Introduction
The White-bellied Heron Ardea insignis, hereafter WBH, is classified as ‘Critically Endangered’ with an estimated global population of 50–249 mature individuals (BirdLife International 2018), and a relatively restricted range. However, recent counts can only account for approximately 60 individuals: 28 in Bhutan, 23 in Myanmar, eight in India and one in China (Price and Goodman Reference Price and Goodman2015). It is now extinct in Nepal and possibly extinct in Bangladesh (BirdLife International 2018). The causes of its population decline and extirpation are largely undocumented, but attributed to rapid loss and degradation of its natural habitat which is mostly caused by development projects such as hydropower construction and mining activities, illegal fishing, deforestation and human-induced forest fires across the range countries (Kushlan and Hafner Reference Kushlan and Hafner2000, BirdLife International 2018, Dema et al. Reference Dema, Towsey, Sherub, Sonam, Kinley, Truskinger, Brereton and Roe2020, WWF 2019).
The WBH is probably a specialist piscivore which spends its daylight hours along riverbanks comprised typically of sand and gravel bars, hunting for fish. The only evidence about the diet of the WBH was from a single stomach analysis that contained crayfish (Baker Reference Baker1926). Hancock and Kushlan's (Reference Hancock and Kushlan1984) examination of its bill morphology predicted that they feed on large-sized fishes, amphibians, small mammals, and reptiles, while RSPN (Reference Pradhan and Frederick2011) supposed that it might feed on many fish species.
The WBH breeds in a conifer Pinus roxburghii as well as broad-leaved trees (Acharja Reference Acharja2020, Khandu et al. Reference Khandu, Gale, Pradhan, Acharja and Bumrungsri2020a). Nesting takes place in the temperate forest within 400–1,430 m asl (Acharja Reference Acharja2020). Also, they have been reported in a wide variety of wetlands, lakes, marshes, and large or small rivers with sand and gravel bars (Choudhury Reference Choudhury2000, King et al. Reference King, Buck, Ferguson, Fisher, Goblet, Nickel and Suter2001, RSPN Reference Pradhan and Frederick2011). The paucity of ecological information such as diet and preferred feeding habitats has impeded the effective implementation of conservation actions (Price and Goodman Reference Price and Goodman2015, Heath Reference Heath2019). Given the current trend in prey depletion due to illegal fishing and aquatic habitat loss and degradation, an assessment of prey types is crucial for ensuring the availability and abundance of preferred prey in their feeding habitats, determining the characteristics of possible future artificial weirs for enhancing feeding habitats as well as providing baseline diet information for captive-bred animals.
We aimed to understand the relationship between the relative abundance of WBH in four different river microhabitat types relative to prey biomass. We predicted that WBH would prefer microhabitats with a varying stock of prey types and biomass, as seen in Egretta garzetta which showed a microhabitat preference based on prey composition (Wong et al. Reference Wong, Corlett, Young and Lee2000). We also investigated the types of prey species and sizes preferred by the WBH and factors affecting their foraging activity. We predicted that WBH would prefer prey types and sizes based on profitability and availability (Maccarone and Brzorad Reference Maccarone and Brzorad2002). We hypothesized that microhabitat type, season, time of the day and bird age would affect the foraging activity of the WBH.
Methods
Study area
Bhutan contains nearly 50% of the known WBH population and the greatest number of breeding pairs compared to other range countries. The study was carried out in two major river basins; Punatsangchhu (chhu = river) basin (27°7’23.55”N, 90°4’13.44”E) falling within the jurisdiction of Punakha, Wangduephodrang and Tsirang districts and Mangdechhu basin (27°9’47.88”N, 90°39’49.33”E) of Zhemgang district, in Bhutan (Figure 1). These are the two major habitats where more than 95% of Bhutan's known population of WBH occurs, yet both river basins have been subjected to four large hydropower projects and numerous small to large scale sand and gravel mining activities. From a single agrometeorological station located in Tsirang district about 30 km from the farthest transect, the total annual rainfall recorded was 1,359 mm, and the temperature ranged from 12°C to 21°C (NCHM 2017). Punatsangchhu basin is an important habitat for large numbers of migratory waterbirds such as Tadorna ferruginea and Phalacrocorax carbo as well as threatened and near-threatened species such as Aythya nyroca and Haliaeetus leucoryphus. Tetrameles nudiflora and Syzygium spp. dominate the riverine forest vegetation found at an elevations of < 370 m along the Punatsangchhu (Ghemiray Reference Ghemiray2016). Mangdechhu basin falls within Zhemgang district which contains the main habitat of the WBH selected for this study. From a single agrometeorological station located in Zhemgang district near the center of the sampling transect, total annual rainfall was 1,438 mm, and the temperature ranged from 14°C to 21°C (NCHM 2017). It supported 10 globally threatened bird species including Tragopan blythii, Aceros nipalensis and Spelaeornis caudatus (Dorji Reference Dorji2011). Mixed broadleaved and conifer forest with Daubanga grandiflora, Syzygium formusa and Pinus roxburghii as dominant species are found along this basin (Tshering Reference Tshering2016).

