Introduction
Multiple sclerosis (MS) is a neurodegenerative and demyelinating disorder of the central nervous system (CNS) that occurs in the pediatric age range in 3–5% of those with the disease (Ghezzi et al., Reference Ghezzi, Deplano, Faroni, Grasso, Liguori, Marrosu and Zaffaroni1997; Krupp, Banwell, & Tenembaum, Reference Krupp, Banwell and Tenembaum2007). Cognitive impairment is reported to occur in 30–50% of pediatric-onset MS patients with greatest deficits in “fluid” skills such as attention, information processing, and executive skills (Amato et al., Reference Amato, Goretti, Ghezzi, Lori, Zipoli, Portaccio and Trojano2008; MacAllister, Belman, & Milazzo, Reference MacAllister, Belman and Milazzo2005; Till et al., Reference Till, Ho, Dudani, Garcia-Lorenzo, Collins and Banwell2012).
Maturation of cognitive abilities may be vulnerable to the diffuse neuronal and axonal damage associated with pediatric MS, particularly for skills that have yet to be established (Anderson et al., Reference Anderson, Jacobs, Spencer-Smith, Coleman, Anderson, Williams and Leventer2009). However, mixed findings have been reported in pediatric MS studies examining how the developmental stage of the child at the time of disease onset influences outcome. Relative to older age at disease onset, young age at disease onset has been associated with greater cognitive impairment (Banwell & Anderson, Reference Banwell and Anderson2005; MacAllister et al., Reference MacAllister, Belman and Milazzo2005) and lower IQ (Amato et al., Reference Amato, Goretti, Ghezzi, Lori, Zipoli, Portaccio and Trojano2008). However, in some studies (e.g., MacAllister et al., Reference MacAllister, Belman and Milazzo2005; Till et al., Reference Till, Ho, Dudani, Garcia-Lorenzo, Collins and Banwell2012), the effect of age at onset is no longer significant after accounting for disease duration. More recently, a study of 26 pediatric MS patients did not find an association between age at onset, nor disease duration, on performance-based measures of executive functions (Holland, Graves, Greenberg, & Harder, Reference Holland, Graves, Greenberg and Harder2014).
Timing of assessment is also critical as deficits may emerge later in development as children “grow into the deficit.” Indeed, older age at testing has been associated with worse cognitive outcome over a 2-year follow-up of pediatric-onset MS patients (Amato et al., Reference Amato, Goretti, Ghezzi, Lori, Zipoli, Moiola and Trojano2010). Thus, evaluation of cognitive performance in pediatric-onset MS patients must consider age at disease onset, time from disease onset, chronological age at the time of assessment, and the increasing complexity of expected normative cognitive capacity with increasing age.
Studies in pediatric MS have emphasized information processing and attention as being most sensitive to detect failure of age-expected changes over time (Amato et al., Reference Amato, Goretti, Ghezzi, Lori, Zipoli, Moiola and Trojano2010; MacAllister, Christodoulou, Milazzo, & Krupp, 2007; Marin, Banwell, & Till, Reference Marin, Banwell and Till2012; Till et al., Reference Till, Racine, Araujo, Narayanan, Collins, Aubert-Broche and Banwell2013). For example, over a 1-year follow-up interval, pediatric patients with MS revealed less age-expected maturation in information processing and visual scanning as compared with age-matched controls (Till et al., Reference Till, Racine, Araujo, Narayanan, Collins, Aubert-Broche and Banwell2013). However, an understanding of the maturation of specific cognitive skills and the impact of age at disease onset on their maturation remains unclear because of the limited number of longitudinal cognitive studies using the same instruments over multiple assessments.
