INTRODUCTION
Infections involving the capsalid monogenean, Neobenedenia girellae (Monogenea: Monopisthocotylea: Capsalidae) (Hargis, Reference Hargis1955; Yamaguchi, Reference Yamaguchi1963), a parasite of seawater fishes, has become a serious problem in cultured and aquarium fish species in Japan, such as Japanese flounder (Paralichthys olivaceus), red seabream (Pagrus major), yellowtail (Seriola quinqueradiata) and tiger puffer (Takifugu rubripes) (Ogawa et al. Reference Ogawa, Bondad-Reantaso, Fukudome and Wakabayashi1995). Heavily infected fish may stop eating and/or swim erratically, and their body colour darkens with concomitant dermal ulceration with haemorrhage on the body surface, especially around the mouth and eyes. Among these N. girellae-susceptible species in Japan, Japanese flounder is thought to be the most susceptible (Ogawa, K: personal communication (1995)); by contrast, the spotted halibut (Verasper variegatus) is comparatively much less susceptible (our unpublished observation) despite the fact that these two species have similar physical characteristics and have similar ecological niches. These halibut and flounder species are flatfish that inhabit sandy/muddy bottoms in coastal waters of Japan.
The infection cycle of N. girellae has been described by Bondad-Reantaso et al. (Reference Bondad-Reantaso, Ogawa, Fukudome and Wakabayashi1995). The free-living ciliate oncomiracidia emerge from eggs at 5–6 days after laying at 25°C; they attach/grow on the fin and the skin surface of host fish, where they require 10–11 days to reach sexual maturity. These parasites cause haemorrhage and inflammation in host fish, which then die from secondary bacterial infection.
In our previous study, we demonstrated that N. girellae larvae express a surface protein referred to as immobilization/agglutination antigen, which caused fish to produce immunoglobulins (Ig) that, when injected into Japanese flounder, immobilize parasites in vitro (Hatanaka et al. Reference Hatanaka, Umeda, Yamashita and Hirazawa2005). By immunological analyses, this immobilization/agglutination antigen was localized to the surface of cilia of N. girellae. A predominant 8 kDa glycoprotein of surface cilia is thought to be a plausible candidate for the immobilization/agglutination antigen (Hatanaka et al. Reference Hatanaka, Umeda, Yamashita and Hirazawa2005). Although it is not yet clear whether fish antibodies against this antigen prevent infection by N. girellae, it has been established that this antigen is recognized by the fish immune system and thus may be useful for vaccination.
Lectins are proteins that recognize and bind to specific carbohydrate moieties of glycoproteins and glycolipids, and they are widely distributed in bacteria, plants and animals (Kilpatrick, Reference Kilpatrick2000). Studies on mammalian lectins and related proteins, collectins and ficolins, have shown that these proteins play important roles in innate immunity (Turner, Reference Turner1996). In some fish species, several lectins have been isolated from serum, cutaneous mucus and gill mucus, and subsequently characterized (Ewart et al. Reference Ewart, Johnson and Ross1999; Mistry et al. Reference Mistry, Honda and Hirose2001; Cammarata et al. Reference Cammarata, Vazzana, Chinnici and Parrinello2001; Tasumi et al. Reference Tasumi, Ohira, Kawazoe, Suetake, Suzuki and Aida2002, Reference Tsutsumi, Tasumi, Suetake and Suzuki2003). In the Japanese eel (Anguilla japonica), cutaneous mucus lectin AJL-2 has both haemagglutinating and E. coli-growth inhibiting activities. Mucus on the surface of fish is thought to be a biochemical barrier against pathogen attack. Although it is believed that anti-pathogenic factors and mechanisms exist in the cutaneous mucus and serum of fish, sound knowledge of fish lectins necessary to establish this biological defence mechanism is lacking.
In the present study, we confirmed that the spotted halibut is less susceptible to infection by N. girellae compared with Japanese flounder. We thus hypothesized that spotted halibut have an anti-parasitic factor(s) against N. girellae. We identified a strong agglutinin against N. girellae larvae in serum from spotted halibut. This agglutinin was found to comprise 2 types of lectin, termed VVL-α and VVL-β (VVLs), which were at a surprisingly high concentration (~2 mg/ml) in halibut serum. We purified these VVLs and determined sugar specificities of them, and demonstrated that they recognize a parasite ciliary surface glycoprotein of approximately 8 kDa, previously noted as a putative agglutination/immobilization antigen of N. girellae larvae (Turner, Reference Turner1996). We also showed that VVLs immobilize and agglutinate N. girellae larvae in vitro.
