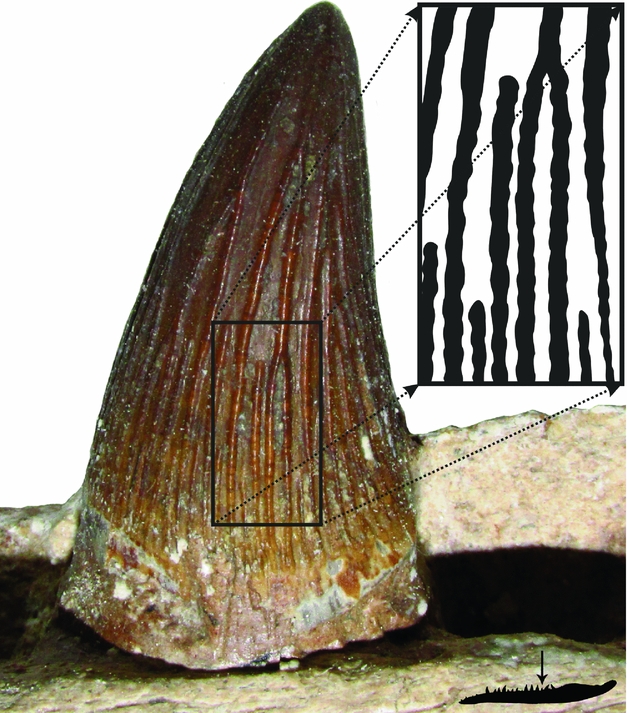
1. Introduction
Megacephalosaurus eulerti Schumacher, Carpenter & Everhart, Reference Schumacher, Carpenter and Everhart2013 is a large brachauchenine pliosaurid that roamed the Western Interior Seaway in what is now Kansas, USA, during the middle phase of the Turonian (Late Cretaceous). The type specimen (FHSM VP-321) was discovered in October 1950, in the strata forming the middle section of the Fairport Chalk Member, Carlile Shale, which falls within the stratigraphic range of the early middle Turonian ammonite Collignoniceras woollgari (Schumacher & Everhart, Reference Schumacher and Everhart2005; Schumacher, Carpenter & Everhart, Reference Schumacher, Carpenter and Everhart2013). FHSM VP-321 consists of a nearly complete skull including the mandible, and an associated material from the anterior part of the cervical section, including three rib heads and a partial neural arch. Additional skeletal material attributed to M. eulerti includes fragments of a skull (UNSM 50136) comprising both maxillae, vomers, palatines, pterygoids, jugals and the right prefrontal (Schumacher, Reference Schumacher2008; Schumacher, Carpenter & Everhart, Reference Schumacher, Carpenter and Everhart2013).
Owing to the reasonable completeness of the type material and its sufficient state of preservation, M. eulerti captures important morphological details present in Late Cretaceous brachauchenine pliosaurids. Here we provide additional information on the dental morphology of Megacephalosaurus and assess the variability observed in its dentition. The results of the present study are expected to facilitate future considerations regarding attribution of isolated tooth crowns to brachauchenine taxa. Such examples include, for instance, the teeth attributed to Polyptychodon Owen, Reference Owen1841a. The type species of Polyptychodon, P. interruptus Owen, Reference Owen1841b, had long been thought to be represented by a rich assemblage of isolated tooth crowns, originally described from the mid-Cretaceous of England (e.g. Owen, Reference Owen1841a,Reference Owenb, Reference Owen and Dixon1850, Reference Owen1851, Reference Owen1860, Reference Owen1861; Seeley, Reference Seeley1869). However, Madzia (Reference Madzia2016) recently concluded that the dental material ascribed to P. interruptus does not show any autapomorphies and hypothesized that it is most likely too variable to represent a single species, prompting a revision of all other specimens attributed to this historical taxon (such studies are currently being undertaken; e.g. Sachs et al. Reference Sachs, Niedźwiedzki, Kędzierski, Kear, Jagt-Yazykova and Jagt2016b,Reference Sachs, Niedźwiedzki, Kędzierski, Kear, Jagt-Yazykova, Jagt, Kear, Lindgren and Sachsc; Madzia & Machalski, Reference Madzia and Machalski2017; Madzia et al. Reference Madzia, Sachs, Evans, Lindgren, Kear and Cau2017; Sachs et al. Reference Sachs, Jagt, Niedźwiedzki, Kędzierski, Jagt-Yazykova and Kear2018), rendering the taxon itself a nomen dubium. Indeed, detailed assessments of the dental morphology of pliosaurids with well-preserved dentitions might be crucial for these studies.
Additionally, we comment on the cranial anatomy of M. eulerti, appraise certain aspects of the morphological traits of this taxon used in phylogenetic studies, and perform multiple runs of phylogenetic analyses of brachauchenines to test the effects of modifications in the sampling of characters associated with teeth.
Institutional abbreviations. BHN – Musée-sur-Mer, Boulogne, France; CAMSM – Sedgwick Museum of Earth Sciences, University of Cambridge, Cambridge, UK; DORK – Dorking & District Museum, Dorking, Surrey, UK; FHSM – Sternberg Museum of Natural History, Fort Hays State University, Hays, Kansas, USA; GPIT – Geologisch-Paläontologisches Institut der Universität Tübingen, Tübingen, Germany; MNA – Museum of Northern Arizona, Flagstaff, Arizona, USA; MNHN – Muséum National d'Histoire Naturelle, Paris, France; MWGUW – Stanisław Józef Thugutt Geological Museum, Faculty of Geology, University of Warsaw, Warsaw, Poland; NHMUK – Natural History Museum, London, UK; SDSM – Museum of Geology, South Dakota School of Mines and Technology, Rapid City, South Dakota, USA; SMU – Shuler Museum of Paleontology, Southern Methodist University, Dallas, Texas, USA; UNSM – University of Nebraska State Museum, Lincoln, Nebraska, USA; USNM – National Museum of Natural History, Washington, DC, USA.
Tooth position abbreviations. d, dentary tooth; mx, maxillary tooth; pmx, premaxillary tooth. The abbreviations are supplemented with body side indicators (l, left; and r, right) and numbers pointing to the positions in tooth-bearing elements (e.g. lmx05 indicates the fifth tooth in the left maxilla).
2. Material and methods
2.a. Material
This study is based on FHSM VP-321 (Figs 1, 2), the type specimen of Megacephalosaurus eulerti that originates from the lower middle Turonian of the Fairport Chalk Member, Carlile Shale, Colorado Group (Kansas, United States).

Figure 1. FHSM VP-321 (type specimen of Megacephalosaurus eulerti): (a) cranium, (b) left half and (c) right half of the mandible.
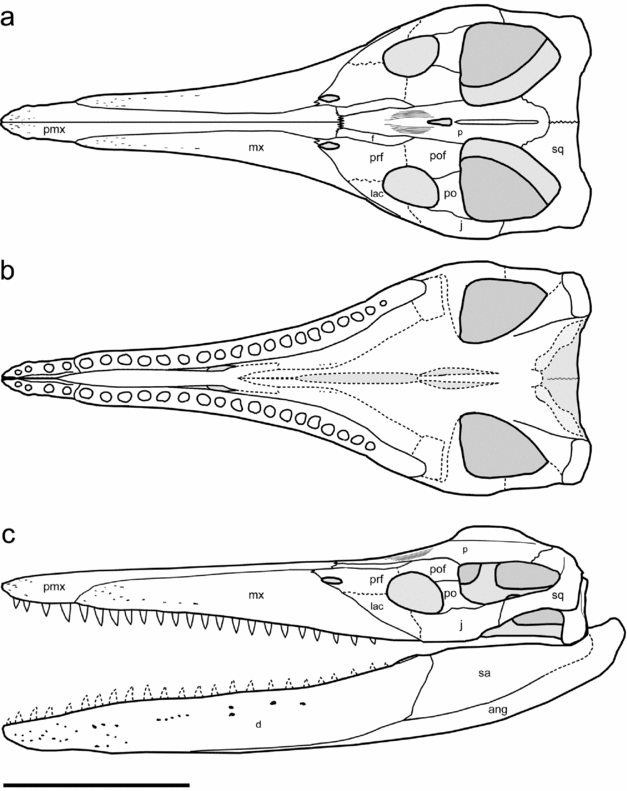
Figure 2. Reconstruction of FHSM VP-321 (type specimen of Megacephalosaurus eulerti): cranium from the dorsal (a) and ventral (b) view, and the whole skull from the left lateral view (c). Abbreviations: ang, angular; d, dentary; f, frontal; j, jugal; lac, lacrimal; mx, maxilla; p, parietal; pmx, premaxilla; po, postorbital; pof, postfrontal; prf, prefrontal; sa, surangular; sq, squamosal. Courtesy of Joschua Knüppe. Scale bar: 50cm.
The dorsal aspects of the cranial morphology were studied directly on FHSM VP-321. The morphological traits observable from the ventral view were evaluated based on the cast of FHSM VP-321 and photographs, because the skull was considered too fragile to be flipped over during the time of visit of one of us (SS).
2.b. The terminology of tooth orientation and morphology
The tooth orientation terminology follows that of Smith & Dodson (Reference Smith and Dodson2003): apical, toward the apices of the tooth crown or the tooth base; basal, toward the cervix dentis; distal, away from the tip of the snout; labial, toward the lips; lingual, toward the tongue; mesial, toward the tip of the snout.