When considering changes over time, it is important to recognize that deficits in one cognitive domain may negatively impact other cognitive skills and their development. For example, in adults with MS, difficulties in working memory may emerge or be exacerbated as a consequence of slow processing speed, a likely consequence of the reliance of working memory on efficient processing (e.g., Archibald & Fisk, Reference Archibald and Fisk2000; DeLuca, Chelune, Tulsky, Lengenfelder, & Chiaravalloti, 2004; Demaree, DeLuca, Gaudino, & Diamond, 1999; Drew, Starkey, & Isler, Reference Drew, Starkey and Isler2009; Leavitt et al., Reference Leavitt, Lengenfelder, Moore, Chiaravalloti and DeLuca2011). How such co-dependency of different cognitive domains is impacted by the onset of MS during the primary maturation of these domains requires longitudinal analyses of pediatric MS patients during the key periods of cognitive maturation.
Another potential predictor of cognitive change relates to the construct of cognitive reserve, which has received much attention as a protective factor in coping with brain insult in adult and pediatric populations (Dennis, Yeats, Taylor, & Fletcher, Reference Dennis, Yeates, Taylor and Fletcher2006; Fay et al., Reference Fay, Yeates, Taylor, Bangert, Dietrich, Nuss and Wright2010; Kesler, Tanaka, & Koovakkattu, Reference Kesler, Tanaka and Koovakkattu2010; Stern, Reference Stern2002). Cognitive reserve refers to an individual’s capacity for adaptive and efficient cognitive processing arising from the sum of genetic cognitive endowment, education, and experience (Stern, Reference Stern2002). This concept is typically estimated using proxies such as education attainment and vocabulary knowledge, but these proxies are not appropriate in children since most have yet to achieve their educational potentials. In children, estimates of pre-insult academic functioning and intelligence (Statz, Reference Statz1993), as well as parental socioeconomic status (Hart, Petrill, Deater Keckard, & Thompson, Reference Hart, Petrill, Deater Keckard and Thompson2007), can be used to estimate reserve capacity. Premorbid learning difficulties are also considered a proxy of cognitive reserve and have been shown to predict risk of memory deficits following traumatic brain injury in children (Farmer et al., Reference Farmer, Kanne, Haut, Williams, Johnstone and Kirk2002).
In the MS literature, adults with a higher cognitive reserve have greater capacity and flexibility to withstand a greater extent of neuropathology, as measured by brain atrophy, before measurable cognitive impairment (Sumowski, Chiaravalloti, Wylie, & DeLuca, Reference Sumowski, Chiaravalloti, Wylie and DeLuca2009; Sumowski, Wylie, DeLuca, & Chiaravalloti, Reference Sumowski, Wylie, DeLuca and Chiaravalloti2010). In other words, those with higher cognitive reserve have been shown to cope better than those with lower reserve even when the magnitude of neuropathology is similar. The cognitive reserve hypothesis has also been extended to include protection against the progression of cognitive impairment in adult MS (Benedict, Morrow, Cookfair, & Schretlen, Reference Benedict, Morrow, Cookfair and Schretlen2010). Another study conducted in adult MS patients over a 3-year interval reported that the presence of moderate cognitive impairment at baseline was the only predictor of further cognitive decline (Amato et al., Reference Amato, Goretti, Ghezzi, Lori, Zipoli, Moiola and Trojano2010). These findings point toward the protective role of a higher initial level of cognitive functioning, or a higher cognitive reserve, in protecting against the negative effect of the disease on cognition. Consistent with the cognitive reserve hypothesis, we have shown that pediatric-onset MS patients whose cognitive abilities remained stable or improved over a 1-year interval were more likely to have higher educated parents (a proxy of higher cognitive reserve) compared to individuals who showed deterioration in function (Till et al., Reference Till, Racine, Araujo, Narayanan, Collins, Aubert-Broche and Banwell2013). These findings raise the question of whether cognitive reserve plays a role in moderating the cognitive trajectories of pediatric-onset MS patients.