MATERIALS AND METHODS
Fish and parasite maintenance
Spotted halibut hatched in our laboratory and Japanese flounder obtained from a commercial seedling producer in Oita Pref. were maintained in a 500 litre polycarbonate tank. N. girellae were cultured in our laboratory as described previously (Umeda and Hirazawa, Reference Umeda and Hirazawa2004; Hirazawa et al. Reference Hirazawa, Mitsuboshi, Hirata and Shirasu2004). Larvae that had hatched within ±12 h of day 8 were used for the experiments.
Infection of host fish with N. girellae
Sixteen uninfected individuals each of spotted halibut and Japanese flounder (12·9±0·78 g and 10·6±0·34 g, respectively) were transferred to the same 100 litre tank. After acclimatization for 1 week, approximately 10 000 hatched N. girellae larvae were put into the tank while the seawater supply was discontinued for 1 h to complete the infection. The trial continued for 12 days after the exposure to parasites. At the end of the experiment, each fish was treated with freshwater to dislodge parasites from the host (Leong, Reference Leong1997), and the number of dislodged parasites was counted under a microscope. The length of both species of fish was measured to calculate the fish surface area (Kobayashi, Reference Kobayashi1980; Kimoto and Sato, Reference Kimoto and Sato2002).
Immobilization/agglutination assays
Ten fish (100–150 g) of spotted halibut and Japanese flounder were sampled, and peripheral blood samples were taken from the caudal vasculature. Serum was prepared from each blood sample, was heat inactivated at 56°C for 30 min, and was stored at −80°C until use. Immobilization assays were performed essentially according to the protocol of Clark et al. (Reference Clark, Dickerson and Findly1988). Seawater (990 μl) containing approximately 100 N. girellae larvae were added to each well of a 24-well plate. Fish serum was serially diluted with seawater (10 μl) and was added to each well. After incubation for 1 h at 25°C, the number of immobilized parasites was counted. under a microscope. The degree of parasite agglutination was calculated as: (number of agglutinated parasites)/(total number of parasites)×100%. Each fish serum was assayed in triplicate to calculate degrees of agglutination.
To determine the identity of specific sugar moieties on spotted halibut lectins, we tested the inhibition of immobilization activity upon addition of sugars, 2 mM and 10 mm: D-glucose, D-mannose, D-galactose, myo-inositol, N-acetyl-D-glucosamine (Glc-NAc) or N-acetyl-D-galactosamine (Gal-NAc).
Purification of lectins
Spotted halibut serum (10 ml) was thawed, and an equal volume of the saturated ammonium sulfate solution was added, followed by gentle stirring on ice for 1 h. After incubation, the solution was centrifuged at 10 000 g for 20 min. The pellet was resuspended in phosphate buffered saline containing 0·9 mm CaCl2 and 0·66 mm MgCl2 (PBS(+)) and dialysed against the same buffer. The solution was applied to a 1 ml column of D-glucose agarose (Sigma-Aldrich) pre-equilibrated with PBS(+). The column was washed with 20 bed volumes of PBS(+), and then the glucose-binding proteins were eluted by PBS(+) containing 200 mm D-glucose. The protein concentration was determined by the Bradford method using a protein assay kit (Bio-Rad) (Bradford, Reference Bradford1976). Bovine serum albumin (BSA) was used as the protein standard.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), deglycosylation and N-terminal amino acid sequencing
SDS-PAGE was performed on 4–20% or 15–25% gradient gels (Laemmli, Reference Laemmli1970). Gels were stained by either Coomassie brilliant blue (CBB) or periodic acid-Schiff (PAS) staining as reported by Zacharius et al. (Reference Zacharius, Zell, Morrison and Woodlock1969). To compare the predicted molecular weight from the cDNA and the observed Mr of the protein from SDS-PAGE analysis, deglycosylation of the lectins by N-glycanase, O-glycanase and sialidase A was carried out using the Enzyme Deglycosylation Kit (PROzyme).
