Introduction
No crustose lichen genus in the Holarctic has been revised in every major sector of the boreal forest. Eastern Asia has long constituted a large knowledge gap in the biogeography of lichens, especially crustose lichens. This is all the more unfortunate due to the central role that eastern Asia is known to have played in the evolution of higher plants (Wen Reference Wen1999; Xiang et al. Reference Xiang, Soltis, Soltis, Steven and Crawford2000; Qian Reference Qian2002), and the need for testable hypotheses in lichen biogeography that include eastern Asia.
Rinodina (Ach.) Gray is one of the better understood genera across the Holarctic, modern monographic treatments being available for the Iberian Peninsula (Giralt Reference Giralt2001), Scandinavia (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002), North America (Sheard Reference Sheard2010) and the saxicolous species of the Old World (Mayrhofer Reference Mayrhofer1984). Though polyphyletic (Helms et al. Reference Helms, Friedl and Rambold2003; Kaschik Reference Kaschik2006; Nadyeina et al. Reference Nadyeina, Grube and Mayrhofer2010; Resl et al. Reference Resl, Mayrhofer, Clayden, Spribille, Thor, Tønsberg and Sheard2016), species-level concepts within Rinodina are becoming increasingly well established and clear outlines of species groups have begun to emerge in conjunction with a refined understanding of crustose-macrolichen transitions. Particularly clear patterns of biogeography have come into focus with the availability of molecular data from different parts of the world (Resl et al. Reference Resl, Mayrhofer, Clayden, Spribille, Thor, Tønsberg and Sheard2016). The present paper is a first attempt at an inclusive treatment of the genus in north-eastern Asia.
The Japanese lichen biota has long been one of the most studied in Asia but its crustose lichen biota is still poorly known. Reports of 25 and 22 species of Rinodina, respectively, in two earlier checklists (Kurokawa Reference Kurokawa2003; Harada et al. Reference Harada, Okomoto and Yoshimura2004) were trimmed back in the synonymized list of Kurokawa & Kashiwadani (Reference Kurokawa and Kashiwadani2006), who collated data showing that several taxa belong in other genera (e.g. Buellia) and accepted 18 species of Rinodina for Japan. Two new corticolous species have recently been added to the lichen biota of Japan, R. chrysidiata Sheard (Lendemer et al. Reference Sheard, Lendemer, Spribille and Tønsberg2012) and R. buckii Sheard (Sheard et al. Reference Sheard, Lendemer, Spribille and Tønsberg2012), bringing the total to 20 species. This compares to 33 species for the British Isles (Giavarini et al. Reference Giavarini, James and Purvis2009), to which should be added R. intermedia Bagl. (Mayrhofer et al. Reference Mayrhofer, Sheard, Grassler and Elix2001), making a total of 34 species for a comparable maritime archipelago only 65% of the area of Japan.
Fewer species have been reported from Korea. Rinodina membranifera (Hue) Zahlbr. was the one Rinodina (except Dimelaena oreina (Ach.) Norman, which the authors included in Rinodina) reported in the only two Korean checklists published to date (Hur et al. Reference Hur, Koh and Harada2005; Moon Reference Moon2013). Rinodina chrysidiata and R. buckii were added to the list of known lichen biota by Lendemer et al. (Reference Lendemer, Sheard, Thor and Tønsberg2012) and Sheard et al. (Reference Sheard, Lendemer, Spribille and Tønsberg2012), respectively. Joshi et al. (Reference Joshi, Kondratyuk, Crişan, Jayalal, Oh and Hur2013) reported R. oleae Bagl. as new to South Korea. Kondratyuk et al. (Reference Kondratyuk, Lökös, Tschabanenko, Haji Moniri, Farkas, Wang, Oh and Hur2013) reported R. fimbriata Körb., R. oleae (as new to Korea a second time), R. polyspora Th. Fr., R. pyrina (Ach.) Arnold, R. sophodes (Ach.) A. Massal. and R. teichophila (Nyl.) Arnold as new to South Korea. Aptroot & Moon (Reference Aptroot and Moon2014) reported a further four species: R. cinereovirescens (Harm.) Zahlbr. R. laevigata (Ach.) Malme, R. placynthielloides Aptroot and R. xanthomelana Müll. Arg. Finally, Kondratyuk et al. (Reference Kondratyuk, Lökös, Halda, Haji Moniri, Farkas, Park, Lee, Oh and Hur2016) reported R. xanthophaea (Nyl.) Zahlbr., making a total of 14 species for Korea.
Twenty-three species of Rinodina are accepted as occurring in the three main sectors of the Russian Far East (arctic, northern and southern forested) in the most recent Russian checklist (Urbanavichus & Andreev Reference Urbanavichus and Andreev2010). However, this tally missed or discounted pre-2010 reports from the “far eastern” sectors of Russia of R. gennarii Bagl. (Bredkina et al. Reference Bredkina, Dobrysh, Makarova and Titov1992; Chabanenko Reference Chabanenko2002), R. polyspora and R. trevisanii (Hepp) Körb. (Chabanenko Reference Chabanenko2002). Two further species, R. kozukensis (Vain.) Zahlbr. and R. teichophila, were added by Skirina (Reference Skirina2010), while Galanina et al. (Reference Galanina, Yakovchenko, Tsarenko and Spribille2011) reported R. excrescens Vain. from numerous localities in the Russian Far East, Sheard et al. (Reference Sheard, Lendemer, Spribille and Tønsberg2012) reported R. buckii from the region, Lendemer et al. (Reference Lendemer, Sheard, Thor and Tønsberg2012) included Russian localities in their description of R. chrysidiata, and Urbanavichene & Palice (Reference Urbanavichene and Palice2016) reported R. efflorescens from the Stanovoye Nagor’e Mountains, making a total of 32 previously reported species.
Materials and Methods
This study is based primarily on collections by the authors, with the exception of JWS. Type specimens of all corticolous species described from Japan have been examined. For Japan, the northern island of Hokkaido has been the region most densely sampled. In far eastern Russia, collections have primarily been made from Zabaikalskiy Krai, Khabarovskiy Krai, Primorskiy Krai, Sakhalin Island and the Kamchatka Peninsula. The vast majority of collections examined were newly made. Unfortunately, it was not possible to freely borrow material that is the basis for previous reports in the literature, such as in Chabanenko (Reference Chabanenko2002) or Skirina (Reference Skirina2010), because of onerous restrictions on the mailing of scientific material by the Russian postal service.
Habit observations of specimens were made using a Wild M5 stereomicroscope. Thallus measurements were taken at ×25 magnification and rounded to the nearest 0·05 mm. Internal ascomatal measurements were made on vertical sections (c. 25 µm thick) cut with a Leitz freezing microtome, at ×50 magnification to an accuracy of 5 µm using a Wild M20 compound microscope. Ascospore measurements were taken at ×500 magnification using a Wild vernier micrometer (scale of 0·1 µm) to an accuracy of 0·5 µm. Ascospore (hereafter “spore”) types are defined in Sheard (Reference Sheard2010). Measurements are quoted as the range between the 25th and 75th percentiles with 5th and 95th percentile outliers indicated in brackets when full species descriptions are given. Observations of ascospore wall structure were made with an oil immersion lens at a combined magnification of ×1250. It is imperative to measure mature spores, which are determined by density of pigmentation and retention of internal lumen. Ascospore wall structure and lumen shape in freshly collected specimens (up to two or three years old) was revealed by heating slide preparations of water-mounted sections over a spirit burner. For species with a conglutinate hymenium this also has the effect of aiding the release of ascospores from the ascus on application of gentle pressure to the cover slip.
Standard reagents (K, C, P) were used sparingly when testing thalli for secondary product reactions. Polarized light (PL) is particularly useful in testing for low concentrations of cortical atranorin. It can also be used for locating crystals of gyrophoric acid and pannarin. Thin-layer chromatography (TLC) was carried out according to Culberson & Kristinsson (Reference Culberson and Kristinsson1970), Culberson (Reference Culberson1972) and Menlove (Reference Menlove1974). All three solvents were used, with glass plates in solvent C to allow for the detection of fatty acids.
Species occurrence mapping was performed on 2·5 min resolution global altitude base layer made available as part of WorldClim (Hijmans et al. Reference Hijmans, Cameron, Parra, Jones and Jarvis2005) using the sp (Pebesma & Bivand Reference Pebesma and Bivand2005) and rasterVis R packages (https://github.com/oscarperpinan/rastervis). Locality names from Russia were rendered using the BGN/PCGN romanization protocol. Locality lists for species do not include all specimens studied, with the exception of the paratype lists for the two newly described species.
Results
We confirmed a total of 43 species as occurring in the study area, including two species new to science: Rinodina hypobadia Sheard (Japan and Russia) and R. orientalis Sheard (Japan, South Korea and far eastern Russia). Of these, we record nine species for the first time from the region: R. dolichospora Malme, R. endospora Sheard, R. freyi H. Magn., R. macrospora Sheard, R. megistospora Sheard & H. Mayrhofer, R. metaboliza Vain., R. sicula H. Mayrhofer & Poelt, R. subminuta H. Magn. and R. willeyi Sheard & Giralt. These are all corticolous species with the exception of R. sicula, which is saxicolous. We include four other species reported from our region by Mayrhofer (Reference Mayrhofer1984) in his monograph on saxicolous Rinodina species (R. cervina Vain., R. compensata (Nyl.) Zahlbr., R. kozukensis and R. teichophila), although we did not study them.
Our findings suggest that Rinodina species diversity has been underestimated in the countries of this region. Whereas 20 species had been reported previously for Japan, we now report 25. Two species have been reduced to synonymy (R. akagiensis Vain., R. melancholica Zahlbr.=R. ascociscana (Tuck.) Tuck.), one species accepted in the current checklist, R. tsunodae Yas. ex Räs., is a previously reported synonym of R. kozukensis, four are considered unconfirmed (R. exigua (Ach.) S. Gray, R. milvina (Wahlenb.) Th. Fr., R. pyrina and R. sophodes) and 11 are new for Japan (Rinodina excrescens, R. freyi, R. gennarii, R. hypobadia, R. intermedia, R. megistospora, R. orientalis, R. polyspora, R. septentrionalis, R. subminuta and R. willeyi). Fourteen species were previously reported for Korea and we also accept 14, albeit with modifications to the list. We reject reports of R. laevigata and R. xanthomelana, four other species are considered unconfirmed (R. fimbriata, R. membranifera, R. pyrina and R. sophodes) and six are new to South Korea (R. ascociscana, R. moziana (Nyl.) Zahlbr., R. orientalis, R. subalbida (Nyl.) Vain., R. subminuta and R. subparieta (Nyl.) Zahlbr.). Finally, while 32 species had previously been reported from the regions of the Russian Far East, we accept 35 species. One previous report is based on a misidentification, although we have found potentially authentic material (R. oleae). For 12 previously reported species, including several of the most widely reported names in the Russian Far East, no authentic material could be confirmed: R. archaea (Ach.) Arnold, R. bischoffii (Hepp) A. Massal., R. calcarea (Arnold) Arnold, R. colobinoides (Nyl.) Zahlbr., R. confragosa (Ach.) Körb., R. exigua, R. melanconia Vain., R. milvina, R. olivaceobrunnea Dodge & Baker, R. pyrina, R. sophodes and R. trevisanii. Fifteen more species are new to the Russian Far East, and 13 of these (all except those denoted by a *) are new to the whole of Russia: R. ascociscana, R. dolichospora, R. endospora, R. freyi, R. hypobadia, R. macrospora, R. megistospora, R. metaboliza*, R. orientalis, R. polyspora*, R. sicula, R. subalbida, R. subminuta, R. tenuis Müll. Arg. and R. willeyi.
We place 14 previously published names on our list of species for which material has not been seen: R. archaea, R. bischoffii, R. calcarea, R. colobinoides, R. confragosa, R. exigua [incl. f. laeviuscula], R. fimbriata, R. melanconia, R. membranifera, R. milvina, R. olivaceobrunnea, R. pyrina, R. sophodes and R. trevisanii. In several of the latter cases, names have almost certainly been widely misapplied (e.g. R. archaea, R. exigua, R. sophodes and R. trevisanii were probably applied to species now shown to be common in the region such as R. freyi and R. subminuta), while in other cases the underlying reports may well be valid but specimens were not available for study. In two additional cases (R. laevigata and R. xanthomelana) we have studied the voucher material behind recent reports from the region and found them to be misidentified.
One of the striking patterns that emerges from the study of far eastern material of Rinodina is the close relationship with the genus in North America. Three of the new records reported here have western North American–eastern Asian distributions: the corticolous species R. endospora, R. macrospora and R. megistospora. Six species have the better known eastern North American–eastern Asian distribution, two of which are shown, based on this study, to provide older names than those currently in use. These species are R. ascociscana (syn. R. akagiensis, R. melancholica, Japan), R. buckii, R. chrysidiata, R. subminuta, R. tenuis (syn. R. adirondackii H. Magn., North America) and R. willeyi. Rinodina cinereovirens (Vain.) Vain. (syn. R. turfacea var. cinereovirens (Vain.) H. Mayrhofer) is also reported as new to North America.

Annotated Species List
Rinodina ascociscana (Tuck.) Tuck.
Genera Lich.: 124 (1872).—Psoroma ascociscana Tuck., Amer. J. Arts & Sci., ser. 2 25: 424 (1858); type: USA: [New Hampshire: Grafton Co.,] very common on trunks in the White Mountains, 1843, E. Tuckerman s. n. (FH—lectotype; COLO, FH, UC, US—isolectotypes).
New synonyms: Rinodina akagiensis Vain., Bot. Mag. Tokyo 35: 62 (1921); type: Japan, Kosuke Prov. [Gunma Pref.], Mons Akagi, 20 Dec 1918, A. Yasuda 348 (TUR-V 08741—holotype!).
Rinodina melancholica Zahlbr., Bot. Mag. Tokyo 41: 361 (1927); type: Japan (Nippon media), [Nagano/Gifu Pref. borders] Mount Norikura, August 1905, Faurie 6865 (W—holotype!).
Rinodina ascociscana is characterized by its ochraceous to brown, usually continuous, glossy thallus surface, narrowly attached apothecia often with radially striate thalline margins at maturity, and particularly by its very large spores, up to 43·5×18·5 µm (Thor 32526, UPS) which belong to the Physcia–Physconia-type and are sometimes larger than those quoted by Sheard (Reference Sheard2010). Zeorin is reported for the first time in one of thirteen specimens (Kashiwadani 38575, TNS).
The holotype of R. akagiensis is a relatively young specimen with a poorly developed thallus. The spores are entirely typical of R. ascociscana in their structure and size (29·0–39·0×14·5–18·0 µm, n=10). The only characters that are somewhat atypical are the narrow margins and slightly convex discs of most of the apothecia. The brown thallus, narrowly attached apothecia with radially cracked margins and the large spores (28·0–32·0×15·0–18·0 µm, n=10) of the R. melancholica holotype indicate that this taxon is also synonymous with R. ascociscana. Again, the thallus is not well developed and the spores are either immature or over-mature but fall within the range quoted by Sheard (Reference Sheard2010).
The species occurs in mixed deciduous forest in Honshu, Japan from 380–1480 m, on Cheju Island, Korea from 750–1600 m, in Gangwon Province from 380 m to the subalpine zone at 1660 m, and in Primorskiy Krai, Russia at low elevations (Fig. 1A). Rinodina ascociscana has previously been considered endemic to eastern North America (Sheard Reference Sheard2010; Lendemer et al. Reference Lendemer, Tripp and Sheard2014).

Fig. 1 Distribution of Rinodina species in north-eastern Asia. A, R. ascociscana; B, R. buckii; C, R. chrysidiata (solid circles: previous records; open circles: new records); D, R. cinereovirens; E, R. conradii (circles), R. gennarii (triangles); F, R. excrescens (solid circles: previous records; open circles: new records).
Selected specimens examined. Japan: Hokkaido: Iburi Prov., Muroran-shi, 42°18'N, 140°58'E, 1904, U. Faurie 6083 (TNS); Mount Tarumae, 42°41'N, 141°21'E, 1977, H. Kashiwadani 14478 (TNS); Ishikari Prov., near Mt. Arashiyama, Asahikana City, 43°47'N, 142°18'E, A. Shimizu 1541 (TNS with R. subalbida); Kitami Prov., Shari-gun, Mount Rausu, 44°04'N, 145°12'E, on Quercus crispula, 1983, H. Kashiwadani 19950 (TNS); Kushiro Prov., Akan-gun, Lake Akan, 43°26'N, 144°08'E, on Quercus crispula, 1995, Y. Ohmura 1903 (TNS); Tokachi Prov., Ashoro-gun, Lake Onneto, 43°23'N, 143°59'E, on Fraxinus mandshurica var. japonica, 1995, Y. Ohmura 1776 (TNS). Honshu: Kozuke Prov., Mt. Akagi, 36°33'N, 139°12'E, K. Tsunoda 463, 468, 508 (TNS); Etchu Prov. (Toyama Pref.), Nakashinkawa-gun (Nakaniikawa-gun), Tateyama-cho, 30 km ESE of Toyama, Syomyo-zaka, on deciduous tree, alt. 1160–1220 m, 36°34'N, 137°31'E, 1994, G. Thor 12608 (TNS & UPS); Mutsu Prov. (Aomori Pref.), Shimokita-gun, Sai-mura, Mt. Nuidoiwa-yama (Nuidoishi-yama), on Fagus crenata, alt. 380–626 m, 41°19'N, 140°51'E, 1994, G. Thor 11746 (TNS & UPS); Shimotsuke Prov. (Tochigi Pref.), Nikko City, 13 km NW of Nikko and 5 km ESE of Yumoto Village, elev. 1610 m, 36°47'56·3''N, 139°28'56·6''E (WGS84,±30 m), 2015, Thor 32526 (UPS); Shinano Prov. (Nagano Pref.), Ohmachi-city, 16 km NW Shinano-Ohmachi, Ohgisawa, on deciduous tree, alt. 1420–1480 m, 36°33'N, 137°43'E, 1994, G. Thor 12728 (TNS & UPS).—Russia: Primorsky Krai: Vladivostok Botanical Gardens, 43°09'N, 131°53'E, on Quercus mongolica, 14 vii 2003, I. A. Galanina (VLA); Khasanskiy District, vicinity of Paset, 42°28'N, 130°48'E, on Quercus mongolica, 14 iv 2002, I. A. Galanina (VLA); Sikhote-Alin’ Mountains, Sikhote-Alin’skiy Zapovednik, Terneyskiy Rayon, 54 km (air line) NW of Plastun, along brook Kaban’, 45°08·286'N, 135°53·158'E, on Abies nephrolepis, 2007, T. Spribille 23861, 23865 (GZU); Sikhote Alin, Terney District, valley of Taratay brook, on the slope of mountain ridge, 45°44'42·03''N, 136°36'11·29''E, on Tilia amurensis, 14 viii 2010, E. Kuznetsova (LECB 12-53).—South Korea: Cheju Island: Eorimok trail, NW slope of Mt. Halla, dead deciduous tree, alt. 1600–1000 m, 33º23'N, 126º31'E, 2001, G. Thor 17161 (AK & UPS); Namcheju-gun, Namwon-up, Songpanak trail, east slope of Mt. Halla, on dead deciduous tree, alt. 750–1500 m, 33°23'N, 126°37'E, 2001, G. Thor 17400, 17429 (on Fraxinus), 17444 (on Acer) (AK & UPS). Gangwon Prov.: Pyeonchang-gun, Mt. Odae, Odaesan Nat. Park, on Quercus, alt. 1560 m, 37°47'N, 128°32'E, 2014, A. Aptroot 72645 (ABL); Yangyang-gun, Ser-myun, Osaeck-ri, southern part of Sorak Mts, Sorak-san Nat. Park, south slope of Mt. Dachong, on deciduous tree. alt. 700–1400 m, 38°05·30–06·45'N, 128°27·00–30'E, 2006, G. Thor 20248 (AK & UPS); from 2·5 km NNE of Osaeck Village to timberline, c. 3·5 km NNE of Osaeck Village, subalpine, on dead Abies sp., alt. 1400–1600 m, 38°06·45–07·00'N, 128°27·30–28·00'E, 2006, G. Thor 20325 (AK & UPS with R. buckii); 38°07'N, 128° 27'E, on Betula in subalpine, 1400–1660 m, G. Thor 20300 (UPS, with R. chrysidiata and R. xanthophaea), 20318 (UPS, with R. chrysidiata); Inje-gun, Buk-myun, Yongdae-ri, inner part of Sorak Mts, Sorak-san Nat. Park, from Backdam (Paekdam) temple and along road in Backdam Valley towards Yongdae-ri Village on Quercus sp., alt. 450–550 m, 38°09·85–10·25'N, 128°22·50'E, 2006, G. Thor 20679, 20702 (AK, with fertile R. chrysidiata); on Quercus sp., alt. 380–420 m, 38°10·25–11·00'N, 128°22·30–22·00'E, 2006, G. Thor 20863 (AK & UPS).
Rinodina balanina (Wahlenb.) Vain.
Ark. Bot. 8(4): 69 (1909).—Lichen balaninus Wahlenb., Flora Lappon.: 426 (1812); type: [Norway, Finnmark] In petris insulae sinus Altensis, 13 [May]1802, Wahlenberg s. n. (UPS—lectotype, Mayrhofer & Moberg Reference Mayrhofer and Moberg2002).
Rinodina balanina is characterized by its brown, marginally effigurate, verrucose, often isidiate thallus and is always found on maritime ornithocoprophilous rocks, mostly north of the Arctic Circle (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002). This species was first reported from the Arctic Ocean coast of Chukotka by Almquist (Reference Almquist1879, from Tiapka near Cape Serdtse-Kamen), subsequently by Vainio (Reference Vainio1909: “in rupe gneissacea in peninsula Jinretlen”), and perhaps based on these reports also by Andreev et al. (Reference Andreev, Kotlov and Makarova1996) and Urbanavichus & Andreev (Reference Urbanavichus and Andreev2010). We have not been able to locate the specimens. These appear to be the only records for the Beringian region as the species has not been reported from Alaska or the North American Pacific coast (Sheard Reference Sheard2010).
Rinodina buckii Sheard
Herzogia 25(2): 126 (2012); type: USA, North Carolina, Swain Co., Great Smoky Mountains National Park, Hyatt Ridge Trail between Beech Gap Trail and Enloe Creek Trail, on fallen branch, 6 Aug. 2009, J. C. Lendemer 19269 & E. A. Tripp (NY—holotype).
This species was recently described from the Appalachian Mountains of eastern USA, Japan, South Korea and far eastern Russia (Sheard et al. Reference Sheard, Lendemer, Spribille and Tønsberg2012). Additional records for eastern North America were mapped in Lendemer et al. (Reference Lendemer, Tripp and Sheard2014). It was previously confused with R. willeyi Sheard & Giralt due to the similarly sized spores, sorediate thallus and chemistry (pannarin, usually with zeorin). The spores of R. buckii, however, belong to the Teichophila-type, are more heavily pigmented and possess a better developed torus. The margins of the thallus are characterized by small, discrete areoles that quickly develop central verrucae upon which the soredia develop. The soredia are, therefore, characteristically raised. They are also larger (40–65 µm in diam.) than those of R. willeyi (20–30 µm in diam.) and further differ in being initiated in the centre of the areoles rather than at their margins. As the areoles get larger and the soredia more dense, the two species become more similar in their vegetative characteristics, except for the smaller size and the often lighter coloured soredia of R. willeyi. This study of further Asian specimens does not change the spore size quoted in the type description of R. buckii.
The form of Rinodina excrescens with lobed areoles (Sheard et al. Reference Sheard, Lendemer, Spribille and Tønsberg2012) might also be mistaken for R. buckii, particularly when blastidia are present (characteristically minute and therefore easily confused with soredia). This form may develop bullate verrucae from the centre of the areoles, thereby mimicking the verrucae of R. buckii. In the case of fertile thalli, R. excrescens is easily distinguished from R. buckii by its smaller, Physcia-type spores. In addition, the thallus of R. excrescens typically has a glossy surface, a brownish tinge and rarely includes zeorin, whereas R. buckii has a matt surface, is always a shade of grey and typically contains zeorin. Rinodina buckii is related to R. subalbida by spore size, structure (Teichophila-type) and thallus chemistry but the latter species is never sorediate and its epihymenium typically contains crystals of pannarin which are not present in R. buckii.
In eastern North America, Rinodina buckii occurs at low to middle elevations (225–1535 m). In Hokkaido it is found from close to sea level to 750 m, at higher elevations (1300–1600 m) in South Korea and at 340 m for the single Russian record (Sheard et al. Reference Sheard, Lendemer, Spribille and Tønsberg2012). Its distribution in Asia is shown in Fig. 1B.
Specimens not previously reported. Japan: Hokkaido: Kitami Prov., Esashi-gun, Esashi-cho, 2 km S of Honcho, on Larix, alt. 10–20 m, 44°55'N, 142°35'E, 1995, G. Thor 14251 (UPS with R. subparieta); Shari-gun, Shari-cho, Shiretoko Nat. Park, path along small stream NE of Iwaobetsu hot-spring hotel (Onsen), 44°07'N, 145°05'E, alt. 240−280 m, on ± mossy, horizontal log, 1995, T. Tønsberg 22845 (BG with R. tenuis, R. excrescens); 9 km NE of Utoro Village, along trail around Shiretoko-goko Lakes, on Taxus cuspidata, alt. 260 m, 44°07'N, 145°05'E, 1995, G. Thor 14294 (UPS); Teshio Prov., Rumoi-gun, Obira-cho, 21 km ENE of Obira at coast, along E and upper trail from Tengunotaki Waterfall to parking area, 44°04'N, 141°55'E, alt. 130−150 m, on Abies sachalinensis, 1995, T. Tønsberg 21985 (BG with R. willeyi), 21997 (BG with type of R. hypobadia); Teshio-gun, Toyotomi-cho, 35 km NNW of Teshio, along small road 2·5 km from coast, 45°12'N, 141°36'E, alt. 10–20 m, on Quercus serrata var. grosseserrata, 1995, T. Tønsberg 22224 (BG); Toyotomi-cho, Rishiri-Rebun-Sarobetsu Nat. Park, 23 km NNW of Teshio at coast, Wakasakanai area, S of the road from coast to Toyotomi, on Abies sachalinensis, alt. 20 m, 45°05'N, 141°39'E, 1995, G. Thor 13636 (UPS with R. excrescens); Tokachi Prov., Kato-gun, Kamishihoro-cho, just W of road 273, 6 km S of Mikuni tunnel through Mt. Mikuni-yama, 43°32'N, 143°09'E, alt. 680 m, on Abies sachalinesis, 1995, T. Tønsberg 23050 (with R. aff. oleae; BG). Honshu: Prov. Tohoku (Prefecture Akita), 11 km NE of Lake Tazawa, 2 km SSW Tsuru-no-yu Onsen, on old Quercus, alt. 616 m, 39°47·303'N, 140°46·405'E (WGS84, ±3 m), 2013, G. Thor 29792 (UPS).—South Korea: Gangwon Prov.: Yangyang-gun, Ser-myun, Osaeck-ri, southern part of the massif Sorak Mts, Sorak-san Nat. Park, south slope of Mt. Dachong subalpine, on dead Abies sp., alt. 1400–1600 m, 38°06·45–07·00'N, 128°27·30–28·00'E, 2006, G. Thor 20325 (AK & UPS with R. ascociscana).
Rinodina cervina Vain.
Bot. Mag. (Tokyo) 35: 61 (1921); type: Japan, Prov. Kozuke, in rupe, 9. 3. 1918, Yasuda 359 (TUR-V 8650— holotype, Mayrhofer Reference Mayrhofer1984).
This is a saxicolous species known only from Japan and not seen by us. The spores are probably Pachysporaria-type I (Sheard Reference Sheard2010), as indicated by the apical satellite lumina found in the most mature spores illustrated by Mayrhofer (Reference Mayrhofer1984). The species is otherwise distinguished by an orange pigment in the medulla, K+ red-violet.
Rinodina chrysidiata Sheard
Lichenologist 44: 179 (2012); type: USA, North Carolina, Clay Co., Nantahala National Forest, 1–1·5 mi N of US 64 on Buck Creek Rd, c. 5 mi NE of Shooting Creek, mesic upland forest, on Liriodendron, 10 November 2007, J. C. Lendemer 10425 (NY—holotype; BG—isotype).
New synonym: Rinodina xanthophaea f. isidiosa Pczelkin, Novosti sistematiki nizshikh rastenii 24: 166 (1987); type: USSR Far East [Russia: Primorsky Krai:] Sikhote-Alin Nature Reserve, [45°02'N, 136°20'E], oak stand near the seashore in the surroundings of Lake Khuntam, on oak bark, 1982, A. V. Pczelkin (LE—holotype!; KW—isotype not seen).