Changes in cognition need to be evaluated at multiple time points and over a lengthy time interval spanning critical developmental periods. Growth curve modeling (often implemented using mixed-effects or multilevel modeling) is a flexible method for the analysis of this type of longitudinal data because, in addition to providing information about how an outcome changes over time with respect to both the rate of change and level (i.e., outcome at any specific point in time), it can provide information about how certain predictors may moderate change for individual patients. Growth curve models offer several advantages over traditional methods for analyzing longitudinal data such as repeated-measures ANOVA. Most importantly, multilevel growth modeling explicitly recognizes that there is likely to be heterogeneity across individuals with respect to change over time, whereas repeated-measures ANOVA assumes that the rate of change is constant across individuals. Another advantage is the ability to estimate change over time even when the data set is not perfectly balanced (Singer & Willett, Reference Singer and Willett2003). Longitudinal analysis of the entire dataset is still possible even if individuals vary in the number of time points to which they contributed data or if the spacing of data points differs between study participants. Given the heterogeneity in the number of evaluations for each patient, a multilevel growth model is fitted to the entire sample without excluding an individual who has one or more missing time points. Growth curve modeling also offers the possibility of estimating nonlinear change processes (e.g., quadratic change). Furthermore, the opportunity to examine correlates of change is particularly relevant to the study of pediatric-onset MS because this type of analysis can provide insight into early identification of patients who are at risk, which is essential for targeting individuals for rehabilitation.
The present study uses growth curve analysis to understand cognitive maturation over childhood and adolescence. In particular, this study addresses how younger age at disease-onset and cognitive reserve impacts performance trajectories on two neuropsychological measures used to assess visual scanning, information processing, and working memory. Cognitive reserve capacity was estimated using the patient’s baseline Full Scale IQ and parental social status. We hypothesized that younger age at disease onset and lower cognitive reserve would have a negative impact on the developmental progression of these specific cognitive skills.
Methods
Participants
Participants included 35 pediatric-onset MS patients who were assessed from year 2000 to 2012 and were between the ages of seven and 18 years at first assessment. Participants were recruited from the Pediatric MS Clinic at The Hospital for Sick Children in Toronto, Canada as part of a longitudinal research study that was initiated in 2007 and involved three serial assessments over a three year period. Because some of these patients underwent cognitive testing between 2000 and 2001 as part of an earlier study (n=3; Banwell & Anderson, Reference Banwell and Anderson2005) and a later study (n=7; 2011–2013), data from these additional assessments were also included. Participants were included if they were diagnosed with clinically definite MS before age 18, were English speaking, had no history of head injury, no neurologic impairment that could interfere with cognitive testing (e.g., visual impairment), and no history of alcohol or illicit drug abuse. All patients were evaluated more than four weeks from any recent relapse or corticosteroid therapy and were not preselected for neuropsychological evaluation on the basis of cognitive complaints. Since depression, anxiety, and problems of attention can co-occur with MS, patients were not excluded if they reported a history of mood disorder or ADHD.
Procedures
Neuropsychological evaluations were conducted at baseline and up to four more assessments, with each evaluation separated by a minimum of 1 year. Of the 35 participants, seven completed only one assessment, five completed two assessments, 13 completed three assessments, seven completed four assessments, and three completed five assessments. While all of the participants included in this study were invited to return for re-testing, some of the patients missed re-assessments due to refusals, inability to locate the patient, scheduling problems, or moving to a city that was more than three hours from the greater Toronto area. To avoid bias that may result from partial datasets, all data were included in the analysis.
Participants received a $10 lunch voucher and a $25 gift certificate. Parking costs were covered for each appointment. Participants received a written report and verbal feedback by a clinical neuropsychologist following each assessment. Ethical approval was granted by the Institutional Research Ethics Board for collection and storage of clinical data for longitudinal data analysis. All participants provided written consent/assent for participation in the study.
