Case report
NA is a 9-year-old boy from Oman, diagnosed with B-thalassaemia major. He was on regular blood transfusion, which was given every 4 weeks. At the age of 2 years, iron chelation therapy was started initially with deferasirox, which was not tolerated and replaced with deferiprone at 75 mg/kg/day. The dose of deferiprone was escalated gradually to 85 mg/kg/day and subsequently to 100 mg/kg/day to control serum ferritin levels.
For 6 years, with deferiprone therapy, he maintained normal neutrophil counts ranging between 3.6 and 6.3×109/L. In December, 2016, he presented to the Pediatric Day Care Unit for regular transfusion. Routine laboratory investigations revealed neutropaenia (0.5 109/L). Deferiprone was stopped and replaced with deferasirox at 25 mg/kg/day. Follow-up complete blood count obtained 5 days later revealed a further drop in the neutrophil count to 0.4×109/L, though he remained afebrile and clinically healthy. He developed fever and right-sided facial cellulitis 4 days later. Clinical examination revealed a tender swelling measuring 4–5 cm over the right mandible. Ultrasound scanning revealed increased thickness of subcutaneous soft tissue with minimal oedema and no definite collection or abscess. X-ray of the mandible ruled out osteomyelitis of the jaw. Concomitantly, analysis of the child’s blood sample showed haemoglobin at 11.2 g/dl, platelet count of 502×109/L, white blood cell count of 2.3×109/L, and neutrophil count of 0×109/L. Other laboratory investigations revealed C-reactive protein at 66 mg/L, serum sodium at 136 mmo/L, and potassium at 4 mmol/L; and normal results were obtained from liver-function tests. Blood samples were sent for culture, which was reported later as negative, and the child was put on empirical antibiotics, namely tazocin and vancomycin. As he presented with spiking high-grade fever together with agranulocytosis, treatment with filgrastim at 10 μg/kg/day was started. Fever was gradually controlled and facial swelling and erythema improved. His neutrophil count remained in the severe