This recently described, golden yellow, xanthone-containing species reproduces vegetatively by means of isidia and has only rarely been found fertile (Lendemer et al. Reference Lendemer, Sheard, Thor and Tønsberg2012). The type of R. xanthophaea f. isidiosa is enigmatic. Its thallus is densely isidiate and typical of R. chrysidiata in every respect. However, three large apothecia are present, c. 0·60 mm diam., and typical of R. xanthophaea in their external morphology. Two relatively thick (hand, not microtome) sections were taken from the one, previously sampled apothecium. Anatomical measurements were all within the range quoted for R. xanthophaea by Lendemer et al. (Reference Lendemer, Sheard, Thor and Tønsberg2012). The Physcia-type spores were also typical of R. xanthophaea, their measurements, (21·5–)23·5–26·5(–28·5)×12·0–13·5 µm (n=20), falling within the range of R. xanthophaea quoted by Lendemer et al. (Reference Lendemer, Sheard, Thor and Tønsberg2012) and possessing identical l/w ratios of (1·6–)1·8–2·1(–2·2). These spores have a very different lumen structure from those of the single poorly fertile specimen examined of R. chrysidiata, belonging to Pachysporaria-type I, newly reported here (Thor 20702) from South Korea.
Pczelkin (Reference Pczelkin1987) states that R. xanthophaea f. sorediosa and f. isidiosa grow together in the Sikhote-Alin Nature Reserve “but do not establish intermediate forms”, and further that the f. isidiosa is the more common. The lack of intermediate forms is supported by our observation (JWS) that in one of the apothecial sections taken from the type, the isidiate thallus could be seen to be growing on top of the thallus supporting the apothecium and separated from it by a dark tissue, suggestive of a prothallus. Our interpretation of these facts is that the holotype of the f. isidiosa is a mixed specimen of R. xanthophaea and R. chrysidiata, the thallus of the former having been overgrown by the latter. Since the isidiate thallus predominates in the holotype of R. xanthophaea f. isidiosa, and the isidia are clearly identified in the name of the forma, it is here synonymized with R. chrysidiata.
One poorly fertile specimen has previously been reported from North America with immature spores, tentatively identified as belonging to Pachysporaria-type I (Sheard et al. Reference Sheard, Lendemer, Spribille and Tønsberg2012). Another fertile specimen is reported here (Thor 20702) and is again small. The fertile part of the collection occupies a crevice of the bark substratum (Quercus sp.) and possesses a grey thallus with incipient isidia. The grey colour presumably reflects a shaded microhabitat and is accompanied by darkly coloured (with a hint of yellow pigmentation) and isidiate, sterile thalli on more exposed surfaces. Xanthones were not found by TLC in either thallus type (presumably because of their absence in the fertile material and very low concentrations in the isidiate material). A hand-cut section of the apothecium confirms the Pachysporaria-type I spore designation for this species. The great majority of spores were over-mature. The five mature spores found measured 23·0–27·0×13·5–14·5 µm and possessed prominent tori, consistent with the type description of R. chrysidiata. These spores are larger than the immature spores recorded by Lendemer et al. (Reference Lendemer, Sheard, Thor and Tønsberg2012) and should be considered more representative of the spore size for this species. The few other apothecia present appeared to be over-mature and it was not considered appropriate to sacrifice a second apothecium for microtome sectioning, given the likely outcome of a poor result.
Rinodina citrinisidiata Aptroot & Wolseley from Thailand (Aptroot et al. Reference Aptroot, Saipunkaew, Sipman, Sparrius and Wolseley2007) is probably very closely related to R. chrysidiata. It is reported to have similar-sized isidia with brown tips and Pachysporaria-type spores of similar size, though apparently more elongately-ellipsoid. It differs, however, in the presence of the xanthone thiomelin, rather than secalonic acid A, in the thallus.
Rinodina chrysidiata is previously known from the island of Hokkaido, South Korea and far eastern Russia (Lendemer et al. Reference Lendemer, Sheard, Thor and Tønsberg2012). It occurs at 550 m elevation in the Russian localities and up to 1600 m in the mountains of South Korea (see Fig. 1C).
Specimens not previously recorded. Russia: Khabarovskiy Krai: foothills of Etkil’-Yankanskiy Mountains, Amgun’ River region, 9·7 km N of Berozovyy, on Picea, elev. 550 m, 51°46·203'N, 135°41·010'E, 2009, C. Printzen 11801 (FR); Sonakh River, Amgun’ River region, c. 10 km NW main Berezovyy-Badzhal route, on Quercus mongolica, elev. 340 m, 51°31·345'N, 135°13·304'E, 2009, C. Printzen 11918 (FR). Primorsky Krai: Lazo Nature Reserve, Tretylog, along the River Perekatnaya, 43:11N 13:58E, 500 m, on Quercus, R. Moberg 9746 (with R. xanthophaea, UPS).—South Korea: Gangwon Prov.: Gangrun City, Mt. Odae, Odaesan Nat. Park, alt. 240 m, 37°49·25'N, 128°42'E, 2014, A. Aptroot 72642 (ABL); Inje-gun, Buk-myun, Yongdae-ri, inner part of the massif Sorak Mts, Sorak-san Nat. Park, c. 1·5 km NW Backdam (Paekdam) temple, on Quercus sp., alt. 550–450 m, 38°09·85–10·25'N, 128°22·50'E, 2006, G. Thor 20702 (AK, UPS fertile with R. ascociscana); alt. 420–380 m, 38°10·25–11·00'N, 128°22·30–22·00'E, 2006, G. Thor 20863 (AK & UPS); Yangyang-gun, Ser-myun, Osaeck-ri, southern part of the massif Sorak Mts, Sorak-san Nat. Park, south slope of Mt. Dachong, on Quercus sp., alt. 1600–1400 m, 38°07·10–06·45'N, 128°27·25–26·00'E, 2006, G. Thor 20475 (AK & UPS); c. 3–5 km SW of the shelter, on dead Abies sp., alt. 1400–1350 m, 38°06·45–28'N, 128°26·00–25·10'E, 2006, G. Thor 20621 (AK with R. subalbida).
Rinodina cinereovirens (Vain.) Vain.
In Nyl. & Norrl., Herb Lich.Fenn. 560 (1921) (UPS—neotype, Mayrhofer & Moberg (Reference Mayrhofer and Moberg2002); H—isoneotype). Lecanora sophodes var. cinereovirens Vain., Meddeland. Soc. Fauna Fl. Fenn. 2: 56 (1878); type: Finland, Tavastia australis, Asikkala, Kailanneimi, prope Tuomisoja, 1863, J. P. Norrlin s. n. (not found); Rinodina turfacea var. cinereovirens (Vain.) H. Mayrhofer, Mayrhofer & Moberg Rinodina - Nordic Lichen Flora 2: 68 (Reference Mayrhofer and Moberg2002).
New synonym: Rinodina turfacea var. ecrustacea (Vain.) H. Olivier, Mem. Soc. Sci. Nat. Cherbourg 37: 163 (1909). Lecanora turfacea var. archaea f. ecrustacea Vain., Meddeland. Soc. Fauna Fl. Fenn. 6: 153 (1881); type: [Russia: Karelia], ad corticem salicis in regione abietina montis Päänuorunen, 1878, E. A. Vainio (TUR-V 8803—holotype, Mayrhofer & Moberg Reference Mayrhofer and Moberg2002).
Thallus thin, light grey or sometimes brownish grey, of scattered, convex verrucae, to 0·2–0·3 mm diam., rarely merging to form larger areoles; surface plane, matt; margin indeterminate; prothallus lacking; vegetative propagules absent.
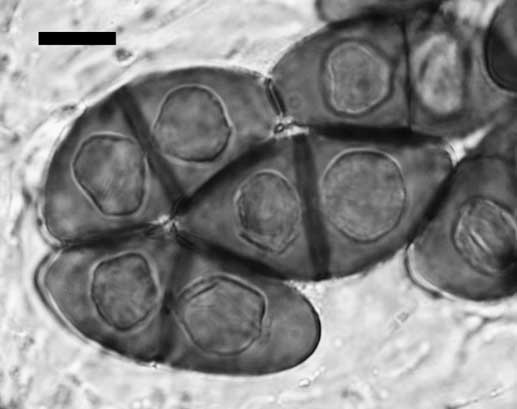
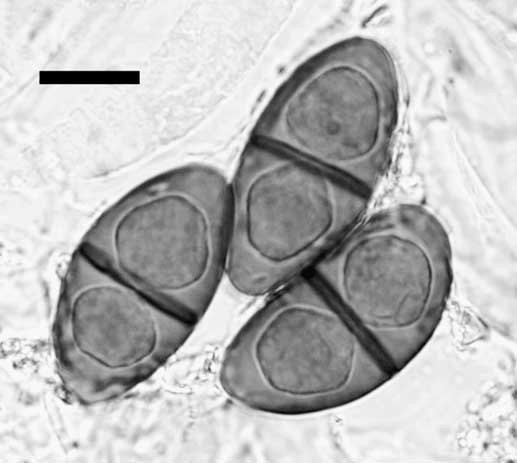
Apothecia quickly differentiating on scattered areoles, rarely contiguous, becoming narrowly attached, sometimes almost stipitate, to 0·60–1·00 mm diam.; disc black, rarely light grey or orange pruinose, plane, rarely concave but sometimes becoming convex or half-globose; thalline margin concolorous with thallus, to c. 0·10 mm wide, entire and typically persistent; excipular ring absent. Thalline exciple 40–70 µm wide laterally; cortex 5–10 µm wide; epinecral layer sometimes present, c. 5 µm wide; crystals absent in cortex, present in medulla (sphaerophorin); cortical cells 4·5–7·0 µm wide, pigmented or not; algal cells 11·0–14·5 µm diam.; thalline exciple 80–120 µm wide below; cortex expanded to 20–60 µm wide, hyphae intricate, very rarely becoming somewhat columnar; proper exciple c. 10 µm wide laterally, expanding to 20–40 µm wide above, often pigmented light brown. Hymenium 90–120 µm high, not inspersed; paraphyses 2·0–2·5 µm wide, not strongly conglutinate, apices expanded to 4·0–6·0 µm wide, dark brown capitate, immersed in diffuse pigment forming a dark red-brown epihymenium, rarely with surface crystals. Asci 60–80×17–24 µm; ascospores 4 or 8 per ascus, type A development, Physcia-type, (21·5–)23·0–25·5(–27·5)×(10·0–)11·5–13·5(–14·0) µm, l/w ratio (1·7–)1·8–2·1(–2·3), n=235, lumina angular, becoming less so but apical walls remaining thick; torus well developed (Fig. 2); walls not or hardly ornamented. Hypothecium hyaline, 30–45 µm deep.

Fig. 2 Physcia-type ascospores of Rinodina cinereovirens, Zabaikal’skiy Krai, Sokhondinskiy Biosphere Zapovednik, 23 July 2006, L. S. Yakovchenko (VLA). Note the broadly ellipsoid spore shape and prominent torus in this 8-spored ascus. Compare with Rinodina turfacea, Fig. 172 in Sheard (Reference Sheard2010). Scale=10 μm.
Pycnidia not observed.
Chemistry. Spot tests all negative, medulla UV (lw) + blue-white. Secondary metabolites: sphaerophorin and satellites in medulla by TLC.
Rinodina cinereovirens is closely related to R. turfacea but has been raised to the level of species because it is distinguished by spores that are frequently only 4 per ascus, possess a shorter median length, a more broadly ellipsoid shape and more bluntly rounded apices (Fig. 2). Upper extremes of spore size for R. cinereovirens, nevertheless, overlap with those of R. turfacea. Apothecia of R. cinereovirens tend to be smaller, possessing discs that are rarely concave but which may often become convex, a character never found in healthy R. turfacea. Rarely, this species may also possess apothecia with orange pruina, as occasionally found in R. turfacea. Rinodina cinereovirens is also similar to R. turfacea in its expanded lower cortex which, however, is usually less deep and has an intricate rather than columnar structure (but note that Mayrhofer & Moberg (Reference Mayrhofer and Moberg2002) described the lower cortex of R. turfacea as being intricate). The species is further differentiated by being limited to corticolous or lignicolous habitats rather than being found primarily on moss and decaying vegetation on the ground.
Magnusson (Reference Magnusson1947) remarked that the apothecium margins of R. cinereovirens were wider than those of R. turfacea but our observations suggest that they appear relatively broad only because of the mostly smaller size of their apothecia. In comparing the two species, it must also be recognized that immature spores of R. cinereovirens (i.e. those that are not fully pigmented) are frequently more elongately-ellipsoid than fully mature spores, which are more darkly pigmented. Furthermore, over-mature spores in which the lumen structure begins to deform, unaccountably, appear to shrink in length, thus making them even more broadly-ellipsoid. It is, therefore, imperative when comparing spore dimensions to control for spore development stage by measuring only those spores that are fully developed and pigmented but not over-mature.
Four-spored asci are also frequent in the western North American corticolous species Rinodina badiexcipula which Sheard (Reference Sheard2010) suggested might be related to R. turfacea on account of its relatively large, Physcia-type spores, pigmented proper exciple and similar chemistry. This species, however, is distinct from R. cinereovirens in its darker thallus colour, thicker apothecial margins and its unusual type of spore development, which is unique within the genus, in that apical wall thickening is delayed until after pigmentation is initiated. This is well illustrated in Figure 21 of Sheard (Reference Sheard2010), as is the asynchronous spore development, another frequent feature of this species.
The combination R. cinereovirens (Vain.) Vain. was listed as a nomen nudum by Lamb (Reference Lamb1963) because neither a basionym nor a description was given in Nyl. & Norrl. Herb. Lich. Fenn. 560. However, in using the name at species rank Vainio was clearly, if indirectly, referencing his own previous variety that he described in 1878, and a reference to the protologue was not required at the time (T. Ahti & A. Sennikov, pers. comm. 2016). The combination as (Vain.) Vain. should therefore be regarded as valid.
Rinodina cinereovirens has a boreal distribution in the study area (Fig. 1D). In the east it occurs into the dwarf shrub tundra as high as 1290 m and in the west at 885–1795 m, the highest elevations being in the subalpine zone. It has previously been reported in our study area from Kamchatka by Himelbrant et al. (Reference Himelbrant, Stepanchikova and Kuznetsova2009), the central Russian Far East by Urbanavichus & Andreev (Reference Urbanavichus and Andreev2010) and the Stanovoye Nagor’e highlands by Chesnokov & Konoreva (Reference Chesnokov and Konoreva2015). It was also reported from Baikal Zapovednik, which is west of our study area, by Urbanavichene & Urbanavichus (Reference Urbanavichene and Urbanavichus1998). The species was not separated from R. turfacea by Sheard (Reference Sheard2010) in North America but is now known to occur in Newfoundland (Ahti 33943b, 34963a, H), New Brunswick (Clayden 18111, NBM), Wapusk National Park, northern Manitoba (Piercey-Normore 9832, WIN), northern Ontario (Brinker 42159a, SASK) and Alaska (Tønsberg 43542, 43762, 43948, 44056, 44106a, 44140, BG).
Selected specimens examined. Russia: Kamchatka Krai: Bystrinsky District, Bystrinsky Nat. Park, “Oxinskye” hot springs, 56°17'06''N, 159°11'07''E, alt. 656 m, on lignum, 19 vii 2003, E. Kuznetsova (LECB 12–059); Mil’kovo District, Kamchatka River basin, SW slope of Tolbachik Volcano, c. 40–43 km SE of Kozyrevsk, 55°43'59''N, 160°11'33''E, alt. 683 m, on lignum of Larix cajanderi, 10 viii 2006, D. Himelbrant (LECB 12-066); Mil’kovo District, Kronotsky Nature Reserve, Levaya Schapina River basin, 55°08'19''N, 159°59'07''E, alt. 330 m, on Sorbus sibirica, 5 viii 2009, D. Himelbrant & I. Stepanchikova (LECB 12-018); Ust’-Bol’sheretsk District, Bannaya River basin, bank of the Bannaya River, 52°54'25''N, 157°30'12''E, alt. 244 m, on Alnus hirsuta, 6 viii 2002, D. Himelbrant & E. Kuznetsova (LECB 12-021); Ust’-Kamchatsk District, Kamchatka river basin, c. 24 km SE of Kozyrevsk, SW slope of Ushkovsky Volcano, c. 6 km N to Studenaya River, 55°57'39''N, 160°16'34''E, alt. c. 1290 m, on lignum, 16 viii 2004, D. Himelbrant & E. Kuznetsova (LECB 12-057); Kamchatka river basin, WSW slope of Shiveluch Volcano, c. 30 km NE of Kluchi, right bank of dry river Baydarnaya, 56°34'12''N, 161°12'23''E, alt. 805 m, on Alnus fruticosa, 24 viii 2002, D. Himelbrant & E. Kuznetsova (LECB 12-011); Kamchatka river basin, W slope of Ushkovsky Volcano, c. 24 km E of Kozyrevsk, 7·8 km N of Studenaya River, 55°57'39''N, 160°14'42''E, alt. 1042 m, on Salix pulchra, 14 viii 2004, D. Himelbrant & E. Kuznetsova (LECB 12-047); Ust’-Kamchatsk District, Yelovka River basin, right bank of Yelovka River near estuary of Levaya River, 56°53'00''N, 160°55'06''E, alt. 160 m, 25 viii 2003, D. Himelbrant & E. Kuznetsova (LECB 12-051, 12-012). Khabarovskiy Krai: Bogorodskoe-De Kastri route, 12 km ESE of town of Bulava, on short spur road leading to Kadi Lake, arm of Amur River, on twigs of Picea, elev. 114 m, 51°55·219'N, 140°35·682'E, 2009, C. Printzen 11666 (FR). Magadanskaya Oblast’: Olskiy District, the foot of Kamenniy Ridge, near the tourist base Magtur, 59°45'24·5''N 149°39'17·9''E, 9 m alt., on Alnus, base of the oldest trunk, 2013, I. A. Galanina M-13-178-3 (VLA). [Sakhalinskaya Oblast’:] Sakhalin Island, vicinity of Yuzhno-Sakhalinsk, mud volcano, mixed forest, 47°04'7·8''N, 142°36'30·6''E, 194 m alt., on Abies, 2012, A. K. Ezhkin R16\13 (VLA). Zabaikal’skiy Krai: Sokhondinskiy Biosphere Zapovednik, 2 km N of Agutsakan forest station, 49°36·611'N, 111°19·477'E, alt. 1125 m, 23 vii 2006, L. S. Yakovchenko (VLA); Sokhondinskiy Biosphere Zapovednik, road between forest stations Shergen-Daban and Ust’-Bablashniy, right bank of Burecha River valley, on Picea and Salix, 49°44·945'N, 110°50·822'E, alt. 1464 m, 2 vii 2007, L. S. Yakovchenko (VLA); Aginskiy Buryatskiy Autonomous Okrug, Alkhanay Nat. Park, 3·5 km E of ranger station ‘Ara-Ilya’, fold ‘Niznyaya Tangaya’, on Pinus, 50°56'35''N, 113°14'32''E, alt. 883 m, 6 vii 2006, L. S. Yakovchenko (VLA).
Rinodina cinereovirescens (Harm.) Zahlbr.
Cat. Lich. Univ. 7: 496 (1931).—Lecanora cinereovirescens Harm., Ann. Cryptog. Exot. 1: 329 (1928); type: “Indochina”, Ba-Lang, Demange (M—lectotype, Mayrhofer Reference Mayrhofer1984).
This saxicolous species is distinguished by its Pachysporaria-type II spores, 14·5–18·0× 7·0–8·5 µm, with very narrow tori, irregularly shaped lumina becoming ± rounded, and with unornamented walls (Mayrhofer Reference Mayrhofer1984). It was reported from Korea by Aptroot & Moon (Reference Aptroot and Moon2014).
Specimen examined. South Korea: Gyeongsangbuk-do: Cheongsong-gun Distr., Budong-myeoun, Sangui-ri, Mt. Juwang, from Jaha bridge to Juwang Cave, 36°23'42''N, 129°09'0635''E, 330–520 m alt., siliceous rock, A. Aptroot 71005 (M).
Rinodina compensata (Nyl.) Zahlbr.
Cat. Lich. Univ. 7: 499 (1931).—Lecanora compensata Nyl. Lich. Japon.: 41 (1890); type: Japan, Mitso, Oct. 1879, Almquist s. n. (S—lectotype, Mayrhofer Reference Mayrhofer1984).
This is another saxicolous species from Japan that was not seen by us except the specimen accompanying the lectotype of Rinodina subalbida (Nyl.) Vain. (Nagasaki, 1979, Almquist, S) referred to by Mayrhofer (Reference Mayrhofer1984). Its apothecia are characterized by possessing a fine-grained episamma and the spores have a clear but narrow torus belonging to the Pachysporaria-type (I?).
Rinodina conradii Körb.
Syst. lich. Germ.: 123 (1855); type: [Poland], Conradsthal near Salzbrunn, near Hirschberg and on the south slope of Gellhornberges, s.d., J. Flotow s. n. (L—lectotype, Mayrhofer & Moberg Reference Mayrhofer and Moberg2002).
This species is primarily ground dwelling on moss or plant remains and is characterized by its type B spore development and persistently 3-septate spores (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002; Sheard Reference Sheard2010). It can only be mistaken for R. intermedia, which possesses type A spore development and 3-septate spores that mature to become submuriform. Rinodina intermedia also has a complex chemistry whereas R. conradii lacks secondary metabolites (Mayrhofer et al. Reference Mayrhofer, Sheard, Grassler and Elix2001). The distribution of R. conradii in the study area is shown in Fig. 1E. It has previously been recorded for far eastern Russia from Popov Island in Primorskiy Krai (Skirina Reference Skirina1996; Chabanenko Reference Chabanenko2002), from Kamchatka (Himelbrant et al. Reference Himelbrant, Stepanchikova and Kuznetsova2009) and from the Stanovoye Nagor’e highlands (Chesnokov & Konoreva Reference Chesnokov and Konoreva2015).
Specimens examined. Russia: Kamchatka Krai: Ust’-Bol’sheretsk District, Bystraya Bol’shaya River basin, 53°05'42''N, 156°53'06''E, alt. 140 m, on Betula ermanii lignum, 18 viii 2002, D. Himelbrant & E. Kuznetsova (LECB k-404); Pravyy Kikhchik river basin, Kuev River valley, on S. udensis, alt.140 m, 53°34'16''N, 156°40'58''E, 26 vii 2004, D. Himelbrant (LECB k-406); Mil’kovo District, Kronotsky Nature Reserve, Levaya Schapina river basin, SW part of Askhachny Ridge, S slope, 55°08'29''N, 159°58'17''E, alt. 340 m, 12 viii 2009, D. Himelbrant & I. Stepanchikova (LECB 12-006). Zabaikal’skiy Krai: Sokhondinskiy Biosphere Zapovednik, 2 km N of Agutsakan forest station, on Betula, 49°36·611'N, 111°19·477'E, alt. 1125 m, 23 vii 2006, L. S. Yakovchenko (VLA).
Rinodina dolichospora Malme
Bihang. Kongl. Svenska Vetensk.-Akad. Handl. 28(1): 28 (1902); type: Brazil, Matto Grosso, Morro Grande, 20 December 1893, G. Malme 2159 (S—lectotype, Mayrhofer et al. Reference Mayrhofer, Kantvilas and Ropin1999).
Rinodina confinis Sampaio, Bolet. Soc. Broter. Ser. 2 2: 177 (1924); type: Portugal, Minho, Póvoa do Lanhoso, S. Gens, August 1919, G. Sampaio s. n. (UPS—syntype, Giralt et al. Reference Giralt, Kalb and Mayrhofer2009).
An ochraceous, subsquamulose thallus and Pachysporaria-type I spores measuring 23·0–28·5×12·5–16·5 µm (n=20) characterize the single collection. Polygonal lumina are present in the early stages of spore development but neither oil globules, as reported by Mayrhofer et al. (Reference Mayrhofer, Kantvilas and Ropin1999), nor apical satellite lumina were observed. The erumpent apothecia of the single specimen seen may relate to its relatively thick thallus. Such apothecia were not commented on by either Sheard (Reference Sheard2010) or Mayrhofer et al. (Reference Mayrhofer, Kantvilas and Ropin1999) but the latter authors also report rather thick thalli. The erumpent apothecia are similar to the description of those for R. elixii H. Mayrhofer et al. but the spores of that species, although large, belong to the Physcia-type (Mayrhofer et al. Reference Mayrhofer, Kantvilas and Ropin1999).
The single record is from the extreme south of far eastern Russia, close to the short border with Korea, and represents a new record for Russia (Kotlov Reference Kotlov2008). The species has a widespread but very scattered distribution around the world, being known from its type and other localities in Brazil, south-western Europe (as R. confinis, Giralt et al. Reference Giralt, Kalb and Mayrhofer2009), the Mississippi Delta region and southern Appalachians in the USA (Sheard Reference Sheard2010), and also from coastal New South Wales, Australia (Mayrhofer et al. Reference Mayrhofer, Kantvilas and Ropin1999). Most recently, the species has been found at higher elevations in the Great Smoky Mountains, southern Appalachians, USA (Lendemer et al. Reference Lendemer, Harris and Tripp2013, Reference Lendemer, Tripp and Sheard2014). The species seems to have a wide ecology, which might help explain its worldwide distribution.
Specimen collected. Russia: Primorsky Krai: Khasanskiy District, vicinity of Paset, 42°28'N, 130°48'E, oak forest (Quercus mongolica) on Q. mongolica, 14 iv 2010, I. A. Galanina (VLA).
Rinodina efflorescens Malme
Svensk Bot. Tidskr. 21: 251 (1927); type: Sweden, Västergötland, Habo, St. Kärr, cort. Fagi, 11 July 1923, G. O. Malme s. n. (S—holotype [online image!], studied by T. Tønsberg).
This corticolous species is characterized by its discrete soralia, Physcia-type spores (when fertile) and the presence of pannarin, zeorin and secalonic acid A. The soralia are typically yellowish in colour due to the pigment secalonic acid A.
Although we have not seen any specimens, R. efflorescens was recently reported from within the western limits of our study area by Makryy and Lishtva (on Kodar Ridge in the Vitimsky Zapovednik, Stanovoye Nagor’e Mountains, cited by Urbanavichene & Palice Reference Urbanavichene and Palice2016) and in the Baikal Reserve (op. cit., citing fertile material). We expect that it will be found to be more widespread in our study area, this notion possibly being strengthened by the presence of species such as R. subparieta and R. excrescens, with which it occurs in North America (Sheard Reference Sheard2010).
Rinodina endospora Sheard
Bryologist 105: 658 (2002); type: USA, California, Santa Clara Co., Mount Hamilton Range, San Antonio Valley, on Quercus lobata with R. santae-monicae, 4 August 1968, Alice Howard s. n. (UC—holotype).
Rinodina endospora is characterized by its relatively large Dirinaria-type ascospores. Typical of this spore type, the spores show type B development, are often swollen at the septum (more so in KOH) and possess septal discs. The spore size of (20·5–)22·0–24·0×(9·0–)9·5–10·5(–11·5) μm (n=40) agrees with that of specimens from western North America (Sheard & Mayrhofer Reference Sheard and Mayrhofer2002; Sheard Reference Sheard2010) but they become more elongately-ellipsoid (l/w ratio (2·0–)2·1–2·6(–2·7)). The spores may be quite pointed at intermediate stages of development. The asci are also primarily 4-spored. In view of the asynchronous spore development reported in the original species description, this new finding is not surprising. The characteristic flecking of the epinecral layer on the dark brown apothecium margins in North American collections is poorly developed. However, we consider this unimportant because the development of an epinecral layer is one of the most variable morphological characters in the genus. There were no other detectable anatomical differences between the present material and North American collections.
Rinodina endospora is probably related to R. metaboliza which also has Dirinaria-type spores and is present in the same collection. In addition to possessing larger spores, R. endospora generally has a better developed thallus that is also darker and typically has a brownish tinge. Previously considered to be a western North American endemic species, R. endospora is now reported from Russia for the first time. It is the first of three western North American–East Asian disjunct species listed here.
Specimens collected. Russia: Kamchatka Krai: Ust’-Bol’sheretsk District, Pravyy Kikhchik river basin, vicinity of Mokushka River, 53°32'56''N, 156°41'07''E, alt. 235 m, floodplain forest, Chosenia arbutifolia, 22 vii 2004, Himelbrant (LECB 12-010 with R. metaboliza, R. subminuta).
Rinodina excrescens Vain.
Ann. Acad. Sci. Fenn., Ser. A 27: 84 (1928); type: Russia, (Siberia Occidentali) Konda, ad lignum putridum in pineto prope Leunsk, s. d., E. Vainio s. n. (TUR-V 08798—holotype).