Figure 1. Location of the Punatsangchhu (left) and Mangdechhu (right) basins, Bhutan.
Foraging activity
We conducted surveys for ~ 300 days (25 days/month) from 25 February 2018 until 10 January 2019 to locate and observe WBH. An area count survey (Kushlan Reference Kushlan2011) with a systematic approach (Dorge et al. Reference Dorge, Högstedt and Lislevand2014, Fu et al. Reference Fu, Chen, Dowell and Zhang2016) was employed to locate the focal species. With the help of two to four experienced local birdwatchers, we scanned both sides of small and large rivers where the WBH was known to forage, between 06h00 and 17h00. Riverbanks and roads running parallel to rivers were used as transects, covering an average of 4 km along riverbanks on foot and 30 km along roads by vehicle per day. Continuous focal animal sampling was chosen to observe the foraging activity due to its suitability for the study and minimal bias (Altmann Reference Altmann1974, Rose Reference Rose1999). Only 2-hr observations were videoed per individual per day due to limited battery power. After spotting a bird, observations were made from a hide which was ≥ 100 m from the focal individual considering the wariness of the bird. Binoculars (10 x 42) and a 20–60x monocular spotting scope were used to observe the birds. Foraging bouts were recorded on a prepared worksheet and simultaneously filmed using a 500-mm zoom lens with a 2x converter for future reference and re-evaluation of the data. All observations were made under favourable weather conditions (no rain or strong wind).
The independent variables collected included microhabitat type, season, time of the day and age of the bird. Adapting the habitat classification from Fasola (Reference Fasola1994) and Campos and Lekuona (Reference Campos and Lekuona2001), we grouped the foraging habitat into four microhabitats (pool, pond, riffle, and run; Figure 2). A pool was defined as shallow to deep water (0.5–1 m) with a smooth surface and low average velocity of ~ 0.15 mps. Rate of flow or velocity was determined through a float method following Michaud and Wierenga (Reference Michaud and Wierenga2005). A pond was defined as a naturally formed temporary water body along the riverbank which was not connected directly to the main river. The water was mostly static and relatively shallow (< 0.5 m). Riffles were defined as shallow water usually consisting of multiple channels with depth (0.2–0.5 m) and moderate average velocity (0.25 mps) agitated by rocks. Runs were defined as faster running water with higher average velocity (0.34 mps) and greater depth (> 1 m) typically at the center of main river channels. We grouped our observations into four seasons prevalent in Bhutan: winter (December–February), spring (March–May), summer (June–August) and autumn (September–November). Time of day was divided into three periods: morning (06h00–10h00), midday (10h01–14h00), and afternoon (14h01–16h00). WBH age was grouped into adults and juveniles. Adult WBH have more extended lace-like plumes on the nape than the juveniles (BirdLife International 2018). The juveniles have shorter white scapulars than adults. The adults have a white underbelly while juveniles have a brownish underbelly. Sex determination was not possible to infer in the field due to an innate lack of dimorphism.

Figure 2. Four microhabitat types used by White-bellied Herons: (a) A riffle with two herons (circled) actively foraging, (b) A pool with a heron grasping a fish (Schizothorax richardsonii), (c) A pond with a heron's head bent forward and (d) A run with a heron standing and waiting for prey.
Microhabitat use and biomass catch per unit effort (CPUE)
The foraging habitat use was determined by direct observation of the WBH in each microhabitat from our daily surveys. Thus, the relative abundance of the WBH in each microhabitat was estimated by dividing the number of observations of WBH in each microhabitat by the total observations of WBH in all microhabitats (Jing et al. Reference Jing, Ma, Li, Li and Chen2007, Gyimesi et al. Reference Gyimesi, Franken, Feige and Nolet2012). The CPUE (g/h) of each species of fish caught during the sampling period was calculated by dividing the total catch in biomass (g), by total sampling effort (h) (Ghosh and Biswas Reference Ghosh and Biswas2017).