The morphological traits exposed on the outer surface of the enamel are described using the nomenclature advocated by Zverkov et al. (Reference Zverkov, Fischer, Madzia and Benson2018): apicobasal ridges, longitudinally running enamel ridges of variable apicobasal extent; they can be developed around the entire circumference; usually semicircular or triangular in cross-section; ridglets, subtle enamel structures commonly developed between adjacent apicobasal ridges or on an unridged enamel surface; the roughness of the ridglets varies from being near-smooth to producing a distinct vermicular pattern (see Madzia, Reference Madzia2016, fig. 7).
The distinction between isodonty/anisodonty and homodonty/heterodonty is often somewhat arbitrary and the terms are used interchangeably (Sassoon, Foffa & Marek, Reference Sassoon, Foffa and Marek2015). Here, we use isodonty/anisodonty when speaking of size of the dentition and homodonty/heterodonty when assessing its morphology.
2.c. Tooth crown assessability
The dental material of FHSM VP-321 is generally well preserved, though some teeth have been slightly labiolingually compressed, with parts of the crowns being displaced and the enamel lost. In the premaxillae, tooth crowns can be assessed in lpmx03 and lpmx04. In the maxillae, assessable tooth crowns are present in the positions lmx01–19 (entire tooth row). However, the assessability of these teeth is largely limited to the labial surfaces, as the lingual sides are either very poorly preserved or inaccessible (Fig. 3). The dentary teeth are somewhat problematic. FHSM VP-321 consists of the left dentary, with most teeth seemingly preserved in their respective tooth positions. However, as George F. Sternberg's original notes say, only ‘ten or more teeth’ were in the alveoli and the rest of them ‘scattered around the quarry’. Thus, Schumacher, Carpenter & Everhart (Reference Schumacher, Carpenter and Everhart2013: 617) hypothesized that some of those teeth were likely added before the specimen was mounted. Therefore, the tooth crowns in the lower jaws cannot be assessed with regard to the alveoli they are attached to but can still be considered within the morphological variability of the tooth crowns preserved in or associated with the type skull.

Figure 3. A segment of the left maxilla showing the preservation of the lingual sides of the tooth crowns (lmx05–lmx15). Scale bar: 5cm.
2.d. Phylogenetic analyses
The phylogenetic analyses were performed through TNT 1.5 (Goloboff, Farris & Nixon, Reference Goloboff, Farris and Nixon2008; Goloboff & Catalano, Reference Goloboff and Catalano2016), using a modified version of the dataset of Fischer et al. (Reference Fischer, Benson, Zverkov, Soul, Arkhangelsky, Lambert, Stenshin, Uspensky and Druckenmiller2017), which in turn is based on the data sampling first published by Benson & Druckenmiller (Reference Benson and Druckenmiller2014). Except for the modifications applied based on the present study (commented on below), the dataset was supplemented by the addition of three taxa: Pliosaurus almanzaensis (O'Gorman, Gasparini & Spalletti, Reference O'Gorman, Gasparini and Spalletti2018), Pliosaurus patagonicus (Gasparini & O'Gorman, Reference Gasparini and O'Gorman2014; O'Gorman, Gasparini & Spalletti, Reference O'Gorman, Gasparini and Spalletti2018) and Rhaeticosaurus mertensi (Wintrich, Reference Wintrich2017). The final version of the dataset includes 99 operational taxonomic units (OTUs) and 270 characters (see online Supplementary Material at http://journals.cambridge.org/geo).
Gómez-Pérez & Noè (Reference Gómez-Pérez and Noè2017) recently described a new pliosaurid, Acostasaurus pavachoquensis, from the lower Barremian of Colombia. Based on its cranial anatomy, the taxon was suggested to represent a brachauchenine, though the attribution was regarded as ambiguous by the authors (Gómez-Pérez & Noè, Reference Gómez-Pérez and Noè2017: 31). We decided to exclude Acostasaurus from the present analyses due to the ongoing preparation of the type specimen. Thus, if included, the scores would not reflect all morphological features present in the taxon. Nevertheless, the study assessing the phylogenetic placement of the pliosaurid is currently in preparation by Noè and Gómez-Pérez (Gómez-Pérez & Noè, Reference Gómez-Pérez and Noè2017: 30). Still, relevant dental characters of A. pavachoquensis are compared to those of brachauchenines and discussed within the phylogenetic context and the scope of the present study.
For the sake of clarity, and owing to the fact that the changes we have made to the M. eulerti OTU are based on our revision of the dental morphology and cranial anatomy of that taxon, descriptions of the applied modifications and particular runs of the phylogenetic analyses are provided in Section 6.
2.d.1. Settings
The memory was set to 10000 trees. The dataset was first analysed using the ‘New Technology’ search with Sectorial Searches and Tree fusing (Goloboff, Reference Goloboff1999), and 100 search replicates; saving all shortest trees reconstructed. Then we performed the ‘Traditional search’ through the tree bisection and reconnection (TBR) branch swapping using the trees from RAM. Bootstrap values were calculated from the analysis of 1000 replicates, with output as frequency differences (GC).
3. Systematic palaeontology
PLESIOSAURIA de Blainville, Reference de Blainville1835
PLIOSAURIDAE Seeley, Reference Seeley1874
THALASSOPHONEA Benson & Druckenmiller, Reference Benson and Druckenmiller2014
BRACHAUCHENINAE Benson & Druckenmiller, Reference Benson and Druckenmiller2014
Megacephalosaurus Schumacher, Carpenter & Everhart, Reference Schumacher, Carpenter and Everhart2013
Type species. Megacephalosaurus eulerti Schumacher, Carpenter & Everhart, Reference Schumacher, Carpenter and Everhart2013
Age. Early middle Turonian, Late Cretaceous.
Diagnosis. As for the type and only species.
Megacephalosaurus eulerti Schumacher, Carpenter & Everhart, Reference Schumacher, Carpenter and Everhart2013
Reference Carpenter1996 Brachauchenius lucasi Carpenter, p. 261
Reference Schumacher and Everhart2005 Brachauchenius lucasi Schumacher & Everhart, p. 38
Reference Albright, Gillette and Titus2007 Brachauchenius lucasi Albright, Gillette & Titus, p. 37
Reference Schumacher2008 Brachauchenius Schumacher, p. 215
Reference Schumacher, Carpenter and Everhart2013 Megacephalosaurus eulerti Schumacher, Carpenter & Everhart, p. 617
Reference Benson, Evans, Smith, Sassoon, Moore-Faye, Ketchum and Forrest2013 Brachauchenius eulerti Benson et al., p. 30, figure 23
Reference Fischer, Arkhangelsky, Stenshin, Uspensky, Zverkov and Benson2015 Megacephalosaurus eulerti Fischer et al., p. 4
Reference Cau and Fanti2016 Brachauchenius eulerti Cau & Fanti, p. 959, figure 6
Reference Angst and Bardet2016 Megacephalosaurus eulerti Angst & Bardet, p. 449
Reference Páramo-Fonseca, Gómez-Pérez, Noè and Etayo-Serna2016 Megacephalosaurus eulerti Páramo-Fonseca et al., p. 99
Reference Madzia2016 Megacephalosaurus eulerti Madzia, p. 2
Reference Fischer, Benson, Zverkov, Soul, Arkhangelsky, Lambert, Stenshin, Uspensky and Druckenmiller2017 Megacephalosaurus eulerti Fischer et al., p. 1670
Reference Gómez-Pérez and Noè2017 Megacephalosaurus eulerti Gómez-Pérez & Noè, p. 16.
Type specimen. FHSM VP-321, almost complete skull including the mandible (Figs 1, 2), with an associated material from the anterior part of the cervical section that includes three rib heads and a partial neural arch.
Type locality and horizon. Near Fairport, NW Russell County, Kansas, USA; middle section of the Fairport Chalk Member, Carlile Shale, Colorado Group; lower middle Turonian; Collignoniceras woollgari ammonite zone (Schumacher & Everhart, Reference Schumacher and Everhart2005; Schumacher, Carpenter & Everhart, Reference Schumacher, Carpenter and Everhart2013).
Emended diagnosis. Modified from Schumacher, Carpenter & Everhart (Reference Schumacher, Carpenter and Everhart2013): Large brachauchenine pliosaurid plesiosaur (skull length >150 mm) differing from Brachauchenius lucasi in the following characters: pretemporal length of the palate significantly longer; temporal fenestrae shorter; frontals interdigitate in the premaxilla/parietal suture in M. eulerti, whereas the frontals of B. lucasi do not contact posterior termination of premaxillae (a thin wedge of bone extends anteriorly between premaxillae and maxillae); premaxilla/parietal suture near the posterior border of the external nares in M. eulerti, whereas in B. lucasi it extends more posteriorly to the midway between external nares and orbits; vomers longer and internal nares positioned further posteriorly than in B. lucasi; posterior termination of vomers visible, medial palatine alae occur further posteriorly than in B. lucasi; long slit-like anterior interpterygoid vacuity present whereas it is absent in B. lucasi; at least some cervical ribs double-headed, whereas all cervical ribs single-headed in B. lucasi.