Cognitive assessment
Two standardized and widely used neuropsychological measures that are known to be sensitive to change in pediatric-onset MS (Portaccio et al., Reference Portaccio, Goretti, Lori, Zipoli, Centorrino, Ghezzi and Amato2009) were chosen from a larger battery (see Till et al., Reference Till, Ghassemi, Aubert-Broche, Kerbrat, Collins, Narayanan and Banwell2011 for a description). The measures included the Symbol Digit Modalities Test (SDMT) - oral version (Smith, Reference Smith2002) and the child and adult version of the Trail Making Test (TMT). The SDMT, which involves speeded verbal transcription of symbol-number pairings, was administered to assess perceptual speed and visual search. The total number of correct items within a 90-s time limit was used as the dependent outcome given that the exact same test is administered at each evaluation. The test–retest reliability for the SDMT is strong (r>0.80) (Benedict et al., Reference Benedict, Duquin, Jurgensen, Rudick, Feitcher, Munschauer and Weinstock-Guttman2008). Raw scores were analyzed instead of standardized scores to avoid the challenges related to interpreting changes in standardized scores that are influenced by norms that differ by stratified sampling methods (i.e., child norms are stratified by sex whereas adult norms are stratified by education level) and are based on different populations.
Parts A and B of the TMT were administered according to guidelines presented by Reitan (Reference Reitan1959), and total time in seconds was recorded. Part B of the TMT (TMT-B) was used to assess working memory, in addition to perceptual/motor-speed and visual search, which are cognitive mechanisms that are shared with Part A of the TMT (TMT-A) (Sánchez-Cubillo et al., Reference Sánchez-Cubillo, Periáňez, Adrover-Roig, Rodríguez-Sánchez, Ríos-Lago, Tirapu and Barceló2009). If the interaction between the predictor and TMT-B was significant, then performance on the TMT-A was used to statistically control for the shared abilities between TMT-A and TMT-B, allowing for a more pure assessment of working memory. The TMT-B requires the participant to keep alphabetical and numerical sequences in mind while alternating between numbers and letters (i.e., 1→A→2→B) and simultaneously searching for the correct stimulus on a page, whereas the TMT-A requires the participant to search for and connect sequential numbers on a page (i.e., 1→2→3). Reliability for the TMT in adolescents is adequate, reported to be 0.65 in healthy adolescents (Barr, Reference Barr2003), and has been reported to be less reliable in the 9–14 year age range (Strauss, Sherman, & Spreen, Reference Strauss, Sherman and Spreen2006). The child version of the TMT (15 stimuli) was used for participants aged 14 and under whereas the adult version of the test (25 stimuli) was used for persons 15 years of age and older. Because two different versions of the TMT were used that differ in terms of the length and complexity of the trails, Z-scores were analyzed instead of time-to-completion scores.
Full Scale IQ (FSIQ) was calculated using the four subtest estimate of the Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler, Reference Wechsler1997), except for three individuals who were assessed before 2007 using the Wechsler Intelligence Scale for Children – Third Edition (WISC-III; Wechsler, Reference Wechsler1991). The General Ability Index (GAI) was used for these three individuals. The correlation coefficient between the WASI and WISC-III is 0.81 for FSIQ (Wechsler, 1991), despite variation between test items across the two tests. The GAI and FSIQ of the WISC-III are also highly correlated (0.98) in a Canadian WISC-III standardization sample (Weiss, Saflofske, Prifitera, Chen, & Hildebrand, Reference Weiss, Saklofske, Prifitera, Chen and Hildebrand1999). All cognitive testing was conducted by experienced psychometrists supervised by a clinical neuropsychologist.
Assessment of covariates
Age at first MS attack (defined as disease onset), total number of relapses (defined as physician-confirmed presence of a new neurologic symptom or deficit persisting for 24 h), and medications were retrieved from a clinical database. Social status, which is a proxy for socioeconomic status, was estimated using the Barratt Simplified Measure of Social Status (BSMSS) (Barratt, Reference Barratt2006). The BSMSS is based on average years of parental education and occupation of each parent.