Prey availability
Fish prey sampling was carried out in both Mangdechhu and Punatsangchhu basins. Thirteen different 100-m thalweg lengths approximately 500 m from where the WBH was sighted foraging were randomly selected for fish sampling, following the methods of Arunachalam (Reference Arunachalam2000) and Johnson and Arunachalam (Reference Johnson and Arunachalam2009). Fish sampling was carried out on 18 different days with the help of local fishermen and field assistants. Cast nets (10 and 20 mm mesh) and electrofishing gear (6 V) were used in different microhabitats to reduce bias arising due to the type of gear used (Ghosh and Biswas Reference Ghosh and Biswas2017). Cast nets and electrofishing gear were employed for a total of 18 days (5 hrs/day on average) equally in the four microhabitats as much as possible. Fish sampling was carried out when the herons were not using the immediate area to minimize human disturbance. The caught fish were counted, measured (total length, girth, and weight) and released after two hours to prevent double counting (Dorji Reference Dorji2016). Fish species identification was carried out with the help of the published checklist of fishes from the local regions (Gurung et al. Reference Gurung, Dorji, Tshering and Wangyal2013, NRCRLF Reference Tshering, Wangchuk, Dorji, Norbu and Philipp2017) and photos were taken to further validate their identification with the help of an experienced ichthyologist.
Based on our field observations, WBH exhibit foraging behaviours similar to other herons. Stand and wait is a common behaviour where the bird stands at one location typically lasting more than five minutes with its neck either retracted or fully erect. Walking slowly or stalking is also exhibited frequently by the WBH while scanning for potential prey. Hopping or foot paddling are seldom employed. WBH catch prey through both impaling as well as grasp capture. To understand the foraging activity patterns and efficiency of WBH, five variables were collected: pacing rate, striking rate, capture rate, success rate, and intake rate. For foraging details, the number of steps/min was defined as the pacing rate. The striking rate was calculated as the total number of strikes divided by total observed duration, whereas the capture rate was calculated by using the total number of captures divided by total duration of the observations. The number of prey captured per strike was defined as the success rate. Intake rate was calculated as the prey biomass consumed by the bird divided by the total observation time. A feeding attempt made to capture prey using a deliberate forward movement of the head was defined as a strike.
A volume index was adopted to calculate the relative biomass of the ingested prey by squaring the length of the prey (Sato and Maruyama Reference Sato and Maruyama1996) since the body shape was roughly the same for almost all the fish species. Prey size was estimated in relation to the average length of the bird’s bill (16.4–19 cm) (HeronConservation 2019, RSPN 2020) and grouped into three classes small (< 10 cm), medium (10–20 cm), and large (> 20 cm), (Campos and Lekuona Reference Campos and Lekuona1997).
Statistical Analyses
We analysed data for normality using the Shapiro-Wilk tests and found that none of the variables were normally distributed. Therefore, we chose nonparametric statistics to analyse our data. Kruskal-Wallis tests were used to analyse the feeding activity variables against their factors (microhabitat, season, and time of the day) and relative abundance with the microhabitat types. Dunn’s test (with Bonferroni correction) was applied as a post-hoc test to make comparisons among the groups. For microhabitat, season and time of day, the average (± SE) values were reported following Choi and Yoo (Reference Choi and Yoo2011). Spearman’s correlation test was used to test for correlations between the relative abundance of WBH and prey biomass CPUE. Mann-Whitney U tests were used to analyse foraging activity differences between the adult and juvenile WBH. For the analysis of differences between age groups, values were reported as medians and the first and third quartiles (hereafter Q1–Q3) following Jakubas and Manikowska (Reference Jakubas and Manikowska2011). All analyses were performed using R software version 3.5.2 (R Development Core Team 2018).