Eluates containing approximately 20 μg of reduced protein were subjected to SDS-PAGE, and the protein bands were transferred to Pall fluoro trans W (PVDF) membranes (Nippon Genetics Co., Ltd) in a Trans-blot SD semi-dry transfer cell (Bio-Rad). Protein bands were visualized with CBB, and bands appearing at ~15 kDa and ~18 kDa were sequenced on a PPSQ-21 protein sequencer (Shimadzu Biotech).
Molecular cloning of cDNA of lectins (VVLs)
Total RNA was extracted from heart, kidney, liver, testis, ovary, spleen, gill, brain, skin, muscle, leukocytes and erythrocytes using the RNeasy Mini kit (Qiagen). First-strand cDNA was synthesized using a 5′/3′ rapid amplification of cDNA ends (RACE) kit (Roche Diagnostics) for 3′-RACE. Based on N-terminal amino acid sequencing, the degenerate primer, VVL-NP (5′-AAYTGYCCNATGTTYGGTT-3′) was synthesized. The first cDNA was amplified by polymerase chain reaction (PCR) in a 50 μl reaction containing 25 μl of HotStarTaq Master Mix (Qiagen), 10 pm degenerate primer and anchor primer, specific 3′-end of synthesized cDNA for 3′-RACE, and 1 μl of cDNA template. PCR amplification was performed as follows: an initial denaturation at 94°C for 15 min, then 30 cycles of 94°C for 0·5 min, 55°C for 1 min and 72°C for 2·5 min, followed by final elongation at 72°C for 10 min. For 5′-RACE, first-strand cDNA was synthesized using the 5′-Full RACE Core Set (Takara Bio, Inc.) with a 5′-phosphorylated primer, VVL-RT (5′-CACCAGGTTGGCTCCCTCTGACACAC-3′) for VVLs. The first-strand cDNA was amplified by PCR in a 50 μl reaction containing 25 μl of HotStarTaq Master Mix, 10 pm primer sets, VVL-A1 primer (5′-CACGTACTTGTAGCAGCGGTTGTTGAAGC-3′) and VVL-S1 primer (5′-ACACAGATGAACTGGGCTGATGCAGAGC-3′), and 1 μl of cDNA template. PCR amplification was performed as follows: an initial denaturation at 94°C for 15 min, then 30 cycles of 94°C for 0·5 min, 58°C for 1 min and 72°C for 2 min, followed by final elongation at 72°C for 10 min. The nucleotide sequences obtained by 3′- and 5′-RACE over the coding regions of VVLs were confirmed by PCR using specific primers: VVL-OF-1 (5′-CAGATCATCTCCA ACCCCTCGTCC-3′) and VVL-OR-1 (5′-GCATTTGTATCTTTATTGCTGAGGAC-3′). Amplified products were subcloned into plasmid pHSG299 (Takara Bio, Inc.) and used to transform JM109 competent cells (Takara Bio, Inc.). At least 10 clones for each organ were sequenced on a 3100 DNA sequencer (Applied Biosystems).
Subtype separation by chromatofocusing and iso-electric focusing
Eluted lectins from D-glucose columns were further isolated on a Mono P 5/50 chromatofocusing column (GE Healthcare Biosciences) with the pH interval 5·2–3·5. Isoelectric focusing (pH 4–7) was done using IEF Gel (Tefco) with the pI standard marker (Invitrogen Corp.).
Isoform identification
VVL-α and -β (see Results section) were reduced and subjected to SDS-PAGE (VVL-α) or isoelectric focusing (VVL-β). Protein bands derived from each heterodimer were cut from the gels and the proteins tryptically digested. Tryptic peptides derived from each band or spot were submitted for identification by MALDI-TOF mass fingerprinting using Q-TOF Micro (MICROMS). The peptide masses obtained by MALDI analysis were also subjected to search protein databases.
Indirect lectin-fluorescence staining
N. girellae larvae were fixed in 1% formaldehyde (v/v), washed with distilled water, and then spread onto glass slides and air dried. After blocking non-specific binding sites with spotted halibut serum lacking VVLs, purified VVLs (or biotin-labelled lectins with 50 mm D-glucose at final concentration, as a negative control) were diluted 1:100 in the blocking solution and added and incubated for 2 h at room temperature. Amino groups in VVLs were labelled with biotin using EZ-Link Sulfo-NHS-LC-LC-Biotin (Pierce). The slides were washed with PBS(+), and then Streptavidin–Alexa Fluor 488 conjugate (diluted 1:400; Invitrogen Corp.) was added. After incubation, the slides were washed and mounted under cover-slips. The parasites were examined using a confocal laser scanning microscope (FLUOVIEW FV300; Olympus Optical Co.).