4. Dental morphology of M. eulerti
4.a. Tooth count
The premaxillae bear four alveoli each. The right maxilla bears 18 alveoli, and the left one 19. Only the left dentary has a fully assessable alveolar section. It shows 24 tooth positions, with the symphyseal segment encompassing the first six teeth. The number of alveoli in the right dentary is unknown.
4.b. General morphology and size
All tooth crowns are conical (subcircular in cross-section; Fig. 4a), slightly curved linguodistally, and possess a pointed apex. The upper jaw dentition is subisodont (all teeth being approximately the same size). The size variability in the lower jaw teeth cannot be assessed. However, its subisodont character is likewise indicated.

Figure 4. The highlighted details show the cross-sectional shape of a non-flattened tooth crown (a), gradual change in the shape of the apicobasal ridges from a subcircular (apically placed arrow) to near-triangular (basally placed arrow) cross-section (b) and branching ridges (indicated by arrows) in the middle section of a tooth crown (c). All three tooth crowns are attached to the left dentary (d). The scale bar (10cm) applies for (d).
Obtaining precise measurements of the preserved tooth crowns is impossible due to numerous breakages and labiolingual compression of the crowns, slight displacements of some parts and unknown basal extent of the enamel. Nevertheless, approximate values are provided in the online Supplementary Material at http://journals.cambridge.org/geo.
4.c. Enamel structural elements
All assessable tooth crowns possess a similar pattern of enamel structural elements. For a better perception of the character distribution, the most distinguishable elements are discussed separately.
4.c.1. Apicobasal ridges
In the teeth attached to the left dentary, the apicobasal ridges are developed around the entire circumferences of all fully assessable tooth crowns. Nothing suggests that the morphology of the ridges in the maxillae differed. However, the lingual sides of the crowns in the left maxilla are not accessible and thus cannot be assessed (Fig. 3). The morphology, density and distribution of the ridges varies. All reach the cervix dentis and run up to the apical half to one-fourth of the crowns. Though it is impossible to count the exact number of the apicobasal ridges in most teeth due to preservational reasons, it seems that approximately half of all ridges reach the apex or the apicalmost region of the crowns. Near the base of the crown, most ridges are less pronounced and possess a near-triangular cross-section. In some crowns, the ridges are ‘folded’, somewhat resembling indistinct and irregular denticle-like structures (Fig. 5). Apically, they become more protruding and their cross-section is semicircular (Fig. 4b). Mesiolabially, the ridges are slightly more distantly spaced than their linguodistal counterparts, though the density of the ridges varies (labially, there are approximately 13–16 ridges per cm) and depends on the presence of additional morphological features, such as the branching ridges which ‘force’ the ridges to be less densely packed at the mid-crown. Some tooth crowns, especially those attached to the left dentary, include distinctively branching apicobasal ridges (up to five branching ridges in a tooth crown; Fig. 4c). This feature, however, does not seem to be characteristic for a particular surface or apicobasal segment, though they are most pronounced at the mid-length of the crowns. No branching ridges have been observed in the premaxillary teeth. However, this might be due to a low number of assessable tooth crowns preserved in the premaxillae.
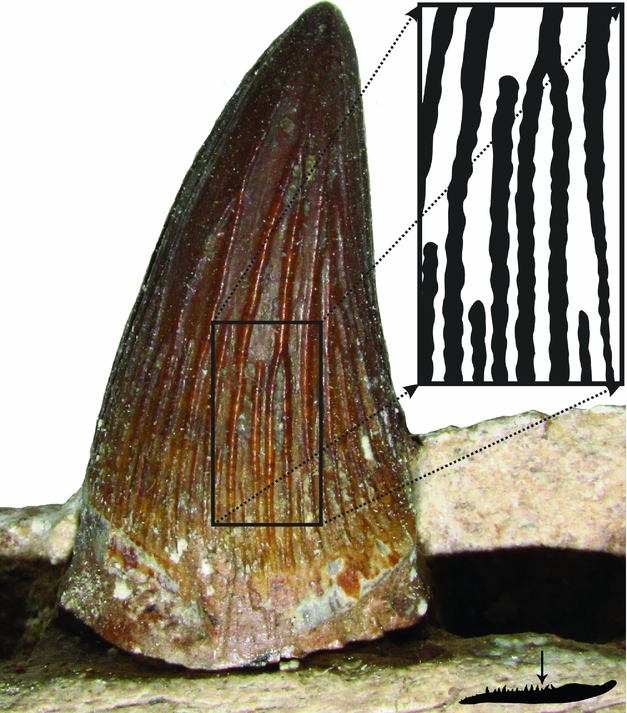
Figure 5. A tooth crown attached to the left dentary (position indicated with black arrow) showing ‘folded’ apicobasal ridges (highlighted field).
4.c.2. Ridglets
When the apicobasal ridges are rather loosely packed (usually at the mid-length of the crowns and more apically), the enamel surface exposed between adjacent ridges possesses rather smooth vermicular ridglets.
4.c.3. Carinae
None of the apicobasally oriented ridges can be regarded as a carina. These structures are therefore absent.
4.d. Tooth wear
In the vast majority of tooth crowns with well-preserved apices, the tips do not exhibit apparent tooth wear. In rare cases, however, the occlusal wear is present (Fig. 6a). The preservation of the specimen does not allow for conclusive assessment of the origin of apical breakages observed in some tooth crowns. Still, the possibility that some have occurred during the lifetime of the individual seems plausible in the case of some tooth crowns with otherwise well-preserved enamel (Fig. 6b).

Figure 6. Two tooth crowns attached to the left dentary (positions indicated with black arrows) showing the occlusal wear (a) and a breakage that potentially occurred during the lifetime of the individual (b). Note the differing curvature of the crowns confirming the positions of the tooth crowns cannot be considered genuine (labial surfaces of the crowns indicated with white arrows). Scale bars: 1cm.
4.e. Comparisons to dentitions of other thalassophoneans
The dentition of M. eulerti is subisodont, as in Brachauchenius (Albright, Gillette & Titus, Reference Albright, Gillette and Titus2007), Luskhan itilensis (Fischer et al. Reference Fischer, Benson, Zverkov, Soul, Arkhangelsky, Lambert, Stenshin, Uspensky and Druckenmiller2017) and possibly Stenorhynchosaurus munozi (Páramo-Fonseca et al. Reference Páramo-Fonseca, Gómez-Pérez, Noè and Etayo-Serna2016), but unlike that of Acostasaurus pavachoquensis (Gómez-Pérez & Noè, Reference Gómez-Pérez and Noè2017), ‘Kronosaurus’ boyacensis (McHenry, Reference McHenry2009), Kronosaurus queenslandicus (McHenry, Reference McHenry2009), Liopleurodon ferox (Noè, Reference Noè2001), Peloneustes philarchus (Noè, Reference Noè2001; Ketchum & Benson, Reference Ketchum and Benson2011), Pliosaurus spp. (Sassoon, Noè & Benton, Reference Sassoon, Noè and Benton2012; Benson et al. Reference Benson, Evans, Smith, Sassoon, Moore-Faye, Ketchum and Forrest2013; Sassoon, Foffa & Monk, Reference Sassoon, Foffa and Marek2015) and Simolestes vorax (Noè, Reference Noè2001), which exhibit extensive regional partitioning.
Comparisons of tooth counts are often problematic because they require tooth-bearing elements with reasonably complete alveolar sections. Nevertheless, FHSM VP-321 possesses four alveoli in each premaxilla, as in MNA V9433 (Albright, Gillette & Titus, Reference Albright, Gillette and Titus2007), Acostasaurus pavachoquensis (Gómez-Pérez & Noè, Reference Gómez-Pérez and Noè2017), Kronosaurus queenslandicus (McHenry, Reference McHenry2009) and possibly Stenorhynchosaurus munozi (Páramo-Fonseca et al. Reference Páramo-Fonseca, Gómez-Pérez, Noè and Etayo-Serna2016), but unlike in Liopleurodon ferox (5; Noè, Reference Noè2001), ‘Kronosaurus’ boyacensis (5; Hampe, Reference Hampe1992), Simolestes vorax (5?; Noè, Reference Noè2001), Pliosaurus spp. (5–6?; Benson et al. Reference Benson, Evans, Smith, Sassoon, Moore-Faye, Ketchum and Forrest2013), Peloneustes philarchus (6; Ketchum & Benson, Reference Ketchum and Benson2011), Makhaira rossica (6; Fischer et al. Reference Fischer, Arkhangelsky, Stenshin, Uspensky, Zverkov and Benson2015) and Luskhan itilensis (7; Fischer et al. Reference Fischer, Benson, Zverkov, Soul, Arkhangelsky, Lambert, Stenshin, Uspensky and Druckenmiller2017).