Results
Feeding microhabitats
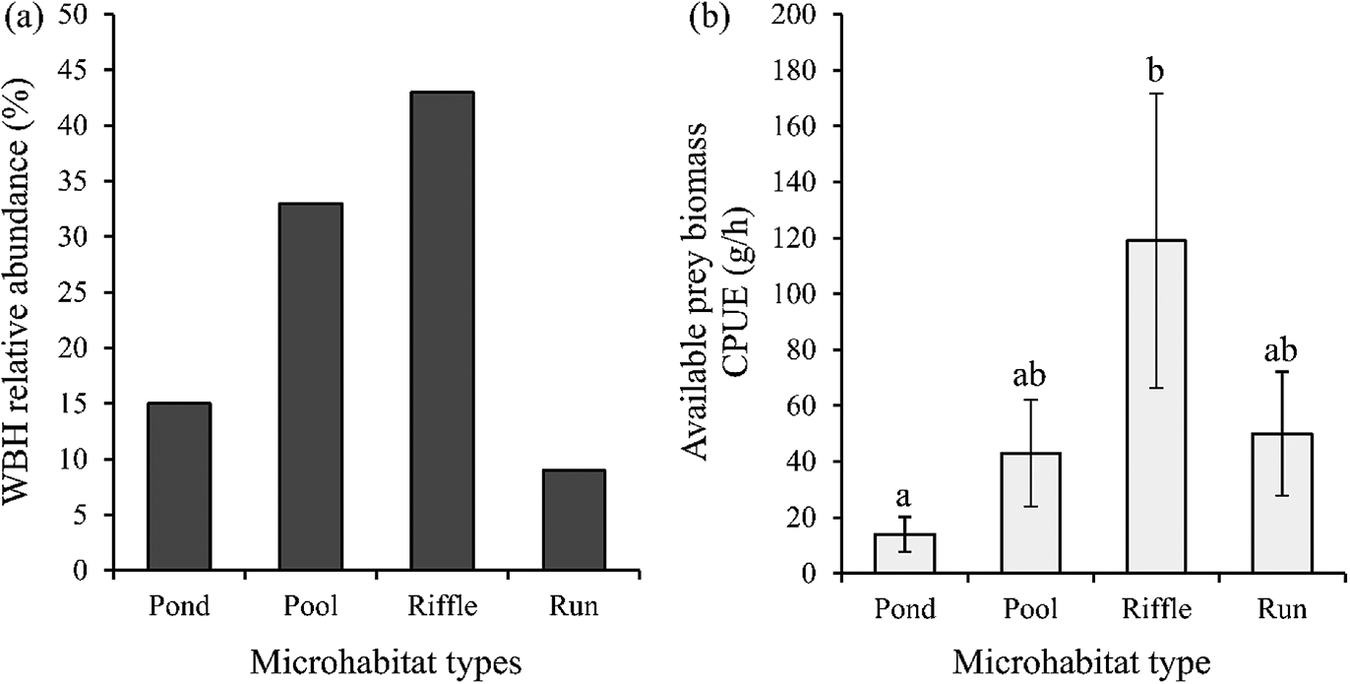
A total of 3,777 min of active foraging was observed. Each feeding bout lasted for 5–58 min. We recorded the presence of 42 runs, 42 pools, 34 riffles and 20 ponds of available microhabitat. The available microhabitats were located independently from each other although along the same longitudinal path of the river as the transects. Riffles were used most commonly with 80 feeding observations, followed by pools with 62 feeding observations. Twenty-eight feeding observations were made in ponds and only 17 in the runs. The relative habitat use of the WBH was determined from a total of 187 feeding observations completed in four microhabitats (Figure 3a). The highest relative abundance was observed in riffles (43%) followed by the pools (33%), followed by ponds (15%) and runs (9%). There was a significant difference in the use of microhabitats between pools and ponds ( χ2 = 253, df = 3, P < 0.001), between riffles and ponds (χ2 = 253, df = 3, P < 0.001), riffles and pools (χ2 = 253, df = 3, P < 0.001), runs and riffles (χ2 = 253, df = 3, P < 0.001) and between runs and pools (χ2 = 253, df = 3, P < 0.001).

Figure 3. (a) Relative abundance of White-bellied Herons in four types of microhabitats. Values are given as a percentage. (b) Available prey biomass catch per unit effort (CPUE) (grams/hour) in the four microhabitats based on sampling with cast nets and electrofishing gear. Values are given as means (±SE). Significant differences among variables are labeled with different letters above the bars based on a Kruskal-Wallis test at P < 0.05.
Microhabitat type was associated with significantly different pacing rates and intake rates of the WBH, but not the other measures of foraging behaviour (Table 1). There was a significantly higher pacing rate of the WBH in the pools compared to the runs (χ2 = 9.52, df = 3, P < 0.05). For biomass intake rate, there was a significantly higher rate in the riffles compared to the ponds (χ2 = 9.84, df = 3, P < 0.05). Although no statistically significant difference was observed among microhabitats for success rate (χ2 = 4.56, df = 3, P = 0.2), it tended to be higher in the riffles, pools and runs compared to the ponds (Table 1).
Table 1. Effect of microhabitat types on the foraging activities of White-bellied Herons expressed as means (±SE). Superscripts with the same letter indicates no significant difference using Dunn’s post-hoc test with Bonferroni correction (P > 0.05). Numbers in the parenthesis are sample sizes of the behaviors in the given habitat type, NS, not significant; Kruskal-Wallis test results for the effect of microhabitat on the given foraging behavior are listed at the bottom of each column.