The thallus morphology of the Rinodina excrescens collections exhibits the same range of variation as the species in eastern North America; from bullate areoles, which may be blastidiate/sorediate, to plane areoles with sublobate margins (Sheard Reference Sheard2010). The apothecia usually become narrowly attached, their discs are often pruinose (pannarin crystals) and the lecanorine margins are often flexuose and sometimes incomplete (crenulate). When fertile, the species has Physcia-type ascospores that are quite variable in size, as is often the case with species that may also reproduce by vegetative means. The spore measurements overlap with those from North American material but tend to be longer, (16·0–)17·5–19·5(–21·5)×(8·0–)8·5–9·5(–10·0) µm (n=75), l/w ratio (1·8–)1·9–2·2(–2·4). The species has previously been characterized by the presence of pannarin as the single detectable secondary metabolite, which is also present in the epihymenium. A new finding is that, very occasionally, zeorin accompanies pannarin in the thallus (Thor 14292, Tønsberg 22845a, 22904).
In its typical form, Rinodina excrescens is difficult to confuse with other species because of the bullate nature of the thallus. The species has been compared to the recently described eastern North American species, R. bullata Sheard & Lendemer (Sheard et al. Reference Lendemer, Sheard, Thor and Tønsberg2012), but the bullate areoles of that species are smaller, loosely attached to the substratum and contain atranorin rather than pannarin. Lobate specimens may mimic young, presorediate stages of species such as R. subparieta, R. buckii and R. willeyi, but these are distinguished by their grey rather than brownish colour, presence of atranorin rather than pannarin in the first species and, typically, the additional presence of zeorin in the latter two.
Rinodina excrescens is a new record for Japan where it occurs at elevations of up to 720 m in Hokkaido. It was recently reported as widely distributed in the Russian Far East by Galanina et al. (Reference Galanina, Yakovchenko, Tsarenko and Spribille2011) and Yakovchenko et al. (Reference Yakovchenko, Galanina, Malashkina and Bakalin2013). Its range within the study area is substantially expanded by the records reported here (Fig. 1F). The species is elsewhere known from the Great Lakes region and eastern North America, where it is widely distributed but not common (Sheard Reference Sheard2010), and from southern Europe where it is scattered and very rare (Galanina et al. Reference Galanina, Yakovchenko, Tsarenko and Spribille2011).
Specimens examined and not previously published. Japan: Hokkaido: Ishikari Prov., Kamikawa-gun, Kamikawa-cho, Obako River valley, E of Obako Gorge Tourist Centre, 43°42'N, 143°01'E, alt. 710–720 m, on Alnus, 1995, T. Tønsberg 23122 (BG); Kitami Prov., Soya-gun, Sarufutsu-mura, 12 km NW of Hamatonbetsu Town, 8 km SE of Poronuma Lake, 1·5 km from sea, on Picea glehnii, alt. 30 m, 45°13'N, 142°15'E, 1995, G. Thor 14188 (UPS); Shari-gun, Shari-cho, Shiretoko Nat. Park, 7 km NE of Utoro Village, N and S of small road to Iwaobetsu hot-spring hotel (Onsen) 2 km (road distance) from jct to Shiretoko-goko Lakes, 44°06'N, 145°05'E, alt. 130 m, on Abies sachalinensis, 1995, T. Tønsberg 22775a, 22775b (BG); Shiretoko Nat. Park, 9 km NE of Utoro Village, along trail around Shiretoko-goko Lakes, on Abies sachalinensis, alt. 260 m, 44°07'N, 145°05'E, 1995, G. Thor 14292 (UPS); Kushiro Prov., Akkeshi-gun, Hamanaka-cho, 55 km E of Kushiro City, 3 km E of Hichiripputo Lake, just S of road following coast, on Betula sp., alt. 60 m, 43°02'N, 145°03'E, 1995, G. Thor 14440 (UPS with R. subparieta); on Alnus sp., G. Thor 14450 (UPS with R. xanthophaea and R. buckii); Nemuro Prov., Notsuke-gun, Betsukai-cho, 100 km ENE of Kushiro City, N shore of Furenko Lake, just NW of road 244, on Betula sp., alt. 5–10 m, 43°22'N, 145°15'E, 1995, G. Thor 14425 (UPS with R. willeyi); Shiretoko Peninsula, Menashi-gun, Rausu-cho, 15 km SW of Sakae City, on Quercus mongolica, alt 30 m, 43°49'N, 145°04'E, 1995, T. Tønsberg 22904 (BG); Teshio Prov., Teshio-gun, Toyotomi-cho, Rishiri-Rebun-Sarobetsu Nat. Park, 23 km NNW of Teshio at coast, Wakasakanai area, 1 km from coast, on Abies sachalinensis, alt. 20 m, 45°05'N, 141°39'E, 1995, G. Thor 13636 (UPS with R. buckii). Honshu: Shimotsuke Prov. (Tochigi Pref.), Nikko City, 14 km WNW of Nikko and 4 km SE of the village Yumoto, field station Nikko Shizen Fureai House, on old Larix kaemperi, elev. 1400 m, 36°46'11·2''N, 139°27'21·8''E (WGS84, ± 100 m), 2015, Thor 32286 (UPS).—Russia: Khabarovskiy Krai: Chegdomyn-Sofiysk route, c. 19 km NE of the Bureya River ferry crossing at Shakhtinskiy, 76 km (air line) NNE of Chegdomyn, between Umal’ta and Nimakan Rivers, 51°47·546'N, 133°21·122'E, lignicolous on snag, 658 m elev., 2009, T. Spribille 31600 (GZU); Amgun’ River region, 18·7 km SW of Berezovyy, on Larix gmelinii, elev. 222 m, 51°33·598'N, 135°28·956'E, 2009, C. Printzen 11870 (FR). [Sakhalinskaya Oblast’:] Sakhalin Island, Dolinsk District, Pchelinnaia River, 47°21'06·1''N, 142°52'28·6''E, 10 m alt., on Abies, 2012, A. K. Ezhkin R20\13 (VLA); Sakhalin Island, Susunayskiy Ridge, vicinity of Yuzhno-Sakhalinsk, Parkovaia Mountain, 46°58'23·6''N, 142°45'30·9''E, 207 m alt., on Abies, 2012, A. K. Ezhkin R21\13 (VLA); 46°58'26·15''N, 142°45'26·12''E, 198 m alt., on Picea, 2010, A. K. Ezhkin 11R/11 (VLA).
Rinodina freyi H. Magn.
Acta Horti Gotob. 17: 236 (1947); type: Switzerland, Graubünden, Engadin, Zernez, alte Zaunlatten, 1500 m, 16.VIII.1924, E. Frey s. n. (G—holotype); Berner Oberland, Diemtigtal, Grimmialp, 1280 m, 8 July 1934, E. Frey s. n. (G—paratype).
Rinodina glauca Ropin, Herzogia 9: 807 (1993); type: [Italy: Südtirol:] Ehrenburg, Bruneck, on Populus tremula - Arnold Lich. exs. 1654 (M pro parte—lectotype as R. ramulicola Kernstock ex Arnold, Verh. K. K. Zool.-Bot. Ges. Wien 46: 132 (1896) - nom. illeg.).
This species new to Japan and Russia is characterized by its relatively small, darkly pigmented, Physcia-type ascospores with a heavy torus and lack of secondary substances. The spore measurements from the specimens examined from Hokkaido are often larger ((15·5–)16·0–18·5(–20·5)×(7·5–)8·0–9·0(–9·5) µm (n=40)) but overlap with those published for North American material ((12·0–)15·0–16·0(–18·5)×(6·0–)7·5–8·0(–9·0) µm (Sheard Reference Sheard2010)). The species is further characterized by its contiguous, broadly attached apothecia and plane areoles.
In North America, the species is very common and a characteristic pioneer of the twigs of a wide range of shrubs and trees across the southern boreal zone (Sheard Reference Sheard2010). It is typically located in the axils of twig branches and on leaf scars, and other substratum sites that retain moisture. The species has been confused with Rinodina septentrionalis Malme (Giralt & Mayrhofer Reference Giralt and Mayrhofer1995; Sheard Reference Sheard2010), which has very similar spores, but the apothecia of that species are more scattered and more narrowly attached. The thallus of R. septentrionalis typically consists of small, usually isolated, convex areoles. Despite the differences between mature thalli, the two species are difficult to separate when immature. Rinodina freyi is widespread in Central Europe (Giralt & Mayrhofer Reference Giralt and Mayrhofer1995) and, as reported here, in the northern half of north-eastern Asia (Fig. 3A). It has also been reported from a site in western Mongolia, just west of the mapping area, by Hauck et al. (Reference Hauck, Tønsberg, Mayrhofer, de Bruyn, Enkhtuya and Javkhlan2013).

Fig. 3 Distribution of Rinodina species in north-eastern Asia (cont.). A, R. freyi; B, R. hypobadia; C, R. megistospora (circles), R. metaboliza (triangles), R. moziana (squares); D, R. oleae (circles), R. polyspora (triangles), R. sicula (squares); E, R. orientalis; F, R. septentrionalis.
Specimens examined. Japan: Hokkaido: Kitami Prov., Esashi-gun, 9 km S of Homatonbetsu, on Alnus sp., alt. 50 m, 45°03'N, 142°23'E, 1995, G. Thor 14200 (TNS & UPS); on Salix, G. Thor 14208 (TNS with R. polyspora); Rishiri-to Island, Rishiri-gun, Rishirifuji-cho, Oniwaki area, 500 m N of Numaura Village, on Abies sachalinensis, alt. 15 m, 45°07'N, 141°17'E, 1995, G. Thor 13943 (UPS with R. buckii and R. subparieta); Shari-gun, Shari-cho, Shiretoko Nat. Park, NW slope of Shiretoko Peninsula c. 10 km NE Utoro Town, along trail from Iwaobetsu hot-spring hotel (Onsen) to Mt. Rausu-dake, on Abies sachalinensis twigs, alt. 395 m, 44·1065°N, 145·09207°E, 2010, G. Thor 23686 (UPS with R. subalbida and R. subminuta); Wakkanai City admin. area, 45°28'N, 141°57'E, on Alnus, G. Thor 14014 (TNS, UPS); Kushiro Prov., Akkeshi-gun, 2 km S Sakaki-machi, 43°06'N, 145°06'E, on Quercus, K. H. Moon 4195 (TNS); Kawakami-gun, around Kayanuma Hot Springs, 43°00'N, 1425°00'E, on Abies, H. Kashiwadani 39036 (TNS).—Russia: Kamchatka Krai: Mil’kovo District, Kamchatka River basin, SW slope of Tolbachik Volcano, c. 40–43 km SE of Kozyrevsk, 55°43'56''N, 160°11'39''E, alt. 689 m, on Populus suaveolens, 9 viii 2006, D. Himelbrant (LECB 12-036); Mil’kovo District, Kronotsky Nature Reserve, Levaya Schapina River basin, right bank of Ipuin River, 55°06'05'N, 159°59'22''E, alt. 280 m on Crataegus chlorosarca, 10 viii 2009, D. Himelbrant & I. Stepanchikova (LECB 12-034 with R. metaboliza); Ust’-Bol’sheretsk District, Pravyy Kikhchik River basin, 53°34'39''N, 156°41'17''E, alt. 229 m, on Crataegus chlorosarca branch, 26 vii 2004, D. Himelbrant (LECB 12-060). Khabarovskiy Krai: Komsomolsk-De Kastri route, Sushko Mountain, 9 km SW of De Kastri, 51°26·743'N, 140°38·357'E, 53 m elev., on Alnus at seashore, 2009, T. Spribille 30686 (GZU). Magadanskaya Oblast’: Ola District, vicinity of Arman, bridge over River Armani, 59°40'17·5''N, 150°10' 05·8″E, 45 m alt., on Chosenia, 2013, I. A. Galanina M-13-186-1 (VLA); Ola District, vicinity of Magadan City, 60°24'49·5''N, 151°30'49·04''E, 13 m alt., on Chosenia, 2013, I. A. Galanina M-13-210-1 (VLA). Primorskiy Krai: Sikhote-Alin’ Mountains, Oblachnaya Mountain, 20 km (air line) E of Yasnoye, 43°40·519'N, 134°12·855'E, on Rhododendron mucronulatum, 2007, T. Spribille 23578 (GZU). [Sakhalinskaya Oblast’:] Sakhalin Island, Dolinsky District, Makuy stream, 47º16'N, 142º42'E, twig of Picea, 2008, N. A. Tsarenko & S. V. Nesterova S-W-25-02E-2 (VLA); Sakhalin Island, Dolinsky District, 107 km of federal highway north of Firsovo, 47°49'17''N, 142°30'17·62''E, 12 vii 2008, A. V. Bogacheva & N. A. Tsarenko (VLA); Sakhalin Island, Dolinsky District, Makuy stream, 47°16'N, 142°42'E, on Betula, 2008, S. V. Nesterova & N. A. Tsarenko W-2008-25-03E (VLA); Sakhalin Island, Lyutoga River, 48 km west of Yuzhno-Sakhalinsk, 46°49'N, 142°18'E, on Betula, 10 viii 2006, I. A. Galanina (VLA). Zabaikal’skiy Krai: Sokhondinskiy Biosphere Zapovednik, 2 km N of Agutsakan forest station, on Betula, 49°36·611'N, 111°19·477'E, alt. 1125 m, 23 vii 2006, L. S. Yakovchenko (VLA); Sokhondinskiy Biosphere Zapovednik, road between forest stations Shergen-Daban and Ust’-Bablashniy, right bank Burecha River valley, on Picea, 49°44·945'N, 110°50·822'E, alt. 1464 m, 2 vii 2007, L. S. Yakovchenko (VLA); Aginskiy Buryatskiy Autonomous Okrug, Alkhanay Nat. Park, 3·5 km E of ranger station ‘Ara-Ilya’, fold ‘Niznyaya Tangaya’, on Pinus, 50°56'35''N, 113°14'32''E, alt. 883 m, 6 vii 2006, L. S. Yakovchenko (VLA).
Rinodina gennarii Bagl.
Comment. Soc. Crittog. Ital. 1: 17 (1861); type: Italy, Liguria, Mte. Faiallo nell’ Apennino di Voltri, s. d., F. Baglietto s. n. (MOD—lectotype, Mayrhofer & Moberg Reference Mayrhofer and Moberg2002).
This saxicolous species is well characterized by its relatively small ascospores with type B development (but the development type is often difficult to find) and septal swelling in KOH, therefore belonging to the Dirinaria-type (Sheard Reference Sheard2010). The records reported here represent a new addition to the lichen biota of Japan for this primarily coastal species with a worldwide distribution (Trinkaus et al. Reference Trinkaus, Mayrhofer and Matzer1999). The species was previously reported from Kunashir Island (Kuril Islands, Russia) off the north tip of Hokkaido (Bredkina et al. Reference Bredkina, Dobrysh, Makarova and Titov1992; Chabanenko Reference Chabanenko2002). Kotlov (Reference Kotlov2008) refers to specimens on bark and rock and therefore a mixture of two species. Rinodina gennarii is now often included in R. oleae (Kaschik Reference Kaschik2006; Giavarini et al. Reference Giavarini, James and Purvis2009) but in our opinion this species is restricted to corticolous substrata. Rinodina gennarii is primarily a species of maritime rocks but also occasionally grows on lignicolous maritime pilings. These lignicolous specimens have a different habit to R. oleae (Sheard Reference Sheard2010) but are also relatively infrequent so that their variability is not well known. According to our records, Rinodina gennarii is infrequent in north-eastern Asia; we have verified specimens only from Japan (Fig. 1E) but it is likely under-documented.
Specimens examined. Japan: Hokkaido: Kitami Prov., Rishiri-to Island, 1 km SW of Hondomari, 45°15'N, 142°13'E, on maritime rocks, G. Thor 13933 (TNS); Shari-gun, Shari-cho, Utoro Town, downtown Utoro, on vertical rock 100 m from the seashore, alt. 5–10 m, 44°04·2110'N, 144°59·3293'E, 2010, G. Thor 23795 (UPS); Kushiro Prov., Kushiro-cho, 14 km ESE of Kushiro City, small fishing village at seashore, on mortar, alt. 2 m, 42°56·2938'N, 144°29·4431'E, 2010, G. Thor 25904 (UPS).
Rinodina hypobadia Sheard sp. nov.
MycoBank No.: MB 819994
Thallus thin, light to dark grey. Apothecia erumpent, then broadly attached. Hypothecium reddish or chestnut brown; vegetative propagules absent. Ascospores Dirinaria-type, (12·5–)14·5–16·5(–18·5)×(6·5–)7·0–8·5(–10·0) µm, lumina Physcia–Physconia-like, spores mostly lightly pigmented at maturity, a few immature spores inflated at septum, others inflated on application of KOH; torus absent; walls not ornamented. Secondary metabolites pannarin and zeorin by TLC, pannarin crystals also present in epihymenium.
Type: Japan, Hokkaido, Teshio Prov., Rumoi-gun, Obira-cho, 21 km ENE of small town of Obira at coast, along E and upper trail from Tengunotaki Waterfall to parking area, 44°04'N, 141°55'E, alt. 130–150 m, mixed deciduous/Abies sachalinensis forest, on A. sachalinensis, 28 May 1995, T. Tønsberg 21997, with Rinodina buckii (BG—holotype).

Fig. 4 Rinodina hypobadia, Japan, Hokkaido, Teshio Prov., Rumoi-gun, Obira-cho, T. Tønsberg 21997 (BG—holotype). A, habit, note the continuous thallus and erumpent young apothecia; B, Dirinaria -type ascospores; C, section through apothecium in transmitted light; D, the same section viewed in polarized light, the pigmented hypothecium prominent in both. Scales: A=1 mm; B=10 µm; C & D=50 µm. In colour online.
Thallus thin, light to dark grey, continuous, becoming rimose but not areolate; surface plane, matt; margin determinate, in part delimited by a narrow, dark brown, fimbriate prothallus; vegetative propagules absent (Fig. 4A).
Apothecia erumpent, then broadly attached, finally slightly constricted at base; mostly not contiguous, to 0·40–0·80 mm diam.; discs black, slightly pruinose (more obvious when moist), persistently plane; thalline margin concolourous with thallus, 0·05–0·10 mm wide; excipular ring absent. Thalline exciple 40–80 µm wide; cortex poorly organized, c. 10 µm wide; epinecral layer 5–15 µm wide; crystals present in cortex and medulla; cortical cells obscured by crystals, to 3·5–5·5 µm wide, hyaline; algal cells to 8·0–14·0 µm long; proper exciple 5–10 µm wide laterally, mostly red-brown pigmented, expanded to 10–20 µm wide above. Hymenium (25–)60–100(–120) µm high, not inspersed; paraphyses 2·0–2·5 µm wide, conglutinate, apices expanded to 3·0–4·0 µm wide, lightly capitate, immersed in a dispersed pigment forming a red-brown epihymenium with crystals present. Asci Lecanora-type, 30–60×13–20 µm; ascospores 8 per ascus, type A or B development, Dirinaria-type, (12·5–)14·5–16·5(–18·5)×(6·5–)7·0–8·5(–10·0) µm (n=110), l/w ratio (1·7–)1·8–2·1(–2·3); lumina Physcia- to Physconia-like (Fig. 4B), spores mostly immature and hyaline, rarely becoming dark brown, a few immature spores inflated at septum, others inflated only after application of KOH, finally lightly pigmented, darker when over-mature, then strongly constricted at the septum; torus absent; walls not ornamented. Hypothecium reddish or chestnut brown (Fig. 4C & D), (25–)50–80 µm deep.
Pycnidia raised, asymmetric, to 0·30 mm wide. Conidia bacilliform, c. 3·5×1·0 µm.
Chemistry. Spot tests K−, C−, P+ cinnabar (red needles under the microscope). Secondary metabolites: pannarin and zeorin in thallus by TLC, pannarin crystals also present in epihymenium (Fig. 4D).
Etymology. The species is named for its pigmented hypothecium (‘hypo-’, Greek for below; ‘badius’, Latin for chestnut brown).
This new species is characterized by its erumpent apothecia, strongly pigmented hypothecium, Dirinaria-type spores and the presence of pannarin in the thallus and epihymenium. The only other Rinodina species known with a pigmented hypothecium is R. sheardii Tønsberg but this species has Pachysporaria-type I spores, a very different chemistry (Elix & Tønsberg Reference Elix and Tønsberg1999) and is also sorediate (Sheard Reference Sheard2010). The spores of R. hypobadia are extremely variable in shape and size, and most examined were probably immature because they were unpigmented. Over-mature spores are mostly strongly constricted and are not included in the measurements quoted above. Rather few specimens have been examined so the above description should be regarded as preliminary. Rinodina hypobadia is infrequent in north-eastern Asia, where we have recorded it only from Hokkaido, Primorskiy Krai and southern Sakhalin Island (Fig. 3B).
Paratypes examined. Japan: [Hokkaido:] Teshio Prov., Rumoi-gun, Obira-cho, 21 km ENE of small town of Obira at coast, along E and upper trail from Tengunotaki Waterfall to parking area, 44°04'N, 141°55'E, alt. 130–150m, mixed deciduous/Abies sachalinensis forest, on A. sachalinensis, 1995, T. Tønsberg 21995 with R. subparieta (BG); Tomamae-gun, Shosanbetsu-mura, 16 km NE of the small town of Haboro at coast, along Wakabanosawa stream at Wakaba bridge, marsh dominated by Alnus and Salix, on Salix sp., alt. 100 m, 44°24'N, 141°54'E, 1995, G. Thor 13538 (TNS & UPS).—Russia: Primorsky Krai: Khasanskiy District, Zarubino, 42°38'N, 141°44'E [sic; should be 131°04'E!], on Tilia, 10 v 2010, I. A. Galanina (VLA). Sakhalinskaya Oblast’: Sakhalin Island, Susunayskiy Ridge, vicinity of Yuzhno-Sakhalinsk, Ostraia Mountain, mixed forest, south-eastern exposure, 46°58'38·85''N, 142°46'37·41''E, 344 m alt., on Populus, 2011, A. K. Ezhkin 20R-11 (VLA).
Rinodina intermedia Bagl.
Comment. Soc. Crittog. Ital. 1: 315 (1863); type: [Switzerland], Sulle rupi conferte da leg. strato di muschi alla Madonna del Sasso, Locarno, Aug. 1862, F. Baglietto s. n. (MOD—holotype).
This ground-dwelling species is easily recognized by its subsquamulose thallus, 3-septate to submuriform ascospores with type A development, and a unique chemistry (Mayrhofer et al. Reference Mayrhofer, Sheard, Grassler and Elix2001; Sheard Reference Sheard2010). These authors compared Rinodina intermedia with R. conradii, which has strictly 3-septate spores with type B spore development and an absence of secondary metabolites. The two species were considered to be allopatric in North America, being separated by their distribution but also by elevation where their distributions overlap in Colorado. Rinodina intermedia occurs at lower elevations in this state than R. conradii, as it also does in the Himalayas. Interestingly, the two species have now been found together in one locality south of Lake Baikal and when seen side by side their morphologies are distinguished by the thinner thallus and smaller, more clustered apothecia of R. conradii.
Sheard (Reference Sheard2010) considered R. intermedia to have a Sonoran distribution with northern outliers in North America but it is widespread across the world in xeric habitats at low latitudes and is absent from high latitudes in both the Northern and Southern Hemispheres where R. conradii is typically found (Mayrhofer et al. Reference Mayrhofer, Sheard, Grassler and Elix2001). The species has previously been recorded from Russia (Kotlov Reference Kotlov2008; including reports from Ol’khon Island in Lake Baikal by Makryy (Reference Makryy2008) and from Buryatia by Urbanavichene & Urbanavichus (Reference Urbanavichene and Urbanavichus2008)) and is a new addition to the lichen biota of Japan (Kurokawa & Kashiwadani Reference Kurokawa and Kashiwadani2006). Although this species is usually terricolous, the Japanese record is corticolous; this substratum is otherwise known for this species only from the Himalayas (Mayrhofer et al. Reference Mayrhofer, Sheard, Grassler and Elix2001).
Specimens examined. Japan: Honshu: Shimotsuke Prov. (Tochigi Pref.), Nikkoyumoto, on Quercus magnolia, 1400 m, A. Henssen 29254j (H).—Russia: Zabaykal’skiy Krai: Sokhondinskiy Biosphere Reserve, surroundings of the Agutsa patrol cabin, valley of the River Kumyl-Aliya, below Glubokaya on the right bank of the river, 49°41'12·6''N, 111°26'0·04''E, 1180 m elev., on mossed-over rock, 2009, L. S. Yakovchenko 101 (VLA); ibid., upper reaches of the River Ingod, left bank of the river, 3 km above the winter shelter hut “Ingod” on the bank, deep canyon at woodland edge, facing river, 49°56'47''N, 111°11'03·9''E, 1307 m elev., on soil accumulations in depressions of walls, in lower (more xerophytic) part of canyon, 2009, L. S. Yakovchenko 131 (VLA).
Rinodina kozukensis (Vain.) Zahlbr.
Cat. Lich. Univ. 7: 524 (1931).—Melanaspicilia kozukensis Vain. Bot Mag. (Tokyo) 35: 61 (1921); type: Japan, Prov. Kozuke, in rupe, 26 Feb 1918, Yasuda (TUR-V 9106—holotype, Mayrhofer Reference Mayrhofer1984).
Rinodina tsunodae Yas. ex Räs., J. Jap. Bot. 16: 141 (1940); type: Japan, Prov. Kozuke, saxicola, 21 Jan. 1920, Yasuda 442 (H—holotype, Mayrhofer Reference Mayrhofer1984).
A saxicolous species described from Japan with spores of the Pachysporaria- to Milvina-type, according to Mayrhofer (Reference Mayrhofer1984). Its thallus is thin, continuous to rimose-areolate and light to middle grey. The species has not been seen by us but was recently reported as new to Russia by Skirina (Reference Skirina2010) from two islands in Peter the Great Bay in the Sea of Japan.
Rinodina luteonigra Zahlbr.
Bot. Mag. Tokyo 41: 360 (1927); type: Japan, [Nagasaki Pref.:] Tsushima [Island], May 1901, Faurie 3952 (W—holotype!, accompanied by R. subalbida (Nyl.) Vain.).
Thallus thin at margin, continuous, thicker in centre, golden yellow; surface plane becoming rugose, matt; prothallus not seen; vegetative propagules absent.
Apothecia erumpent remaining broadly attached, frequent but mostly not contiguous, to c. 0·60 mm diam.; disc black, slightly concave to persistently plane; thalline margin concolourous with thallus, entire, persistent, c. 0·10 mm wide; excipular ring dark brown, confluent to raised (best seen when moist). Thalline exciple c. 80 µm wide, hyaline; cortex and epinecral layer not evident; crystals filling margin; marginal hyphae not pigmented, to c. 5·5 µm wide; algal cells to c. 10·5 µm wide; thalline exciple to c. 110 µm wide below; proper exciple lightly pigmented, c. 10 µm wide laterally, expanding to c. 50 µm wide at periphery. Hymenium c. 160 µm high, not inspersed; paraphyses c. 2·0 µm wide, conglutinate, apices to c. 3·5 µm wide, lightly pigmented, immersed in a dispersed pigment forming a dark, red-brown epihymenium. Asci 80–100×27–42 µm; ascospores 8 per ascus, type A development, Pachysporaria-type I, (29·5–)31·0–34·5(–37·5)×(15·0–)16·0–17·5(–18·5) µm (n=40), l/w ratio (1·7–)1·9–2·1(–2·2); lumina somewhat angular to subpolygonal during development, becoming ± spherical, lacking well-defined canals, walls persistently thick, some mature spores developing apical satellite lumina; torus well developed at maturity (Fig. 5); walls not ornamented. Hypothecium c. 35 µm deep, hyaline.

Fig. 5 Pachysporaria -type I ascospores of Rinodina luteonigra, Japan, Nagasaki Pref., Tsushima Island, May 1901, Faurie 3952 (W—holotype). Note the large size and heavy tori of these spores. Scale=10 μm.
Pycnidia not seen.
Chemistry. Thiomelin and zeorin detected by TLC.
A full description is given above since the species is otherwise known only from Zahlbruckner’s original, brief type description. Rinodina luteonigra has a similar habit to R. lepida (Nyl.) Müll. Arg. (syn. R. flavonigella Tuck.) and like the synonym of the latter species it is named for the contrast between the pigmented thallus and black apothecial discs. The major secondary substances for the two species are also the same. The ascospores of both belong to Pachysporaria-type I but those of R. luteonigra (Fig. 5) are much larger than those of R. lepida and some older spores also develop apical satellite lumina. The spores are also larger than those of R. adirondackii, once thought to be the species with the largest ascospores belonging to this spore type (Sheard Reference Sheard2010), although the spores of neither species are as large as those of the subsequently described R. megistospora Sheard & H. Mayrhofer (Sheard et al. Reference Sheard, Knudsen, Mayrhofer and Morse2011). The pigmentation and spore type of R. luteonigra and R. lepida both suggest a relationship with the Southern Hemisphere saxicolous species R. thiomela (Nyl.) Müll. Arg. and R. xanthomelana Müll. Arg.
The distribution of R. luteonigra is not known but given its type locality on Tsushima Island it is likely to be subtropical. This would appear to echo the over-representation of species with Pachysporaria-type I spores in the lichen biota of the southern part of continental USA (Sheard Reference Sheard2010).