The maxillary tooth count of FHSM VP-321 (lmx=19; rmx=18) is the same as that of Brachauchenius specimen MNA V9433 (Albright, Gillette & Titus, Reference Albright, Gillette and Titus2007) but probably lower than in most species ascribed to Pliosaurus, which are commonly estimated to have around 23–25 maxillary teeth (e.g. Knutsen, Reference Knutsen2012; Sassoon, Noè & Benton, Reference Sassoon, Noè and Benton2012; Benson et al. Reference Benson, Evans, Smith, Sassoon, Moore-Faye, Ketchum and Forrest2013; O'Gorman, Gasparini & Spolletti, Reference O'Gorman, Gasparini and Spalletti2018). It is also much lower than in Stenorhynchosaurus munozi, which has at least 29 functional alveoli in each maxilla (Páramo-Fonseca et al. Reference Páramo-Fonseca, Gómez-Pérez, Noè and Etayo-Serna2016), and probably slightly lower than in Acostasaurus pavachoquensis (lmx >19; rmx >15), in which, however, neither maxilla is complete (Gómez-Pérez & Noè, Reference Gómez-Pérez and Noè2017). It is also lower than in Liopleurodon ferox (~23; Noè, Reference Noè2001) and significantly lower than in Peloneustes philarchus (30–31; Ketchum & Benson, Reference Ketchum and Benson2011).
The dentary tooth count (ld=24) is similar to that of MNA V9433 (25 dentary teeth; Albright, Gillette & Titus, Reference Albright, Gillette and Titus2007) but seems to be significantly lower than in most species attributed to Pliosaurus: P. brachydeirus: >35, P. kevani: 36–37; however, P. carpenteri: 27, P. westburyensis: ~27 (Benson et al. Reference Benson, Evans, Smith, Sassoon, Moore-Faye, Ketchum and Forrest2013), P. almanzaensis: ld > 26, rd > 24 (O'Gorman, Gasparini & Spolletti, Reference O'Gorman, Gasparini and Spalletti2018). The dentary tooth count is also lower than in Liopleurodon ferox (25–(?)28; Noè, Reference Noè2001) and ‘Pliosaurus’ andrewsi (32; Noè, Reference Noè2001), and significantly lower than in Peloneustes philarchus (36–44; Ketchum & Benson, Reference Ketchum and Benson2011). In the specimens attributed to Simolestes vorax, however, the dentary tooth count seems to be unusually variable (19–29; Noè, Reference Noè2001). Interestingly, the dentary tooth count of A. pavachoquensis is lower than in M. eulerti (ld=22; rd=21; Gómez-Pérez & Noè, Reference Gómez-Pérez and Noè2017).
The tooth count in the symphyseal segment, which is often used as a diagnostic feature (see e.g. Noè, Smith & Walton, Reference Noè, Smith and Walton2004; Benson et al. Reference Benson, Evans, Smith, Sassoon, Moore-Faye, Ketchum and Forrest2013), is 6, as in MNA V9433 (Albright, Gillette & Titus, Reference Albright, Gillette and Titus2007), Liopleurodon ferox (Noè, Reference Noè2001), and similar to Kronosaurus queenslandicus (6 and a half; McHenry, Reference McHenry2009). However, it is much lower than in Makhaira rossica (>10; Fischer et al. Reference Fischer, Arkhangelsky, Stenshin, Uspensky, Zverkov and Benson2015), Stenorhynchosaurus munozi (≥10; Páramo-Fonseca et al. Reference Páramo-Fonseca, Gómez-Pérez, Noè and Etayo-Serna2016) and most species attributed to Pliosaurus: P. brachydeirus: >11, P. kevani: 14–15?, P. westburyensis: ~9?, P. carpenteri: 9; P. ‘portentificus’: 8 (Benson et al. Reference Benson, Evans, Smith, Sassoon, Moore-Faye, Ketchum and Forrest2013); P. almanzaensis: ≥9 (O'Gorman, Gasparini & Spolletti, Reference O'Gorman, Gasparini and Spalletti2018). Only P. patagonicus and P. rossicus, with 6 symphyseal teeth and ~25 dentary teeth in total, seem to have a similar dentary tooth count to that of M. eulerti (Gasparini & O'Gorman, Reference Gasparini and O'Gorman2014). The number of symphyseal alveoli is also substantially lower than in ‘Pliosaurus’ andrewsi (12; Noè, Reference Noè2001) and Peloneustes philarchus (13–15; Ketchum & Benson, Reference Ketchum and Benson2011). However, it is higher than in Simolestes vorax (5; Noè, Reference Noè2001) and Acostasaurus pavachoquensis (5 and a half; Gómez-Pérez & Noè, Reference Gómez-Pérez and Noè2017).
The dentition is subhomodont. No significant differences are observed in the shape of the tooth crowns and the development and distribution of the outer enamel structures throughout the tooth rows. The tooth crowns of M. eulerti are all subcircular in cross-section and do not possess carinae, as in Liopleurodon ferox (Tarlo, Reference Tarlo1960), ‘Pliosaurus’ andrewsi (Tarlo, Reference Tarlo1960), Acostasaurus pavachoquensis (Gómez-Pérez & Noè, Reference Gómez-Pérez and Noè2017), ‘Kronosaurus’ boyacensis (Hampe, Reference Hampe1992), Kronosaurus queenslandicus (Schumacher, Reference Schumacher2008; McHenry, Reference McHenry2009), Brachauchenius lucasi (Liggett et al. Reference Liggett, Shimada, Bennett and Schumacher2005; Albright, Gillette & Titus, Reference Albright, Gillette and Titus2007), ‘Polyptychodon’ hudsoni (D.M., pers. obs.), and the assemblage of isolated tooth crowns once attributed to ‘Polyptychodon’ (Madzia, Reference Madzia2016; Madzia & Machalski, Reference Madzia and Machalski2017), but differ from the teeth of Gallardosaurus iturraldei (Gasparini, Reference Gasparini2009), Pliosaurus spp. (Knutsen, Reference Knutsen2012; Benson et al. Reference Benson, Evans, Smith, Sassoon, Moore-Faye, Ketchum and Forrest2013; Gasparini & O'Gorman, Reference Gasparini and O'Gorman2014; O'Gorman, Gasparini & Spolletti, Reference O'Gorman, Gasparini and Spalletti2018), Makhaira rossica (Fischer et al. Reference Fischer, Arkhangelsky, Stenshin, Uspensky, Zverkov and Benson2015), Stenorhynchosaurus munozi (Páramo-Fonseca et al. Reference Páramo-Fonseca, Gómez-Pérez, Noè and Etayo-Serna2016) and Luskhan itilensis (Fischer et al. Reference Fischer, Benson, Zverkov, Soul, Arkhangelsky, Lambert, Stenshin, Uspensky and Druckenmiller2017), which are carinated and subtrihedral to trihedral, and the tooth crown of the Crimean pliosaurid (Zverkov, Reference Zverkov, Baraboshkin and Bykov2015), which is carinated and trihedral-to-‘trapezoid’ in its cross-sectional shape. The apicobasal ridges of some teeth are branching, as in Brachauchenius lucasi (Albright, Gillette & Titus, Reference Albright, Gillette and Titus2007), ‘Polyptychodon’ hudsoni (Madzia, Reference Madzia2016), Liopleurodon ferox (Noè, Reference Noè2001), and a few teeth once attributed to ‘Polyptychodon’ (Madzia, Reference Madzia2016; Madzia & Machalski, Reference Madzia and Machalski2017), but unlike in all other thalassophoneans. However, as noted by Madzia (Reference Madzia2016), the ‘Polyptychodon’ assemblage is most likely of multispecies character, and in the vast majority of ‘Polyptychodon’ tooth crowns the ridges do not branch.
5. Remarks on the cranial anatomy of M. eulerti
5.a. Measurements
FHSM VP-321 is dorsoventrally flattened but some morphological traits are measurable. However, all values should be regarded as approximate.
Anteroposterior length of the skull = ~1530 mm; anteroposterior length of the premaxillae (measured along the dorsal margin; to the premaxilla-parietal suture) = ~930 mm; maximum mediolateral width of the left premaxilla (measured perpendicular to the median suture) = ~70 mm; maximum anteroposterior length of the left maxilla (measured as the shortest distance between the ventralmost premaxilla–maxilla and maxilla–jugal contacts) = ~865 mm; maximum anteroposterior length of the left external naris = ~50 mm; maximum mediolateral width of the left external naris = ~17 mm; anteroposterior length of the pineal foramen = ~15 mm; width of the pineal foramen (measured perpendicular to the anteroposterior length) = ~14 mm; anteroposterior length of the postpineal segment of the skull = ~350 mm; maximum anteroposterior length of the parietal crest = ~230 mm; minimum distance between the temporal fenestrae (measured perpendicular to the anteroposterior axis of the skull) = ~230 mm; maximum width of the squamosal complex (measured perpendicular to the anteroposterior axis of the skull) = ~615 mm.
5.b. Remarks
Owing to its reasonably complete nature, sufficient state of preservation and its status as one of the youngest brachauchenines, the morphology of M. eulerti provides important insights into the course of the character evolution in the last pliosaurids.