Prey availability
At least 12 species of fish belonging to three families were identified in this study (Table 2). The family Cyprinidae was dominant in the heron’s foraging habitats. Although Neolissochilus hexagonolepsis was the most commonly sampled fish species, the mean CPUE was relatively low (31 ± 8 g/h). Whereas the mean CPUE for Salmo trutta (407 ± 166 g/h) and Labeo pangusia (287 ± 49 g/h) were among the highest, their frequencies of capture were very low (six and seven). Schizothorax richardsonii was one of the most commonly sampled fishes with a relatively high CPUE (149 ± 50 g/h).
Table 2. Summary of the available fish species sampled from the three rivers where the White-bellied Herons were found foraging most frequently. Catch per unit effort (CPUE) (grams/hour) values are given as means (±SE).

River codes: PC Punatsangchhu, MC Mangdechhu.
A significant difference in the mean available prey biomass catch per unit effort (CPUE) was observed between the microhabitat types (Figure 3b). The highest biomass CPUE was observed in riffles (118 ± 5 g/h) while the lowest was found in the ponds and the difference was statistically significant (14 ± 5 g/h) (χ2 = 12.50, df = 3, P < 0.01). No other significant differences were found among microhabitats in relation to CPUE. A weak, but significant positive correlation was observed between the available biomass CPUE and WBH relative abundance (rs = 0.22, P < 0.01).
Diet composition
From the 97 prey items caught by WBH, 95% were fish, of which 71% could be identified to the genus level (Table 3). The remainder (5%) could not be identified because the species was too small or the observer too far away to allow identification. The most commonly consumed food by the WBH belonged to the genus Schizothorax (64%). Snow trout Schizothorax richardsonii constituted 28% of the observed prey, while unidentified Schizothorax spp. accounted for 36% of the total diet. Because of the prey’s often distinctive morphology, observations made in proximity (≤ 100 m) often enabled us to make identification at the species level. Fish belonging to genus Garra contributed only 3% of the total consumed fish. Schizothorax spp. were the only fish prey exploited during all months of the year by the WBH.
Table 3. Summary of monthly observations of the prey species consumed by White-bellied Herons.

Season
The foraging activity of the WBH differed significantly among the seasons (Table 4). There was a significantly higher pacing rate in autumn than the spring (χ2 = 7.9, df = 3, P < 0.05), while no significant differences were observed among the other seasons. The striking rate was significantly higher during the spring than the others (χ2 = 37.9, df = 3, P < 0.001). However, the capture rate was significantly higher during the summer than the spring (χ2 = 10.1, df = 3, P < 0.05). The success rate did not differ between the seasons (χ2 = 5.4, df = 3, P = 0.14) and there was no significant difference in biomass intake rate across all seasons (χ2 = 5.9, df = 3, P = 0.11) (Table 4).
Table 4. Differences between seasons in the foraging activity of White-bellied Heron expressed as means (±SE). Superscripts with the same letters indicates no significant difference using Dunn’s post-hoc test with Bonferroni correction (P > 0.05). Numbers in the parenthesis are sample sizes for the birds observed in each season, NS, indicates not significant. Kruskal-Wallis test results for the effect of seasons on the given foraging behavior are listed at the bottom of each column.

Time of day
The foraging activity of the WBH differed with time of day (Table 5). The capture rate was significantly higher in the morning compared to midday periods (χ2 = 12.05, df = 2, P < 0.01), with WBH capturing five times more fish in the morning than midday. There was no significant differences in capture rates observed among other times. The success rate was significantly higher in the morning compared to midday (at least three times more efficient in catching fish) (χ2 = 13.53, df = 2, P < 0.001). Therefore, the intake rate was significantly higher during the morning than the midday period (χ2 = 9.64, df = 2, P < 0.01). Consequently, the biomass consumption per unit time was more than two-fold higher in the morning than the midday period. No other differences were observed in the biomass consumption. There was no significant difference in pacing (χ2 = 0.3, df = 2, P = 0.86) and striking rates (χ2 = 2.6, df = 2, P = 0.27) with the time of the day.
Table 5. Effect of time of day on the foraging activity of the White-bellied Herons expressed as means (± SE). Superscripts with the same letter indicates no significant difference after Dunn's post-hoc test with Bonferroni correction (P > 0.05). Numbers in the parenthesis are sample sizes, NS, not significant. Kruskal-Wallis test results for the effect of time of day on the given foraging behavior are listed at the bottom of each column.