neutropaenia range for 13 days, from 20 December to 1 January, with concomitant lymphopaenia for 3 days (1.3–1.8×109/L). After three doses of filgrastim, irregular heart rate was noticed on routine clinical examination. The child was asymptomatic and afebrile; he had a heart rate of 118/minute with extra beats, blood pressure of 116/73 mm Hg, and an oxygen saturation of 100%; his peripheral pulsations were well-felt. On auscultation, he had normal heart sounds and there was an ejection click+ and frequent extrasystoles+. An electrocardiogram was obtained and revealed frequent premature ventricular contractions (Fig 1). Serum sodium (139 mmol/L) and potassium levels (3.8 mmol/L) were normal, as were the levels of serum calcium (2.34 mmol/L), phosphorous (1.45 mmol/L), and magnesium (0.8 mmol/L).
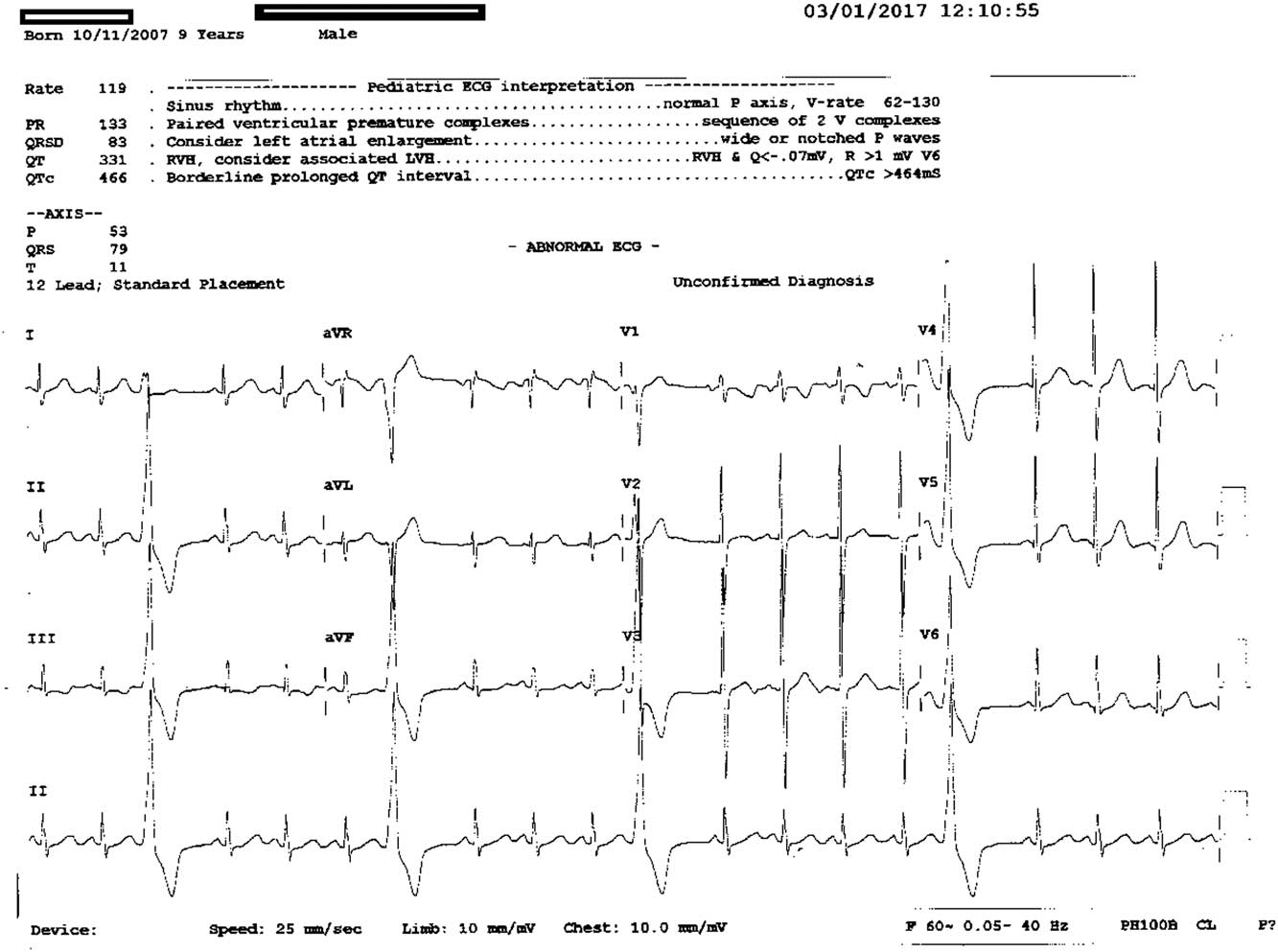
Figure 1 12 leads ECG showing premature ventricular contractions.
Echocardiography was carried out, and revealed normal intracardiac anatomy, normal chamber dimensions, and contractility. Holter monitoring for 24 hours ruled out any runs of ventricular tachycardia. The patient was monitored for 22–39 hours, revealing a maximum heart rate of 156 bpm, minimum heart rate of 82 bpm, very frequent premature ventricular contractions in isolation, and frequent bigeminy and trigeminy with no couplets, triplets, or runs of ventricular tachycardia. In addition, 21 premature atrial contractions were seen in isolation. A cardiac exercise electrocardiogram was obtained 2 weeks later; the baseline showed few premature ventricular contractions and occasional trigeminy and quadrigeminy (Fig 2). He exercised midway through stage IV of the Bruce Protocol. With an increasing grade of exercise, the ectopics totally disappeared – there were no couplets, triplets, quadruplets, or any runs of ventricular tachycardia. The recovery phase showed a return of ventricular ectopies with little trigeminy and bigeminy. This pattern is considered very suggestive of benign monomorphic ventricular ectopies with bigeminy and trigeminy.