The vast majority of the recent phylogenetic studies dealing with the interrelationships within Plesiosauria has been based on the dataset assembled by Benson & Druckenmiller (Reference Benson and Druckenmiller2014) which covers the most distinctive members of all currently recognized plesiosaur clades (see e.g. Benson et al. Reference Benson, Evans, Smith, Sassoon, Moore-Faye, Ketchum and Forrest2013; Fischer et al. Reference Fischer, Arkhangelsky, Stenshin, Uspensky, Zverkov and Benson2015; Cau & Fanti, Reference Cau and Fanti2016; Otero, Reference Otero2016; O'Gorman, Reference O'Gorman2016; Sachs, Hornung & Kear, Reference Sachs, Hornung and Kear2016a; Fischer et al. Reference Fischer, Benson, Zverkov, Soul, Arkhangelsky, Lambert, Stenshin, Uspensky and Druckenmiller2017; O'Gorman et al. Reference O'Gorman, Otero, Hiller, Simes and Terezow2017a,Reference O'Gorman, Panzeri, Fernández, Santillana, Moly and Reguerob; Sachs, Hornung & Kear, Reference Sachs, Hornung and Kear2017; Sachs & Kear, Reference Sachs and Kear2017; Serratos, Druckenmiller & Benson, Reference Serratos, Druckenmiller and Benson2017; Fischer et al. Reference Fischer, Benson, Druckenmiller, Ketchum and Bardet2018; O'Gorman, Gasparini & Spolletti, Reference O'Gorman, Gasparini and Spalletti2018; Otero, Soto-Acuña & O'Keefe, Reference Otero, Soto-Acuña and O'Keefe2018). The cranial anatomy of M. eulerti is evaluated here within the context of Benson & Druckenmiller's (Reference Benson and Druckenmiller2014) phylogenetic dataset. We took the M. eulerti OTU from a recent study focused on the origin and interrelationships of the brachauchenine pliosaurids (Fischer et al. Reference Fischer, Benson, Zverkov, Soul, Arkhangelsky, Lambert, Stenshin, Uspensky and Druckenmiller2017), reviewed all character states and rescored those for the following nine characters:
5.b.1. Character 25
Maxilla, posterior extent of maxillary tooth row: around orbital midlength or more anteriorly (0); ventral to postorbital bar (1); ventral to temporal fenestra midlength (2). Scored as ‘0’. The posterior extent of the maxillary tooth row reaches the anterior margin of the orbit (Fig. 7a). Originally scored as terminating ventrally to postorbital bar (1).
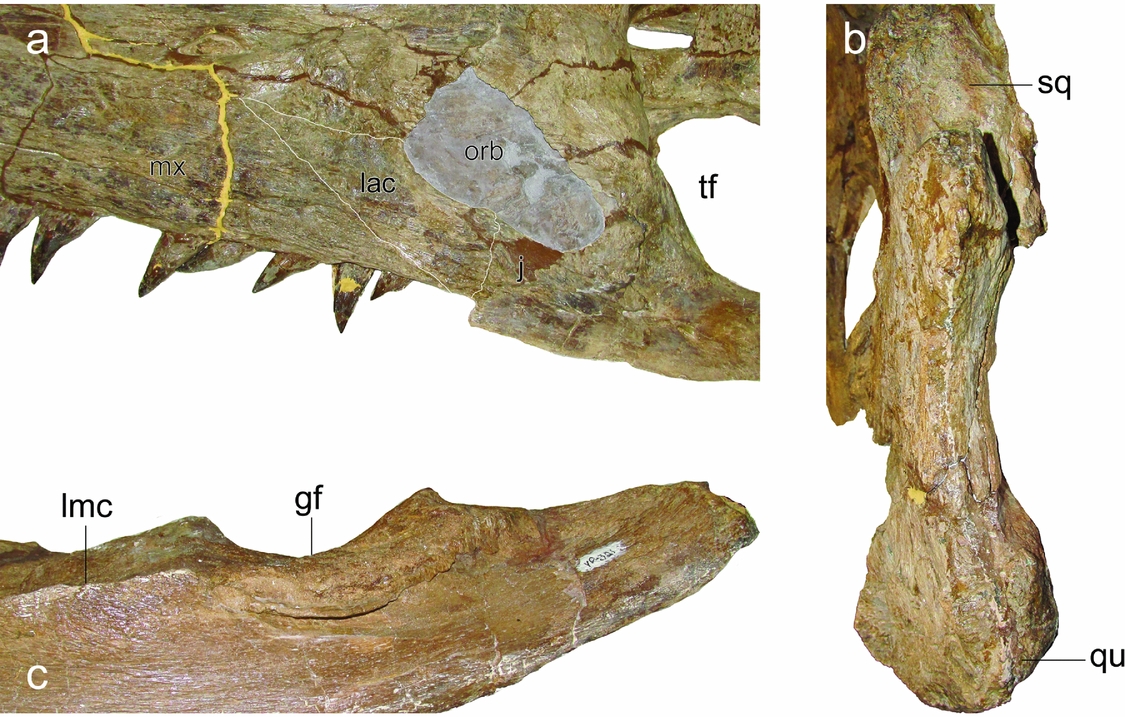
Figure 7. FHSM VP-321 (type specimen of Megacephalosaurus eulerti), illustration of selected revised character states: (a) posterior termination of maxilla at level of anterior edge of orbit (25.0); (b) quadrate–squamosal complex in posterior view – a squamosal-quadrate foramen is absent (59.0); and (c) posterior side of right mandibular ramus with longitudinal medial crest anterior to glenoid fossa (127.1). Abbreviations: gf, glenoid fossa; j, jugal; lac; lacrimal; lmc, longitudinal medial crest; mx, maxilla; orb, orbit; qu, quadrate; sq, squamosal; tf, temporal fenestra. No scale intended.
5.b.2. Character 27
Maxilla participation in internal naris: participates (0); does not participate (1). Scored as ‘0’. Originally scored as ‘unknown’ (?).
5.b.3. Character 59
Squamosal–quadrate foramen: absent (0); present (1). Scored as ‘0’ (Fig. 7b). Originally scored as ‘unknown’ (?).
5.b.4. Character 86
Parasphenoid, ventral surface anteriorly: covered by pterygoids anterior to the posterior interpterygoid vacuities (0); visible through V-shaped notch in posterior pterygoid contact anterior to posterior interpterygoid vacuities (1). Originally scored as ‘0’. However, precise determination of the character state is difficult due to slight displacement of the elements (Fig. 8). As such, we scored it as ‘unknown’ (?).

Figure 8. FHSM VP-321 (type specimen of Megacephalosaurus eulerti), detail of the palatal segment in ventral view showing slight displacement of the elements. Abbreviations: aiv, anterior interpterygoid vacuity; piv, posterior interpterygoid vacuity; ps, parasphenoid; pt, pterygoid.
5.b.5. Character 114
Structure of the dentary along the ventral surface of the mandibular symphysis: no ventral elaboration (0); forms raised ventral platform or sharp keel/ridge adjacent to symphysis (1). There is no elaboration of the dentary along the ventral surface of the mandibular symphysis (0). Originally scored as ‘unknown’ (?).
5.b.6. Character 127
Surangular, fossa and longitudinal crest on medial surface anterior to glenoid: prominent longitudinal crest forms ventral margin of deep, dorsomedially facing surangular fossa (0); prominent longitudinal crest forms medial margin of mediolaterally expanded dorsal surface of surangular bearing shallow, dorsally facing fossa (1); crest and surangular fossa weak or absent, dorsal portion of surangular ‘blade-like’ (2); dorsolaterally facing fossa bounded laterally by a sharp crest (3). Scored as ‘1’ (Fig. 7c). Originally scored as ‘unknown’ (?).
5.b.7. Character 128
Coronoid length and morphology: long, approaching or participating in symphysis (0); small, superficial element that is often disarticulated and thus not preserved, but represented by a facet on the surangular (1). The coronoid is long and probably participates in the symphysis (0). Originally scored as ‘unknown’ (?).
5.b.8. Character 129
Prearticular, large dorsomedian trough or rugosity: absent or weak (0); present (1).There is no dorsomedian trough or rugosity on the prearticular (0). Originally scored as ‘unknown’ (?).
5.b.9. Character 138
Number of maxillary teeth: 12–17 (0); 20–25 (1); >28 (2). In the previous versions of Benson & Druckenmiller's (Reference Benson and Druckenmiller2014) dataset, M. eulerti was scored for state ‘1’ (20–25). However, FHSM VP-321 had 18 teeth in the right and 19 in the left maxilla. Thus, none of the states covers the taxon. Considering that scoring tooth counts is often problematic (see also Section 7.c), we provisionally keep two different scores for this character, ‘0’ and ‘1’, and explore the effects of these settings (see Section 2.d).
6. Parsimony analyses
We performed two runs of unweighted parsimony analyses with all multistate characters set as unordered, and additional two runs with a subset of multistate characters set as ordered (for rationale see e.g. Brazeau, Reference Brazeau2011; Madzia & Cau, Reference Madzia and Cau2017; see online Supplementary Material at http://journals.cambridge.org/geo for list of characters and their settings).
In all these four runs, we rescored eight character states in the M. eulerti OTU (25, 27, 59, 86, 114, 127, 128 and 129; see above for descriptions of the applied modifications). Two runs instead of a single run of each unweighted parsimony analysis (‘unordered’ and ‘ordered’) were performed to test the effects of the application of different scores for character 138 in M. eulerti and the Brachauchenius specimen MNA V9433, which share the same maxillary tooth count (lmx=19; rmx=18) and which cannot be scored for the current version of Benson & Druckenmiller's (Reference Benson and Druckenmiller2014) dataset as none of the states covers them.