Extraction of membrane cilia proteins
Ciliary membrane proteins of N. girellae were solubilized in Triton X-114 (Sigma-Aldrich) at 0°C and isolated by phase separation at 32°C as previously described (Hatanaka et al. Reference Hatanaka, Umeda, Yamashita and Hirazawa2005), and essentially as described by Border (Reference Border1981) and Dickerson et al. (Reference Dickerson, Clark and Findly1989). Extracted proteins were precipitated by adding 9 vols of acetone, and then resuspended in 100 μl of 10 mm Tris-HCl (pH 7·5) and stored at −80°C until use.
Lectin blot
Protein bands from SDS-PAGE were electroblotted onto PVDF membranes. Filters were blocked by incubation with spotted halibut serum (diluted 1:10) lacking VVLs. Then, the filters were incubated with 5 μg of biotin-labelled lectins in PBS(+). After incubation, filters were washed with PBS(+) and then incubated with Streptavidin-AP (alkaline-phosphatase) conjugate (Roche Diagnostics) diluted 1:25 000 in PBS(+). Filters were finally washed and developed in NBT-BCIP substrate (Roche Diagnostics).
Characterization of haemagglutination activity of VVLs
Haemagglutinating activity was assayed by the 2-fold serial dilution method using rabbit erythrocytes in 96-well microtitre plates. Fifty μl of diluted samples were allowed to react with the same volume of a 2% suspension of rabbit erythrocytes. After incubation at room temperature for 30 min, the activity was determined as the lowest concentration of sample solution giving visible agglutination. The following saccharides (200 mm each) were examined: D-mannose, myo-inositol, D-xylose, lactose monohydrate, N-acetyl-D-glucosamine, D-galactose, L-a-fucose, N-acetyl-D-galactosamine, D-glucose.
RESULTS
Degree of infection of spotted halibut and Japanese flounder by N. girellae
Twelve days after exposure to N. girellae larvae, the parasite was found on dorsal, anal and caudal fins and the surface of the mouth and eye regions of both spotted halibut and Japanese flounder. Dermal ulcerations with haemorrhage were mainly found around the mouth of Japanese flounder. The number of the parasites on spotted halibut and Japanese flounder was 63·0±26·1 (N) and 282·6±72·5 (N/N), respectively (Fig. 1A), and the number of parasites per surface area was 1·4±0·6 (N/cm2) and 6·8±2·5 (N/cm2), respectively (Fig. 1B). In both cases, the number of parasites on spotted halibut was significantly lower (Student's t-test, P<0·01) compared with Japanese flounder.

Fig. 1. The number of Neobenedenia girellae on the surface of the spotted halibut (Verasper variegatus) and Japanese flounder (Paralichthys olivaceus). Six specimens of each species were infected with the parasites. After 2 days, the number (A) and population density (B) of parasites on the surface of fish were assessed. Statistically significant differences are indicated by ** (P<0·01, Student's t-test).
Agglutination/immobilization activities of fish serum
In the presence of spotted halibut serum, N. girellae larvae immediately ceased to swim and, after a few minutes, began to form large aggregates and settle to the bottom of the wells (Fig. 2A, B). Spotted halibut serum at a 1:100 dilution caused agglutination of live parasites, whereas a 1:100 dilution of Japanese flounder serum caused little agglutination. The rate of agglutination in the groups treated with the halibut serum was significantly higher than that in the groups treated with Japanese flounder serum (Fig. 2C). Agglutination in spotted halibut serum was suppressed significantly by adding D-glucose, D-galactose or myo-inositol at a concentration of 2 and 10 mm. In this respect, D-glucose was especially effective (Fig. 3).

Fig. 2. Immobilization activity of serum from spotted halibut (Verasper variegatus) or Japanese flounder (P. olivaceus) against N. girellae larvae. Parasite agglutinated by spotted halibut serum (A and B). The parasites were incubated with fish sera (diluted 1:100) for 1 h at room temperature (C).