Rinodina macrospora Sheard
Bryologist 105(4): 665 (2002); type: Canada, British Columbia, Vancouver Island, Botanical Beach, 4 km SW of Port Renfrew, on Picea sitchensis behind beach, 14 August 1975, W. J. Noble 5371 (UBC—holotype; SASK—isotype).
This species is characterized by its large Physcia-type spores, cortical atranorin and its habitat which is limited to either driftwood or conifer trees on maritime foreshores. The ascospores of this single collection are of similar length but are more narrowly ellipsoid ((26·5–)28·0–32·0(–33·0)×(12·5–)13·0–13·5(–14·0) µm (n=20), l/w ratio 2·1–2·4(–2·5)) than North American collections (Sheard & Mayrhofer Reference Sheard and Mayrhofer2002; Sheard Reference Sheard2010). The spore walls are also less heavily ornamented. These differences might be due to the relatively early developmental stage of the single sample we collected and report here.
New to far eastern Asia in coastal Khabarovskiy Krai. This is the second example of a species with a western North American–East Asian disjunct distribution. It should be searched for on Sakhalin Island and Hokkaido.
Specimen examined. Russia: Khabarovskiy Krai: Sea of Japan, Tabo Bay (Bukhta Tabo), north of De Kastri, rocky promontory on north side of bay, 51°37·685'N, 140°53·098'E, 26 m, corticolous on exposed Picea jezoensis on coastal cliff, 2009, T. Spribille 30831 (GZU).
Rinodina manshurica Räsänen
Arch. Soc. Zool. Bot. Fenn. ‘Vanamo’ 5: 27 (1950); type: [China], Manshuria (Mantšukuo), Schitonhzusik, on Tilia manshuricae, 9 Oct. 1923, I. Korejev (H—holotype!).
Thallus thin, grey-brown, rimose, becoming rimose-areolate; areoles to c. 0·80 mm wide; surface plane, matt; margin indeterminate; prothallus absent; vegetative propagules absent.
Apothecia erumpent, remaining broadly attached, frequent but mostly not contiguous, to c. 0·50 mm in diam.; disc black, becoming convex; thalline margin concolourous with thallus, entire, c. 0·05 mm wide, becoming excluded; excipular ring absent. Thalline exciple c. 30 µm laterally; cortex not evident; epinecral layer c. 5 µm wide; crystals absent in medulla; algal cells to 13·5 µm long; thalline exciple c. 20 µm wide below; epinecral layer c. 10 µm wide; proper exciple not distinguishable laterally, to c. 20 µm wide at periphery. Hymenium c. 70 µm high, not inspersed; paraphyses c. 2·0 µm wide, apices to c. 3·0 µm, lightly capitate forming a light brown epihymenium, lacking a dispersed pigment. Asci c. 50×20 µm; ascospores 8 per ascus, development type not observed, Dirinaria-type, (14·0–)15·5–16·0(–16·5)×(7·5–)8·0–8·5 µm (n=20), l/w ratio (1·7–)1·9–2·0(–2·1), some slightly swollen at septum, more so in KOH; lumina Physcia-like, septal disc more conspicuous in KOH, sometimes becoming pigmented; torus absent; walls not ornamented. Hypothecium c. 40 µm deep, hyaline.
Pycnidia not seen.
Chemistry. Spot tests all negative; secondary metabolites not tested by TLC.
Rinodina manshurica is known only from its type and is characterized by small, erumpent apothecia and small spores with Physcia-like lumina. The apothecial characters are reminiscent of R. subminuta H. Magn. but on close examination the spores belong to the Dirinaria- rather than to the Physcia-type, and they are also smaller. The spores of R. manshurica are very slightly swollen at the septum and slightly more so in KOH. Additionally, the spores lack a torus and many possess septal discs, all characters of the Dirinaria- rather than Physcia-type spores. The spore development type would be expected to be type B but immature, non-septate spores were not observed. Rinodina metaboliza also has Dirinaria-type spores with Physcia-like lumina but its spores are larger and have more obvious septal swellings, and its apothecia are never erumpent. Rinodina manshurica is almost certainly closely related to R. oleae and may ultimately come to be included within that species. At present, R. manshurica can be distinguished from the material assigned here to R. aff. oleae by the smaller mean spore size in the single sample of R. manshurica based on a relatively small number of measurements from local material (n=20 in R. manshurica and n=33 in R. aff. oleae). The small erumpent apothecia also bring to mind R. orientalis, but the slightly longer spores of this species develop a torus and possess lumina that can sometimes mimic the Pachysporaria-type II spores in shape, although they are more angular and belong to the Physcia-type.
Rinodina megistospora Sheard & H. Mayrhofer
Bryologist 114(3): 460 (2011); type: USA, Oregon, Curry Co., Siskiyou National Forest, Oak Flat off road along Rogue River, near Agness [probably on Quercus], 20 Oct 1996, C. C. Bratt 9969 (SBBG—holotype; SASK—isotype).
Thallus thin to thick, light grey, initially of isolated areoles, 0·25–0·55 mm wide, with minute, radiating lobules 0·15–0·20 mm wide, areoles quickly coalescing to become continuous; surface verrucose, with overlapping, minute lobules, matt; margin determinate; prothallus absent; vegetative propagules absent.
Apothecia broadly attached, frequent, sometimes contiguous, to 0·70–1·0 mm diam.; disc dark brown to black, becoming convex to half globose; thalline margin concolourous with thallus, entire, 0·05–0·10 mm wide, becoming excluded; excipular ring usually present at first, raised. Thalline exciple 55–90 µm wide laterally, cortex poorly organized, 10–15 µm wide; epinecral layer typically absent; crystals present in cortex (atranorin), absent in medulla; cortical cells 3·0–6·5 µm diam., not pigmented; algal cells 9·0–14·5 µm long; thalline exciple 65–90 µm wide below, cortex 15–20 µm wide; proper exciple lightly pigmented orange-brown, 10–20 µm wide laterally, expanding to 15–30(–50) µm at periphery, concolourous with epihymenium. Hymenium 120–160 µm high, some intrusion of hypothecial inspersion at base; paraphyses 2·0–2·5 µm wide, branched, conglutinate, apices expanded to 3·5–4·5 µm, lightly pigmented, immersed in a dispersed pigment forming a dark, orange-brown epihymenium. Asci with immature spores, 90–120×30–35 µm, spores easily released; ascospores 4–8 per ascus, type A development, Pachysporaria-type I, (34·5–)37·0–40·5(–43·0)×(17·0–)18·0–20·5(–21·5) µm (n=175), l/w ratio (1·7–)1·9–2·1(–2·3), some immature spores with transient polygonal lumina, over-mature spores may possess apical satellite lumina; torus narrow; walls very lightly ornamented. Hypothecium 60–120 µm deep, hyaline except lightly pigmented at base, inspersed.
Pycnidia not seen.
Chemistry. Spot tests K+ yellow, C−, P+ faint yellow. Secondary metabolites: atranorin confirmed by TLC and two unknown compounds, A5, B5, C6 & A5, B6, C6.
A new description is given above to provide a better measure of character range than was possible in the original description, which was based on the type specimens alone (Sheard et al. Reference Sheard, Knudsen, Mayrhofer and Morse2011). The original description is not changed in any substantive way by the addition of new data from far eastern Asia. Rinodina megistospora is characterized by its very large, Pachysporaria–type I spores, the presence of atranorin in the cortex and two unknown substances which are consistently present. The light grey thallus, convex apothecia and general habit of R. megistospora are reminiscent of the terricolous R. mniaroeiza (Nyl.) Arnold, which also contains cortical atranorin. However, R. mniaroeiza is an oro-arctic, terricolous species with smaller, Physcia-type spores (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002; Sheard Reference Sheard2010).
Only six other Rinodina species have ascospores averaging >30 µm in length (Sheard Reference Sheard2010). These are R. ascociscana, R. macrospora Sheard, R. roscida (Sommerf.) Arnold (all with Physcia-type spores), R. oregana H. Magn. (Dirinaria-type spores) and R. tenuis (Pachysporaria-type I spores). The first three and the last of these species are reported here for far eastern Asia. Rinodina tenuis is distinguished from R. megistospora by its thin, plane thallus and presence of medullary pannarin rather than cortical atranorin. Rinodina luteonigra from southern Japan also has Pachysporaria-type I spores in this size range but is easily distinguished by its yellow pigmented thallus.
Previously known only from a Quercus stand at the type locality in southern Oregon, in a relatively high rainfall region close to the coast, Rinodina megistospora is now also recorded from near coastal localities at low elevations in Hokkaido, Japan (on unknown phorophytes) and on Fagus on Shikoku Island, Kochi Prov. The species also occurs on Betula and Picea in the Sikhote Alin’ Mountains, Primorsky Krai and in the Khomi Mountains, Khabarovskiy Krai, suggesting that it might be widespread but infrequent on the mainland (Fig. 3C). Rather than being a western North American endemic as originally thought, R. megistospora must now be regarded as a disjunct species of the Klamath-Siskiyou Mountain region, a region well known for its floristic diversity (Coleman & Kruckeberg Reference Coleman and Kruckeberg1999). It is the third species in this study belonging to the Eastern Asiatic–western North American group of disjunct species.
Specimens examined. Japan: Hokkaido: Ishikari Prov., Mt. Arashiyama, Asahikawa City, 43°47'N, 142°18'E, on Quercus crispula, 2004, A. Shimizu 1559 (TNS); Kitami Prov., Shari-gun, Shari-cho, Shiretoko Nat. Park, NW slope of Shiretoko Peninsula c. 10 km NE of Utoro Town, along trail from Iwaobetsu hot-spring hotel (Onsen) to Mt. Rausu-dake, on deciduous tree, alt. 388 m, 44·10611°N, 145·09242°E (WGS84, by GPS), 2010, G. Thor 23974 (UPS); Shiretoko Nat. Park, NW slope of Mt. Rausu along trail from Iwaobetsu hot-spring hotel (Onsen) to summit, alt. 600 m, 44°06·000'N, 145°06·300'E, 2010, A. Frisch 10/Jp56, 10/Jp84 (UPS); Teshio Prov., Teshio-gun, Wakasakanai, 45°42'N, 141°44'E, 1980, H. Kashiwadani 16169 (TNS). Shikoku: Kochi Pref., Ino-cho Town, along path from road to small and steep hill Mt. Komochi-gon’gen, on Fagus crenata, alt. 1610–1630 m, 33º46'49–54''N, 133º11'16–19''E (WGS84), 2006, G. Thor 21398 (UPS).—Russia: Khabarovskiy Krai: Komsomolsk-De Kastri route, Khomi Mountains, about halfway between Chernyy Mys and Tsimmermannovka, 51°05·418'N, 138°57·113'E, on Betula costata, 529 m elev., 2009, T. Spribille 30550 (GZU). Primorskiy Krai: Sikhote-Alin’ Mountains, Oblachnaya Mountain, 20 km (air line) E of Yasnoye, 43°40·435'N, 134°12·759'E, on Picea jezoensis, 2007, T. Spribille 23515 (GZU, with R. xanthophaea).
Rinodina metaboliza Vain.
Ann. Acad. Sci. Fenn., Ser. A 27: 87 (1928); type: [Russia:] Siberia, Tobolsk, Levusch ad corticem Salicis, 1880, E. Vainio s. n. (TUR-V 8490—holotype).
This species is characterized by its type B ascospore development, Dirinaria-type ascospores measuring 17·5–21·5×9·0–11·0 µm (n=30) with septal swelling, enhanced in KOH. The spores can very easily be mistaken for the Physcia-type on first sight because of their relatively angular lumina. The species also lacks detectable secondary substances.
Rinodina metaboliza is a boreal species known from North America (Sheard Reference Sheard2010), Russia (Magnusson Reference Magnusson1947; Kotlov Reference Kotlov2008) and Scandinavia (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002) but has not previously been reported from far eastern Asia. We report it from Kamchatka and the Transbaikal region (Fig. 3C).
Specimens examined. Russia: Kamchatka Krai: Mil’kovo District, Kronotsky Nature Reserve, Levaya Schapina River basin, right bank of Ipuin River, 55°06'05''N, 159°59'22''E, alt. 280 m, on Crataegus chlorosarca, 10 viii 2009, D. Himelbrant & I. Stepanchikova (LECB 12-034 with R. freyi); Ust’-Bol’sheretsk District, Pravyy Kikhchik River basin, vicinity of Mokushka River, 53°32'56''N, 156°41'07''E, alt. 235 m, on Chosenia arbutifolia, 22 vii 2004, D. Himelbrant (LECB 12-010 with R. endospora and R. subminuta). Zabaikal’skiy Krai: Sokhondinskiy Biosphere Zapovednik, 2 km N of Agutsakan forest station, on Larix, 49°36·611'N, 111°19·477'E, alt. 1125 m, 23 vii 2006, L. S. Yakovchenko (VLA); Sokhondinskiy Biosphere Zapovednik, road between forest stations Shergen-Daban and Ust’-Bablashniy, right bank of Burecha River valley, on Picea, 49°44·945'N, 110°50·822'E, alt. 1464 m, 2 vii 2007, L. S. Yakovchenko (VLA with R. septentrionalis).
Rinodina mniaroea (Ach.) Körb.
Syst. Lich. Germ.: 126 (1855).—Lecanora mniaroea Ach., Syn. Lich.: 339 (1814); type: [Switzerland:] Helvetia, supra muscos, J. C. Schleicher 91a (H-ACH 1136A—lectotype, Mayrhofer & Moberg Reference Mayrhofer and Moberg2002).
This species was reported for all sectors of the Russian Far East by Urbanavichus & Andreev (Reference Urbanavichus and Andreev2010). Although we have not seen any specimens from our study area, the species is widespread across oro-arctic regions of the Northern Hemisphere. Rinodina mniaroea is characterized by apothecia that become convex, excluding the thalline margins, and by its large Physcia-type spores (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002; Sheard Reference Sheard2010). Three chemotypes have previously been recognized as varieties (Moberg & Mayrhofer 2002) but as a result of molecular studies (Resl et al. Reference Resl, Mayrhofer, Clayden, Spribille, Thor, Tønsberg and Sheard2016) these have recently been raised to species status as R. cinnamomea (Th. Fr.) Räsänen with skyrin and other anthraquinones in the lower medulla, R. mniaroeiza (Nyl.) Arnold with cortical atranorin, and R. mniaroea lacking a diagnostic secondary chemistry although variolaric acid is often present. All might be expected to occur in the study area. Rinodina turfacea is distinguished by its persistently plane apothecial discs, persistent margins and the presence of sphaeophorin. Rinodina roscida is another oro-arctic species similar to R. mniaroea and R. turfacea but typically has a pruinose thallus and apothecial discs, larger spores, lacks sphaerophorin and occurs in calcareous rather than acidic terrain (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002; Sheard Reference Sheard2010).
The specific epithet has usually been cited as ‘mniaraea’ in the belief that the name ‘mniaroea’ was an error made by Acharius. However, there is no evidence that this is the case and Acharius was consistent in his usage. Original orthography may not be changed other than through conservation and so Acharius’ original spelling must be used (Coppins & Kirk 2017, pers. comm.). This also applies to the name ‘mniaroeiza’.
Rinodina mongolica H. Magn.
Lichens from central Asia II. Sino-Swedish Expedition Publ. 22. XI Botany 2: 36 (1944); type: Mongolia australis, Beli-miao, [on cortex accompanied by Candelariella aurella], 41°30'N, 110°10'E, 24 Oct. 1929, B. Bohlin 109a (S—lectotype, designated here).
Thallus mostly thin, grey to ochraceous, areolate; areoles to 0·70 mm wide; surface rugose, matt; margin indeterminate; prothallus not seen.
Apothecia broadly attached, becoming somewhat sessile, scattered or contiguous, up to 0·80 mm diam.; disc black, slightly concave at first, becoming plane, never convex; thalline margin concolourous with thallus, to 0·10 mm wide, entire; excipular ring absent.Thalline exciple c. 80 µm wide laterally; cortex c. 10 µm wide; epinecral layer c. 10 µm wide; crystals absent in cortex and medulla; cortical cells to c. 6·5 µm wide, pigmented; algal cells large, to 26·5 µm long; thalline exciple c. 100 µm wide below, cortex not expanded; proper exciple c. 10 µm wide laterally, expanded to 25–40 µm wide above. Hymenium c. 100 µm high, not inspersed; paraphyses 2·5–3·0 µm wide, apices to 5·5–6·5 µm wide, reddish brown capitate, without dispersed pigment, forming a reddish brown epihymenium. Asci c. 50×15–20 µm; ascospores type A development (very rarely type B), Dirinaria-type, (15·0–)15·5–18·0(–9·0)×(7·5–)8·0–8·5(–9·0) µm (n=40), l/w ratio (1·8–)1·9–2·1(–2·3), few immature spores seen, then slightly swollen at septum, more so in KOH, some with septal wall thickening; lumina Physconia-like, without septal wall thickening at maturity, darkly pigmented at septum but torus absent; walls not ornamented. Hypothecium c. 80 µm deep, hyaline.
Pycnidia not seen.
Chemistry. Spot tests all negative.
This new description agrees with that of Magnusson (Reference Magnusson1944) but is more complete. The lectotype is abundantly fertile but the ascospore structure was initially difficult to determine due to dehydration. After soaking an apothecium in water overnight and heat treatment of a squash preparation, the spores were observed to be Physconia-like, without wall thickening at the septum or apices at maturity. The few young spores were observed to be swollen at the septum, the swelling becoming more apparent after treatment with KOH. The spore structure is therefore similar to Rinodina pyrina and a taxonomic relationship is further suggested by the large algal cells (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002; Sheard Reference Sheard2010; Sheard et al. Reference Sheard, Knudsen, Mayrhofer and Morse2011). Magnusson does not make the comparison with R. pyrina but states that he ventured to describe the species “because of its pale yellowish grey, velvety thallus and thin walled, uniform spores”. The thallus is thicker than is typical for R. pyrina and the ochraceous colour is also unusual for that species. The epihymenium is reddish brown but the pigment is present in the capitate apical cells rather than being diffuse around the apical cells as in species with Physcia- and Physconia-type spores.
The spores of Rinodina mongolica are longer than is typical for R. pyrina and more comparable in this respect to R. imshaugii (see Table 1 in Sheard et al. Reference Sheard, Knudsen, Mayrhofer and Morse2011). However, they lack the persistent apical thickening that is often found in R. imshaugii and their l/w ratio includes broadly-ellipsoid spores (like R. pyrina). The thallus and apothecium morphology is also quite unlike that of R. imshaugii and it therefore seems likely that R. mongolica is a good species, although poorly known since it is based on the type alone. A second specimen (Bohlin 109b, S) is poorly fertile and therefore unhelpful.
Rinodina moziana (Nyl.) Zahlbr.
Cat. Lich. Univ. 7: 544 (1931). Lecanora moziana Nyl., Lich. Japon.: 40 (1890); type: Japan, [Kyushu], Mitso (= Mozi or Moji), 20 Oct 1879, Almquist (H-Nyl 28851 as L. mitsoana, Mayrhofer Reference Mayrhofer1984—lectotype!, H-Nyl 28859 as L. mitsoana—topotype!).
=Rinodina destituta (Nyl.) Zahlbr. Cat. Lich. Univ. 7: 510 (1931).—Lecidea destituta Nyl., Sertum Lich. Trop. Labuan et Singapore, Accedunt Observ.: 41 (1891); type: USA, Illinois, super saxa arenaria, s. d., J. Wolf s. n. (H-NYL 10210—holotype!). See Sheard (Reference Sheard2010) for other synonyms listed under this name.
Rinodina discolorans var. japonica Räsänen. J. Jap. Bot. 16: 141 (1940); Lamb, Ind. Nom. Lich.: 646 (1963)=Rinodina discolor var. japonica (Räsänen.) Sato, Ind. Plant. Nippon. 4: 116 (1943); type: Japan, Kosuke Prov., 7 Dec. 1920, Tsunoda 444 (H—holotype), 9 Mar 1921, Tsunoda 445 (H—paratype).
Rinodina vezdae H. Mayrhofer, J. Hattori Bot. Lab. 55: 473 (1984); type: Vězda Lich. Bohem. exs. 113 (as R. discolor) ČSSR: Moravia occid., Vev. Bitýška, ad saxa dioritica in valle fluvii Svratka, c. 350 m, 12. 12. 1956, A. Vězda s. n. (M—holotype!).
Rinodina moziana is one of only five saxicolous species recorded by us for the region. The original collections of R. moziana are very similar to specimens of R. destituta (including R. vezdae) as understood by Sheard (Reference Sheard2010). Consequently R. destituta is here reduced to synonymy with R. moziana. Both original collections are very small, the lectotype being similar to the ‘ochrocea’ morphotype of R. destituta as described by Sheard (Reference Sheard2010). The topotype is closest to the most typical state of R. destituta, the ‘vezdae’ morphotype. They both possess cortical atranorin, a thalline apothecial margin becoming partly or completely carbonized, a proper exciple with an aeruginose pigment at its upper margin, and relatively large Mischoblastia-type spores. Spore measurements, and their other characteristics, also correspond well with R. destituta: (20·0–)21·5–23·0(–24·0)×12·0–12·5(–13·0) µm (n=30), l/w ratio (1·6–)1·7–1·8(–2·0). The larger spores are swollen at the septum, with a narrow torus at maturity, and their walls are unornamented.
Rinodina moziana, now known from both Japan and Korea (including a specimen reported as R. teichophila; Aptroot & Moon Reference Aptroot and Moon2014), has a wide distribution in eastern North America (as R. destituta, Lendemer et al. Reference Lendemer, Tripp and Sheard2014) and is also found in Central and Southern Europe (reported as R. vezdae, H. Mayrhofer Reference Mayrhofer1984; Giralt Reference Giralt2010). The species is also rather common in Australia (Kaschik Reference Kaschik2006). Its distribution in far eastern Asia is southern and so far confined to islands (Fig. 3C).
Specimens examined. Japan: Bonin Island: Chichi-jima Island, Mt. Yoake, on exposed siliceous rocks, elev. 300 m, 27°4'58·404''N, 142°13'22·511''E, 2013, A. Frisch, Y. Ohmura & H. Kashiwadani 13/Jp62 (UPS).—South Korea: Cheju Island: Namcheju-gun, Andok-myon, 20 km west of town of Sogwip’o, south of Road 12, Andok Valley, on rock in stream, alt. 85 m, 33º16'N, 126º21'E, 2001, G. Thor 17305 (UPS); Andok-myon, Sagye-ri, Mt. Dansan, steep exposed hill, alt. 50 m, 33°14'N, 126°17'E, 2001, G. Thor 17377 (UPS).
Rinodina aff. oleae Bagl.
Mem. Reale Accad. Sci. Torino, Ser. 2, 17: 403 (1857); type: Italy, Liguria, Granarolo, 1855, F. Baglietto s. n. (MOD—lectotype, Kaschik Reference Kaschik2006).
The thallus of this corticolous species is continuous, becoming rimose-areolate, and the apothecia are erumpent at first and reminiscent of R. subminuta but with smaller apothecia and ascospores, (15·5–)16·5–18·0(–19·0)×(6·5–)8·0–9·0(–9·5) µm (n=33), l/w ratio (1·8–)1·9–2·3(–2·6). Some spores are inflated at the septum, and more so in KOH. The absence of a torus and presence of a septal disc with Physcia-like lumina suggest that the spores belong to the Dirinaria-type. However, type B spore development was not observed in our material. The very obvious erumpent apothecia and relatively large spores are not typical of R. oleae (Giralt Reference Giralt2001) and there is, therefore, some doubt about this identification. As noted previously, the saxicolous R. gennarii is sometimes included in R. oleae (Kaschik Reference Kaschik2006; Giavarini et al. Reference Giavarini, James and Purvis2009).
The corticolous Rinodina oleae is widespread in southern Europe (Giralt Reference Giralt2001). In North America the species is not well known and has a widely scattered distribution (Sheard Reference Sheard2010). The species was previously reported from the southern Russian Far East by Urbanavichus & Andreev (Reference Urbanavichus and Andreev2010) but these records belong to the saxicolous R. gennarii; the vouchers behind a more recent report, from Khabarovskiy Krai (Yakovchenko et al. Reference Yakovchenko, Galanina, Malashkina and Bakalin2013), were not available for this study. It has also been reported from Korea by Joshi et al. (Reference Joshi, Kondratyuk, Crişan, Jayalal, Oh and Hur2013). Four of the five East Asian records, including one new to China, are found in a narrow region near the three-corners area where Russia, China and North Korea meet; we also report the species as new to Japan from Hokkaido (Fig. 3D).
Specimens examined. China: Jilin Prov.: Changbaishan Nat. Park, 41°54'N, 127°53'E, on Larix, 22 vi 2010, I. A. Galanina; on Betula, 27 vi 2010, I. A. Galanina (both VLA).—Japan: Hokkaido: Tokachi Prov., Kato-gun, Kamishihoro-cho, just W of road 273, 6 km S of Mikuni tunnel through Mt. Mikuni-yama, 43°32'N, 143°09'E, alt. 680 m, on Abies sachalinensis, 1995, T. Tønsberg 23050 (BG with R. buckii).—Russia: Primorskiy Krai: Hankayskiy District, vicinity of Pogranichniy, 44°23'N, 131°25'E, on Q. mongolica, 11 viii 2010, I. A. Galanina (hb. VLA); Khasanskiy District, vicinity of Zarubino, 42°37'N, 131°04'E, on Q. mongolica, 20 v 2001, I. A. Galanina; 10 v 2010, I. A. Galanina (both VLA); Shkotovsky District, vicinity of Shkotovo, 43°19'N, 132°21'E, on Fraxinus, 28 v 2010, I. A. Galanina (VLA).
Rinodina orientalis Sheard sp. nov.
MycoBank No.: MB 819995
Thallus grey to dark grey-brown, thin, continuous, rarely becoming thick, rimose-areolate. Apothecia erumpent, remaining broadly attached, margins often poorly formed; disc black, plane, often becoming strongly convex excluding the thalline margin. Ascospores (rarely 4) 8 per ascus, Physcia-type, (14·5–)16·0–18·0(–19·5)×(6·5–)7·0–8·5(–9·0) µm, lumina often with relatively rounded angles (Pachysporaria-like); torus developing late. Secondary metabolites absent.
Type: Japan, Hokkaido, Kitami Prov., Shari-gun, Shari-cho, Shiretoko National Park, NW slope of Shiretoko Peninsula c. 10 km NE of Utoro Town, along the trail from Iwaobetsu hot-spring hotel (Onsen) to Mt. Rausu-dake, old-growth montane forest dominated by Quercus crispula (syn. Q. mongolica) but also with Acer mono, Betula ermanii, Prunus nipponica and Sorbus sp., on Sorbus sp. (close to plot A2), alt. 611 m, 44·10228°N, 145·10025°E (WGS84, by GPS), 12 July 2010, G. Thor 24138 (UPS—holotype).

Fig. 6 Rinodina orientalis, Japan, Hokkaido, Kitami Prov., Shari-gun, Shari-cho, Shiretoko National Park, G. Thor 24138 (UPS— holotype). A & B, habit: A, showing young erumpent apothecia with plane discs; B, broadly attached apothecia with convex discs. C, Physcia-type ascospores. Scales: A=500 µm; B=200 µm; C=10 µm. In colour online.
Thallus grey to dark grey-brown, thin, continuous, rarely becoming thick, rimose-areolate (Fig. 6A); areoles to 0·50–0·80 mm wide; surface plane or scabrid becoming rugose, matt; margin indeterminate; prothallus not seen; vegetative propagules absent.
Apothecia erumpent (Fig. 6A), remaining broadly attached, to 0·30–0·65(–0·80) mm diam., margin c. 0·05 mm wide, often poorly formed; disc black, plane, often becoming strongly convex, excluding the thalline margin (Fig. 6B); excipular ring rarely evident, then lighter brown than disc and confluent with thalline margin. Thalline exciple 40–80 µm wide; cortex 5–15 µm wide, typically with epinecral layer 5–10 µm wide; crystals absent in cortex and medulla; cortical cells to 3·5–4·5(–6·0) µm wide, typically pigmented; algal cells to 10·0–15·0(–20·5) µm long; proper exciple 5–10 µm wide laterally, sometimes pigmented, expanded to 20–40 µm above. Hymenium 70–100 µm high, typically conglutinate; paraphyses 2·0–3·0 µm wide, apices lightly capitate, immersed in dispersed pigment forming a dark, red-brown epihymenium. Asci 55–60×12–15(–18) µm, Lecanora-type; ascospores 8 per ascus (rarely 4), type A development, Physcia-type (Fig. 6C), (14·5–)16·0–18·0(–19·5)×(6·5–)7·0–8·5(–9·0) µm (n=118), l/w ratio (1·9–)2·0–2·4(–2·6), sometimes slightly inflated at the septum at first, not more so in KOH, later rarely slightly constricted; lumina often with relatively rounded angles (Pachysporaria-like); torus developing late; walls not ornamented. Hypothecium hyaline, 30–50 µm deep, to 80 µm deep when disc convex.
