages collected in surveys of naturally infected newts were linked with adults of P. robusta and of M. terdentatum. Our data contribute significantly to the building of a molecular library, for a better understanding of life cycles and host specificity of helminths infesting the widely distributed host, L. vulgaris.
Parastrigea robusta
Digenean cysts found in most newt individuals are difficult to associate with a distinct species because P. robusta, S. sphaerula and S. strigis are found to infest L. vulgaris in the wild, and Holostephanus volgensis in experimental trials (Vojtková, Reference Vojtková1966; Vojtková & Vojtek, Reference Vojtková and Vojtek1972; Shimalov et al., Reference Shimalov, Shimalov and Shimalov2001). As the morphological features of strigeid metacercaria cysts in amphibian hosts are not reliably distinctive at the species level (Nieawiadomska, Reference Niewiadomska1970), they are often lumped together to a single larval genus Tetracotyle (e.g. Vojtková, Reference Vojtková1963a, Reference Vojtkováb; Vojtková & Roca, Reference Vojtková and Roca1994). Morphometric features of the cysts seem to distinguish among potential taxa infesting L. vulgaris (Odening, Reference Odening1965, Reference Odening1967; Vojtková, Reference Vojtková1966), but variability within and among host species is not fully explored, leading to a considerable uncertainty. Using the molecular markers of morphologically identified adult parasites from the definitive host, the mallard (A. platyrhynchos), the metacercaria cysts of newts were confirmed as P. robusta cysts.
Commonly, the first intermediate host species of P. robusta in the wild are the planorbid snails Anisus vortex, Gyraulus albus, Planorbis planorbis and Segmentina nitida (Odening, Reference Odening1965; Vojtek, Reference Vojtek1989), none of which has been recorded until present in the breeding ponds of this surveyed newt population (Sinsch & Breuer, Reference Sinsch and Breuer2018). Ongoing molecular research will reveal whether or not the locally common Gyraulus parvus is a new first intermediate host species in the local P. robusta life cycle. Second intermediate host species in the wild are the newts Triturus cristatus and L. vulgaris (both present at the study site) and the brown frogs Rana arvalis and R. temporaria (Odening, Reference Odening1965; Vojtková & Vojtek, Reference Vojtková and Vojtek1972; Vojtek, Reference Vojtek1989). Unlike T. cristatus in the Czech Republic, none of the crested newts reproducing in pond 1 was infested by P. robusta metacercariae (Sinsch, unpubl. obs. (2017) on ten individuals), whereas 73% of the smooth newts were infested. The observed infestation rate contrasts sharply with only 2.5% in R. arvalis (Zhigileva & Kirina, Reference Zhigileva and Kirina2015) and the absence of P. robusta in the brown frogs of the Berlin and Cologne regions (Spieler, Reference Spieler1990; Andreas, Reference Andreas2007). Definitive hosts of P. robusta in the wild are A. platyrhynchos, Aythya ferina, A. fuligula and Tachybaptus ruficollis (Szidat, Reference Szidat1929; Bykhovskaya-Pavlovskaya, Reference Bykhovskaya-Pavlovskaya1962; Dubois, Reference Dubois1968; Sitko, Reference Sitko1969; Macko, Reference Macko1974; Storer, Reference Storer2000; Sitko et al., Reference Sitko, Faltýnková and Scholz2006). The type host A. platyrhynchos is common in our study area, whereas other potential hosts are absent. In conclusion, the local life-cycle variant of P. robusta in the ponds surveyed includes a not yet evidenced planorbid snail, to the best of our knowledge probably G. parvus, exclusively smooth newts as the host of metacercariae and mallards as the definitive host.
Oswaldocruzia filiformis
Four species of Oswaldocruzia nematodes of doubtful validity have been reported from the intestines of L. vulgaris: O. duboisi, O. filiformis, O. goezei and O. molgeta (Vojtková, Reference Vojtková1963a; Avery, Reference Avery1971; Ben Slimane et al., Reference Ben Slimane, Durette-Desset and Chabaud1993). Morphological variability of diagnostic features led to the proposal that the latter three are conspecific and should be referred to as O. filiformis (Moravec & Vojtková, Reference Moravec and Vojtková1975). Consequently, our study links the molecular identification of O. filiformis solely to morphologically identified O. molgeta, which is most probably a junior synonym of O. filiformis. We consider O. duboisi as erroneously assigned O. filiformis in a single L. vulgaris specimen of the collection of the Natural History Museum of Paris (Ben Slimane et al., Reference Ben Slimane, Durette-Desset and Chabaud1993). Oswaldocruzia goezei reported from smooth newts in Poland (Taranko-Tulecka, Reference Taranko-Tulecka1959) and the Czech Republic (Vojtková, Reference Vojtková1963a) was later assigned to O. filiformis (Moravec & Vojtková, Reference Moravec and Vojtková1975). In conclusion, O. filiformis remains the only confirmed and published Oswaldocruzia nematode infesting L. vulgaris as definitive host so far. The observed infestation rates of 5–6% in the studied newts are low compared with those of L. vulgaris in the Czech Republic (37.3%, Barus & Groschaft, Reference Barus and Groschaft1962; 16.6–75.8%, Vojtková, Reference Vojtková1963a) and in Greece (33%, Sattmann, Reference Sattmann1990).
Megalobatrachonema terdentatum
Despite being a commonly observed nematode in the intestine of many European amphibian species (Vojtková, Reference Vojtková1963a; Vojtková & Roca, Reference Vojtková and Roca1996), this is the first study to provide DNA sequences for M. terdentatum. Molecular markers proved to be particularly useful to identify the morphologically indistinct larval stages found moving through the body cavity of several of the newts examined (Petter & Chabaud, Reference Petter and Chabaud1971). The observed infestation rates of 25–29% in the studied newts are in agreement with those in L. vulgaris in the Czech Republic (16.6–31%, Vojtková, Reference Vojtková1963a).
Cosmocercid nematode species
Two distinct cosmocercid nematode species were found in the intestine of one and two newts, respectively. As the more abundant species was morphologically identified as C. longicauda, our molecular data deposited in GenBank represent the first link of a DNA sequence with this nematode species. In contrast, the other nematode sequence cannot be assigned to a species because the only specimen detected was not examined morphologically for species identification. Given that the specimen does not belong to a not yet recorded parasite of L. vulgaris, possible species identities are C. ornata (Shimalov et al., Reference Shimalov, Shimalov and Shimalov2001) or O. brevicaudatum (e.g. Barus & Groschaft, Reference Barus and Groschaft1962). The maximum likelihood tree (fig. 4) does not indicate a close relationship between C. longicauda and the unidentified nematode, so that it appears more likely to be O. brevicaudatum than C. ornata. Further collection of specimens will help to overcome this unresolved identity. The observed infestation rates (C. longicauda) of 2–5% in the studied newts are considerably lower compared to those of L. vulgaris in the Czech Republic (34.4%, Barus & Groschaft, Reference Barus and Groschaft1962). If the unidentified nematode was O. brevicaudatum, the observed infestation would be comparable to that found in the Czech Republic (2.2%, Barus & Groschaft, Reference Barus and Groschaft1962).
In conclusion, the parasitic helminth diversity using smooth newts as intermediate or definitive hosts is lower than previously perceived (28 instead of 31 species), including two Acanthocephala and Cestoda each, 14 Trematoda and ten Nematoda species.
Acknowledgements
We thank Jiljí Sitko for valuable comments on the host spectrum of Strigeidae.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals. Permits for the collection of newts were issued by the Struktur- und Genehmigungsbehörde Nord.