Statistical methods
Growth curve models were estimated using the mixed-effects modeling approach implemented with the PROC MIXED procedure in SAS (version 9.3). Because individual profile trajectories for each participant were visualized and suggested a linear time effect, models with higher-order polynomial terms for non-linear change were not estimated.
Unconditional growth models were first estimated to describe within-subjects initial status and rate of change on the unadjusted SDMT raw scores and TMT-B Z-scores, respectively, over multiple time points. Conditional models were then estimated for the SDMT and TMT-B longitudinal outcomes to assess the impact of covariates on these cognitive trajectories. Separate conditional models were fitted for each of three time-invariant covariates (age of disease onset, baseline FSIQ scores, and BSMSS score), with particular focus on whether there was an interaction between time and the covariate, which would indicate that the covariate moderated change over time. For all analyses, disease duration (in years, defined from time of first attack) was used as the measure of time to allow for comparison of the rate of cognitive decline among individuals who experienced the disease for the same duration. This method allows one to identify whether differences in the rate of cognitive decline are distinct from the effect of disease progression. Use of “disease duration” as the time variable also allows one to interpret the trajectory intercept as corresponding to the value of the dependent variable at disease onset. Standardized score on the TMT-A (at each evaluation) was entered as a covariate in the conditional model for TMT-B to statistically control for the abilities that are shared between the two tests, and thus to allow for a more pure assessment of the working memory component that is unique fto TMT-B. Two main factors are involved in the growth curve modeling: the ‘random intercept’, which represents the initial status, or level, of the cognitive outcome at time zero (i.e., first presentation of disease-related symptoms), and the “random slope”, which represents the rate of linear change per year in cognitive outcome from the time of disease onset. A two-tailed alpha level of 0.05 was used to judge statistical significance.
RESULTS
Sample Characteristics
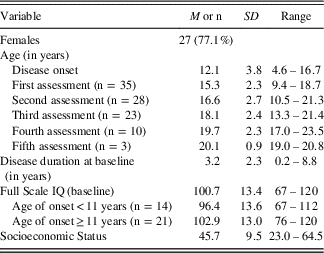
Table 1 lists the characteristics of the MS cohort. Most of the participants (n=27; 77.1%) in the study were female, consistent with the higher female preponderance reported in pediatric MS literature (Boiko, Vorobeychik, Paty, Devonshire, & Sadovnik, Reference Boiko, Vorobeychik, Paty, Devonshire and Sadovnik2002). Mean age at disease-onset was 12.1 years (SD=3.8; range: 4.6–16.7). Mean age at baseline evaluation was 15.3 years (SD=2.3; range: 9.4–18.7 years), reflecting an average disease duration of 3.2 years (SD=2.3; range: 0.2–8.8 years) at the time of initial testing. The mean age at subsequent evaluations is shown in the table; the sample size at each evaluation gets smaller as only 23 (65.7%) of the patients underwent three or more evaluations. The mean FSIQ at baseline was 100.7 (SD=13.4; range from 67–120). Mean FSIQ was 96.4 (SD=13.6; range from 67–112) for young-onset and 102.9 (SD=13.0; range from 76–120) for older-onset patients. This difference in baseline FSIQ between the young- versus the older-onset patients was not significant, controlling for disease duration (F(2,32)=2.01; p=.17). The mean years of parental education level was 15.5 years (SD=2.1; range: 11.0–19.5 years). Using the BSMSS, mean social status score was 45.7Footnote 1 (SD=9.5; range: 23.0–64.5).