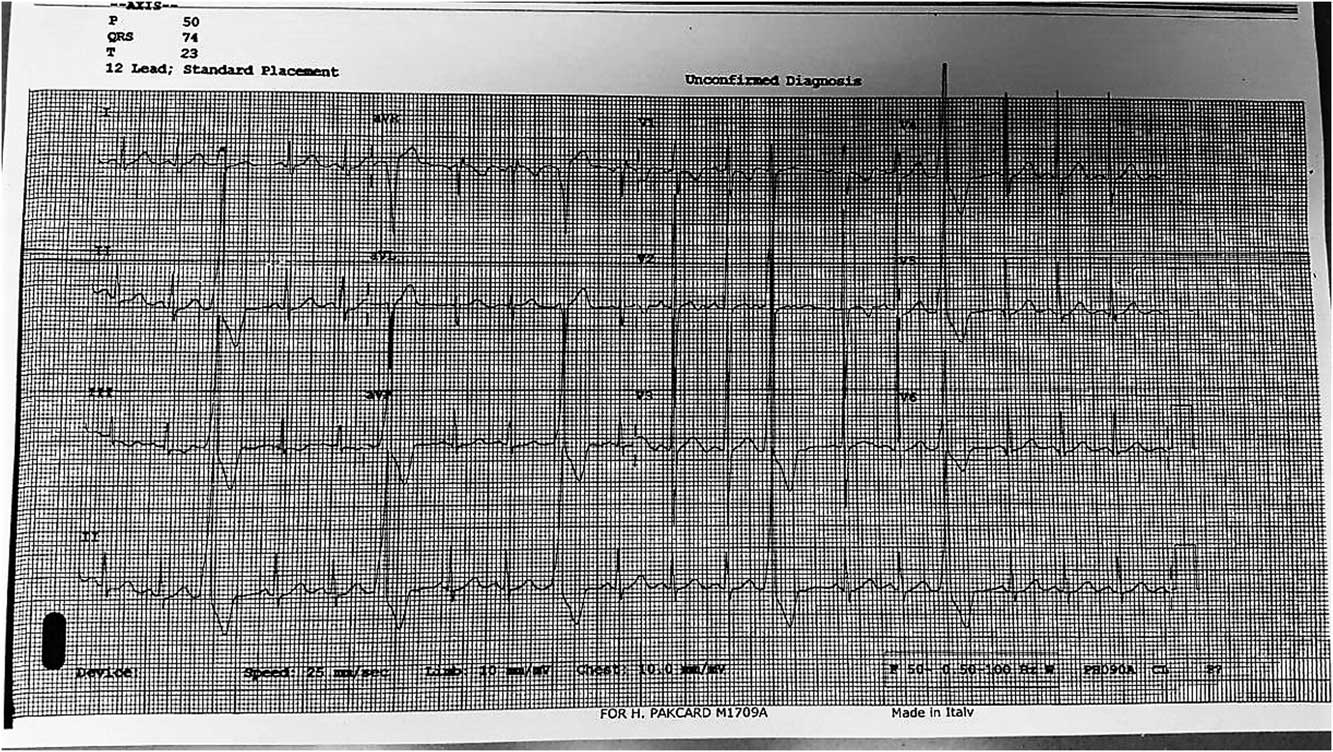
Figure 2 ECG showing premature ventricular contractions, with occasional trigeminy and quadrigeminy.
Cardiac T2*MRI was carried out and reported a low-cardiac-iron status with cardiac T2* of 32.5 ms, hepatic T2* of 13.6 ms, and estimated hepatic iron at 2.1 mg/g dry weight. There was no evidence of myocarditis or cardiomyopathy, and no fatty infiltration was noted. On follow-up, the arrhythmia persisted for 2 weeks after discontinuation of filgrastim. Electrocardiogram was carried out 1 week later and revealed normal tracing.
Discussion
We report on the case of a 9-year-old patient with B-thalassaemia major treated with filgrastim for deferiprone-induced agranulocytosis who developed frequent premature ventricular contractions.