(1) Two runs of parsimony analyses (one ‘unordered’ and one ‘ordered’) had the character 138 scored for state ‘1’ (as in Fischer et al. Reference Fischer, Benson, Zverkov, Soul, Arkhangelsky, Lambert, Stenshin, Uspensky and Druckenmiller2017). Therefore, in these two runs the state ‘1’ is redefined to cover the OTUs with 18–25 maxillary teeth (instead of the original 20–25).
(2) Other two runs of both parsimony analyses (again one ‘unordered’ and one ‘ordered’) had the character scored for state ‘0’ because comparisons of the dentition of M. eulerti with those of other thalassophoneans show that the last brachauchenines had a lower tooth count in the maxillae than their predecessors. Hence, the state ‘0’ is redefined in these runs to cover OTUs with 12–19 maxillary teeth (instead of the original 12–17).
6.a. Results
In the first run of ‘unordered’ parsimony analysis (1stUOPA; M. eulerti and MNA V9433 scored as ‘1’), the ‘New Technology’ search inferred 18 most parsimonious trees (MPTs) of length 1535 (CI=0.258; RI=0.678). The ‘Traditional search’ using the trees from RAM reconstructed 10000 MPTs (maximum memory). The ‘New Technology’ search in the second run of ‘unordered’ parsimony analysis (2ndUOPA; M. eulerti and MNA V9433 scored as ‘0’) reconstructed 27 trees of length 1536 (CI=0.258; RI=0.677). The ‘Traditional search’ inferred again 10000 MPTs.
In the first run of ‘ordered’ parsimony analysis (1stOPA; M. eulerti and MNA V9433 scored as ‘1’), the ‘New Technology’ search reconstructed 27 MPTs of length 1618 (CI=0.245; RI=0.684). The ‘New Technology’ search in the second run of ‘ordered’ parsimony analysis (2ndOPA; M. eulerti and MNA V9433 scored as ‘0’) inferred 25 MPTs of length 1619 (CI=0.245; RI=0.684). In both these runs, the subsequent ‘Traditional search’ using the trees from RAM inferred 10000 MPTs.
All four runs produced a large polytomy at the base of the Pliosaurus + Brachaucheninae node and in the smallest clade containing the mid- to Late Cretaceous brachauchenines Kronosaurus queenslandicus and Brachauchenius lucasi (Fig. 9). The Decay Index (Bremer support) and bootstrap values calculated for both these nodes were very low (DI: 1; bootstrap support <0.05) and do not merit additional discussion.
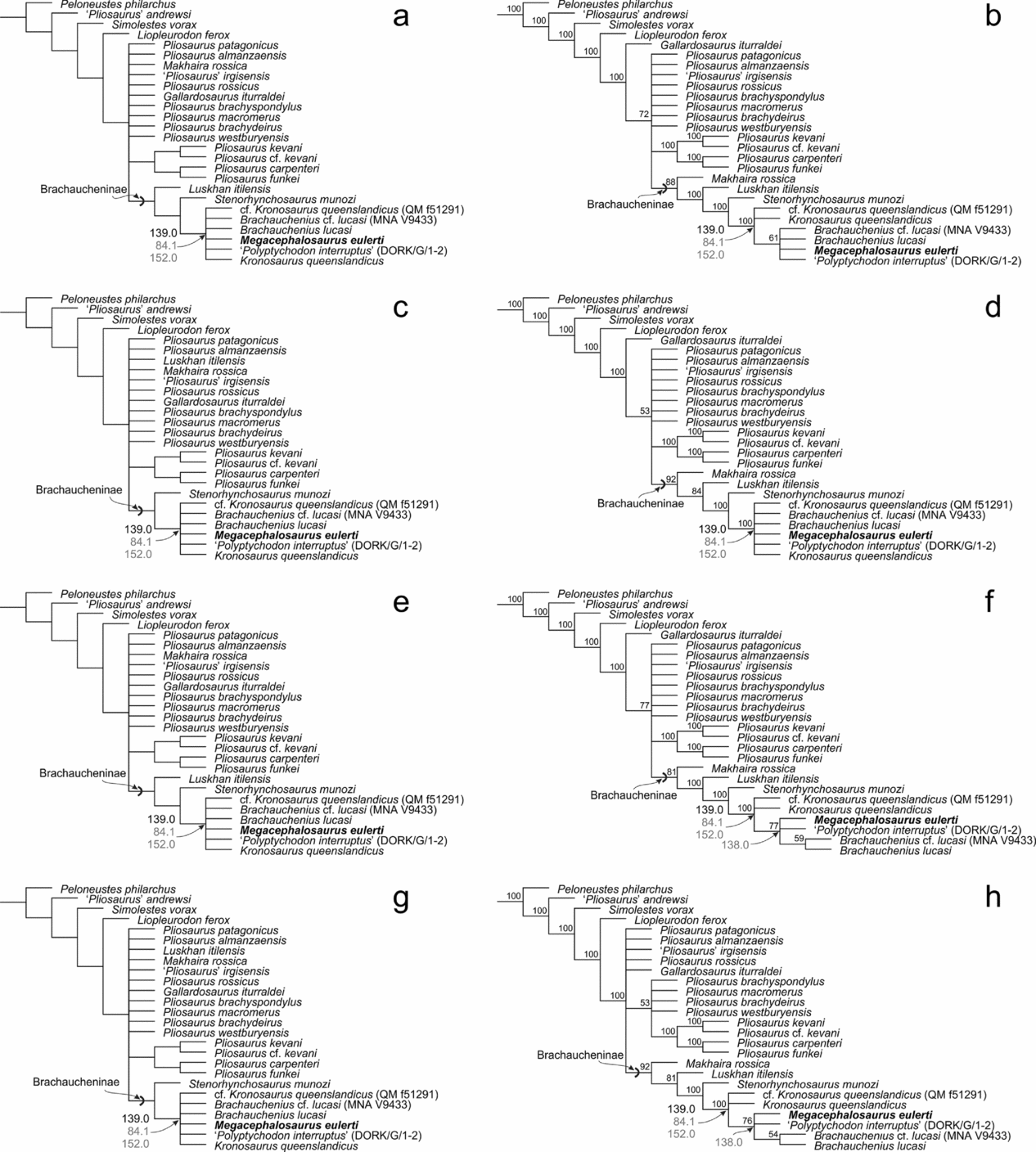
Figure 9. The tree topologies showing inferred phylogenetic relationships among thalassophonean pliosaurids with unambiguous (black) and ambiguous (grey) synapomorphies of mid- to Late Cretaceous brachauchenines, focusing especially on tooth-associated characters discussed in the text: (a) strict consensus tree of the 1stUOPA, (b) majority rule consensus tree of the 1stUOPA, (c) strict consensus tree of the 1stOPA, (d) majority rule consensus tree of the 1stOPA, (e) strict consensus tree of the 2ndUOPA, (f) majority rule consensus tree of the 2ndUOPA, (g) strict consensus tree of the 2ndOPA and (h) majority rule consensus tree of the 2ndOPA.
The Kronosaurus + Brachauchenius node can be diagnosed by a single unambiguous synapomorphy (139: 1→0; i.e. presence of subcircular rather than subtrihedral/trihedral cross-sectional shape of the teeth) and additional two ambiguous synapomorphies (84: 2→1; i.e. posterior termination of parasphenoid just anterior to basioccipital–basisphenoid contact on ventral surface of the basicranium; and 152: 1→0; i.e. reduction in the number of cervical vertebrae).
The modifications of scores for character 138 did not add much to the overall resolution of the latest brachauchenines (Fig. 9e–h) though a major subset of MPTs resulting from 2ndUOPA and 2ndOPA mapped the reduction in tooth count (138: 1→0) as the only synapomorphy of the least inclusive clade containing M. eulerti and MNA V9433 (often reconstructed together with DORK/G/1-2, the ‘Dorking specimen’ of ‘Polyptychodon interruptus’, and B. lucasi; both of which, however, have the character 138 scored as ‘unknown’ due to their insufficient state of preservation).
7. Discussion
7.a. Variability in the dentition of Megacephalosaurus and the taxonomic utility of branching ridges
The dental morphology of Megacephalosaurus eulerti exhibits a low degree of variability, with the most striking differences being observable in the development of the apicobasal ridges. The distribution of the ridges around the crown circumference, their density and the apicobasal extent are near-uniform throughout the dentition. However, some of the crowns possess a distinctive branching pattern of the ridges, which contrasts with the non-branching ridges in other tooth crowns.
The character and distribution of the apicobasal ridges is known to be diagnostic for some taxa (e.g. Tarlo, Reference Tarlo1960), and branching ridges alone have already been used as a character distinguishing Brachauchenius from the classic European taxon ‘Polyptychodon’ (VonLoh & Bell, Reference VonLoh and Bell1998; Angst & Bardet, Reference Angst and Bardet2016). Still, the taxonomic utility of this feature seems to be largely elusive as some late Albian–Cenomanian teeth once assigned to the ‘Polyptychodon’ assemblage (CAMSM TN 1716.5 and MWGUW ZI/60/001; Madzia, Reference Madzia2016; Madzia & Machalski, Reference Madzia and Machalski2017; respectively), or the type tooth of Liopleurodon ferox (BHN 3R 197; Noè, Reference Noè2001), also show occasional branching ridges near the base of the tooth crowns. At the same time, in most teeth of M. eulerti, the presence of branching ridges cannot be confirmed.