Table 1 Demographic and clinical characteristics of the study sample (n=35)
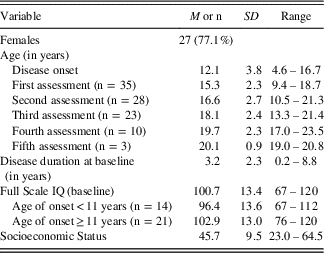
Note. Full Scale IQ estimated using the WASI (Wechsler, Reference Wechsler1997), except for three individuals who were assessed before 2007 using the WISC-III (Wechsler, Reference Wechsler1991); Full Scale IQ was estimated for these individuals using only the Verbal Comprehension Index and the Perceptual Reasoning Index scores. Socioeconomic status estimated by the Barratt Simplified Measure of Social Status (BSMSS) (Barratt, Reference Barratt2006). Total score on this social status measure can range from 8 (very low) to 66 (very high).
Visual inspection of individual growth trajectories on the TMT-B and SDMT revealed considerable variability with respect to both the individuals’ initial scores and how scores changed over time. Some patients showed decline, some showed improvement, and others showed a more stable pattern, although the trajectories all showed an approximate linear form. Variability in initial status and rates of change was explicitly modeled next by the growth curve models.
Unconditional Models: Examination of Within-Subject Change Over Time
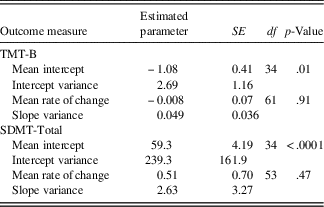
The results of the unconditional model are summarized in Table 2. Mean initial status, as determined by the mean of the random intercepts, was Z=−1.08 on the TMT-B and 59.3 (raw score units) on the SDMT. These values indicate that when disease duration is extrapolated to a value of zero (i.e., time of first clinical symptom), performance on the TMT-B is slightly below average whereas the SDMT score falls within the average range (as determined by converting the raw score to a scaled score for the average age of the sample). Regarding rate of cognitive change, the estimated mean of the random slopes representing change over time was not significant for either TMT-B or SDMT performance. In other words, on average, the “growth,” or rate of change in the study sample is stable with increasing disease duration. However, there are substantial individual differences in the rate of change, as indicated by the standard deviation of the random slopes, which equals 0.22 for TMT-B and 1.62 for SDMT. Consequently, this variability in the growth curves may be predicted from theoretically meaningful variables (e.g., age at disease onset, cognitive reserve factors).
Table 2 Unconditional model: change over time
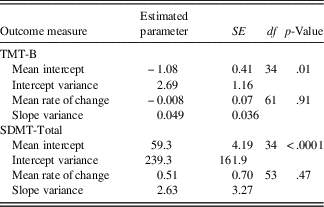
Note. SDMT=Symbol Digit Modalities Test - oral version (Smith, Reference Smith2002), total raw score used; TMT-B=Trail Making Test - Part B (Reitan, Reference Reitan1959), Z-score used.
Conditional Models: Correlates of within Subject Parameters
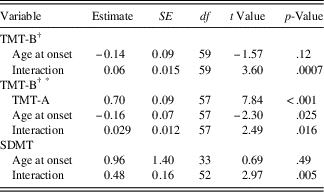
Results of the conditional model investigating the moderation of TMT-B and SDMT by age at disease onset are shown in Table 3a. The effect of age at onset on the random intercept, that is, the level of the cognitive outcome at disease onset, was not significant for either TMT-B (estimate=-0.14; SE=0.09; p=.12) or SDMT (estimate=0.96; SE=1.40; p=.49), indicating that participants with different disease onset ages started at roughly the same point for each test. However, on the TMT-B, there was a significant interaction between age at disease onset and time (estimate=0.06; SE=0.02; p<.001), indicating that age of onset predicts the slope of the TMT-B trajectories, such that later age of disease onset is associated with greater increases in TMT-B scores over time. The conditional model examining the moderation of TMT-B by age at disease onset was then repeated by entering TMT-A as a covariate to examine the impact of age at disease onset on the development of working memory (as opposed to other cognitive skills that are shared with TMT-A). Using this covariate, both the random intercept (estimate=-0.16; SE=0.07; p=.025) and the interaction between age at disease onset and time were significant (estimate=0.029; SE=0.012; p=.016). These findings indicate that patients who were older at disease onset tended to have higher scores on the working memory component of the TMT-B at disease onset and greater increases in TMT-B scores over time. Likewise, there was a significant interaction between age at disease onset and time for SDMT (estimate=0.48; SE=0.16; p=.005), such that later age of onset is associated with greater increases in SDMT scores over time. These interactions are represented in Figure 1, which illustrates that individuals with a younger age at disease onset experienced smaller increases compared to individuals with an older age at disease onset.