Age
A total of 163 feeding bouts for adults and 24 for juvenile WBH were analysed to compare their feeding activities. There was no statistically significant difference between the pacing rates (Mann-Whitney U test, Z = 0.5, P = 0.65) of adults (median = 23 steps/min, Q1–Q3 = 0–35) and juveniles (median 22 steps/min, Q1–Q3 = 0–37) (Figure 4a). There was a statistically significant difference in the striking rate (Mann-Whitney U test, Z = –3.4, P < 0.001) between adults (median = 0.13 strikes/min, Q1–Q3 = 0.04–0.43) and juveniles (median = 0.6 strikes/min, Q1–Q3 = 0.3–1.2) (Figure 4b), with the striking rate of juvenile herons being four times higher than the adults. However, the capture rate (Mann-Whitney U test, Z = 1.7, P = 0.24) did not differ between adults (median = 0.04 captures/min, Q1–Q3 = 0–0.07) and juveniles WBH (median = 0 captures/min, Q1–Q3 = 0–0.06) (Figure 4c). The success rate (Mann-Whitney U test, Z = 2.6, P < 0.05) was significantly higher for the adults (median = 0.1 success/strike, Q1–Q3 = 0–1) compared to the juveniles (median = 0 success/strike, Q1–Q3 = 0–0.05) with the adults acquiring more than thrice as many fish as juveniles (Fig 4d). Consequently, the biomass intake rate of adults (median = 2 g/min, Q1–Q3 = 0–20) was significantly higher (Mann-Whitney U test, Z = 0.5, P < 0.05) than juveniles (median = 0 g/min, Q1–Q3 = 0–2.5) (Figure 4e).

Figure 4. Effect of age on the foraging activities of the White-bellied Herons expressed as medians (Q1–Q3). Significant differences among variables are labeled with different letters based on Mann-Whitney tests at P < 0.05.
Discussion
Overall, we found some notable differences in the use of the four principal microhabitats by WBH as well some clear differences regarding season, time of day, and age. The higher relative abundance of the WBH in riffles and pools may be attributed to a number of factors, but primarily higher prey biomass CPUE in these microhabitats as well as the likelihood of foraging success. The prey availability, distribution, and abundance influence the microhabitat use and selection in wading birds including most ardeid species (Kersten et al. Reference Kersten, Britton, Dugan and Hafner1991). The higher capture and success rates in riffles and pools suggests that these are likely the most suitable microhabitats for the WBH. Riffles were used more commonly by the WBH than other microhabitats despite the lower availability of these habitats compared to runs and pools. The shallow water in the riffles may have also increased prey visibility and vulnerability (Gawlik Reference Gawlik2002). Similar studies carried out on other waders found that most wading birds showed microhabitat use and selection in relation to water levels (Bancroft et al. Reference Bancroft, Gawlik and Rutchey2002, Maccarone and Brzorad Reference Maccarone and Brzorad2005, Baschuk et al. Reference Baschuk, Koper, Wrubleski and Goldsborough2012, Renken et al. Reference Renken, Thompson and Maccarone2016). Overall, the WBH has relatively long legs with tibio-tarsus measuring 25–30 cm and bill (18–20 cm) (RSPN 2020) which probably makes the bird a versatile forager in habitats with varying water depths (Grant Reference Grant1968, Baker Reference Baker1979).
The WBH in the pools, riffles and the ponds had higher pacing rates compared to the runs probably because these habitats were mostly surrounded by open areas of sand and gravel bars which likely enabled them to scan through more foraging points in a relatively shorter period of time compared to the runs. However, the WBH in runs were found standing on rocks while waiting for prey to appear. Their movements were also limited to the exposed surface area of the rocks. Thus, the WBH took more time to catch fish in runs when compared to other microhabitats based on the comparatively lower observed capture rates. Overall, although herons in the pools had the highest pacing rates and tended to have the higher intake rates, there was not a clear relationship between pacing rate and intake rate as in other studies (Papakostas et al. Reference Papakostas, Kazantzidis, Goutner and Charalombidou2005).
The genus Schizothorax was the most important prey item in the diet of the WBH; it was the dominant prey consumed in all the seasons of the year, indicating that Schizothorax spp. were available to the WBH year-round. RSPN (Reference Pradhan and Frederick2011) carried out prey sampling using cast nets and suggested that snow trout Schizothorax richardsonii and brown trout Salmo trutta were the only two species large enough to be captured by the WBH. During our prey sampling we also recorded larger sized fish most commonly from the genus Schizothorax, thus the size of the fish and their availability likely explain the dominance of Schizothorax spp. in the diet. The dominance of these species in the WBH diet, suggests that this might be a target for restocking, if it is needed (see below) and also an important food source for captive-bred birds.