Fig. 3. Monosaccharide-mediated inhibition of immobilization activity of spotted halibut serum. Statistically significant differences from the control value are indicated by * (P<0·05) and ** (P<0·01) (Student's t-test). Neobenedenia. girellae larvae were incubated with the serum (diluted 1:100) for 1 h at room temperature in the absence (control) or presence of various monosaccharides (2 or 10 mm).
Purification of VVLs from serum
Lectins in spotted halibut serum were purified by liquid chromatography using a D-glucose-coupled agarose column. Judging from the amount of lectins in the eluate, we estimated that the concentration of these lectins in spotted halibut serum (VVLs) was 1·92±0·55 mg/ml (n=5). Purified lectins also immobilized live parasites in vitro, and agglutination of live parasites occurred in the presence of purified lectins (1 μg/ml). On the other hand, flow-through proteins from the D-glucose column did not immobilize live parasites in vitro. SDS-PAGE under non-reducing conditions yielded 2 protein bands of ~33 kDa and ~30 kDa, which we named VVL-α and VVL-β, respectively (Fig. 4A, lane 1), and only the VVL-α band was visualized by PAS staining (Fig. 4A, lane 2). On the other hand, SDS-PAGE reducing conditions yielded 2 protein bands of ~18 kDa and ~15 kDa (Fig. 4B). This result showed that both VVL-α and VVL-β are composed of 2 subunits. To determine the molecular mass of the VVLs component, the glycoprotein was enzymatically deglycosylated using N-glycanase (PNGase F), O-glycanase and sialidase A. As shown in Fig. 4C for VVLs, addition of N-glycanase yielded a clear mobility shift for the ~18 kDa protein band on reducing SDS-PAGE, and these VVLs components had similar peptide masses of ~15 kDa. N-terminal amino acid sequencing of VVLs components yielded similar results: NH2-Ser-Asp-Gly-Pro (Glu)-Glu-Leu-Gln-Leu-Gln (Glu)-Arg-Gly-Asn (Asp)-Cys-Pro (Asp)-Met-Phe-Trp-Phe-Ser-Phe for the ~18 kDa protein band, and NH2-Ser-Asp-Gly-Pro-Glu-Leu-Gln-Leu (Gln)-Gln-Arg-Gly-Asn (Asp)-Cys (Ser)-Pro (Asp)-Met-Phe-Trp-Phe-Ser-Phe for the ~15 kDa protein band, respectively.

Fig. 4. SDS-PAGE analysis of fractions from a D-glucose affinity column during the purification of VVLs. The 200 mm D-glucose eluate (protein: 5 μg/lane) was resolved under non-reducing conditions on a 4–20% gradient polyacrylamide gel and visualized using CBB (A, lane 1) or by PAS staining (A, lane 2), or resolved under reducing conditions (B). Mobility shifts of VVLs analysed by reducing SDS-PAGE after incubation with individual enzymes (N-glycanase, O-glycanase and sialidase A) or combinations thereof (C).
Molecular cloning of VVL cDNA
Four types of cDNA fragments of VVLs were obtained by 3′-RACE using degenerate primer synthesized based on N-terminal amino-acid sequencing. Subsequently, 5′-untranslated regions of VVLs were isolated by 5′-RACE using specific primers. The cDNA of VVLs contained a 489-bp open reading frame encoding 163 amino acids including a signal peptide of 17 amino acids (GenBank Accession numbers for VVL isoform-1, -2, -3 and -4 are AB220916, AB220917, AB274523 and AB274524, respectively) (Fig. 5). Two cDNA sequences had a potential N-linked carbohydrate site (Asp123-Phe124-Thr125), and thus one of these clones was thought to represent the VVL-α-1 gene (Fig. 6). The mannose-type carbohydrate-binding motifs, Glu121-Pro122-Asn123 (EPN) and Glu121-Pro122-Ser123 (EPS), were found in isoform-1, -3 and -4, and isoform-2, respectively (Fig. 6). The Trp142-Asn143-Asp144 (WND) motif, which is important in the binding of both carbohydrate and Ca2+, was identified near the C-terminal region of the carbohydrate recognition domain (CRD) in these isoforms (Fig. 6). VVL isoform-1, -3 and -4, and VVL isoform-2 have the highest similarity with Japanese eel mucus lectin AJL-2 (AB050703), and Japanese eel serum lectin eCL-2 (AB060538), respectively. The estimated pI based on the deduced amino-acid sequences of putative mature peptides of isoform-1, -2, -3 and -4 were 5·34, 4·85, 4·60 and 5·01, respectively.