Pycnidia not seen.
Chemistry. Spot test all negative. Secondary metabolites absent by TLC.
Etymology. Named after its wide distribution in far eastern Asia.
This new corticolous species is characterized by its Physcia-type spores with a narrow torus developing late and lacking wall ornamentation. Due to the rounded nature of the spore lumina, particularly in early development, and rare swelling at the septum, it was first thought that the spores might belong to the Dirinaria-type. However, the spores do not inflate further in KOH, and possess type A development. Asci may rarely develop only four spores, as in the holotype (Fig. 6C). The late developing torus and red-brown colour of the epihymenium both suggest that the spores should be interpreted as belonging to the Physcia-type (Sheard Reference Sheard2010). The apothecia are initially erumpent and very reminiscent of Rinodina subminuta H. Magn. but the discs of mature apothecia often become strongly convex excluding the thalline margin, characters that are absent in healthy R. subminuta. In addition, the spore structure of R. subminuta is more typical of the Physcia-type, with more angular lumina during development, a more obvious torus and larger spore size (averaging 18·5–20·0 µm long; Sheard Reference Sheard2010).
Rinodina orientalis is found in montane to subalpine forest in Japan which, together with the tendency for some spores to be inflated at the septum, immediately invited comparison with the European R. ventricosa Hinteregger & Giralt (Hinteregger Reference Hinteregger1994). However, this species does not have erumpent apothecia and its spores are larger ((18·0–)18·5–20·0(–21·0)×8·5–9·5(–10·5) µm (n=40)) with persistently very angular lumina, and more frequent and more obvious septal swelling. The two specimens (28 May 2010, I. A. Galanina; 10 May 2010, I. A. Galanina, both VLA) from southern Primorsky Krai, Russia do not have obvious erumpent apothecia and should be regarded as tentative identifications of this species. In addition to the morphological similarities with R. subminuta, R. orientalis also has a similar elevational range, except that it has not been found below 600 m on Hokkaido, Japan. This new species is widespread in eastern Asia as far west as the Mongolian border region (Fig. 3E).
Paratypes examined. Japan: Hokkaido: Kitami Prov., Shari-gun, Shari-cho, Shiretoko National Park, NW slope of Shiretoko Peninsula c. 10 km NE of Utoro Town, along trail from Iwaobetsu hot-spring hotel (Onsen) to Mt. Rausu-dake, on Sorbus commixta, alt. 1021 m, 44·08942°N, 145·11675°E (WGS84, by GPS), 2010, G. Thor 24529, 24532 (UPS); on Weigela middendorffiana at trail, alt. 840 m, 44·05447°N, 145·06740°E (WGS84, by GPS), 2010, G. Thor 24793 (UPS); on Betula ermannii, alt. 1000 m, 44·09°N, 145·12°E (WGS84, estimated from GPS), 2010, G. Thor 25600 (UPS); Shiretoko N.P., NW slope of Mt. Rausu along trail from Iwaobetsu hot-spring hotel (Onsen) to summit, alt. 600 m, 44°06·000'N, 145°06·300'E, 2010, A. Frisch 10/Jp21 (UPS); alt. 1200 m, 44°04·940'N, 145°07·682'E, 2010, A. Frisch 10/Jp26 (UPS). Honshu: Shimotsuke Prov. (Tochigi Pref.), Nikko City, 14 km WNW of Nikko and 4 km SE of Yumoto Village, the property surrounding the field station Nikko Shizen Fureai House, on Malus toringo, elev. 1400 m, 36°46'11·2''N, 139°27'21·8''E (WGS84, ±100 m), 2015, Thor 32262 (UPS); Shinano Prov. (Nagano Pref.), Ohmachi-city, 16 km NW Shinano-Ohmachi, Ohgisawa, along path near river 100 m S to 500 m W of Ohgisawa, on Salix sp., alt. 1420–1480 m, 36°33'N, 137°43'E, 1994, G. Thor 12723 (TNS & UPS).—Russia: Primorsky Krai: Chkotovskiy District, Chkotovo, 43°19'N, 132°21'E, 28 v 2010, I. A. Galanina; Khankaiskiy District, shore of Lake Khanka, near Turiy Rog, 45°00'N, 132°25'E, on Quercus dentata, 24 vi 2005, L. S. Yakovchenko; on Quercus mongolica, 10 v 2010, I. A. Galanina (both VLA). [Sakhalinskaya Oblast’:] Kuril Islands, Iturup Island, vicinity of Baranski Volcano, 45°5'26·57''N, 147°58'56·75''E, 461 m alt., on Acer, 17 viii 2013, A. K.Ezhkin (VLA); Sakhalin Island, Nevelskiy Pass, 46°44'35·92''N, 142°6'21·02''E, 425 m alt., on Sorbus, 6 xi 13, A. K. Ezhkin (VLA); Sakhalin Island, vicinity of Yuzhno-Sakhalinsk, Susunayskiy Ridge, Mt. Bolshevik, 47°56'31·7''N, 142°48'21·1''E, 95 m alt., on Fraxinus, 2012, A. K. Ezhkin R26\13 (VLA); Sakhalin Island, vicinity of Yuzhno-Sakhalinsk, Novo-Aleksandrovka, valley of stream Krasnosel’skiy, on Salix, 47°02'4·2''N, 142°43'34·8''E, 48 m alt., 2012, A. K. Ezhkin R18\13 (VLA); Sakhalin Island, vicinity of Yuzhno-Sakhalinsk, Susunayskiy Ridge, Ostraia Mountain, 46°58'39·2''N, 142°46'24·4''E, 237 m alt., on Populus, 2011, A. K. Ezhkin R3/13 (VLA); Sakhalin Island, vicinity of Yuzhno-Sakhalinsk, Susunayskiy Ridge, River Rogatka, 46°58'36·6''N, 142°49'07·4''E, 282 m alt., on Salix, 2011, A. K. Ezhkin R4/13 (VLA); Sakhalin Island, vicinity of Yuzhno-Sakhalinsk, Susunayskiy Ridge, Krasnaia Mountain, 46°56'8·5''N, 142°49'50·3''E, 737 m alt., on Acer, 2012, A. K. Ezhkin R6/13 (VLA); Sakhalin Island, Susunayskiy Ridge, vicinity of Yuzhno-Sakhalinsk, Parkovaia Mountain, 46°58'26·15''N, 142°45'26·12''E, 100 m alt., on Acer, 2011, A. K. Ezhkin 22R-11 (VLA); on Populus, 2011, A. K. Ezhkin 23R-11 (VLA); Sakhalin Island, Susunayskiy Ridge, vicinity of Yuzhno-Sakhalinsk, Turgenev Mountain, 47°00'33·6''N, 142°48'35·7''E, 592 m alt., on Salix, 2012, A. K. Ezhkin R9/13 (VLA). Zabaikal’skiy Krai: Sokhondinskiy Biosphere Zapovednik, 2 km N of Agutsakan forest station, Agutsakan River flood plain, on Larix, 49°36·611'N, 111°19·477'E, alt. 1125 m, 23 vii 2006, L. S. Yakovchenko (VLA with R. metaboliza and R. endospora).—South Korea: Gangwon Prov.: Gangrun City, Mt. Odae, Odaesan Nat. Park, on Fraxinus, alt. 240 m, 37°49'N, 128°42'E, 2014, A. Aptroot 72654 (ABL); Pyeonchang-gun, Mt. Odae, Odaesan Nat. Park, on Quercus, alt. 1230–1560 m, 37°47'N, 128°32'E, 2014, A. Aptroot 72750; Phoenix Park, on Quercus, alt. 650 m, 37°35'N, 128°19'E, 2014, A. Aptroot 72606 (both ABL); Yangyang-gun, Ser-myun, Osaeck-ri, southern part of the massif Sorak Mts, Sorak-san National Park, south slope of Mt. Dachong, along trail from shelter c. 500 m WNW of the top of Mt. Dachong to village at Hangyeryong Pass, c. 1–3 km SW of the shelter, on Acer palmatum, alt. 1600–1400 m, 38°07·10–06·45'N, 128°27·25–26·00'E, 2006, G. Thor 20390, 20416 (on Quercus sp.) (AK & UPS); 500 m SW of shelter, on dead shrub in alpine heath, alt. 1600 m, lat. 38·11833, long. 128·45417, G. Thor 20365 (UPS).
Rinodina oxydata (A. Massal.) A. Massal.
Neag. Lich.: 19 (1854).—Mischoblastia oxydata A. Massal. Ric. Lich. Crost.: 42 (1852); type: Italy, Verona, in oppido Lavagno (ad saxa basalti), s. d., A. Massalongo s. n. (VER—holotype).
The only specimen of this species examined from the region was very small but possessed the typically thin thallus, pigmented apothecial margins and Mischoblastia-type ascospores (immature, 16·0–18·5×9·5–10·5 µm, n=5) characteristic of Rinodina oxydata. The apothecial section showed crystals under PL suggestive of atranorin but they could not be specifically located to the cortex. The specimen was too small to be sampled for TLC. Rinodina moziana is similar but is distinguished by its larger spores and thicker thallus.
Rinodina oxydata is widely distributed throughout the world, being recorded from Europe, southern Africa, Australasia, South America (Kaschik Reference Kaschik2006) and North America (Sheard Reference Sheard2010; Lendemer et al. Reference Lendemer, Tripp and Sheard2014). The species was previously reported from Japan (Kurokawa & Kashiwadani Reference Kurokawa and Kashiwadani2006) and Russia (Kotlov Reference Kotlov2008) and it was listed for the southern Russian Far East by Urbanavichus & Andreev (Reference Urbanavichus and Andreev2010).
Specimen examined. Russia: Primorskiy Krai: Shkotovsky District, vicinity of Shkotovo, oak forest (Quercus mongolica, Betula dahurica, Fraxinus mandshurica), 43°19'N, 132°21'E, on tufa, 28 v 2010, I. A. Galanina (VLA).
Rinodina placynthielloides Aptroot
Fungal Diversity 14: 40 (2003); type: Taiwan, Taichung Co., 30 km ENE Taichung, 5 km NW Kukwan, along mountain trail, 1000–1300 m alt., 51RTG9279, on granite of wall along path, 22 Oct 2001, Aptroot 53541 (B—holotype).
Thallus thin, grey-brown, areolate, areoles to c. 0·60 mm wide; surface plane, matt; prothallus very thin, black, entire to fimbriate; vegetative propagules abundant, blastidia, darker brown than thallus, quickly covering surface of areoles, (30×60–)55×80(–70×100) µm, budding soredia, (30–)35–40(–50) µm.
Apothecia broadly attached, infrequent, some contiguous, c. 0·45 mm diam.; disc dark brown, persistently plane; margin concolorous with thallus, entire, c. 0·05 mm wide; excipular ring not evident. Thalline exciple c. 60 µm wide laterally; cortex c. 10 µm wide, epinecral layer absent; crystals absent in cortex and medulla; cortical cells c. 4·5 µm wide, pigmented; algal cells c. 11 µm wide; thalline exciple 90–100 µm wide below; proper exciple not evident laterally, c. 30 µm wide at surface. Hymenium c. 80 µm high, not inspersed; paraphyses c. 2·5 µm wide, not conglutinate, apices to c. 4·0 µm wide, forming a brown epihymenium. Asci c. 60×19 µm; ascospores (4–)8 per ascus, development type not observed, Pachysporaria-type II, (13·0–)13·5–14·5(–18·0)×(7·0–)7·5–8·5(–11·5) µm (n=22), l/w ratio (1·6–)1·7–1·8(–1·9), development often asynchronous; lumina rounded from start, torus present, walls not ornamented. Hypothecium hyaline, c. 60 µm deep.
Pycnidia not seen.
Chemistry. Spot tests all negative. Secondary metabolites absent by TLC (Aptroot & Sparrius Reference Aptroot and Sparrius2003).
The measurements cited above were taken from a hand-cut section, rather than a microtome section, in order to conserve the limited number of apothecia present. The type description states that the asci are 4-spored. Only one such ascus was seen and represents the extreme state of the frequently asynchronous spore development in the asci. This ascus had spores at the maximum of the size range quoted above and they were also pointed as opposed to their more typical bluntly-ellipsoid shape. The type description also mentions lecideine apothecia, but these apothecia belong to a small thallus of Rinodina moziana with Mischoblastia-type spores that is also present on the type specimen. The spore measurements might also have been taken from these apothecia since they are mostly larger than those quoted above for R. placynthielloides. The thallus of R. moziana is a light grey colour and contains atranorin crystals but it also hosts abundant germinating soredia from the surrounding thalli of R. placynthielloides, thus explaining the apparent confusion between the two species. Although the holotype is a mixture of two species, the blastidiate thalli are dominant and there can be little doubt that the type description refers to R. placynthielloides rather than R. moziana.
Aptroot & Sparrius (Reference Aptroot and Sparrius2003) refer to the vegetative propagules as isidia but, when viewed under the compound microscope, these can clearly be seen to bud off soredia and are more typical of blastidia. These blastidia quickly cover the surface of the areoles and are the defining character of this interesting species. As demonstrated by their presence on the surface of the accompanying R. moziana thallus, the blastidia and their soredia are clearly very effective at colonizing adjacent substrata.
Rinodina placynthielloides was reported from South Korea by Aptroot & Moon (Reference Aptroot and Moon2014).
Additional specimen examined. South Korea: Prov. Chungchongbuk-do: Danyang-gun, Daegang-myun, Sonam Valley, 36°54'N, 128°20'E, 230 m alt., siliceous rock, 2007, Aptroot 67688 (ABL).
Rinodina polyspora Th. Fr.
Nova Acta Reg. Soc. Sci. Uppsala, Ser. 3, 3: 226 (1861); type: Switzerland, bei Zurich, 1853, P. Hepp s. n. (Hepp Fletchen Eur. 77 as Psora sophodes (UPS—lectotype [Giralt & Mayrhofer Reference Giralt and Mayrhofer1994]; BERN—isotype).
The 16-spored ascus is characteristic for this species although asci with 8 spores are also rarely found (Sheard Reference Sheard2010). The ascospores belong to the Physcia-type but are often over-mature, then lose their internal structure. The thallus is usually grey-brown and the apothecia are broadly attached, often becoming convex. The species is not to be confused with R. polysporoides which also possesses 16-spored asci but has Dirinaria-type spores (Giralt & Mayrhofer Reference Giralt and Mayrhofer1994).
Rinodina polyspora has recently been reported from Korea by Joshi et al. (Reference Joshi, Kondratyuk, Crişan, Jayalal, Oh and Hur2013). This species is new to the lichen biota of Japan (Fig. 3D). The species was previously recorded from the Russian Far East by Chabanenko (Reference Chabanenko2002). It is also found in western and eastern North America (Sheard Reference Sheard2010) and Europe (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002).
Specimens examined. Japan: Hokkaido: Kitami Prov., Esashi-gun, Hamatonbetsu-cho, 9 km S of Hamatonbetsu, on Salix sp. at the river, alt. 50 m, 45°03'N, 142°23'E, 1995, G. Thor 14208 (TNS & UPS); Soya-gun, Sarufutsu-mura, 12 km NW of Hamatonbetsu, 8 km SE of Poronuma Lake, 1·5 km from the sea, 45°13'N, 142°15'E, alt. 30 m, on Picea glehnii, 1995, T. Tønsberg 22756 (BG with R. subminuta); Esashi-gun, Esashi-cho, 2 km S of Honcho, 44°55'N, 142°35'E, alt. 10–20 m, on Acer, 1995, T. Tønsberg 22753 (BG with R. subminuta).—Russia: Sakhalinskaya Oblast’: Yuzhno-Sakhahlinsk District, Yuzhno-Sakhalinsk, Botanical Garden, on Salix hultenii, elev. 80–90 m, 46°56'37·1''N, 142°45'54·1''E, 2004, C. Printzen 9107 (FR).
Rinodina roscida (Sommerf.) Arnold
Verh. K. K. Zool.-Bot. Ges., Wien 37: 133 (1887). Basionym: Lecanora roscida Sommerf., Suppl. Flora Lappon.: 97 (1826); type: [Norway: Nordland], Saltdalen, moss on calcareous rocks, 1824, S. C. Sommerfelt s. n. (O—lectotype, Mayrhofer & Moberg Reference Mayrhofer and Moberg2002).
This species is reminiscent of Rinodina turfacea in its oro-arctic distribution, its presence among mosses or on decaying vegetation on the ground and its large Physcia-type spores. It differs in its light grey thallus lacking sphaerophorin, pruinose apothecial discs and being limited to calcareous habitats. Its spores are also significantly larger than those of R. turfacea (Sheard Reference Sheard2010). Rinodina roscida is widespread in the oro-arctic of the Northern Hemisphere (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002) and has previously been recorded from the arctic portion of the Russian Far East (Makarova & Katenin Reference Makarova and Katenin1983; Urbanavichus & Andreev Reference Urbanavichus and Andreev2010).
Specimens examined. Russia: Magadanskaya Oblast’: Omsukchanskiy District, c. 500 km north-eastward from Magadan, foothills of Kilganskie Range, vicinity of mining camp Dzuletta, 61°11'39·8''N, 153°58'49·8''E, alt. 1480 m, upper part of the slope with calcareous rocks, on mosses, 2012, Yakovchenko M-12-Ca-1, M-12-Ca-2 (VLA).
Rinodina septentrionalis Malme
Svensk Bot. Tidskr. 6: 920 (1913); type: Sweden, Jämtland, Undersåker, 1912, G. O. Malme s. n., on Salix and Alnus, Malme, Lich. Suec. Exs. 290 (UPS—lectotype, Mayrhofer & Moberg Reference Mayrhofer and Moberg2002; COLO—isolectotype!).
This species is characterized by its scattered, narrowly attached apothecia, its small, scattered, convex areoles and by its relatively small, Physcia-type ascospores measuring (15·5–)16·5–18·5(–19·5)×(7·0–)7·5–8·0(–8·5) µm (n=40), l/w ratio (1·9–)2·1–2·4(–2·6), with prominent tori. The spore size of our collections is comparable to that quoted by Mayrhofer & Moberg (Reference Mayrhofer and Moberg2002) and Sheard (Reference Sheard2010), and as indicated previously the spores cannot be separated from those of R. freyi. Thalli of this last species consist of plane areoles and are mostly found associated with leaf scars and twig axils or other sites where water tends to be retained on the bark substratum. Nevertheless, immature thalli of these two species are difficult to separate.
Rinodina septentrionalis was previously recorded in our study area from the Sikhote-Alin’ Mountains by Chabanenko (Reference Chabanenko2002, based on an earlier unpublished report by Insarov & Pchelkin, specimen in herbarium “MGU”, not seen), Kamchatka (Himelbrant et al. Reference Himelbrant, Stepanchikova and Kuznetsova2009), the lower reaches of the Amur (Yakovchenko et al. Reference Yakovchenko, Galanina, Malashkina and Bakalin2013) and the Stanovoye Nagor’e highlands (Chesnokov & Konoreva Reference Chesnokov and Konoreva2015). It is an oro-arctic species in North America (Sheard Reference Sheard2010), Scandinavia and Siberia (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002). The records reported here are the first from Japan and it is significant that they are from relatively high elevations. The species is widely distributed in the study area, especially north of 45° (Fig. 3F) and has also been reported from Mongolia (Hauck et al. Reference Hauck, Tønsberg, Mayrhofer, de Bruyn, Enkhtuya and Javkhlan2013).
Selected specimens examined. Japan: Honshu: Shimotsuke Prov. (Tochigi Pref.), Nikko City, 20 km WNW of Nikko, SW shore of Lake Karikomi, on branch found on the ground, elev. 1635 m, 36°49'26·1''N, 139°25'53·8''E, 2015, G. Thor 32127; 13 km NW of Nikko and 5 km ESE of Yumoto Village, 40 m SE of the end of the dirt road, on Alnus sp. at small stream, elev. 1610 m, 36°47'56·3''N, 139°28'56·6''E, 2015, G. Thor 32537 (both UPS).—Russia: Kamchatskyi Krai: Elizovo District, Kronotsky Nature Reserve, Pikhtovaya Growe, 54°08'N, 159°56'E, alt. 25–50 m, Abies gracilis, 2009, L. I. Rassokhina (LECB 12-055); Mil’kovo District, Kamchatka River basin, SW slope of Tolbachik Volcano, c. 40–43 km SE of Kozyrevsk, near Bubochka hill, 55°43'54''N, 160°11'27''E, alt. 680 m, on Alnus fruticosa, 10 viii 2006, D. Himelbrant (LECB 12-016 with R. sibirica); Mil’kovo District, Kronotsky Nature Reserve, Levaya Schapina River basin, mouth of Ipuin River, 55°06'55''N, 159°57'45''E, alt. 280 m, old Crataegus chlorosarca, 8 viii 2009, D. Himelbrant & I. Stepanchikova (LECB 12-058 with R. sibirica); Ust’-Bol’sheretsk District, Bannaya River basin, bank of Bannaya River, 52°54'25''N, 157°30'12''E, alt. 244 m, Alnus hirsuta, 6 viii 2002, D. Himelbrant & E. Kuznetsova (LECB 12-056); Ust’-Bol’sheretsk District, Bystraya Bol’shaya River basin, 53°04'51''N, 156°54'38''E, alt. 61 m, on Betula ermanii branch, 18 viii 2002, D. Himelbrant & E. Kuznetsova (LECB 12-049); Ust’-Bol’sheretsk District, Pravyy Kikhchik River basin, 53°34'23''N, 156°41'05''E, alt. 236 m, on Crataegus chlorosarca branch, 4 viii 2004, D. Himelbrant & E. Kuznetsova (LECB 12-043); Ust’-Kamchatsk District, Yelovka River basin, right bank of Yelovka River near the estuary of Levaya River, 56°53'00''N, 160°55'06''E, alt. 160 m, on Picea ajanensis branch, 25 viii 2003, D. Himelbrant & E. Kuznetsova (LECB 12-044). Khabarovskiy Krai: Bogorodskoe-De Kastri route, 200 m N of junction to Savinskoe, 2·7 km E of Amur River bank, on Larix gmelinii, elev. 64 m, 52°09·954'N, 140°24·597'E, 2009, C. Printzen 11641 (FR); Komsomolsk-De Kastri route, Khomi Mountains, c. 20 air km E of Chernyy Mys, hills on north bank of Salasu River, 51°05·896'N, 138°46·303'E, on Picea twig, 316 m elev., 2009, T. Spribille 30496 (GZU). Magadanskaya Oblast’: Magadan Reserve, 60°44'26''N, 146°08'17''E, on Alnus, 17 vii 2010, E. A. Zheludeva (VLA). [Sakhalinskaya Oblast’]: Sakhalin Island, road to mud volcanoes near village of Klyuchi, top of the ridge, 47°11'N, 142°35'E, on Larix, 2005, A. V. Galanin S-C1-05, I. A. Galanina S-06-C1-1 (VLA); Sakhalin Island, Susunayskiy Ridge, near Yuzhno-Sakhalinsk, Ostraia Mountain, 46°58'38·29''N, 142°46'14·43''E, 350 m alt., on Sorbus, 2011, A. K. Ezhkin 17R-11 (VLA); Sakhalin Island, Dolinsky District, N of Firsovo, 47°38'N, 142°34'E, on dry twig, 2008, N. A. Tsarenko & S. V. Nesterova S-W-27-02E-3 (VLA); Sakhalin Island, Nogliksky District, vicinity of Val, bank of River Evay, 52°19'N, 143°03'E, 2009, A. V. Bogacheva & V. Y. Barkalov S-09-83-1 (VLA); Sakhalin Island, Smirnihovsky District, vicinity of Pobedino, 49°49'N, 142°46'E, on twig, 2009, N. A. Tsarenko S-W08-18-02NW-6 (VLA); Sakhalin Island, Tymovsky District, S of Palevo, 50°36'33''N, 142°41'27''E, 20 ix 2008, N. A. Tsarenko (VLA). Zabaikal’skiy Krai: Aginskiy Buryatskiy Autonomous Okrug, Alkhanay Nat. Park, 3·5 km E of ranger station ‘Ara-Ilya’, fold ‘Niznyaya Tangaya’, on Pinus, 50°56'35''N, 113°14'32''E, alt. 883 m, 6 vii 2006, L. S. Yakovchenko (VLA); Sokhondinskiy Biosphere Zapovednik, 2 km N of Agutsakan forest station, on Betula, 49°36·611'N, 111°19·477'E, alt. 1125 m, 23 vii 2006, L. S. Yakovchenko (VLA); Sokhondinskiy Biosphere Zapovednik, road between forest stations Shergen-Daban and Ust’-Bablashniy, right bank Burecha River valley, on Picea, 49°44·945'N, 110°50·822'E, alt. 1464 m, 2 vii 2007, L. S. Yakovchenko (VLA).
Rinodina sibirica H. Magn.
Svensk Bot. Tidskr. 30: 261 (1936); type: [Russia:] Siberia, Jenisejsk, Kolmogorova, 59°30'N lat., 3 Oct 1876, M. Brenner 462c (S—lectotype (f. typica H. Magn.) Sheard Reference Sheard2010).
Rinodina sibirica is characterized by apothecia that may be erumpent when young but quickly become narrowly attached, sometimes almost stipitate, and the discs of older apothecia may become convex (even half globose) and/or pruinose due to the surface accumulation of hymenial gelatin (Sheard Reference Sheard2010). The ascospores are intermediate between the Physcia- and Physconia-types, possessing rather narrow lumen canals in immature spores and becoming darkly pigmented with a prominent torus. The spore type and structure suggest that R. sibirica might be related to the R. archaea species aggregate, although it was not included in the study of that group by Mayrhofer & Sheard (Reference Mayrhofer and Sheard2007). It is perhaps most closely related to R. orculata Poelt & M. Steiner which has smaller but otherwise very similar spores. In the present collections it was noted that spore development may be asynchronous and this may lead to the full development of only four spores per ascus. Rinodina sibirica may be similar in its external morphology to R. cinereovirens but it lacks sphaerophorin and, therefore, crystals in the medulla are absent. The spores of R. sibirica are also smaller, averaging 20·5–21·5×10·0–10·5 µm (Sheard Reference Sheard2010), than those of R. cinereovirens which average 23·0–25·5×11·5–13·5 µm, as quoted above.
Previously known from subarctic North America (Sheard Reference Sheard2010) and Siberia (Magnusson Reference Magnusson1947), and previously reported from the central Russian Far East (Urbanavichus & Andreev Reference Urbanavichus and Andreev2010). The distribution of R. sibirica is similar to that of R. freyi and R. septentrionalis, occupying a band across the northern half of our study area (Fig. 7A).

Fig. 7 Distribution of Rinodina species in north-eastern Asia (cont.) A, R. sibirica; B, R. subminuta; C, R. subparieta (solid circles: sorediate thalli; open circles: fertile non-sorediate thalli); D, R. turfacea (solid circle=record from soil; open circles=lichenicolous records on Lobarina scrobiculata); E, R. willeyi; F, R. xanthophaea (solid circles=previous records; open circles=new records).