Pradhan et al. (Reference Pradhan, Norbu and Frederick2007) showed that nesting took place in March to early June, while the summer (June–August) and autumn (September–November) seasons coincide with feeding of fledglings (RSPN Reference Pradhan and Frederick2011, Acharja Reference Acharja2020) when the parents need to provide food to the juveniles and themselves, as seen with other ardeids (Fasola Reference Fasola1984, Moreno Reference Moreno1984, McGuire Reference McGuire1986, Martin Reference Martin1987). We observed that adults continue to feed the juveniles that had fledged perhaps until as late as October. This may be the reason for their higher biomass intake rates during the summer and autumn seasons compared to winter and spring. In contrast, our field observations indicated that WBH mostly avoided the swollen and muddy mainstream river and foraged in pools and temporary ponds near the riverbanks which might have resulted in lower success rates in the summer.
While there is no concrete evidence of predation on adult WBH, there are likely several competitors. Great Cormorants Phalacrocorax carbo are possibly the main competitors during the winter-spring seasons when large migrant flocks of these birds are present in WBH habitats. Otters are also perhaps one of the principal mammalian competitors throughout the season. However, the type of interaction between these species and the WBH is yet to be ascertained.
WBH, like many ardeids had varying feeding intensity in relation to time of day (Lo and Fordham Reference Lo and Fordham1986) with foraging peaks during the morning and afternoon, and reduced feeding around midday (Kushlan Reference Kushlan1976). This study found that WBH was more efficient in foraging during the morning and afternoon with comparatively higher capture and success rates relative to midday hours, although some other ardeids have shown no significant difference in their foraging efficiency in relation to time of day (Papakostas et al. Reference Papakostas, Kazantzidis, Goutner and Charalombidou2005, Choi and Yoo Reference Choi and Yoo2011). The glare of the sunlight may have impeded their foraging success during the midday as is the case with birds that hunt for food over water (Krebs and Partridge Reference Krebs and Partridge1973). WBH was also observed to avoid the hotter midday hours by shading itself on a nearby perch, thus reducing their foraging time.
Juvenile WBH chiefly foraged in pond and pool microhabitats. While they were occasionally seen exploring riffles, they were never seen using the run microhabitat in the entire study period. This suggests that either juveniles were inefficient in exploiting the fish from the runs or habitat conditions were not favourable for the less experienced juveniles. It is likely that herons may adopt varying foraging techniques to maximize their foraging efficiency with regard to habitat conditions and prey characteristics (Dimalexis et al. Reference Dimalexis, Pyrovetsi and Sgardelis1997, Gwiazda and Amirowicz Reference Gwiazda and Amirowicz2006). Although the striking rate was significantly higher in juvenile WBH than the adults, capture, success, and biomass intake rates were comparatively lower in juveniles. This suggests that juvenile herons are less efficient foragers than adults, likely due to less experience in catching and handling prey (Draulans and Van Vessem Reference Draulans and Van Vessem1985, Marchetti and Price Reference Marchetti and Price1989) which is also associated with less developed sensorimotor faculties (Cezilly and Boy Reference Cezilly and Boy1988).
Overall, our results imply that WBH exhibit both habitat and food specialist foraging behaviour in contrast to the generalist feeding behaviours of other species in this genus. For instance, Purple Heron Ardea purpurea, which is the most closely related species to the WBH (Xi et al. Reference Xi, Bin and Xu2018, Klinsawat et al. Reference Klinsawat, Khandu, Bumrungsri, Sukmak, Chaichanathong, Kaolim and Wajjwalku2019) was found using agricultural lands and rivers for foraging and their diet comprised numerous prey species including insects, reptiles, amphibians and crustaceans (Campos and Lekuona Reference Campos and Lekuona1997, Reference Campos and Lekuona2001). Hancock and Kushlan (Reference Hancock and Kushlan1984) assumed that besides fish, WBH might also consume amphibians, reptiles, or small mammals. However, WBH were observed to forage only in freshwater bodies and their sole diet is probably fish in these basins. Therefore, it is particularly important to protect their existing riverine habitats from further degradation and loss and enhance their food resources to increase the carrying capacity of the current habitats of WBH for their long-term survival. Thus, it is also reasonable to assess whether supplementary diet provisioning through the creation of few artificial weirs and ponds restocked with the native fish species would be beneficial to WBH and their river ecosystems.