Fig. 5. cDNA nucleotide sequences and deduced amino acid sequence of VVL isoform-1. Underlining shows residues determined by N-terminal amino acid sequencing. Arrows indicate locations and orientation of primers. Based on this sequence, degenerate primer VVL-NP primer was synthesized, and cDNA clones for VVL isoform-1, -2, -3 and -4 were then obtained by PCR, using VVL-NP primer and adaptor primer. For 5′-RACE, first-strand cDNA was synthesized with a 5′-phosphorylated primer VVL-RT primer, and amplified by PCR, using VVL-A1 and VVL-S1 primers. The overlapping sequence was reconfirmed by PCR, using VVL-OF-1 and VVL-OR-1 primers. An asterisk marks the stop codon, a potential N-terminal signal peptide is italicized and polyadenylation signal (AATAAA) is boxed.

Fig. 6. Amino-acid sequence comparison of VVL isoforms. Residues forming carbohydrate-binding motifs (EPN and EPS), and WND motif, which is important in binding of both carbohydrate and Ca2+ is shaded.
VVL separation by chromatofocusing column
VVLs were subsequently submitted to chromato-focusing column chromatography to be isolated. The chromatogram successfully yielded 2 peaks (Fig. 7A), the first and second peak containing VVL-β and VVL-α, respectively (Fig. 7B). The isoelectric focusing of VVL-α under reducing conditions showed a single protein band with a pI of ~4·6 (Fig. 7C, lane 1), and showed 2 protein bands with pIs of ~4·8 (VVL-α-1) and ~4·6 (VVL-α-2) and similar to VVL-β showed 2 protein bands with pIs of ~4·8 (VVL-β-1) and ~4·6 (VVL-β-2) (Fig. 7C, lanes 2 and 3), after being enzymatically deglycosylated using N-glycanase. These results suggest that both VVL-α and VVL-β form heterodimers via disulfide bonds, and are composed of the same subunits, with 1 subunit having a pI of ~4·8 (VVL-α-1) of VVL-α with an N-linked sugar chain(s).

Fig. 7. Elution profile of chromatofocusing column chromatography and isoelectric focusing of VVLs. The 200 mm D-glucose eluate was applied to a chromatofocusing column (A). Elution of proteins was monitored by absorbance at 280 nm. Bars indicate collected fractions for VVL-α and -β. Purified VVL-α (B, lane 1) or -β (B, lane 2) by chromatofocusing column chromatography resolved under non-reducing conditions. Isoelectric focusing of VVL-α before (C, lane 1) or after incubation with N-glycanase (C, lane 2), or VVL-β (C, lane 3) under reducing conditions.
Isoform identification by mass spectrometry
Table 2 details the tryptic peptides derived from each subunit by MALDI-TOF mass spectrography. The peptide fragment, Tyr-Val-Ala-Thr-Gln-Met-Asn-Trp-Ala-Asp-Ala-Glu-Leu-Asn-Cys-Val-Ser-Glu-Gly-Ala-Asn-Leu-Val-Ser-Ile-His-Ser-Leu-Asp-Glu-Glu-Asn-Phe-Val-Lys, derived from VVL-α-1 and VVL-β-1 is specific to the deduced amino acid sequence of the isoform-1 cDNA. In addition, all peptide fragments derived from VVL-α-1 and VVL-β-1 were coincident with the deduced amino-acid sequence of the isoform-1 cDNA. In case of VVL-α-2 and VVL-β-2, peptide mass fingerprints provided insufficient information for isoform identifications.
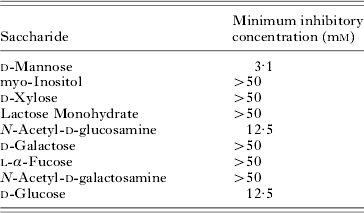
Table 1. Saccharide inhibition of haemagglutination activity of VVL-α from spotted halibut serum
(Inhibition of haemagglutination activity against rabbit erythrocytes of VVL-α. VVL-β showed identical results with VVL-α.)