Selected specimens examined. Russia: Kamchatka Krai: Bystrinsky District, Bystrinsky Nat. Park, Gorgochan Pass, 55°51'48''N, 158°38'40''E, alt. 686 m, on Betula platyphylla, 18 viii 2003, D. Himelbrant & E. Kuznetsova (LECB 12-042); Mil’kovo District, Kamchatka River basin, SW slope of Tobalchik Volcano, c. 40–43 km SE of Kozyrevsk, near the Bubochka hill, 55°43'54''N, 160°11'27''E, alt. 680 m, on Alnus fruticosa, 10 viii 2006, D. Himelbrant (LECB 12-016 with R. septentrionalis); Mil’kovo District, Kronotsky Nature Reserve, Levaya Schapina River basin, right bank of Ipuin River, 55°06'05''N, 159°59'22''E, alt. 280 m, 10 viii 2009, D. Himelbrant & I. Stepanchikova (LECB 12-020, 12-025, with R. subparieta); Kronotsky Nature Reserve, Levaya Schapina River basin, mouth of Ipuin River, 55°06'55''N, 159°57'45''E, alt. 280 m, on old Crataegus chlorosarca, 8 viii 2009, D. Himelbrant & I. Stepanchikova (LECB 12-058 with R. subparieta, R. septentrionalis); Ust’-Kamchatsk District, Kamchatka River basin, WSW slope of Shiveluch Volcano, c. 31 km NE of Kluchi, 56°33'60''N, 161°12'19''E, alt. 721 m, on Betula ermanii, 24 viii 2002, D. Himelbrant & E. Kuznetsova (LECB 12-033). Khabarovskiy Krai: Komsomolsk-De Kastri route, Khomi Mountains, 14 km ESE of Reshayushchiy, 51°23·030'N, 139°41·948'E, on Abies nephrolepis, 132 m elev., 2009, T. Spribille 30597 (GZU); Moni station on BAM railroad, along main route between Komsomolsk and Berezovyy, 34 km (air line) SE of crossing of Amgun’, 51°28·322'N, 136°07·591'E, on Betula, 349 m elev., 2009, T. Spribille 31277 (GZU); foothills of Etkil’-Yankanskiy Mountains, Amgun’ River region, 9·7 km (air line) due N of Berozovyy, 51°46·193'N, 135°41·045'E, on Picea twigs, 550 m, 2009, T. Spribille 31285 (GZU), C. Printzen 11795, 11813 (FR); Sonakh River, Amgun’ River region, c. 7·6 km NW of main Berezovyy-Badzhal route, 51°30·411'N, 135°14·111'E, on Alnus twigs, 342 m elev., 2009, T. Spribille 31389 (GZU); Sonakh River, Amgun’ River region, c. 7·6 km NW main Berezovyy-Badzhal route, on Rhododendron, elev. 330 m, 51°30·363'N, 135°14·221'E, 2009, C. Printzen 11880 (FR); Bureinskiy Zapovednik, upper reach of Pravaya Bureya River, Tsarskaya Dorogа, c. 650 m N of patrol cabin ‘Staraya Medvezhka’, 52°09·002'N, 134°19·035'E, on ?Alnus, 872 m, 2009, T. Spribille 31737 (with R. subparieta), 31761 (both GZU). Magadanskaya Oblast’: vicinity of Klepka, 59°44'N, 151°29'E, on dry Larix twig, 2002, A. Iamborko M-02-1 (VLA). [Sakhalinskaya Oblast’:] Sakhalin Island, Tymovsky District, 441 km federal highway, 50°32'N, 142°36'E, Betula, 2008, N. A. Tsarenko S-W08-19-03W-7 (VLA). Zabaykalskiy Krai: Nat. Park Alkhanay, Ara-Ilya forest station, steppe, on Betula and Populus, 50°54'06·4''N, 113°09'10·8''E, 8 vii 2006, L. S. Yakovchenko; on Betula, 50°56'35·1''N, 113°14'32·14''E, alt. 883 m, 6 vii 2006, L. S. Yakovchenko; Duldurginskiy District, south-east of Toptanay, 50°44'30''N, 113°51'51''E, 14 vii 2006, L. S. Yakovchenko (all VLA); Sokhondinskiy Biosphere Zapovednik, 2 km N of Agutsakan forest station, Agutsakan River flood plain, on Betula, 49°36·611'N, 111°19·477'E, alt. 1125 m, 23 vii 2006, L. S. Yakovchenko (VLA); Aginskiy Buryatskiy Autonomous Okrug, Alkhanay Nat. Park, 3·5 km E of ranger station ‘Ara-Ilya’, fold ‘Niznyaya Tangaya’, on Pinus, 50°56'35''N, 113°14'32''E, alt. 883 m, 6 vii 2006, L. S. Yakovchenko (VLA).
Rinodina sicula H. Mayrhofer & Poelt
Biblioth. Lichenol. 12: 143 (1979); type: Italy, Sicilia, Messina, Monti Nebrodi, 1978, Hertel & Hertel 19609 (M—holotype).
A rare saxicolous species in Europe (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002; Mayrhofer & Sheard Reference Mayrhofer and Sheard2007) characterized by its Physconia-type ascospores and the presence of gyrophoric acid in the apothecium margin (C+ faint red under the compound microscope, and PL+). The material studied from our area is very poorly developed, lacking a thallus, and only possessing apothecia sitting in the centre of black, radiating and fimbriate prothalli. The spores are larger and more narrowly ellipsoid than the size quoted in the above references (21·0–24·0×9·0–10·0 µm, n=8) but their structure is characteristic of the species. The specimens are too small to be examined for gyrophoric acid using TLC.
Rinodina sicula was previously known from southern Sweden and Denmark, and other scattered localities across Europe (Mayrhofer & Sheard Reference Mayrhofer and Sheard2007). This is a new record for Russia (Fig. 3D).
Specimens examined. Russia: Kamchatka Krai: Tigil’ District, vicinity of Palana, 59°05'N, 159°56'E, vii 2003, Bakalin (LECB 12-038). Magadan Region: Omsukchanskiy District, 500 km NE from Magadan, foothills of Kilganskie Range, vicinity of mining camp Dzuletta, 61°11'43·3''N, 153°58'34·9''E, 1321 m alt., 2012, L. S. Yakovchenko M-12-27-1 (VLA). Zabaikal’skiy Krai: Sokhondinskiy Biosphere Zapovednik, 4 km NE of Agutsakan forest station, 49°43·47'N, 111°66·12'E, 27 vii 2006, L. S. Yakovchenko (VLA).
Rinodina subalbida (Nyl.) Vain.
Bot. Mag., Tokyo 35: 62 (1921).—Lecanora subalbida Nyl., Lich. Japon.: 40 (1890); type: Japan, Vega Expedition (1879) Almquist s. n. (H-Nyl. p.m. 2998—lectotype, designated here).
Thallus light grey, often darker grey to grey-brown, at first comprised of isolated, thin areoles scattered on a dark prothallus, areoles becoming contiguous, usually thick, sometimes merging to form a continuous or typically rimose-areolate thallus; areoles to (0·80–)1·20–1·40 mm diam.; surface sometimes plane, typically becoming rugose or verrucose, matt; margin determinate, delimited by a blackish, continuous to fimbriate prothallus, narrower and entire adjacent to other species; vegetative propagules absent.
Apothecia often erumpent, remaining broadly attached, frequent, contiguous or not, to 0·60–1·20 mm diam.; disc black, concave or plane, sometimes becoming convex, even half spherical, frequently pruinose; thalline margin concolorous with thallus, c. 0·10 mm wide, typically persistent but sometimes becoming excluded when disc half spherical; excipular ring absent, or confluent or raised, rarely forming proper margin when thalline margin incomplete. Thalline exciple (40–)55–80 µm wide laterally; cortex poorly organized, epinecral layer 5–15 µm wide; crystals present in medulla and cortex (pannarin); cortical cells mostly obscured by crystals, to 3·5–4·5 µm wide, not pigmented; algal cells 8·0–14·5 µm long; proper exciple hyaline or light brown, 10–15 µm wide (unless thalline margin absent and then c. 55 µm wide and carbonized), expanding to 20–45 µm wide above. Hymenium hyaline or lightly pigmented, 90–120 µm high, not inspersed; paraphyses 2·0–2·5 µm wide, strongly conglutinate, apices 3·0–4·0 µm wide, hardly pigmented, immersed in dispersed pigment, forming a red-brown epihymenium, crystals (pannarin) typically present. Asci 70–90×23–28 µm; ascospores (4–)8 per ascus, type A development, Teichophila-type, (19·0–)22·0–26·5(–29·0)×(10·5–)12·0–14·0(–15·0) µm (n=189), l/w ratio (1·6–)1·7–2·0(–2·2); lumina Physcia-like at first although with relatively thick lateral walls, finally Pachysporaria-like, not inflated at septum in KOH, often slightly constricted at the septum when mature; torus well developed at maturity (Fig. 9), walls not ornamented. Hypothecium hyaline or light reddish brown, 50–80 µm deep.
Pycnidia not seen.
Chemistry. Spot tests K−, C−, P+ cinnabar. Secondary metabolites: pannarin in cortex and medulla, ± zeorin (in medulla only?), unknown A7, B7 (deep blue in LW), C7 or often B7 only, usually present in absence of zeorin; pannarin also in epihymenium (red needles in alcoholic P), causing pruinosity of discs in older apothecia.
A full description of this corticolous species is given above for the first time. Rinodina subalbida is a rather variable species in its external morphology. The thallus, at maturity, is typically rugose to verrucose and the apothecia, although most commonly plane, may become convex or rarely half-spherical with the exclusion of the thalline margin. The apothecial variation is added to by the presence of disc pruinosity in many mature specimens, reflecting the accumulation of pannarin in the epihymenium. The developmental variablity of the Teichophila-type ascospores is typical (Sheard Reference Sheard2010), the lumina being Physcia-like when immature and later becoming more rounded and Pachysporaria-like.
The habit (but not the colour) of young thalli of Rinodina subalbida (Fig. 9) and the structure of its spores are reminiscent of the recently described R. campestris Sheard & C. A. Morse from the central plains of North America (Sheard et al. Reference Sheard, Knudsen, Mayrhofer and Morse2011). However, R. subalbida is well distinguished by the presence of pannarin in both the thallus and epihymenium. Very rarely (Tønsberg 22750) verrucate thalli approach the bullate morphology of R. excrescens and it is then best distinguished by the presence of zeorin, which is normally absent in R. excrescens, and by the smaller, Physcia-type spores of the latter species. Rinodina subalbida is also similar to R. bolanderi H. Magn., from coastal California and the Georgia Straits area of British Columbia, in its Teichophila-type spores and general habit but differs in its smaller apothecia and thallus containing pannarin rather than atranorin.
The spores of R. subalbida are similar in size and shape to the Teichophila-type spores of R. buckii, but they are more Physcia- and less Pachysporaria-like than the latter species. Another apothecial similarity of the two species is their lightly pigmented hypothecium. Although the thalli of both species are also somewhat similar in being verrucose, R. subalbida is never sorediate. Unlike R. subalbida, pannarin is not present in the epihymenium of R. buckii.
Nylander’s description of the species refers to both corticolous and saxicolous material, causing an obvious challenge for lectotypification. A specimen in S (Nagasaki, 1879, Almquist), mentioned in the type description, is saxicolous but is not conspecific with the corticolous material deposited in H-Nyl. Mayrhofer (Reference Mayrhofer1984) referred the saxicolous specimen to Rinodina compensata. Two other corticolous specimens in H-Nyl. represent young thalli in which the thallus itself is thinner than typical, and also some apothecia are eroded, perhaps eaten away by invertebrates. Both have crystals in their medulla and epihymenium. The spores of both belong to the Teichophila-type and fall within the range of the more luxuriant material measured above. One of these specimens (H-Nyl. 2884) is from a locality (Simonosaki) not listed among the paratypes. The other, H-Nyl. 2998, has no locality reference other than “Japonia, Vega exp.” but is cited as “Jap. p. 40” in the type description. This specimen is accordingly selected here as the lectotype of R. subalbida.
Rinodina subalbida was described from Japan and is new to South Korea and the southern part of far eastern Russia (Fig. 8A).

Fig. 8 Distribution of Rinodina species in north-eastern Asia (cont.) A, R. subalbida; B, R. tenuis.
Selected specimens examined. Japan: Hokkaido: Ishikari Prov., nr. Mt. Arashiyama, Asahikana City, 43°47'N, 142°18'E, A. Shimizu 1541, 1551 (TNS with R. ascociscana); Kitami Prov., Esashi-gun, Esashi-cho, 2 km S of town of Honcho, just SW of road 238, 44°55'N, 142°35'E, alt. 10–20 m, on Abies sachalinensis, 1995, T. Tønsberg 22750 (BG); Rishiri-gun, Rishiro-to Island, Oshidomari area, 45°13'N, 141°13'E, on Picea, G. Thor 13749 (TNS); Shiretoko Nat. Park, 9 km NE of Utoro Village, along trail around Shiretoko-goko Lakes, 44°07'N, 145°05'E, alt. 260 m, on Prunus, 1995, T. Tønsberg 22804 (BG with R. subparieta), 22905 (BG with R. buckii); Kushiro Prov., Akan-gun, Taro-ko to Mt. O-akan trail, 43°27'N, 144°09'E, on Sorbus, Y. Ohmura 1488 (TNS); Akkeshi-gun, Hamanaka-cho, 55 km E of Kushiro City, 3 km E of Hichiripputo Lake, 43°02'N, 145°03'E, alt. 60 m, on Sorbus, 1995, T. Tønsberg 22931, 22932, 22933 with R. willeyi (all BG); Teshio Prov., Teshio-gun, Toyotomi-cho, 35 km NNW of the small town of Teshio along small road 2·5 km from coast, 45°12'N, 141°36'E, alt. 10–20 m, on Quercus serrata var. grosseserrata, 1995, T. Tønsberg 22224 (BG); Tokachi Prov., Ashoro-gun, Lake Onneto, 43°23'N, 143°59'E, on Picea, Y. Ohmura 1794 (TNS). Honshu: Kozuke Prov., Minakami, Takaguwa Hotspring, 34°40'N, 138°59'E, K. Tsunoda 744 (TNS); Bizen Prov. (Okayama Pref.), Maniwa-gun, Kawakami-mura, 1–2·3 km N of Hiruzen Research Institute, on exposed tree at roadside, alt. 500–550 m, 35°18'N, 133°38'E, 1994, G. Thor 12220 (TNS & UPS); Mutsu Prov. (Aomori Pref.), Mt. Hakkoda, c. 3 km E of Kasamatsu Pass along route 103, on Fagus crenata, alt. 850 m, 40°38'N, 140°54'E, 1994, G. Thor 11895 (TNS & UPS with R. xanthophaea and R. subminuta); Kai Prov. (Yamanashi Pref.), Makioka-cho, Yamanashi-City, road to Odarumi Pass, on Salix sp., alt. 1726 m, 35°49·172'N, 138°38·963'E, 2012, G. Thor 28176 (UPS); Prov. Tohoku (Prefecture Akita), Nikko City, 14 km WNW of Nikko and 4 km SE of Yumoto Village, field station Nikko Shizen Fureai House on Malus toringo, elev. 1400 m, 36°46'11·2''N, 139°27'21·8''E, 2015, G. Thor 32322 (UPS); 11 km NE Lake Tazawa, 2 km SSW of Tsuru-no-yu Onsen, on old Quercus, alt. 616 m, 39°47·303'N, 140°46·405'E, 2013, G. Thor 29787 (UPS). Shikoku: Kochi Pref., Ino-cho Town, along path from road to small and steep hill Mt. Komochi-gon’gen, on Hydranga paniculata, alt. 1610–1630 m, 33°46'49–54''N, 133°11'16–19''E, 2006, G. Thor 21364 (UPS).—Russia: Primorskiy Krai: Sikhote-Alin’ Mountains, 25 km (air line) ESE of Yasnoye, hunting/fishing base camp near river, 43°36·058'N, 134°14·315'E, on Picea jezoensis, 2007, T. Spribille 23492 (GZU). [Sakhalinskaya Oblast’:] Sakhalin Island, Nevelskiy Pass, mixed forest, 46°44'35·92''N, 142°6'21·02''E, 425 m alt., on Sorbus, 6 xi 2013, A. K. Ezhkin (VLA).—South Korea: Cheju Island: along the Youngshil trail on west slope of Mt. Halla, on dead Abies koreana, alt. 1650–1700 m, 33°21'N, 126°32'E, 2001, G. Thor 17001 (AK & UPS); rim surrounding crater of Mt. Halla, on dead Berberis amurensis, alt. 1850–1950 m, 33°22'N, 126°33'E, 2001, G. Thor 17057 (AK & UPS); Namcheju-gun, Namwon-up, along Songpanak trail on east slope of Mt. Halla, from Songpanak Nat. Park office to Azalea field shelter, on dead deciduous tree, alt. 750–1500 m, 33º23'N, 126º37'E, 2001, G. Thor 17398 (AK & UPS); Cheju-shi, Odung-dong, Kwanumsa Temple, on deciduous tree, alt. 580 m, 33º25'N, 126º34'E, 2001, G. Thor 17634 (UPS). Gangwon Prov.: Gangrun City, Mt. Odae, Odaesan Nat. Park, on Juglans, alt. 240 m, 37°49'N, 128°42'E, 2014, A. Aptroot 72636; Inje-gun, Mt. Seorak, Seoraksan Nat. Park, on Abies, alt. 1400–1600 m, 38°07'N, 128°27'E, 2014, A. Aptroot 73199 (both ABL); Yangyang-gun, Ser-myun, Osaeck-ri, southern part of the massif Sorak Mts, Sorak-san Nat. Park, south slope of Mt. Dachong, on exposed, dead Abies sp., alt. 1400–1350 m, 38°06·45–28'N, 128°26·00–25·10'E, 2006, G. Thor 20621 (AK with R. chrysidiata).
Rinodina subminuta H. Magn.
Bot. Not. 44 1947; type: USA, New Hampshire, White Mountains, on Acer saccharinum, s. d., E. Tuckerman s. n. (S—lectotype; BG, DUKE, FH, L, UPS, US—isolectotypes, Sheard Reference Sheard2010).
Rinodina erumpens H. Magn., Acta Horti Gotob. 17: 231 (21 Nov., 1947); type: Siberia, Yeniseisk, Gorinskij Volok, on Sorbus aucuparia?, 59.20N, 3 October 1876, M. Brenner 466j (S—lectotype, Sheard Reference Sheard2010).
The morphology and anatomy of the collections reported here are entirely consistent with records from eastern North America (Sheard Reference Sheard2010) including the erumpent apothecia, frequent presence of zeorin, and Physcia-type spores measuring (16·5–)18·0–21·0(–23·0)×(8·5–)9·0–10·0(–11·0) µm (n=144), l/w ratio (1·8–)1·9–2·2(–2·4). Rinodina subminuta should not be confused with the newly described species R. orientalis which possesses a similar but mostly darker grey-brown thallus, erumpent apothecia and smaller Physcia-type spores. A more detailed comparison between the two species is made under that species. Rinodina oleae can also possess erumpent apothecia but has smaller Dirinaria-type spores (with Physcia-like lumina) that tend to inflate at the septum on application of KOH.
Two specimens from Klondike Gold Rush National Historical Park (Tønsberg 39042, 39046) were identified as Rinodina subminuta by JWS in Spribille et al. (Reference Spribille, Pérez-Ortega, Tønsberg and Schirokauer2010). On further examination, they have been shown to belong to R. pallidescens Sheard & Tønsberg. The latter has also been reported by Sheard et al. (Reference Sheard, McCune and Tønsberg2014) from Glacier Bay National Park (Fryday 10242, MSC), another locality in the northern part of the Alaskan Panhandle.
Rinodina subminuta is a new record for far eastern Asia, with a distribution spanning Russia, Japan and the Korean Peninsula (Fig. 7B) that is similar to that of R. ascociscana (Fig. 1A), R. excrescens (Galanina et al. Reference Galanina, Yakovchenko, Tsarenko and Spribille2011; see also Fig. 1F) and R. subparieta (Fig. 7C). It has a wide altitudinal range: in Japan it occurs from sea level to 1200 m in Hokkaido, and 855–1980 m in Honshu, whereas in South Korea its elevational range is 1650–1950 m on the southerly Cheju Island, and 1350–1400 m in the Sorak Mountains of Gangwon Province. It is one of six species in this study that have an exclusively eastern North American–East Asian distribution.
Selected specimens examined. Japan: Hokkaido: Ishikari Prov., nr. Mt. Arashiyama, Asahikana City, 43°47'N, 142°18'E, A. Shimizu 1541, 1551 (TNS with R. ascociscana); Kitami Prov., Esashi-gun, Esashi-cho, 2 km S of town of Honcho, just SW of road 238, 44°55'N, 142°35'E., alt. 10–20 m, on Abies sachalinensis, 1995, T. Tønsberg 22750 (BG); Rishiri-gun, Rishiro-to Island, Oshidomari area, 45°13'N, 141°13'E, on Picea, G. Thor 13749 (TNS); Shari-gun, Shiretoko Nat. Park, 44°07'N, 145°05'E, on Alnus, G. Thor 14342 (TNS); Kushiro Prov., Akan-gun, Taro-ko to Mt. O-akan trail, 43°27'N, 144°09'E, on Sorbus, Y. Ohmura 1488 (TNS); Akkeshi-gun, nr. Sanbanswana, 43°04'N, 140°04'E, S. Arakawa 1548 (TNS); Akkeshi-gun, Hamanaka-cho, 55 km E of Kushiro City, 3 km E of Hichiripputo Lake, 43°02'N, 145°03'E, alt. 60 m, on Sorbus, 1995, T. Tønsberg 22931, 22932, 22933 with R. willeyi (all BG); Teshio Prov., Teshio-gun, Toyotomi-cho, 35 km NNW of the small town of Teshio along small road 2·5 km from coast, 45°12'N, 141°36'E, alt. 10–20 m, on Quercus serrata var. grosseserrata, 1995, T. Tønsberg 22224 (BG); Tokachi Prov., Ashoro-gun, Lake Onneto, 43°23'N, 143°59'E, on Picea, Y. Ohmura 1794 (TNS). Honshu: Kozuke Prov., Minakami, Takaguwa Hotspring, 34°40'N, 138°59'E, K. Tsunoda 744 (TNS); Bizen Prov. (Okayama Pref.), Maniwa-gun, Kawakami-mura, 1–2·3 km N of Hiruzen Research Institute, on exposed tree at roadside, alt. 500–550 m, 35°18'N, 133°38'E, 1994, G. Thor 12220 (TNS & UPS); Mutsu Prov. (Aomori Pref.), Mt. Hakkoda, c. 3 km E of Kasamatsu Pass along route 103, on Fagus crenata, alt. 850 m, 40°38'N, 140°54'E, 1994, G. Thor 11895 (TNS & UPS with R. xanthophaea and R. subminuta); Kai Prov. (Yamanashi Pref.), Makioka-cho, Yamanashi-City, road to Odarumi Pass, on Salix sp., alt. 1726 m, 35°49·172'N, 138°38·963'E, 2012, G. Thor 28176 (UPS); Prov. Tohoku (Prefecture Akita), Nikko City, 14 km WNW of Nikko and 4 km SE of Yumoto Village, field station Nikko Shizen Fureai House on Malus toringo, elev. 1400 m, 36°46'11·2''N, 139°27'21·8''E, 2015, G. Thor 32322 (UPS); 11 km NE of Lake Tazawa, 2 km SSW of Tsuru-no-yu Onsen, on old Quercus, alt. 616 m, 39°47·303'N, 140°46·405'E, 2013, G. Thor 29787 (UPS). Shikoku: Kochi Pref., Ino-cho Town, along path from road to small and steep hill Mt. Komochi-gon’gen, on Hydranga paniculata, alt. 1610–1630 m, 33°46'49–54''N, 133°11'16–19''E, 2006, G. Thor 21364 (UPS).—Russia: Primorskiy Krai: Sikhote-Alin’ Mountains, 25 km (air line) ESE of Yasnoye, hunting/fishing base camp near river, 43°36·058'N, 134°14·315'E, on Picea jezoensis, 2007, T. Spribille 23492 (GZU). [Sakhalinskaya Oblast’:] Sakhalin Island, Nevelskiy Pass, mixed forest, 46°44'35·92''N, 142°6'21·02''E, 425 m alt., on Sorbus, 6 xi 2013, A. K. Ezhkin (VLA).—South Korea: Cheju Island: along the Youngshil trail on west slope of Mt. Halla, on dead Abies koreana, alt. 1650–1700 m, 33º21'N, 126º32'E, 2001, G. Thor 17001 (AK & UPS); rim surrounding crater of Mt. Halla, on dead Berberis amurensis, alt. 1850–1950 m, 33º22'N, 126º33'E, 2001, G. Thor 17057 (AK & UPS); Namcheju-gun, Namwon-up, along Songpanak trail on east slope of Mt. Halla, from Songpanak Nat. Park office to Azalea field shelter, on dead deciduous tree, alt. 750–1500 m, 33°23'N, 126°37'E, 2001, G. Thor 17398 (AK & UPS); Cheju-shi, Odung-dong, Kwanumsa Temple, on deciduous tree, alt. 580 m, 33°25'N, 126°34'E, 2001, G. Thor 17634 (UPS). Gangwon Prov.: Gangrun City, Mt. Odae, Odaesan Nat. Park, on Juglans, alt. 240 m, 37°49'N, 128°42'E, 2014, A. Aptroot 72636; Inje-gun, Mt. Seorak, Seoraksan Nat. Park, on Abies, alt. 1400–1600 m, 38°07'N, 128°27'E, 2014, A. Aptroot 73199 (both ABL); Yangyang-gun, Ser-myun, Osaeck-ri, southern part of the massif Sorak Mts, Sorak-san Nat. Park, south slope of Mt. Dachong, on exposed, dead Abies sp., alt. 1400–1350 m, 38°06·45–28'N, 128°26·00–25·10'E, 2006, G. Thor 20621 (AK with R. chrysidiata).
Rinodina subparieta (Nyl.) Zahlbr.
Cat. Lich. Univers. 7: 554 (1931).—Lecanora subparieta Nyl., Acta Soc. Sci. Fenn. 26: 30 (1900); type: Japan, Itchigômé, 1879, Almquist (H-Nyl. 28856—holotype!).
Rinodina degeliana Coppins, Lichenologist 15: 147 (1983); type: Sweden, Lule Lappmark, Kvikkjokk parish, south-east slope of Nammatj, 66°56'N, 17°42'E, alt 520 m, on Salix bark in Picea woodlands, 28 July 1977, B. J. Coppins 6238 & L. Tibell (E—holotype!; UPS—isotype!).
Rinodina subparieta is distinguished by its thallus structure which is composed of light grey, scattered areoles, with abundant atranorin, mostly with whitish soredia first forming along the areole margins in a labriform or sublabriform manner. The areoles may occasionally merge in later development to form a more continuous thallus and the soredia frequently spread to cover the areole surfaces. Molecular studies (Resl et al. Reference Resl, Mayrhofer, Clayden, Spribille, Thor, Tønsberg and Sheard2016) have shown that the sorediate R. degeliana must be regarded as a synonym of the non-sorediate R. subparieta, which was previously known only from high elevations in Honshu and thought to be endemic to Japan.
A characteristic of Rinodina species possessing vegetative diaspores is that, when fertile, spore size and structure are variable. Rinodina degeliana was originally described as possessing Dirinaria-type spores (Coppins Reference Coppins1983) but was reported to have Physcia-type spores by both Mayrhofer & Moberg (Reference Mayrhofer and Moberg2002) and Sheard (Reference Sheard2010). However, Sheard (Reference Sheard2010) questioned the distinction between the Physcia- and Physconia- spore types, suggesting that specimens exhibiting the Physcia-spore type merely exhibit an arrested stage of spore development, a very common feature of the species in Europe and North America. It is important to note that the type specimens of both R. subparieta and R. degeliana have fully developed Physconia-type spores at maturity and this seems to be a constant feature of the non-sorediate Japanese collections. All spores are also characterized by the possession of an obvious torus and dark, strongly ornamented walls. The molecular phylogeny of Resl et al. (Reference Resl, Mayrhofer, Clayden, Spribille, Thor, Tønsberg and Sheard2016) showed that the eastern North American and the Japanese collections are more closely related to each other than to samples from elsewhere in the Northern Hemisphere, although this relationship was not statistically significant.
Rinodina subparieta is relatively frequent in Hokkaido where it is found from close to sea level to 1100 m elevation. It is less frequent in Honshu and found from 500–2550 m, and the single record from South Korea, from where it is reported for the first time, is at 1350–1400 m. It was previously reported as R. degeliana from Kamchatka (Himelbrant & Stepanchikova Reference Himelbrant and Stepanchikova2011), from the central Russian Far East by Urbanavichus & Andreev (Reference Urbanavichus and Andreev2010) and from the Khentey region of Mongolia by Hauck & Javkhlan (Reference Hauck and Javkhlan2006). Its distribution in our study area (Fig. 7C) is comparable to R. ascociscana, R. excrescens and R. subminuta. As R. degeliana, the species has been reported as widely distributed in the Northern Hemisphere but is rather infrequently collected. It is found in Scotland (Giavarini et al. Reference Giavarini, James and Purvis2009), Scandinavia and Austria (Tønsberg 1992 Reference Tønsberga ; Mayrhofer & Moberg Reference Mayrhofer and Moberg2002), and eastern and western North America (Sheard Reference Sheard2010; Spribille et al. Reference Spribille, Pérez-Ortega, Tønsberg and Schirokauer2010).