Apart from dams and exposed power lines, we have also encountered numerous locations where dredging of sand and gravel from the riverbanks is carried out for commercial as well household consumption in our study area. Uncontrolled sand and gravel mining in the river systems is a serious threat not only to globally threatened species (Menzies et al. Reference Menzies, Rao and Naniwadekar2020) but also native fish and biotic communities (Kanehl and Lyons Reference Kanehl and Lyons1992, Koehnken et al. Reference Koehnken, Rintoul, Goichot, Tickner, Loftus and Acreman2020). Also, construction of access roads and storage sites for sand mining leads to further fragmentation and loss of the riverine vegetation (Kondolf et al. Reference Kondolf, Piégay and Landon2007, Kumar and Kumar, Reference Kumar and Kumar2014), which is crucial for the nesting and roosting of WBH (Khandu et al. Reference Khandu, Gale, Pradhan, Acharja and Bumrungsri2020a, Reference Khandu, Gale, Kinley, Tandin, Shimano and Bumrungsri2020b). Thus, it is imperative that the daily operation of the sand and gravel mining activities is monitored on a regular basis by the relevant agencies and proper restorative and mitigation measures are put in place (Kondolf et al. Reference Kondolf, Piégay and Landon2007). Crushed stone sand should be encouraged for use as an alternative to dredged sand from the rivers. In our study sites, we have also recorded numerous instances of illegal fishing using a variety of gear including cast nets, hook-and-line, gillnets and snare traps which are likely depleting fish communities (Österblom and Bodin Reference Österblom and Bodin2012, Agnew et al. Reference Agnew, Pearce, Pramod, Peatman, Watson, Beddington and Pitcher2009, Beddington et al. Reference Beddington, Agnew and Clark2007) and potentially endangering WBH near these fishing sites. Therefore, better surveillance of illegal fishing with occasional night patrolling by the foresters, and suspension of permitted fish harvesting, especially during their breeding seasons in core habitats (Puntsangchhu and Mangdechhu) is warranted. Establishing fishponds for interested members of the community is supported by the Royal Society for the Protection of Nature (RSPN) to curb illegal fishing and habitat disturbance. Simultaneously, periodic community awareness and social networking especially targeting local fishermen should be carried out by appropriate NGOs and agencies to enhance compliance with regulations (Scholz and Wang Reference Scholz and Wang2006) and to help shift the local people’s attitudes towards conservation interventions (Williams et al. Reference Williams, Child, Dicks, Ockendon, Pople, Showler, Sutherland, Dicks, Ockendon, Petrovan and Smith2019).
Through our personal observations, we also noticed that WBH tend to explore small streams (25–35 m wet width) near (mean distance 74 m) their nest sites (Acharja Reference Acharja2020), especially during the summer seasons when the monsoonal rain floods their usual foraging habitat. These small streams also need protection as they provide valuable foraging habitat when the foraging conditions in their usual habitats along the larger rivers become unfavourable.
Conclusion
Further study is required to understand how the WBH responds to seasonal changes in river microhabitat parameters such as velocity, turbidity, and temperature caused by monsoonal dynamics as well as human disturbance. This study could not account for the seasonal variations and abundance of its prey species, which might affect their daily foraging activity and success. It is also vital that the fish abundance and density in the WBH habitats are monitored periodically to ensure that the WBH are not deprived of their daily dietary needs. A thorough study is also warranted in Punatsangchhu and Mangdechhu basins to assess the direct impacts of dams and sand and gravel mining to WBH and the fish community so that appropriate mitigation measures can be proposed and implemented.
Acknowledgements
This research was made possible through grant support by 29th Overseas Grant Fund of Pro Natura Foundation (Japan), the Rufford Foundation (grant number 26102-1), Royal Society for the Protection of Nature (Bhutan), and a Graduate School thesis grant of Prince of Songkla University. Thailand’s Education Hub Scholarship for ASEAN Countries (THE-AC) also supported PK. We extend our gratitude to Dr. Kitichate Sridith, Dr. Satoshi Shimano, Ugyen Dorji and lab mates of Small Mammals, Birds and Spiders Research Unit of PSU for their guidance and support. Thanks, are also due to Sonam Dorji, Chencho Bidha, Sonam Tshering, Indra P. Acharja, Tenzin, Thinley Dorji, Kinley, Chimi Dorji, and Phurpa for assisting in the field. We would also like to thank Ugyen Wangchuck Institute for Conservation and Environmental Research for according research permission and support. Our immense gratitude goes to Dr Tim Dodman (Associate Editor) and two anonymous reviewers for their thoughtful comments in further improving this manuscript.