Perhaps, there might be some taxonomic utility when branching ridges are observed in a part of the tooth crown other than immediately adjacent to cervix dentis because this section often shows scattered ridges (Madzia, Reference Madzia2016), which might result from growth abnormalities. If correct, the distinctive branching pattern of the apicobasal ridges, as observed in some tooth crowns of Brachauchenius lucasi, Megacephalosaurus eulerti and ‘Polyptychodon’ hudsoni, might have a common origin.
7.b. Implications for assessments of isolated brachauchenine teeth
The knowledge of the variability in the pliosaurid dentitions is particularly relevant when assessing assemblages of isolated tooth crowns. In brachauchenines, it is important especially for the isolated teeth commonly attributed to ‘Polyptychodon interruptus’. This taxon was originally described from the mid-Cretaceous of England based mainly on isolated teeth (e.g. Owen Reference Owen1841a,b, Reference Owen and Dixon1850, Reference Owen1851, Reference Owen1860, Reference Owen1861; Seeley, Reference Seeley1869). Recently, these teeth were reappraised by Madzia (Reference Madzia2016) who concluded that the assemblage is probably too variable to belong to a single species. The low variability observed in the dentition of M. eulerti seems to support this conclusion. For example, Madzia (Reference Madzia2016) observed significant differences in the density and distribution of the apicobasal ridges (Madzia, Reference Madzia2016: Fig. 4). Some ‘Polyptychodon’ specimens further showed a possible taxonomically relevant pattern in the arrangement of the ridges (Madzia, Reference Madzia2016: Fig. 5). Differences were also observed in the appearance of the outer enamel surface. Some ‘Polyptychodon’ teeth exhibited only very slight roughening of the enamel exposed between the apicobasal ridges (Madzia, Reference Madzia2016: Figs 3, 5). In turn, the enamel surface of other tooth crowns was covered by very rough vermicular ridglets (‘striae’ of Madzia, Reference Madzia2016: Fig. 7). Considering these features, the teeth from the ‘Polyptychodon’ assemblage would occupy a much broader morphospace than those of M. eulerti. Therefore, if representing a single taxon, the dental variability of ‘Polyptychodon’ would be much higher than that of its closest relatives.
7.c. Teeth and the character scores in phylogenetic datasets
Scoring teeth in phylogenetic datasets has proven tricky. This is particularly true for characters involving tooth counts, as the number of teeth may drastically change during ontogeny following shifts in dietary preferences (e.g. Wang et al. Reference Wang, Stiegler, Amiot, Wang, Du, Clark and Xu2017). However, this problem can be eliminated after proper recognition of the ontogenetic patterns of a species and when only those characters present in adult specimens are scored in data matrices.
A much broader problem with the tooth count scores was recently brought up by Madzia & Cau (Reference Madzia and Cau2017), who discussed the data sampling used for the inferences of mosasauroid phylogenetic relationships. Madzia & Cau (Reference Madzia and Cau2017) commented on a six-state character describing the dentary tooth count in mosasauroids (‘Dentary tooth number: 20–24 (0); 17–19 (1); 15–16 (2); 14 (3); 13 (4); 12 (5)’). They concluded that the character state definitions do not work for taxa in which the tooth count is considered as one of only a few characters that distinguish them. Specifically, they noted that the gigantic mosasaurine Mosasaurus hoffmannii can be scored for three of those six states and is covered under the same character state as its supposed relative Mosasaurus lemonnieri (state 2) even though all known individuals of the latter have a higher dentary tooth count (16) than M. hoffmannii (13–15).
Plesiosaur phylogenetics seems to be facing a similar problem. Extreme shifts in tooth counts have never been observed in plesiosaurs. However, the specimens attributed to the Callovian (Middle Jurassic) thalassophoneans Simolestes vorax and Peloneustes philarchus show apparent variability in the number of alveoli (Noè, Reference Noè2001; Ketchum & Benson, Reference Ketchum and Benson2011). In S. vorax, for instance, the dentary tooth counts vary between 19 (rd of NHMUK R3170) and 29 (ld and rd of GPIT 3).
As noted in Section 5, Megacephalosaurus cannot be scored for character 138 of Benson & Druckenmiller's (Reference Benson and Druckenmiller2014) dataset because none of the states covers it. The same applies to Brachauchenius specimen MNA V9433 (lower Turonian of Kane County, Utah) which has the same number of maxillary alveoli as FHSM VP-321: 18 in the right and 19 in the left maxilla (Albright, Gillette & Titus, Reference Albright, Gillette and Titus2007). However, FHSM VP-321 and MNA V9433 slightly differ in their dentary tooth counts: the left dentary of FHSM VP-321 has 24 alveoli while both dentaries of MNA V9433 have 25.
In the most recent version of Benson & Druckenmiller's (Reference Benson and Druckenmiller2014) dataset, published by Fischer et al. (Reference Fischer, Benson, Zverkov, Soul, Arkhangelsky, Lambert, Stenshin, Uspensky and Druckenmiller2017), the maxillary tooth count was scored in 14 of the 24 thalassophonean OTUs. Except for Peloneustes philarchus and the late Barremian (Early Cretaceous) brachauchenine Stenorhynchosaurus munozi, which are scored for state 2 (>28 teeth in the maxillae), all other OTUs, including FHSM VP-321 and MNA V9433, were scored for state 1 (20–25 maxillary teeth). Undoubtedly, such state definitions (i.e. tooth-count extents) might cover some uncertainties stemming from the incomplete character of the analysed material and its state of preservation (see Benson et al. Reference Benson, Evans, Smith, Sassoon, Moore-Faye, Ketchum and Forrest2013, table 1). At the same time, however, they do not necessarily reflect the actual differences between OTUs or clades. For example, most species attributed to Pliosaurus would likely be characterized by the upper limits of state 1 (e.g. Knutsen, Reference Knutsen2012; Sassoon, Noè & Benton, Reference Sassoon, Noè and Benton2012; Benson et al. Reference Benson, Evans, Smith, Sassoon, Moore-Faye, Ketchum and Forrest2013; O'Gorman, Gasparini & Spolletti, Reference O'Gorman, Gasparini and Spalletti2018). In turn, the tooth counts in the last brachauchenines would be oscillating around its lower boundary. Still, both these groups, Pliosaurus spp. and last brachauchenines, have been placed within the same state.
Treatment of such characters in phylogenetic datasets is admittedly problematic (see e.g. Wiens, Reference Wiens2001). As such, using tooth-count extents in character definitions and reasonable extrapolations while scoring taxa seems fully justified. Nevertheless, considering that the dentitions of the last brachauchenines clearly differ from those of their predecessors, the character of the changes in teeth should be investigated in more depth and using multiple approaches to character sampling, character construction and the applied analytical methods (see e.g. Ketchum & Benson, Reference Ketchum and Benson2010).
7.d. The evolution of pliosaurid teeth and dentitions
A recent burst of studies involving detailed morphological assessments of pliosaurid teeth (e.g. Fischer et al. Reference Fischer, Arkhangelsky, Stenshin, Uspensky, Zverkov and Benson2015; Zverkov, Reference Zverkov, Baraboshkin and Bykov2015; Madzia, Reference Madzia2016; Fischer et al. Reference Fischer, Benson, Zverkov, Soul, Arkhangelsky, Lambert, Stenshin, Uspensky and Druckenmiller2017; Gómez-Pérez & Noè, Reference Gómez-Pérez and Noè2017; Madzia & Machalski, Reference Madzia and Machalski2017; Zverkov et al. Reference Zverkov, Fischer, Madzia and Benson2018) resulted in a better understanding of their evolutionary history. Specifically, the new studies have revealed substantial differences between teeth of particular pliosaurid taxa and suggest intriguing oscillations in their disparity through time. For instance, Zverkov et al. (Reference Zverkov, Fischer, Madzia and Benson2018) gathered a number of continuous and discrete characters summarizing the overall dental morphologies of the vast majority of diagnosable thalassophonean taxa and analysed their disparity patterns. The results suggested an increased dental disparity across the Jurassic–Cretaceous transitional interval. In post-Barremian taxa, the disparity decreased. At the same time, new pliosaurid dental material described by Zverkov et al. (Reference Zverkov, Fischer, Madzia and Benson2018) challenges the ‘traditional’ hypothesis reconstructed by all recently published phylogenetic analyses (including those presented here) that only one pliosaurid lineage crossed the Jurassic–Cretaceous boundary. In fact, at least two lineages, clearly distinguishable by their tooth crown morphologies, likely persisted into the Cretaceous. Such a possibility has already been discussed by Fischer et al. (Reference Fischer, Arkhangelsky, Stenshin, Uspensky, Zverkov and Benson2015), Zverkov (Reference Zverkov, Baraboshkin and Bykov2015), Madzia (Reference Madzia2016) and Madzia & Machalski (Reference Madzia and Machalski2017). Nevertheless, testing the hypothesis using phylogenetic analyses is currently difficult, especially due to the limited amount of non-dental pliosaurid remains from the Lower Cretaceous.