Selected sorediate specimens examined. Japan: Hokkaido: Ishikari Prov., Kamikawa-gun, Kamikawa-cho, 0·5–1·5 km E of Obako Gorge Tourist Centre, on Abies sachalinensis, alt 720 m, 43°42'N, 143°01'E, 1995, G. Thor 14624 (UPS with R. buckii); Kitami Prov., Esashi-gun, Esashi-cho, 2 km S of Honcho, on Larix, alt. 10–20 m, 44°55'N, 142°35'E, 1995, G. Thor 14251 (UPS with R. buckii); Shiretoko Nat. Park, NW slope of Shiretoko Peninsula c. 10 km NE of Utoro Town, along the trail from Iwaobetsu hot-spring hotel (Onsen) to Mt. Rausu-dake, on Taxus cuspidata, alt. 388 m, 44·10611°N, 145·09242°E, 2010, G. Thor 23978 (UPS); Kushiro Prov., Akkeshi-gun, Akkeshi-cho, 44 km E of Kushiro City, S Akkeshiko Lake, on Quercus mongolica var. grosseserrata, alt. 120 m, 42°60'N, 144°57'E, 1995, G. Thor 14486 (UPS); Nemuro Prov., Shiretoko Peninsula, Menashi-gun, Rausu-cho, just S of road 334 crossing Shiretoko Peninsula, 4 km WNW of Sakae City, Rausu hot spring, on Alnus sp., alt. 130 m, 44°02'N, 145°09'E, 1995, G. Thor 14406 (UPS with R. xanthophaea); Teshio Prov., Rumoi-gun, Obira-cho, 21 km ENE of small town of Obira at the coast, along the trail from parking area to Tengunotaki Waterfall, 44°04'N, 141°55'E, alt. 50–100 m, on Betula, 1995, T. Tønsberg 21995 (BG with R. hypobadia); alt. 130–150 m, on Abies sachalinensis, 1995, T. Tønsberg 21994, 22010 (BG); alt. 160 m, T. Tønsberg 22018 (BG); Teshio-gun, Toyotomi-cho, Rishiri-Rebun-Sarobetsu Nat. Park, 23 km NNW of the small town of Teshio at coast, Wakasakanai area, S of road from coast to the town of Toyotomi, on dead Salix sp., alt. 20 m, 45°05'N, 141°39'E, 1995, G. Thor 13614 (UPS); Tokachi Prov., Kato-gun, Kamishihoro-cho, 6 km S of Mikuni tunnel through Mt. Mikuni-yama, just W of road 273, on dead deciduous tree, alt. 680 m, 43°32'N, 143°09'E, 1995, G. Thor 14581 (UPS). Honshu: Bizen Prov. (Okayama Pref.), Maniwa-gun, Kawakami-mura, 1–2·3 km N of Hiruzen Research Institute, on exposed tree at roadside, alt. 500–550 m, 35°18'N, 133°38'E, 1994, G. Thor 12212 (UPS with R. willeyi); Etchu Prov. (Toyama Pref.), Nakashinkawa-gun (Nakaniikawa-gun), Tateyama-cho, 25 km ESE of Toyama, Bijodaira, path S of Bijodaira bus stop, on old deciduous tree, alt. 960–980 m, 36°35'N, 137°28'E, 1994, G. Thor 12664 (UPS); Kai Prov. (Yamanashi Pref.), Makioka-cho, Yamanashi-City, at road to Odarumi Pass, on Prunus sp., alt. 1726 m, 35°49·172'N, 138°38·963'E, 2012, G. Thor 28169 (UPS); Shimotsuke Prov. (Tochigi Pref.), 20 km WNW of Nikko, 1 km S of Yumoto Village, 36°48'N, 139°26'E, Yudaki Falls, on deciduous tree, alt. 1440–1480 m, 1994, G. Thor 12771 (UPS with R. xanthophaea); 13 km NW of Nikko and 5 km ESE of Yumoto Village, on Alnus sp., elev. 1640 m, 36°47'59·0''N, 139°28'59·2''E, 2015, G. Thor 32481; Prov. Tohoku (Prefecture Akita), W shore of Lake Tazawa, outlook at Katamaeyama Woods Park, on old Castanea japonica, alt. 336 m, 39°42·995'N, 140°37·759'E, 2013, G. Thor 29757 (all UPS).—Russia: Kamchatka Krai: Elizovo District, Kronotsky Nature Reserve, Pikhtovaya Growe, 54°08'N, 159°56'E, alt. 25–50 m, Abies gracilis, 2009, L. I. Rassokhina (LECB 12-055); xii 2010, L. I. Rassokhina (LECB); Mil’kovo District, Kronotsky Nature Reserve, Levaya Schapina River basin, right bank of Ipuin River, 55°06'05''N, 159°59'22''E, alt. 280 m, on Alnus hirsuta and Picea ajanensis, 10 viii 2009, D. Himelbrant & I. Stepanchikova (LECB 12-020 with R. sibirica and R. efflorescens, LECB 12-025 with R. sibirica); Ust’-Bol’sheretsk District, Bannaya River basin, right bank of the Bannaya River, 52°54'40''N, 157°28'28''E, alt. 270 m, on Betula ermanii, 8 viii 2002, D. Himelbrant & E. Kuznetsova (LECB k-445); Ust’-Kamchatsk District, Yelovka River basin, right bank of Yelovka River near estuary of Levaya River, 56°53'00''N, 160°55'06''E, alt. 160 m, on Picea ajanensis branch, 25 viii 2003, D. Himelbrant & E. Kuznetsova (LECB 12-044). Khabarovskiy Krai: Bureinskiy Zapovednik, upper reach of Pravaya Bureya River, Tsarskaya Dorogа, c. 650 m N of patrol cabin ‘Staraya Medvezhka’, 25 km SE of Sofiysk and 2·6 km N (upstream) of patrol cabin ‘Novaya Medvezhka’, 52°09·002'N, 134°19·035'E, on Alnus, 872 m, 2 viii 2009, T. Spribille 31737 (GZU). Sakhalinskaya Oblast’: Sakhalin Island, 48th km of route Yuzhno-Sakhalinsk - Kholmsk, 46º50'N, 142º16'E, on Abies, I. A. Galanina S-C2-06; Sakhalin Island, 2 km north-east from Kluchi, 47°11'N, 142°35', on Abies, I. A. Galanina (VLA); Sakhalin Island, Dolinsky District, 106 km of federal highway, 47°17'N, 142°42'E, on Alnus, A. V. Bogacheva, N. A. Tsarenko S-T19-3 (VLA); Sakhalin Island, Yuzhno-Sakhahlinsk District, Yuzhno-Sakhalinsk, Mt. Tshekhov, on Picea jezoensis, elev. c. 390 m, 46°58'43·0''N, 142°49'24·5''E, 2004, C. Printzen 9179, 9188 (FR). Zabaikal’skiy Krai: Sokhondinskiy Biosphere Zapovednik, 2 km N of Agutsakan forest station, on Betula, 49°36·611'N, 111°19·477'E, alt. 1125 m, 23 vii 2006, L. S. Yakovchenko (VLA); Aginskiy Buryatskiy Autonomous Okrug, Alkhanay Nat. Park, 3·5 km E of ranger station ‘Ara-Ilya’, fold ‘Niznyaya Tangaya’, on Pinus, 50°56'35''N, 113°14'32''E, alt. 883 m, 6 vii 2006, L. S. Yakovchenko (VLA).—South Korea: Gangwon Prov.: Yangyang-gun, Ser-myun, Osaeck-ri, southern part of the Sorak Mts. massif, Sorak-san Nat. Park, the south slope of Mt. Dachong, on dead Abies sp., alt. 1400–1350 m, 38°06·45–28'N, 128°26·00–25·10'E, 2006, G. Thor 20620 (AK & UPS with R. buckii).
Non-sorediate specimens examined. Japan: Honshu: Etchu Prov., Nakasinkawa-gun, 33 km ESE of Toyama, Midagahara along path to Tateyama crater, lat. 36°34'N, long. 137°34'E, alt. 1960–2000 m., on dead, standing, Abies mariesii, 1994, G. Thor 12708 (UPS – duplicate in TNS, not seen); Kai Prov. (Yamanashi Pref.), Kofu-City, along trail between Odarumi Pass and Mt. Kinpu (Kimpō), old-growth, subalpine forest, on Abies mariesii, alt. 2552 m, 35°52·163'N, 138°37·930'E, 2012, G. Thor 27994 (UPS); Makioka-cho, Yamanashi-City, Odarumi Pass, near mountain hut, on Abies mariesii, alt. 2368 m, 35°52·171'N, 138°40·001'E, 2012, G. Thor 28096 (UPS); on Sorbus sp., G. Thor 28120 (UPS).
Rinodina teichophila (Nyl.) Arnold
Flora 46: 329 (1863).—Lecanora teichophila Nyl., Flora 46: 78 (1863); type: [United Kingdom: England:] Cleveland, Ayton, Mudd, Lich. Brit. Exs. 108 as R. exigua var. metabolica Mudd (BM—lectotype, Mayrhofer & Moberg Reference Mayrhofer and Moberg2002).
This saxicolous species is characterized by its large Teichophila-type spores (20·0–32·0×11·0–19·0 µm) which are often swollen at the septum and more so in KOH (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002; Sheard & Mayrhofer Reference Sheard and Mayrhofer2002) and lack of secondary substances in the thallus. It is widespread in the British Isles, scattered in Scandinavia, central and southern Europe, North Africa and Asia Minor (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002) but absent from North America (Sheard Reference Sheard2010). It has also been recorded from Japan (Mayrhofer Reference Mayrhofer1984; Kurokawa & Kashiwadani Reference Kurokawa and Kashiwadani2006), from Korea by Kondratyuk et al. (Reference Kondratyuk, Lökös, Tschabanenko, Haji Moniri, Farkas, Wang, Oh and Hur2013) and recently from four islands in Peter the Great Bay (Russia) in the Sea of Japan (Skirina Reference Skirina2010). The record of Rinodina teichophila reported by Aptroot & Moon (Reference Aptroot and Moon2014) represents the first record of R. moziana from Korea.
Rinodina tenuis Müll. Arg.
Nuov. Giorn. Bot. Ital. 24: 195 (1892); type: Japan, Honshu, Musashi Prov. [= Saitama Pref.], Mt. Buko, Chichibu, 3 April 1891, Yatabe 246 (G—holotype!; TNS—isotype!).
New synonym. Rinodina adirondackii H. Magn., Bot. Not. 48 1947; type: USA, New York, Essex Co., Adirondack Mountains, Chapel Pond, near St. Huberts, 1600 ft., on cedar in gully, 1933, J. L. Lowe 3751 (UPS—holotype!; NY—isotype!).
Thallus thin, light grey, quickly becoming continuous; surface plane, matt; margin indeterminate; prothallus lacking; vegetative propagules absent.

Fig. 9 Teichophila -type ascospores of R. subalbida, Japan, Honshu, Tohoku Prov., 11 km NE Lake Tazawa, Thor 29787, UPS. Note the variable shape of the lumina from Physcia - to Pachysporaria -like and the prominent tori. Scale=10 µm.
Apothecia erumpent, remaining broadly attached, frequent, rarely contiguous, 0·50–0·60 mm diam.; disc black, persistently plane, often eroded (eaten by invertebrates?); thalline margin concolorous with thallus, c. 0·10 mm wide, entire and persistent; excipular ring sometimes present, slightly raised. Thalline exciple (50–)80–100 µm wide laterally, cortex poorly developed, structure occluded by crystals, 5–15 µm wide; epinecral layer absent; crystals in cortex and medulla (pannarin); cortical cells 3·5–4·5 µm wide, not pigmented; algal cells 9·5–11·5 µm long; proper exciple hyaline to yellowish, 10–20 µm wide, expanding to 15–30 µm wide above. Hymenium 100–130 µm high, not inspersed; paraphyses 2·0–3·0 µm wide, conglutinate, apices expanded to 3·0–4·5 µm wide, not or slightly pigmented and immersed in dispersed pigment forming a light, red-brown epihymenium. Asci 80–100×24–35 µm; ascospores (4–)8 per ascus, type A development, Pachysporaria-type I, (27·0–)32·0–35·5(–39·0)×(12·5–)14·5–17·0(–18·5) µm (n=63), l/w ratio (1·7–)2·1–2·3(–2·6); lumina irregularly rounded to subpolygonal in immature spores, becoming Mischoblastia-like, then rounded in mature spores, sometimes with apical satellite lumina when over-mature; torus well developed in mature spores; walls not ornamented (Fig. 10). Hypothecium hyaline, 20–60 µm deep.

Fig. 10 Pachysporaria -type ascospores of R. tenuis, Japan, Honshu, Musashi Prov., Mt. Buko, 3 April 1891, Yatabe 246 (TNS—isotype). Note the well-developed tori and the close to spherical lumina. Scale=10 μm.
Pycnidia not observed.
Chemistry. Spot tests K−, C−, KC−, P+ cinnabar. Secondary metabolites: pannarin in medulla and cortex, observed as crystals under PL and formation of red needles in alcoholic P.
The above description results from a detailed study of the isotype of R. tenuis and the specimens listed below. It is entirely typical of R. adirondackii as known from eastern North America (Sheard Reference Sheard2010, spores not illustrated) with the possible exception of the somewhat larger, but widely overlapping, spore size. The larger size may be due to the spores from 4-spored asci, which have not been reported from North America. Rinodina tenuis was named for its thin thallus but it is not atypically thin in comparison with many other Rinodina species. The spores are larger than typical for R. willeyi, a species with which it could possibly be confused due to its similar chemistry, but that possesses soredia spreading from the areole margins. The type description of R. membranifera (Hue) Zahlbr. from Korea notes its thin, continuous thallus and very large spores, suggesting that this name might be a later homonym of R. tenuis but the type specimen of this species is not readily available for examination.
Rinodina tenuis has a Great Lakes-Appalachian distribution in North America. Although widespread in eastern North America, it is relatively infrequent (Lendemer et al. Reference Lendemer, Tripp and Sheard2014, as R. adirondackii). We have seen very few collections from eastern Asia, and the species seems to have a narrow distribution in the boreal zone of Khabarovskiy Krai, Sakhalin Island, a single record from Hokkaido, and another from Honshu (Fig. 8B). The records from Russia are the first reports from the country.
Specimens examined. Japan: Hokkaido: Kitami Prov., Shari-gun, Shari-cho, Shiretoko Nat. Park, around path along small stream NE of Iwaobetsu hot-spring hotel (Onsen), 44°07'N, 145°05'E, alt. 240–280 m, on ± mossy, horizontal log, 1995, T. Tønsberg 22845b (BG, with R. excrescens). Honshu: Musashi Prov., Mt. Buko, Yatabe 246 (TNS).—Russia: Khabarovskiy Krai: Komsomolsk-De Kastri route, Khomi Mountains, c. 20 air km E of Chernyy Mys, hills on north bank of the Salasu River, 51°05·896'N, 138°46·303'E, on Picea branch, 316 m elev., 2009, T. Spribille 30494 (GZU); Gorelaya Mountain, 51°08·565'N, 139°09·775'E, bases of branches on snag (bark remnants), 460 m elev., 2009, T. Spribille 31214, 31227 (on base of Abies nephrolepis) (both GZU). [Sakhalinskaya Oblast’:] Sakhalin Island, Cholmskiy District, Slepikovskiy promontory, 47°17'28·00''N, 141°59'10·79''E, alt. 10 m, on Picea, 2013, A. K. Ezhkin R5/5, R8/5 (VLA); Sakhalin Island, vicinity of Yuzhno-Sakhalinsk, Mitsulsky Ridge, Mitul Mountain, 47°02'59·5''N, 142°30'39·1''E, 480 m alt., on Abies, 2012, A. K. Ezhkin R8\13 (VLA); Sakhalin Island, Susunayskiy Ridge, vicinity of Yuzhno-Sakhalinsk, Turgenev Mountain, 47°00'42·3''N, 142°48'13·2''E, 602 m alt., on Picea, A. K. Ezhkin R5\13 (VLA).
Rinodina turfacea (Wahlenb.) Körb.
Syst. lich. Germ.: 123 (1855).—Lichen turfaceus Wahlenb., Flora Lappon.: 408 (1812); type: Norway, Finnmark, Insel Kvalja, Hammerfest, 1802, G. Wahlenberg s. n. (UPS—lectotype, Mayrhofer & Moberg Reference Mayrhofer and Moberg2002).
This species typically grows on moss and decaying plants, less often on wood, in oro-arctic environments. It is characterized by its densely contiguous apothecia which are sometimes angular by compression, have narrow, dark brown margins and slightly concave to persistently plane discs, and by a thallus containing sphaerophorin in the medulla. The spores of the single specimen seen growing directly on soil are the same shape and size (pointed apices, 25·5–32·5×11·0–13·0 µm, n=10) as those from North America (Sheard Reference Sheard2010) and Europe (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002), including the presence of lightly pigmented bands around the cells of some mature spores.
Rinodina turfacea has been previously reported to grow on cyanolichens by Magnusson (Reference Magnusson1947, Reference Magnusson1952), who found it on Lobarina scrobiculata (Scop.) Nyl. thalli from Gällivare, Sweden and Peltigera thalli in the Swiss canton of Wallis. Nowhere does this substratum affinity appear to be as pronounced as in the Russian Far East and Alaska, so much so that we have suspected a cryptic, cyanolichen-specific species may be in play. Specimens from Russia growing on L. scrobiculata were previously identified as R. olivaceobrunnea C. W. Dodge & G. E. Baker but this species does not possess sphaerophorin, and hence lacks crystals in the medulla. Rinodina olivaceobrunnea also has smaller spores, which may become Physconia-like, and it has not been found among the present collections, probably because it is another oro-arctic, rather than boreal, species (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002; Sheard Reference Sheard2010). These lichenicolous specimens have frequent and typically widely spaced, small apothecia with spores measuring (20·0–)21·0–24·0(–25·0)×(9·0–)10·0–10·5(–11·0) µm, l/w ratio (1·9–)2·1–2·4(–2·6) (n=50). They are smaller than the mean range for R. turfacea quoted by Sheard (Reference Sheard2010) but have a similar pointed, elongate-ellipsoid shape and angular lumina with wall thickening at the apices and septum. Two well-developed specimens from Alaska (Spribille 27701, 28055b, GZU) have slightly larger spores and when combined with the above collections the range increases slightly to (20·0–)22·0–24·5 (–25·5)×(9·5–)10·0–10·5(–11·0) µm, but retains the same l/w ratio. These Alaskan specimens also possess sphaerophorin (confirmed by TLC). In the absence of molecular evidence (DNA isolation has not been successful to date), we suggest that these specimens growing on L. scrobiculata be provisionally referred to the type variety of R. turfacea, their smaller size perhaps being the result of a suboptimal habitat and possibly a substratum on which they might never reach full maturity. Specimens from Alaska have also been observed on Erioderma pedicellatum (Hue) P. M. Jørg. and Nephroma helveticum Ach. (Spribille 28115 and 27575, respectively, GZU).
Rinodina turfacea is a common species of acidic ground in arctic and oro-arctic North America, northern Scandinavia and Siberia, and has previously been reported for the Russian Far East from Yakutia (Afonina et al. Reference Afonina, Bredkina and Makarova1980), Chukotka (Makarova & Katenin Reference Makarova and Katenin1983), Kamchatka (Himelbrant et al. Reference Himelbrant, Stepanchikova and Kuznetsova2009), the Sikhote-Alin’ Mountains (Chabanenko Reference Chabanenko2002) and Khabarovskiy Krai (Skirina Reference Skirina2012, as ‘turphacea’). Its distribution in far eastern Asia (Fig. 7D) is similar to that of R. cinereovirens, R. septentrionalis and R. sibirica.
Specimens examined on Lobarina scrobiculata. Russia: Kamchatka Krai: Mil’kovo District, Kronotsky Nature Reserve, Levaya Schapina River basin, W slope, 55°08'38''N, 159°59'24''E, alt. 370 m, on Picea ajanensis, 13 viii 2009, D. Himelbrant & I. Stepanchikova (LECB 12-061); Kronotsky Nature Reserve, Levaya Schapina River basin, SW part of Askhachny Ridge, SSE slope, 55°08'07''N, 159°57'23''E, alt. 300 m, on Picea ajanensis, 11 viii 2009, D. Himelbrant & I. Stepanchikova (LECB 12-001 with R. subparieta); Kronotsky Nature Reserve, Levaya Schapina River basin, SW part of Askhachny Ridge, 55°08'54''N, 159°59'38''E, alt. 410 m, on Picea ajanensis, 13 viii 2009, D. Himelbrant & I. Stepanchikova (LECB 12-026 with R. subparieta; LECB 12-023); Ust’-Bol’sheretsk District, Bannaya River basin, right bank of the Bannaya River, 52°54'40''N, 157°28'28''E, alt. 270 m, on Betula ermanii, 8 viii 2002, D. Himelbrant & E. Kuznetsova (LECB k-443); Ust’-Bol’sheretsk District, Pravyy Kikhchik River basin, right bank of Mokushka River, near bridge, 53°32'58''N, 156°41'09''E, alt. 251 m, on Alnus hirsuta, 22 vii 2004, D. Himelbrant (LECB k-442, k-482). Khabarovskiy Krai: Sredniy Khrebet Mountains, Polоsataya Mountain, 45·5 km (air line) NW of Lazarev, between Pravaya Tumi River and Krutoberezniy stream, 52°22·779'N, 140°53·503'E, alt. 212 m, on Abies nephrolepis, 2009, T. Spribille 31025 (GZU). Magadanskaya Oblast’: Omsukchanskiy District, 500 km north-eastward from Magadan, foothills of Kilganskie Range, vicinity of mining camp Dzuletta, 61°11'43·3''N, 153°58'34·9''E, 1321 m alt., 2012, L. Yakovchenko M-12-27-2, M-12-27-3 (VLA). [Sakhalinskaya Oblast’:] Sakhalin Island, Dolinsky District, 108 km of the federal highway, 47°12'N, 142°49'E, on dry twig, 2008, A. V. Bogacheva & N. A. Tsarenko S-T12-3 (VLA); Sakhalin Island, Noglikskiy District, Dagi River, floodplain forest, 52°06'33·5''N, 142°57'26·2''E, 10 m alt., 2012, A. K. Ezhkin R19\13 (VLA). Zabaikal’skiy Krai: Sokhondinskiy Biosphere Zapovednik, 2 km N of Agutsakan forest station, on Betula, 49°36·611'N, 111°19·477'E, alt. 1125 m, 23 vii 2006, L. S. Yakovchenko (VLA); Sokhondinskiy Biosphere Zapovednik, road between forest stations Shergen-Daban and Ust’-Bablashniy, right bank Burecha River valley, on Picea, 49°44·945'N, 110°50·822'E, alt. 1464 m, 2 vii 2007, L. S. Yakovchenko (VLA).
Specimen examined on ground. Russia: Zabaikal’skiy Krai: Aginskiy Buryatskiy Autonomous Okrug, Alkhanay Nat. Park, 3·5 km E of ranger station ‘Ara-Ilya’, fold ‘Nizhnyaya Tangaya’, on soil, 50°56'35''N, 113°14'32''E, alt. 883 m, 6 vii 2006, L. S. Yakovchenko (VLA).
Rinodina willeyi Sheard & Giralt
Herzogia 11: 124 (1995); type: [USA], Massachusetts, Bristol Co., New Bedford, 1874, H. Willey s. n. (US—holotype).
The collections reported here are fully comparable with North American specimens. The margins of young areoles are often minutely lobate, becoming raised (subsquamulose, Sheard (Reference Sheard2010)) before producing marginal soredia (sublabriform soralia). Soredia may then develop over the surface of the areoles and ultimately completely cover them. Rinodina willeyi might be mistaken for R. buckii owing to its sorediate thallus, presence of pannarin and similar-sized spores but, as previously noted, the spores of R. buckii belong to the Teichophila-type rather than the Pachysporaria-type I of R. willeyi. In addition, R. willeyi usually possesses lighter coloured and smaller soredia, (<40 µm in diam.) than R. buckii and, most importantly, these soredia initially develop on the areole margins rather than on verrucae in the centre of the areoles, as in R. buckii (Sheard et al. Reference Sheard, Lendemer, Spribille and Tønsberg2012).
A new finding is that pannarin is not always accompanied by zeorin (Tønsberg 21985, 22104, 22791, 22911, 22918, BG) but care must be taken to ascertain that such specimens do not represent the lobate form of R. excrescens, a species that mostly lacks zeorin. The blastidia and soredia of R. excrescens are associated with raised verrucae (bullate areoles) and are usually either concolorous with the thallus or greenish, rather than being lighter than the thallus which is the typical case for R. willeyi.
Rinodina willeyi is also related to R. tenuis by its Pachysporaria-type I spores (see Fig. 2C & D, Sheard et al. (Reference Sheard, Lendemer, Spribille and Tønsberg2012), not illustrated in Sheard (Reference Sheard2010)). It differs from R. tenuis in the discontinuous thallus, much less erumpent apothecia and the presence of soredia. Also R. willeyi might possibly be mistaken for R. subparieta due to its light-coloured thallus and soredia originating around the areole margins. However, it is well separated from that species by the presence of pannarin rather than atranorin.
Rinodina willeyi is reported here for the first time from Japan and Russia (Fig. 7E); it is found at elevations of up to 300 m in Hokkaido and Kamchatka, and at 500–550 m in Honshu. Similarly to R. tenuis, these records are another addition to the list of eastern Asiatic–eastern North American disjunct species.
Selected specimens examined. Japan. Hokkaido: Kitami Prov., Wakkanai City administrative area, 7 km S of Soyamisaki Cape and 2·5 km from coast, on Alnus sp., alt. 20 m, 45°28'N, 141°57'E, 1995, G. Thor 14013 (UPS); Shari-gun, Shari-cho, Shiretoko Nat. Park, 7 km NE of Utoro Village, N and S of small road to Iwaobetsu hot-spring hotel (Onsen), 2 km (road distance) from jct road to Shiretoko-goko Lakes, 44°06'N, 145°05'E, alt. 130 m, on Prunus, 1995, T. Tønsberg 22789, 22790 (BG); Kushiro Prov., Akkeshi-gun, Hamanaka-cho, 55 km E of Kushiro City, 3 km E of Hichiripputo Lake, just S of road following coast, 43°02'N, 145°03'E, alt. 60 m, on Sorbus, 1995, T. Tønsberg 22933 (with R. subalbida), 22934, 22935b, 22936a (with R. subminuta and R. buckii; BG); Kushiro-gun, Kushiro-cho, 13 km E of Kushiro City, 3 km (road distance) E of Konbumori Village, just S of road following the coast, 42°57'N, 144°33'E, alt. 130 m, on Sorbus, 1995, T. Tønsberg 23018, 23122 (with R. excrescens; BG); ibid., on Acer sp., G. Thor 14531 (UPS); Nemuro Prov., Notsuke-gun, Btsukai-cho, 100 km ENE of Kushiro City, N shore of Furenko Lake, just NW of road 244, 43°22'N, 145°15'E, alt. 5–10 m, on Betula, 1995, T. Tønsberg 22911; ibid., on Quercus, 1995, T. Tønsberg 22918 (both BG); ibid., G. Thor 14425 (UPS, with R. excrescens); Teshio Prov., Rumoi-gun, Obira-cho, 21 km ENE of the small town of Obira at coast, along E and upper trail from Tengunotaki Waterfall to parking area, 44°04'N, 141°55'E, alt. 130–150 m, on Abies sachalinensis, 1995, T. Tønsberg 21985 (BG, with R. buckii). Honshu: Bizen Prov. (Okayama Pref.), Maniwa-gun, Kawakami-mura, 1–2·3 km N of Hiruzen Research Institute, on exposed tree at roadside, alt. 500–550 m, 35°18'N, 133°38'E, 1994, G. Thor 12212 (UPS with R. subparieta); Shimotsuke Prov. (Tochigi Pref.), Nikko City, 15 km WNW of Nikko, along the trail E of Yukawa stream above the Ryuzu Waterfalls N of Lake Chuzenji, elev. 1390 m, 36°45'57·3''N, 139°26'49·4''E, 2015, G. Thor 32094 (UPS).—Russia: Kamchatka Krai: Ust’-Bol’sheretsk District, Pravyy Kikhchik River basin, vicinity of Mokushka River, 53°32'56''N, 156°41'07''E, alt. 235 m, on Chosenia arbutifolia, 22 vii 2004, D. Himelbrant (LECB k-449, with R. subparieta, R. subminuta).
Rinodina xanthophaea (Nyl.) Zahlbr.
Cat. Lich. Univers. 7: 559 (1931).—Lecanora xanthophaea Nyl., Lich. Jap.: 41 (1890); type: Japan, Magayesi,1879, E. Almquist (H–NYL 29084—lectotype; H–NYL 29085—isolectotype pro parte).
New synonym. Rinodina xanthophaea f. sorediosa Pczelkin, Novosti sistematiki nizshikh rastenii 24: 167 (1987); type: [Russia:] USSR Far East, Sikhote-Alin’ Nature Reserve, along the sea, 2 km from the River Belimbe [approximately 45·320937°N, 137·012290°E], on oak bark, 1982, A. V. Pczelkin (LE—holotype!).
This golden yellow, xanthone-containing corticolous species has been discussed in detail by Urbanavichene & Skirina (Reference Urbanavichene and Skirina2011) and Lendemer et al. (Reference Lendemer, Sheard, Thor and Tønsberg2012; Fig. 2 photomicrographs of thallus). The holotype of the forma sorediosa is fertile, possessing large Physcia-type spores, and the thallus is relatively densely covered with the coarse soredia, both typical characters of R. xanthophaea. Other forms may be fertile and lack soredia or, alternatively, may be sorediate and lack apothecia. The species has a similar distribution in far eastern Asia to another xanthone-containing species, R. chrysidiata (Fig. 1C), but has been collected more frequently, particularly in Japan, and unlike R. chrysidiata the species has not been recorded outside eastern Asia. A further xanthone-containing species, R. citrinisidiata, has been described from Thailand and is discussed under R. chrysidiata. Pycnidia have been observed for the first time (Printzen 11853, FR). They are c. 1 mm diam., with an orange-brown ostiole, and contain bacilliform conidia c. 4·0×1·0 µm.