Indirect lectin-staining
Our data seemed to indicate that VVLs react with ciliary proteins of N. girellae larvae and promote agglutination of parasites. To determine whether VVLs recognize ciliary surface saccharides, indirect fluorescence immunostaining was performed using purified VVLs. The surface fluorescence of formalin-fixed N. girellae larvae indicated that VVLs strongly recognize the ciliary surface saccharides (Fig. 8B). Experiments conducted in the presence of 50 mm D-glucose showed little fluorescence (Fig. 8D).

Fig. 8. Indirect lectin-fluorescence staining of Neobenedenia girellae larvae. Formalin-fixed parasites were incubated with biotin-labelled VVLs, followed by alkaline phosphatase–conjugated streptavidin and were visualized by phase-contrast (A and C) or fluorescence microscopy (B and D). C and D show results in the presence of 50 mm D-glucose, which competes for binding to VVLs.
Identification of the glycoprotein recognized by VVLs
Cilia are the predominant feature of the surface of N. girellae larvae. Because membrane compartments of cilia are thought to be associated with immobilization activities, they were extracted by phase separation in the nonionic detergent, Triton X-114. Fig. 9A shows the protein composition of whole cilia (lane 1) and of membrane components of cilia (lane 2), as resolved by SDS-PAGE. The membrane fraction contained a predominant glycoprotein of ~8 kDa. To characterize saccharides that are recognized by VVLs and mediate the agglutination/immobilization of parasites, we performed lectin blot analysis. The ~8 kDa glycoprotein was strongly detected by biotin-labelled VVLs. The ~50 kDa non-specific band (in both lanes 1 and 2) was thought to be the result of binding of streptavidin-alkaline phosphatase.

Fig. 9. SDS-PAGE and lectin-blot analyses of intact cilia and ciliary integral membrane proteins from Neobenedenia girellae larvae using biotin-labelled VVLs. (A) Intact cilia (lane 1) and integral membrane proteins from cilia (lane 2) were solubilized in Laemmli sample buffer, resolved by reducing SDS-PAGE (15 μg/lane) and silver stained. (B) For lectin-blot analysis, 5 μg of ciliary integral membrane proteins were resolved by reducing SDS-PAGE and electroblotted onto a PVDF membrane. Each lane was incubated with biotin-labelled VVLs in the presence (lane 1, as a negative control) or absence (lane 2) of 50 mm D-glucose. The blots were developed with NBT-BCIP. Relative molecular masses of standard markers are indicated on the left. In both panels A and B the position of the 8 kDa immobilization protein is indicated by an arrowhead.
Detection of the sugar specificity of VVLs
Haemagglutination activity of VVL-α against rabbit erythrocytes is shown in Table 1. It was inhibited by N-Acetyl-D-glucosamine, D-glucose and D-mannose. D-mannose was especially effective. VVL-β showed identical results to VVL-α. In both cases, the haemagglutinination activity was diminished by the addition of 5 mm EDTA.
Table 2. Comparison of tryptic peptides for each VVL isoform obtained by MALDI-TOF mass spectrometry

DISCUSSION
We have found that the susceptibility of spotted halibut to N. girellae was low compared with Japanese flounder when the fishes were exposed to this parasite in the same aquarium. In addition, the survival of spotted halibut after infection with N. girellae in the same aquarium was significantly higher than that of Japanese flounder (data not shown). Active feeding by a large population of the parasites on mucus and epithelial cells of host fish can cause haemorrhage and inflammation. Therefore, the agglutinin activity present on the surface of spotted halibut was thought to prevent serious infections mediated by parasites. VVLs recognize a putative agglutination/immobilization antigen on the ciliary surface of N. girellae. In our previous study, we used immunological analyses to show that the 8 kDa glycoprotein on the ciliary surface of N. girellae is a putative immobilization/agglutination antigen and that fish antibodies against this ciliary protein may immobilize N. girellae in vitro within a few minutes (Hatanaka et al. Reference Hatanaka, Umeda, Yamashita and Hirazawa2005). The 8 kDa glycoprotein is thought to be a homologue of immobilization antigen (i-antigen) on the ciliary surface of the fish protozoan parasite Ichthyophthirius multifiliis (Clark et al. Reference Clark, Lin and Dickerson1995). Fish antibodies against i-antigen bind to cilia and also immobilize I. multifiliis in vitro, and are thought to play an important role in protective immunity. In the catfish, Ictalurus punctatus, passive immunization against i-antigen of I. multifiliis by murine monoclonal antibodies caused rapid exit of I. multifiliis from the host fish (Clark et al. Reference Clark, Lin and Dickerson1996). Further, in the spotted halibut VVLs in the mucus, when released after haemorrhage, are thought to force N. girellae to abandon the host fish. On the other hand, VVLs did not agglutinate the other fish parasites, Cryptocaryon irritans and Heterobothrium okamotoi, although these parasite species have been shown to have agglutination/immobilization antigens on their surface (Hatanaka et al Reference Hatanaka, Umeda, Yamashita and Hirazawa2007; Umeda et al. Reference Umeda, Hatanaka and Hirazawa2007).