Deferiprone is an effective oral iron chelator, which plays a particularly important role in removal of the cardiac iron burden in patients with thalassaemia with transfusional iron overload. Agranulocytosis – that is, having a neutrophil count <0.5×109/L – is the most important and severe side effect, reported in 0.5–3.6% of cases.Reference Pontikoglou and Papadaki 1 Deferiprone-induced agranulocytosis developed in our patient 6 years after starting therapy and lasted for 13 days. The reported incidence of agranulocytosis ranged between 1.5 and 5.5%, time to the event ranged between 9 days and 17 years, and the median time to resolution was 11 days.Reference Wali, Al-Shindhani and Daar 2 , Reference Tricta, Uetrecht and Galanello 3
Premature ventricular contractions have been described in 1% of clinically normal people, especially in elderly men, as detected by standard electrocardiogram. Frequent (>60/hour or 1/minute) and complex premature ventricular contractions can occur in 1–4% of apparently healthy subjects.Reference Kennedy, Whitlock and Sprague 4 Possible causes include cardiomyopathy, myocardial infarction, myocarditis, electrolyte abnormalities, drugs such as digoxin, tricyclic antidepressants, etc., hypoxia and hypercapnia, stress, and sleep deprivation. 5
In healthy children, frequent premature ventricular contractions are rare, being even rarer in children below the age of 9 years. Only 2–6% of older children and young adults have more than 50 premature ventricular contractions/24 hours. These premature ventricular contractions are, in general, considered benign, if no underlying cardiac disease is diagnosed and if the premature ventricular contractions are suppressed by exercise.Reference Beaufort-Krol, Dijkstra and Bink-Boelkens 6
It was important in the current case to rule out the presence of a structural cardiac lesion – which was achieved using ECHO – the effect of B-thalassaemia and possible myocardial iron overload – with cardiac MRI T2* ruling out significant cardiac siderosis – electrolyte abnormalities – with serum levels being normal in our patient – and the possible relationship with treatment with filgrastim.
Filgrastim is a recombinant human granulocyte-colony-stimulating factor that promotes proliferation, differentiation, and maturation of neutrophil precursors. Its receptors have been also identified on monocytes, macrophages, and lymphocytes. It stimulates a variety of different cellular activities depending on the cells to which it binds.Reference Sugano, Anzai and Yoshikawa 7
Along with its cell-mobilising property, recently, several studies addressed the effects of filgrastim on the heart, mostly after myocardial infarction. Several studies documented a beneficial effect on the healing process and on post-infarction arrhythmia.Reference Baldo, Davel and Damas-Souza 8 , Reference Baldo, Rodrigues and Mill 9 On the other hand, myocardial infarction, atrial fibrillation, and arrhythmia have been reported in several patients with malignancy receiving filgrastim. In a rat model, Lee et alReference Lee, Chen and Chang 10 attributed its proarrhythmic effect to the activation of nestin and nerve growth factor expression as well as to increased sympathetic reinnervation, which may increase its arrhythmogenic potential.
Interestingly, in a prospective study conducted by Guneysel on 102 neutropaenic patients, two doses of filgrastim at 5 μg/kg for 2 days did not demonstrate any electrocardiogram changes other than a significant reduction in mean heart rate.Reference Guneysel, Onur and Denizbasi 11 This could be explained by the short duration and relatively small dose of filgrastim utilised.
In our case, premature ventricular contractions were absent before initiation of filgrastim therapy and developed after receiving three doses of filgrastim at 10 μg/kg. It was not associated with haemodynamic instability, and did not require medical intervention.
Conclusion and recommendations
Premature ventricular contraction can occur as a side effect of filgrastim even in young patients. Careful monitoring, and routine electrocardiogram tracing should be obtained for all children receiving filgrastim to screen for abnormal rhythm. If detected, a thorough cardiac evaluation should follow. Identifying this potential side effect can prevent its progression to more serious arrhythmia.
Acknowledgement
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. Authors’ Contribution: H.F.N. was directly involved in the clinical care of the patient and detected the abnormal rhythm, collected the data, drafted the initial manuscript, performed literature review, and approved the final manuscript as submitted. N.J. performed the cardiac assessment of the child, including interpretation of electrocardiogram, Holter monitoring, exercise electrocardiogram and ECHO cardiography. He reviewed and revised the manuscript, and approved the final manuscript as submitted. Y.A.W. initially recognised the association of premature ventricular contractions with filgrastim, critically reviewed the manuscript, and approved the final manuscript as submitted.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.