Rinodina xanthophaea was described from Japan (Nylander Reference Nylander1890) and has been previously recorded from Russia (Oxner Reference Oxner [“Oksner”]1948; Pczelkin Reference Pczelkin1987; Chabanenko Reference Chabanenko2002; Skirina Reference Skirina2010, Reference Skirina2012; Urbanavichene & Skirina Reference Urbanavichene and Skirina2011; Rodnikova Reference Rodnikova2012, Reference Rodnikova2013; Yakovchenko et al. Reference Yakovchenko, Galanina, Malashkina and Bakalin2013). The species is found at relatively high elevations in the south of the region: 850–2460 m into the subalpine zone of Honshu, Japan, 1000–1700 m on Cheju Island, 1400–1600 m in Gangwon Prov., South Korea, and further north at 60–300 m on Hokkaido, Japan and 500 m in Primorskiy Krai, Russia (Urbanavichene & Skirina (Reference Urbanavichene and Skirina2011) report 1200–1600 m). It is widespread in the region (Fig. 7F) and has been reported as far west as the Khamar-Daban Mountains in the Baikal region (51°25'34''N, 104°54'26''E; Urbanavichene Reference Urbanavichene2010), from Gora Olocha in the Stanovoye Nagor’e Mountains in Amurskaya Oblast’ (Urbanavichene & Skirina Reference Urbanavichene and Skirina2011, interpreted here as 55·57851°N, 126·53028°E) and from the Jewish Autonomous Region (Urbanavichene & Skirina Reference Urbanavichene and Skirina2011; also Zhurbenko Reference Zhurbenko2014, as the host of Ovicuculispora parmeliae (Berk. & Curt.) Etayo). It was recently reported as new to Korea by Kondratyuk et al. (Reference Kondratyuk, Lökös, Halda, Haji Moniri, Farkas, Park, Lee, Oh and Hur2016).
Additional selected specimens examined (previously unpublished records only; more records are reported in Urbanachivene & Skirina (2011) and Lendemer et al. (Reference Lendemer, Sheard, Thor and Tønsberg2012)). Japan: Hokkaido: Kitami Prov., Rishiri-to Island, Rishiri-gun, Rishirifuji-cho, Oshidomari area, along path from Rishiri-hokuroku campsite, 2·5 km S of the town of Sakae to Mt. Pon, on A. sachalinensis, alt. 300 m, 45°13'N, 141°13'E, 1995, G. Thor 13773 (UPS); Kushiro Prov., Akkeshi-gun, Hamanaka-cho, 55 km E of Kushiro City, 3 km E of Hichiripputo Lake, just S of road following coast, on Alnus sp., alt. 60 m, 43°02'N, 145°03'E, 1995, G. Thor 14450 (UPS with R. excrescens and R. buckii), 14452 (UPS); Nemuro Prov., Shiretoko Peninsula, Menashi-gun, Rausu-cho, just S of road 334 crossing Shiretoko Peninsula, 4 km WNW of Sakae City, Rausu hot spring, on Alnus sp., alt. 130 m, 44°02'N, 145°09'E, 1995, G. Thor 14405, 14406 (UPS both with R. subparieta). Honshu: Kai Prov. (Yamanashi Pref.), Makioka-cho, Yamanashi-City, at road to Odarumi Pass, on Salix sp., alt. 1726 m, 35°49·172'N, 138°38·963'E, 2012, G. Thor 28180 (UPS); Kochi Pref., Ino-cho Town, NE slope of Mt. Iwaguro-yama, along path from the road, on deciduous tree, alt. 1300 m, 33°45'10''N, 133°09'54''E (WGS84), 2006, G. Thor 21344 (UPS); Mutsu Prov. (Aomori Pref.), Mt. Hakkoda, c. 3 km E of Kasamatsu Pass along route 103, on Fagus crenata, alt. 850 m, 40°38'N, 140°54'E, 1994, G. Thor 11895 (TNS & UPS with R. subalbida and R. subminuta); Shimotsuke Prov. (Tochigi Pref.), 20 km WNW of Nikko, 1 km S of the village of Yumoto, 36°48'N, 139°26'E, Yudaki Falls, on deciduous tree, alt. 1440–1480 m, 1994, G. Thor 12771 (UPS with R. subparieta); Shinano Prov. (Nagano Pref.), Minamisaku-gun, along SW trail from Kinpuzan Hut to top of Mt. Kinpu (Kimpō), on Salix sp., alt. 2460 m, 35°52·100'N, 138°37·451'E, 2012, G. Thor 27861 (UPS); Prov. Tohoku (Prefecture Akita), 100 m W of the W shore of Lake Tazawa, outlook at Katamaeyama Woods Park, on old Castanea japonica, alt. 336 m, 39°42·995'N, 140°37·759'E, 2013, G. Thor 29754, 29755 (UPS).—Russia: Kamchatka Krai: Ust’-Bol’sheretsk District, Bannaya River basin, right bank of Bannaya River, 53°05'37''N, 156°53'29''E, alt. 150 m, on old Betula ermanii, 16 viii 2002, D. Himelbrant & E. Kuznetsova (LECB k-240, LECB k-422). Khabarovskiy Krai: Khomi Mountains, De Kastri-Komsomolsk route, between Tsimmermannovka and Chernyy Mys, Gorelaya Mountain, logging road S into mountains, on Betula, elev. 390 m, 51°08·256'N, 139°09·538'E, 2009, C. Printzen 11771, 11778 (FR); base of the Etkil’-Yankanskiy Mountains, Amgun’ River region, 12·3 km N of Berozovyy, headwaters of the stream Lesosechnaya, on Betula, elev. 613 m, 51°47·289'N, 135°39·161'E, 2009, C. Printzen 11836, 11853 (FR); Sonakh River, Amgun’ River region, c. 7·6 km NW of Berezovyy-Badzhal route, logging road, on log of Betula, elev. 330 m, 51°30·363'N, 135°14·221'E, 2009, C. Printzen 11877 (FR). Primorsky Krai: Sikhote Alin, valley of Taratay River, on upper part of mountain ridge, 45°44'42''N, 136°36'11''E, on Pinus koraiensis, 14 viii 2010, E. Kuznetsova (LECB); Sikhote Alin’, vicinity of Kush mountain, valley of Berezovaya River, 46°06'40''N, 136°24'47''E, on Abies nephrolepis, 14 viii 2010, E. Kuznetsova (LECB); Lazo Nature Reserve, Tretylog, along Rv. Perekatnaya, 43:11N, 13:58E, 500 m, on Quercus, R. Moberg 9746 (with R. chrysidiata, UPS); Lazo Nature Reserve, Nogeevskaya Gorge, 43:08N, 134:01E, 500 m, on Picea yuanensis, R. Moberg 9837; on rocks, R. Moberg 9804 (UPS). [Sakhalinskaya Oblast’:] Sakhalin Island, Nevelskiy Pass, on Sorbus, 46°44'35·92''N, 142°06'21·02''E, 425 m alt., 2013, A. K. Ezhkin gps11 (VLA).—South Korea: Cheju Island: along the Eorimok trail on the NW slope of Mt. Halla, on Carpinus, alt. 1000–1600 m, 33°23'N, 126°31'E, 2001, G. Thor 16970 (AK & UPS); on Quercus dentata, G. Thor 16988 (AK & UPS); Amcheju-gun, Namwon-up, along Songpanak trail on east slope of Mt. Halla above Azalea field shelter, on Sorbus commixta, alt. 1500–1700 m, 33°21'N, 126°32'E, 2001, G. Thor 17551, 17559 (AK & UPS). Gangwon Prov.: Inje-gun, Mt. Seorak, Seoraksan Nat. Park, alt. 1400–1600 m, on Abies, 38°07'N, 128°27'E, 2014, A. Aptroot 73207; alt. 1600–1700 m, on Abies, A. Aptroot 73086 (both ABL); Pyeonchang-gun, Mt. Odae, Odaesan Nat. Park, alt. 1300–1560 m, on Quercus, 37°48'N, 128°34'E, 2014, A. Aptroot 72768 (ABL); Yangyang-gun, Ser-myun, Osaeck-ri, southern part of the massif Sorak Mts, Sorak-san Nat. Park, south slope of Mt. Dachong, alt. 1350–1600 m, 38°07·10–06·45'N, 128°27·25–26·00'E, 2006, G. Thor 20470, 20495, 20512, 20515, 20602 (UPS).
Rinodina sp. A
Thallus dark grey to grey-brown, continuous to areolate, areoles to 0·50–0·80 mm wide, verrucose; vegetative propagules absent; prothallus not seen.
Apothecia to 0·60–0·80 mm diam., margins 0·10–0·15 mm wide. Thalline exciple to c. 90 µm wide, crystals in medulla, no detectable cortex. Proper exciple c. 15 µm laterally, expanding to c. 25 µm above. Hymenium to c. 110 µm high; paraphyses c. 3·0 µm wide, apices to c. 4·5 µm wide immersed in a diffuse pigment forming a conglutinate, dark, red-brown epihymenium. Asci c. 75×23 µm, only a few possessing mature ascospores; ascospores 4–8 per ascus, Pachysporaria-type I, (22·0–)23·5–27·(–28·5) ×(10·5–)11·5–14·5(–15·5) µm (n=29), l/w ratio (1·6–)1·9–2·1(–2·2); lumina with very obvious apical canals. Hypothecium pigmented light brown, to c. 40 µm deep.
Pycnidia not seen.
Chemistry. Spot tests K−, C−, P+ cinnabar (red needles under the microscope). Secondary metabolites: pannarin and possible trace of zeorin by TLC.
Due to the scarcity of the collections, the apothecial measurements were taken from hand-cut sections only. Other species with Pachysporaria-type I ascospores have subtropical distributions in North America, with the exception of sorediate species found in the Pacific Northwest region (Sheard Reference Sheard2010). This taxon is of particular interest because ascospores with persistent apical canals are known only in one other species in the genus, Rinodina luteonigra, detailed above. Rinodina flavosoralifera Tønsberg and R. verruciformis Sheard (Sheard Reference Sheard2010) are probably related because, at maturity, their spores possess apical satellite lumina formed at the terminus of transient canals. Rinodina sheardii Tønsberg also has a pigmented hypothecium but is sorediate, the soralia are sometimes distinctly yellow and C+ orange due to the presence of an unknown pigment (xanthone?) and lacks pannarin (Tønsberg 1992 Reference Tønsbergb ). The newly described R. hypobadia also has a pigmented hypothecium and pannarin but its spores are much smaller and belong to the Dirinaria-type.
The species is known only from two collections from the subtropical Cheju Island and one from Gangwon Province, South Korea.
Specimens examined. South Korea: Cheju Island: rim surrounding the crater of Mt. Halla, rocky slope with small trees and shrubs, on dead Betula ermanii, alt. 1850–1950 m, 33º22'N, 126º33'E, 2001, G. Thor 17045 (AK & UPS); Namcheju-gun, Namwon-up, along Songpanak trail on east slope of Mt. Halla above Azalea field shelter, Abies koreana forest with scattered deciduous trees, on Sorbus commixta, alt. 1500–1700 m, 33°21'N, 126°32'E, 2001, G. Thor 17544 (UPS). Gangwon Prov.: Yangyang-gun, Ser-myun, Osaeck-ri, southern part of the massif Sorak Mts, Sorak-san National Park, south slope of Mt. Dachong, along trail from the village of Osaeck towards top of Mt. Dachong, from where trail crosses a small stream c. 1·5 km NNE of the village of Osaeck to c. 2·5 km NNE of the village, on Quercus sp., alt. 1400–1600 m, 38°06·45–07·00'N, 128°27·30–28·00'E, 2006, G. Thor 20302 (AK & UPS).
Species not seen in the present study but reported in the literature
A number of additional species have been reported in the literature from north-eastern Asia that we have not seen and, therefore, have been unable to confirm. Some corticolous taxa in the list are old names that have been widely misinterpreted in the past and may not occur in our area. The species that in our opinion are the most likely to occur in north-eastern Asia are included in the key and are marked by an asterisk (*) below.
*Rinodina archaea (Ach.) Arnold. A widely distributed, primarily lignicolous species in the Northern Hemisphere (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002; Mayrhofer & Sheard Reference Mayrhofer and Sheard2007; Sheard Reference Sheard2010). Previously reported from Russia (Andreev et al. Reference Andreev, Kotlov and Makarova1996; Chabanenko Reference Chabanenko2002; Skirina Reference Skirina2010, Reference Skirina2012; Urbanavichus & Andreev Reference Urbanavichus and Andreev2010; Rodnikova Reference Rodnikova2013) in many localities but possibly a misapplied name for R. sibirica.
*Rinodina bischoffii (Hepp) A. Massal. This species from calcareous rock is accepted for the Russian Far East Arctic by Urbanavichus & Andreev (Reference Urbanavichus and Andreev2010).
Rinodina calcarea (Arnold) Arnold. Another species from calcareous rocks, listed as a rare species occurring on Wrangel Island by Belikovich et al. (Reference Belikovich, Galanin, Afonina and Makarova2006), and accepted for the Russian Far East Arctic by Urbanavichus & Andreev (Reference Urbanavichus and Andreev2010). However, this species has a Mediterranean-Turanian distribution and is unlikely to be present in the Arctic. It is possibly a misidentification of R. calcigena (Th. Fr.) Lynge (H. Mayrhofer 2017, pers. comm.).
Rinodina colobinoides (Nyl.) Zahlbr. An epiphytic species accepted for the Russian Far East by Urbanavichus & Andreev (Reference Urbanavichus and Andreev2010), perhaps based on an earlier report of R. sorediata H. Magn. (a synonym) from Quercus mongolica in the Sikhote-Alin’ Mountains by Insarov and Pchelkin (reported by Chabanenko Reference Chabanenko2002).
*Rinodina confragosa (Ach.) Körb. A saxicolous species reported from the lower reaches of the Amur (Yakovchenko et al. Reference Yakovchenko, Galanina, Malashkina and Bakalin2013), from an island in the Sea of Japan (Rodnikova Reference Rodnikova2012) and previously on Bol’shoi Pelis Island, another island in Peter the Great Bay (Skirina Reference Skirina1996). The species is known from Europe, South Africa, Australia (Kaschik Reference Kaschik2006) and western North America (Sheard Reference Sheard2010). It distinguished by its Physcia-type spores, columnar apothecial lower cortex and the presence of atranorin.
Rinodina exigua (Ach.) S. Gray. This is a corticolous species with Physcia-type spores and a thallus containing atranorin. It is widespread in Europe and adjacent regions (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002) and infrequent in the Coastal Range and Sierra Nevada of California (Sheard Reference Sheard2010). Many primary and secondary literature reports are taken up by Andreev et al. (Reference Andreev, Kotlov and Makarova1996), Chabanenko (Reference Chabanenko2002), Skirina (Reference Skirina2010), Rodnikova (Reference Rodnikova2013) and Urbanavichus & Andreev (Reference Urbanavichus and Andreev2010) from the Russian Far East but the name might have been misapplied.
Rinodina exigua f. laeviuscula (Nyl.) Zahlbr. This taxon was described from Japan but was not studied by Magnusson (Reference Magnusson1947) or by ourselves.
Rinodina fimbriata Körb. Reported from Korea by Kondratyuk et al. (Reference Kondratyuk, Lökös, Tschabanenko, Haji Moniri, Farkas, Wang, Oh and Hur2013) but we have not seen the material. A saxicolous species with large Mischoblastia-type spores found in mesic habitats in Europe and the Great Lakes region of North America (Sheard Reference Sheard2010). It is related to R. moziana and R. oxydata.
Rinodina laevigata (Ach.) Malme. This corticolous species is characterized by its deep, lower apothecial cortex, Physcia-type spores and the lack of secondary substances. It was reported from South Korea by Aptroot & Moon (Reference Aptroot and Moon2014) but the specimens do not belong to R. laevigata and appear to represent at least two species unknown to us.
Rinodina melanconia Vain. A saxicolous species described from Yinretlen, near Kolyushinskaya Bay on the Arctic Ocean coast of Chukotka. The type was studied by Mayrhofer (Reference Mayrhofer1984) and treated as a species excludenda because it was in poor condition. Listed for the Russian Far East by Urbanavichus & Andreev (Reference Urbanavichus and Andreev2010).
Rinodina membranifera (Hue) Zahlbr. This corticolous species was described by Hue (Reference Hue1909) from Korea and explicitly compared to R. ascociscana and R. tenuis. We have not seen type material because it was not available for loan but the type description, which notes the thin thallus and very large spores, suggests that it might be a synonym of R. tenuis. It was the only Rinodina species (except Dimelaena oreina, which was included in Rinodina) in the two Korean checklists (Hur et al. Reference Hur, Koh and Harada2005; Moon Reference Moon2013).
*Rinodina milvina (Wahlenb.) Th. Fr. A saxicolous species with a dark brown thallus and large, Milvina-type spores reported from Scandinavia, Scotland, the mountains of Central and Southern Europe, North Africa and central Asia (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002), and western North America (Sheard Reference Sheard2010). The report from Japan (Kurokawa & Kashiwadani Reference Kurokawa and Kashiwadani2006) needs to be confirmed. Also reported from the central Russian Far East by Urbanavichus & Andreev (Reference Urbanavichus and Andreev2010), but it is not known on what this record is originally based.
*Rinodina olivaceobrunnea Dodge & Baker. Primarily an oro-arctic, muscicolous species with large, Physcia- to Physconia-type spores. The spores are not as large as those of R. turfacea and it also lacks sphaerophorin. Reported from the Arctic Russian Far East (Andreev et al. Reference Andreev, Kotlov and Makarova1996; Urbanavichus & Andreev Reference Urbanavichus and Andreev2010) and likely to be correct.
*Rinodina pyrina (Ach.) Arnold. This corticolous species possesses Buellia-like, Dirinaria-type spores (Sheard et al. Reference Sheard, Knudsen, Mayrhofer and Morse2011) and occurs in the Northern Hemisphere (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002; Sheard Reference Sheard2010), often in dryish habitats, and is probably introduced into Australia (Mayrhofer et al. Reference Mayrhofer, Kantvilas and Ropin1999). The species was reported from several islands in the southern Russian Far East by Chabanenko (Reference Chabanenko2002) and Skirina (Reference Skirina2010) and, probably on this basis, included for the southern Russian Far East by Urbanavichus & Andreev (Reference Urbanavichus and Andreev2010). It was also recently reported from the Stanovoye Nagor’e highlands in the west of our study area (Chesnokov & Konoreva Reference Chesnokov and Konoreva2015) and from Korea by Joshi et al. (Reference Joshi, Kondratyuk, Crişan, Jayalal, Oh and Hur2013). It is included on the most recent checklist of Japanese lichens (Kurokawa & Kashiwadani Reference Kurokawa and Kashiwadani2006).
Rinodina sophodes (Ach.) A Massal. This is a distinctive corticolous species characterized mainly by its brown thallus and Milvina-type spores. It is widely distributed in the British Isles, Central and Southern Europe and Macaronesia, and it is also reported from northern Africa and Asia Minor (Mayrhofer & Moberg Reference Mayrhofer and Moberg2002). It is absent from North America (Sheard Reference Sheard2010). Rinodina sophodes is another old name that has also been reported for Korea (Joshi et al. Reference Joshi, Kondratyuk, Crişan, Jayalal, Oh and Hur2013), Japan (Kurokawa & Kashiwadani Reference Kurokawa and Kashiwadani2006) and the Russian Far East (Chabanenko Reference Chabanenko2002; Kotlov Reference Kotlov2008; Urbanavichus & Andreev Reference Urbanavichus and Andreev2010; Rodnikova Reference Rodnikova2012). Chabanenko (Reference Chabanenko2002, citing Skirina (Reference Skirina1996) and using the name R. cacuminum [Th. Fr.] Malme) and Skirina (Reference Skirina2010) have reported the species from islands in Peter the Great Bay in the Sea of Japan and Skirina (Reference Skirina2012) reported it from Khabarovskiy Krai.
*Rinodina trevisanii (Hepp) Körb. This species is broadly distributed across Scandinavia and Siberia and is scattered in montane localities in the Southern Alps, Asia Minor and western North America (Mayrhofer & Sheard Reference Mayrhofer and Sheard2007). It has been widely reported from the Russian Far East (Chabanenko Reference Chabanenko2002) and from western Mongolia by Hauck et al. (Reference Hauck, Tønsberg, Mayrhofer, de Bruyn, Enkhtuya and Javkhlan2013).
Rinodina xanthomelana Müll. Arg. Specimens reported by Aptroot & Moon (Reference Aptroot and Moon2014) belong to a Buellia species, probably closely related to B. ocellata (Flot.) Körb. but with smaller spores.
Discussion
Biogeographers have long been fascinated by the disjunctive distribution of flowering plants between eastern Asia and eastern North America (Gray Reference Gray1846; Boufford & Spongberg Reference Boufford and Spongberg1983; Wen Reference Wen1999; Xiang et al. Reference Xiang, Soltis, Soltis, Steven and Crawford2000; Qian Reference Qian2002). This “East–East disjunction” has also been noted for bryophytes (Schofield Reference Schofield1969) and lichens (Yoshimura Reference Yoshimura1968; Culberson Reference Culberson1972; Sheard et al. Reference Sheard, Lendemer and Tripp2008, Reference Sheard, Lendemer, Spribille and Tønsberg2012; Galanina et al. Reference Galanina, Yakovchenko, Tsarenko and Spribille2011; Lendemer et al. Reference Lendemer, Sheard, Thor and Tønsberg2012). The present paper adds four additional disjunct species (for a total of six) known for the genus Rinodina alone, and suggests that such disjunctions might prove to be more common than previously anticipated when other crustose genera are studied in detail. In flowering plants, East Asian–western North American disjunctions are less common than East–East disjunctions (Xiang et al. Reference Xiang, Soltis and Soltis1998; Kurokawa Reference Kurokawa2006) due to the late Tertiary and Quaternary orogenies in western North America (Wen Reference Wen1999). We were therefore intrigued to discover three recently described western North American species, Rinodina endospora, R. macrospora and R. megistospora, in far eastern Asia. Rinodina macrospora is a species limited to coastal foreshores in the Pacific Northwest (Sheard & Mayrhofer Reference Sheard and Mayrhofer2002; Sheard Reference Sheard2010) and is now recorded from a similar locality in Khabarovskiy Krai, Russia.
Explanations for such disjunctions in lichens have largely mirrored those provided for flowering plants. Nearly all papers that have discussed this topic start from the assumption that they are results of Tertiary range sunderings (Jørgensen Reference Jørgensen1983; Kärnefelt Reference Kärnefelt1990; Galanina et al. Reference Galanina, Yakovchenko, Tsarenko and Spribille2011; Lendemer et al. Reference Lendemer, Sheard, Thor and Tønsberg2012). However, the emerging molecular evidence does not appear to support an ancient disjunction for the lichen mycobiont, at least at the species level. Instead, genetic data from East–East disjunctions in lichen mycobionts suggest active gene flow. Spribille (Reference Spribille2011) found a strong East–East clade structuring in Mycoblastus sanguinarius, but haplotypes were nearly identical between the Russian Far East and eastern North America, a result inconsistent with long isolation. More recently, Resl et al. (Reference Resl, Mayrhofer, Clayden, Spribille, Thor, Tønsberg and Sheard2016) found sequences of Rinodina subparieta from eastern Asia and eastern North America formed a single clade, with interdigitation of Asian and American samples. Such a result seems hard to reconcile with >12 million years of isolation, regardless of how a lichen reproduces (asexually or sexually) or whether the mycobiont is homo- or heterothallic. Furthermore, genetic evidence supporting reciprocally monophyletic sister species (vicariants) in lichen mycobionts occurring in eastern Asia and eastern North America has been mixed. Of the ten putative East–East vicariants cited by Culberson (Reference Culberson1972), molecular data have subsequently supported only one pair to truly be sister species (Umbilicaria esculenta/mammulata: Davydov et al. (Reference Davydov, Peršoh and Rambold2010)). By contrast, five East–East vicariants mentioned by Culberson that have been studied using molecular methods have been shown not to be each other’s closest relatives (Anzia colpodes/colpota: Wang et al. (Reference Wang, Goffinet, Liu, Liang, Shi, Zhang, Zhang and Wang2015); Cladonia caroliniana/nipponica and C. uncialis/pseudostellata: Stenroos et al. (Reference Stenroos, Pino-Bodas, Weckman and Ahti2015); Cladonia evansii/pseudevansii: Athukorala et al. (Reference Athukorala, Pino-Bodas, Stenroos, Ahti and Piercey-Normore2016); Punctelia rudecta/ruderata: Alors et al. (Reference Alors, Lumbsch, Divakar, Leavitt and Crespo2016)); a further four chemical species pairs in Cladonia cited by Culberson have yet to be sampled with molecular methods. Though not included in previous biogeographical overviews, it should be noted that at least one pair of East–East sister genera is also known (Cetradonia/Gymnoderma: Zhou et al. (Reference Zhou, Wei, Ahti, Stenroos and Högnabba2006)).
It cannot be ruled out that East–East disjunctions at the species level in fungi might in fact be the outcomes of different biogeographical processes than would be expected in vicariants. Spribille (Reference Spribille2011) noted that climate, especially the coincidence of the annual precipitation peak with the warmest quarter of the year, is shared between East Asia and eastern North America, closely tracking the geographical provenance of the corresponding mycobiont sequence data. Climate is also implicated for western North American–European disjuncts in lichen fungi: in both regions, peak precipitation tends to coincide with the coolest time of the year. This raises the possibility that, at least in these cases, current climate may be a more parsimonious explanation for species’ distribution than historical range sundering. It would appear to be consistent with the “everything is everywhere, but the environment selects” hypothesis to explain the distribution of fungi and other microbes; the sexual dispersal propagules of many lichen mycobionts easily fall within the diaspore size range for which this view has been advanced (Baas Becking Reference Baas Becking1934). Genetic studies hold considerably more promise in shedding light on the cause of such distributions than do classical specimen-based studies. The latter bump up against two perennial limitations: morphological studies of lichens can both over- and underestimate genetic divergence of its lecanoromycete symbiont (Crespo & Pérez-Ortega Reference Crespo and Pérez-Ortega2009), affecting hypotheses of geographical patterns of relatedness; and the raw geographical data themselves are still constantly being augmented by newly discovered range extensions, as evidenced by the present paper. Even well-known, comparatively large lichens continue to be discovered in new parts of the world, upending long-standing biogeographical paradigms (e.g. Cornejo et al. Reference Cornejo, Nelson, Stepanchikova, Himelbrant, Jørgensen and Scheidegger2016) and rendering speculation about endemism, especially in crustose lichens, tentative at best.
Although the results of the present study are a substantial advance on previous concepts of Rinodina in north-eastern Asia, much work remains to be done. Within our mapping area we did not have access to Rinodina collections from vast areas, including Amurskaya Oblast’, Magadanskaya Oblast’, the entire Sakha Republic, much of the central Sikhote-Alin’ Mountains, most of the Korean Peninsula and the easternmost provinces of China. Biogeographical hypotheses rely not only on knowledge of species’ occurrence but also on reasonable certainty concerning species’ absence. It is our hope that the present study will make it easier to interpret Rinodina in north-eastern Asia in the future and lay the groundwork for a deeper understanding of the biogeography of this fascinating genus.
The curators of the following herbaria are thanked for loans of specimens: ABL, AK, B, COLO, DUKE, E, FR, G, GZU, H, LE, M, NBM, NY, S, TNS, US and W. GT wishes to thank Akiri Mori, Yokohoma National University, for arranging collecting permits in Shiretoko, Japan (2010) and Tatsuhiro Ohkubo, Utsunomiya University, for arranging collecting permits in Japan (2015). The field trip in South Korea by GT (2006) was financially supported by a grant to K. H. Moon (no. 052-052040) from the Core Environmental Technology Development Project for the Next Generation, funded by the Ministry of Environment of the Korean Government. IS and DH gratefully acknowledge research grant 1.37.151.2014 from St Petersburg State University and grant 14–04–01411 from the Russian Foundation for Basic Research. LSY and IAG were supported by grant 15-29-02382 “Mycobiota Russian Far East: revision of biological diversity as a unique component of the nature of Northeast Asia”. TS gratefully acknowledges the support of Pavel Krestov for his first field trip to Primorskiy Krai in 2007 and Austrian Science Fund grant FWF P21052-B16 for the 2009 field trip, and help from Viktoria Wagner with some of the Russian literature and labels. Work by TS on the current manuscript was supported by Austrian Science Fund grant FWF P25237. TT acknowledges financial support for fieldwork in Japan (Hokkaido) in 1995 from The Scandinavia-Japan Sasakawa Foundation. JWS thanks Teuvo Ahti and Helmut Mayrhofer for helpful discussions on a number of taxonomic and nomenclatural issues connected with this paper. We are grateful to two anonymous reviewers and the editors who contributed substantially to the final manuscript.