In the present study, we have characterized spotted halibut serum lectins, which are simple lectins composed of 163 amino acid residues. These lectins have conserved consensus sequences characteristic of C-type lectincarbohydrate-recognition domains (CRDs). Four consensus cysteine residues, Cys41, Cys114, Cys130 and Cys138 are also conserved, and these are expected to form disulfide bonds (Cys41-Cys138 and Cys114-Cys130). CRDs containing the sequence EPN found in VVL isoform-1, -3 and -4, generally bind mannose or glucose derivatives (Drickamer, Reference Drickamer1992). On the other hand, the sugar binding preference of the EPS-containing motif, found in VVL isoform-2, has not been unequivocally established (Bates et al. Reference Bates, Fournier, Garcia, Valladeau, Durand, Pin, Zurawski, Patal, Abrams, Lebecque, Garrone and Saeland1999; Fujita et al. Reference Fujita, Endo and Nonaka2004).
VVLs are thought to function as ante-antibodies in first line host defence, although knowledge of lectin-based fish defence systems against pathogens remains limited. VVL-α and -β, composed of isoform-1 and -3, was shown to have the highest similarity with Japanese eel mucus lactose-specific lectin AJL-2 which exists in the skin mucus and can agglutinate a marine pathogenic bacterium, Vibrio anguillarum in vitro (Tasumi et al. Reference Tasumi, Ohira, Kawazoe, Suetake, Suzuki and Aida2002). However, the roles of this eel lectin in complement activation or opsonization, remain unclear. In mammals serum mannose-binding lectins (MBLs) are confirmed to activate the lectin pathway of the complement system. In other fish species, cDNAs of an MBL homologue were detected in the Cyprinidae family, and was assumed to activate the lectin pathway (Nakano and Yano, Reference Nakano and Yano1998; Vitved et al. Reference Vitved, Holmskov, Koch, Teisner, Hansen and Skjødt2000). Sugar specificites of VVL-α and -β are similar to those of MBLs and, additionally, VVL-α and -β possess an EPN-motif, which is specific to MBLs. In their sequences, however, a collagen-like sequence (Gly-X-Y) which is specific to MBLs and an MBL homologue in the Cyprinidae family could not be seen in VVL-α and -β sequences. On the other hand, an MBL homologue in the Cyprinidae family possesses a QPD-motif that confers galactose ligand specificity (Drickamer, Reference Drickamer1992; Vitved et al. Reference Vitved, Holmskov, Koch, Teisner, Hansen and Skjødt2000). The sugar specificity of MBL homologues in other teleosts remains unclear. Blue gourami Trichogaster trichopterus serum lectin, which has similar sugar specificity to VVL-α and -β, could serve as an opsonin for the phagocytosis of foreign pathogens. Its sequence has not, however, been established (Fock et al. Reference Fock, Chen, Lam and Sin2000).
Spotted halibut is thought to be less susceptible to N. girellae than other aquacultured fish species (our unpublished data). However, it is still not clear that VVLs really contribute to the lower susceptibility of spotted halibut to this parasite. It is also still unclear why D-mannose did not inhibit immobilizing activity against N. girellae, although haemagglutination activity of VVLs was inhibited by D-mannose. The biological functions of fish lectins remain to be elucidated, and further investigations are needed in order to resolve their roles.
We thank Professor Hisashi Hirano and Dr Tomoko Akiyama (Yokohama City University) for proteomics analysis.