The pliosaurids from the Aptian to Turonian are characterized by a single type of tooth crowns which are conical (subcircular in cross-section). Still, due to our limited knowledge of the Early Cretaceous pliosaurids, we cannot determine at this moment whether the conical-toothed taxa evolved from the trihedral-toothed ones (as suggested by all recently inferred phylogenies, including ours) or whether they represent ‘remnants’ of a distinct lineage with deep Jurassic roots but almost unknown Late Jurassic/Early Cretaceous members, merely represented by isolated tooth crowns (such as those described by Zverkov et al. Reference Zverkov, Fischer, Madzia and Benson2018) and perhaps some non- or poorly diagnosable skeletal elements. Alternatively, both types of tooth crowns might have originated and reversed several times.
The data further suggest that the dentitions of the mid-Cretaceous pliosaurids switched from anisodont to subisodont and reduced the number of alveoli. The precise course and timing of these modifications, however, remains uncertain. Subisodonty is present in the remarkably apomorphic taxon Luskhan from the upper Hauterivian of Russia (Fischer et al. Reference Fischer, Benson, Zverkov, Soul, Arkhangelsky, Lambert, Stenshin, Uspensky and Druckenmiller2017) and possibly also Stenorhynchosaurus from the upper Barremian of Colombia (Páramo-Fonseca et al. Reference Páramo-Fonseca, Gómez-Pérez, Noè and Etayo-Serna2016). However, the dentitions of the lower Barremian taxon Acostasaurus and the Aptian–Albian taxon Kronosaurus are markedly anisodont. Subisodonty then appeared in the common ancestor of the Cenomanian–Turonian taxa Brachauchenius and Megacephalosaurus. It seems, thus, that subisodonty evolved in Luskhan, Stenorhynchosaurus and Brachauchenius + Megacephalosaurus independently.
The reduction in the tooth count, as observed in the youngest brachauchenines, might have appeared already in the taxa with anisodont dentitions. This is indicated by the discovery of Acostasaurus which possesses an anisodont dentition but a tooth count comparable to that of Brachauchenius and Megacephalosaurus. However, better understanding of the peculiar combination of characters present in that taxon, and the morphological disparity in the Early Cretaceous thalassophoneans in general, requires detailed phylogenetic assessments following the full preparation of the type of A. pavachoquensis and additional discoveries of Berriasian–Barremian brachauchenines.
7.e. The distinguishability of the last brachauchenines
Two Late Cretaceous brachauchenine taxa are currently regarded as valid: Brachauchenius lucasi Williston, Reference Williston1903, from the lower or lower middle Turonian of Kansas and Megacephalosaurus eulerti Schumacher, Carpenter & Everhart, Reference Schumacher, Carpenter and Everhart2013, from the lower middle Turonian of Kansas. Another potentially valid taxon, ‘Polyptychodon’ hudsoni Welles & Slaughter, Reference Welles and Slaughter1963, was described from the Turonian of Texas. All these taxa were established based on cranial material (USNM 4989, FHSM VP-321 and SMU 60313, respectively). Further specimens attributed to B. lucasi include skull material (USNM 2361), possibly from the Lake Waco Formation (Turonian), near Austin, Texas (Williston, Reference Williston1907; Schumacher, Carpenter & Everhart, Reference Schumacher, Carpenter and Everhart2013), cranial and postcranial material of two individuals (MNA V9432, MNA V9433) from the lower Turonian of Glen Canyon National Recreation Area, Kane County, Utah (Albright, Gillette & Titus, Reference Albright, Gillette and Titus2007), and an incomplete mandible (MNHN GOU 11) from the middle Turonian of the Goulmima region, Errachidia Province, Morocco (Angst & Bardet, Reference Angst and Bardet2016). In turn, M. eulerti is also known from an incomplete skull (UNSM 50136) that probably originates from the lowermost Turonian of the upper Greenhorn Limestone or the lowermost section of the Fairport Chalk Member, Carlile Shale, Kansas (Schumacher, Reference Schumacher2008; Schumacher, Carpenter & Everhart, Reference Schumacher, Carpenter and Everhart2013). Finally, VonLoh & Bell (Reference VonLoh and Bell1998) reported on four specimens that they assigned to Polyptychodon interruptus. These included a nearly complete mandible with posterior parts of the skull (SDSM 34991), two tooth fragments (SDSM 35004 and SDSM 35005) and fragments of an additional mandible (SDSM 35006); all from different localities falling within the upper Cenomanian Greenhorn Limestone in South Dakota.
Even though the distinctiveness of FHSM VP-321 from the hypodigm of B. lucasi has never been questioned, it was suggested that M. eulerti is also included within Brachauchenius, as B. eulerti (Benson et al. Reference Benson, Evans, Smith, Sassoon, Moore-Faye, Ketchum and Forrest2013; p. 30, fig. 23). In fact, in their phylogenetic analysis, Benson et al. (Reference Benson, Evans, Smith, Sassoon, Moore-Faye, Ketchum and Forrest2013) separated Brachauchenius into three OTUs: B. lucasi, B. eulerti and the specimen MNA V9433 (lower Turonian of Utah; Albright, Gillette & Titus, Reference Albright, Gillette and Titus2007) that was explicitly considered to represent another species of Brachauchenius. Benson et al. (Reference Benson, Evans, Smith, Sassoon, Moore-Faye, Ketchum and Forrest2013) inferred all these OTUs in a polytomy (together with DORK/G/1-2; the ‘Dorking specimen’ of ‘Polyptychodon interruptus’). More recent analyses of brachauchenine phylogenetic relationships (see Fischer et al. Reference Fischer, Arkhangelsky, Stenshin, Uspensky, Zverkov and Benson2015, Reference Fischer, Benson, Zverkov, Soul, Arkhangelsky, Lambert, Stenshin, Uspensky and Druckenmiller2017), including ours that used a modified version of Fischer et al.’s (Reference Fischer, Benson, Zverkov, Soul, Arkhangelsky, Lambert, Stenshin, Uspensky and Druckenmiller2017) data sampling, were not particularly conclusive either. However, a reduced consensus tree inferred by Fischer et al. (Reference Fischer, Benson, Zverkov, Soul, Arkhangelsky, Lambert, Stenshin, Uspensky and Druckenmiller2017, fig. 3B) and two runs of our parsimony analyses (2ndUOPA and 2ndOPA) suggest that B. lucasi and MNA V9433 are more closely related to each other than either is to M. eulerti (Fig. 9f, h).
Considering that the Late Cretaceous brachauchenine record consists mostly of cranial material, one way to improve the resolution of their phylogenetic relationships is to perform an expanded specimen-level phylogenetic analysis which might quantify the differences between particular specimens and test some of the previously proposed hypotheses (such as the conspecificity of FHSM VP-321, the type skull of M. eulerti, and UNSM 50136 – the second specimen referred to the taxon; Schumacher, Carpenter & Everhart, Reference Schumacher, Carpenter and Everhart2013).
8. Conclusions
The Turonian brachauchenine Megacephalosaurus eulerti represents one of the last and best-preserved thalassophonean pliosaurids on record. Therefore, details of its morphological features might be crucial for inferences of phylogenetic relationships of brachauchenine pliosaurids and rates of their morphological evolution shortly before the extinction of the clade.
The study of the dental morphology of M. eulerti shows that its dentition was subisodont and subhomodont, with the most apparent differences being observed in the development of the apicobasal ridges, which tend to branch in some teeth. However, the taxonomic utility of this feature is debatable and perhaps depends on the part of the tooth crown where it develops. Nevertheless, the overall variability in the dentition of M. eulerti is low, which might have an impact on studies assessing assemblages of isolated pliosaurid teeth.
Revision of the cranial anatomy of M. eulerti further allowed a reassessment of some of its morphological characters used in recent phylogenetic studies. We performed four runs of parsimony analyses which inferred a single unambiguous synapomorphy uniting the node comprising mid- to Late Cretaceous brachauchenines (presence of conical teeth with a subcircular cross-sectional shape). The latest brachauchenines (Brachauchenius, Megacephalosaurus) can be also roughly characterized by a switch from anisodont to subisodont dentition and reduction of their tooth count.
However, the origin of brachauchenines and phylogenetic relationships and distinguishability among the last members of the clade still remain somewhat elusive and would probably improve following modifications in character sampling and by using an expanded specimen-level matrix.
Acknowledgements
We would like to thank Mike Everhart (FHSM) for access to FHSM VP-321 and Bruce Schumacher (US Forrest Service, Colorado, USA) for providing us with the original Sternberg's notes. Christina Byrd (FHSM) kindly took additional detailed pictures of FHSM VP-321 for us. We are further indebted to Katrin Sachs (Engelskirchen, Germany) for taking measurements of FHSM VP-321, Roger Benson (University of Oxford, UK) for discussion and clarification of some of the character state scores in M. eulerti and Joschua Knüppe (Ibbenbüren, Germany) for the reconstruction of FHSM VP-321 (Fig. 2). Detailed reviews by Nikolay Zverkov (Lomonosov Moscow State University, Russia) and an anonymous reviewer improved the final version of the manuscript. The study was supported through a Grant for Distinguished Young Researchers (award number 642-2014-3773; Swedish Research Council) to J.L. The programme TNT is made available with the sponsorship of the Willi Hennig Society.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0016756818000523.