Fig. 1 Interaction between age at disease onset and SDMT and TMT-B performance. Predicted trajectories of (A) SDMT and (B) TMT are shown for MS patients with a disease onset age at the 10th percentile (6.7 years; dotted line), 50th percentile (12.3 year; solid line) and 90th percentile (16.3 years; dashed line).
Table 3a Conditional model: moderation of TMT-B and SDMT by age at disease-onset
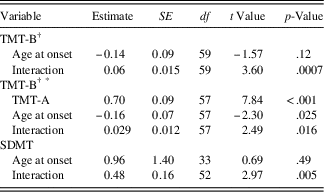
† For TMT, model convergence was not achieved when the residual variance of the intercept factor randomly varied; hence, this parameter was constrained to zero. SDMT=Symbol Digit Modalities Test - oral version (Smith, Reference Smith2002), total raw score used; TMT-B=Trail Making Test - Part B (Reitan, Reference Reitan1959), Z-score used.
* Trail Making Test – Part A (TMT-A) was included as a covariate in the model to control for processing speed, visual scanning and motor speed.
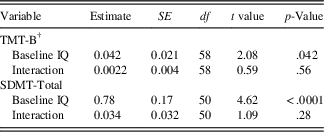
Results of the conditional model examining baseline FSIQ as a covariate are shown in Table 3b. The effect of baseline FSIQ on the initial status of the cognitive outcome was significant for SDMT (estimate=0.78; SE=0.17; p<.0001) and for TMT-B (estimate=0.042; SE=0.021; p=.042), indicating that participants with higher IQ levels tended to have higher scores on these two measures at disease onset. There was no significant interaction between baseline FSIQ and time for either TMT-B (estimate=0.0022; SE=0.004; p=.56) or SDMT (estimate=0.034; SE=0.032; p=.28), indicating that baseline IQ did not predict rates of change in the cognitive outcomes.
Table 3b Conditional model: moderation of TMT-B and SDMT-total by baseline Full Scale IQ

† For TMT, model convergence was not achieved when the residual variance of the intercept factor randomly varied; hence, this parameter was constrained to zero. SDMT=Symbol Digit Modalities Test - oral version (Smith, Reference Smith2002), total raw score used; TMT-B=Trail Making Test - Part B (Reitan, Reference Reitan1959), Z-score used.
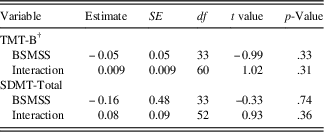
Using BSMSS as a covariate (see Table 3c), there was no significant relationship with the initial status for either TMT-B (estimate=-0.05; SE=0.05; p=.33) or SDMT (estimate=-0.16; SE=0.48; p=.74), indicating that participants from different levels of social status started at similar points on these cognitive measures. Interactions between social status and time were also non-significant for both the TMT-B outcome (estimate=0.009; SE=0.009; p=.31) and the SDMT outcome (estimate=0.08; SE=0.09; p=.36), indicating that social status did not predict rates of change in these cognitive measures.
Table 3c Conditional model: moderation of TMT-B and SDMT-total by social status score
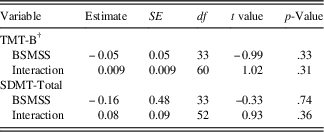
Note. BSMSS=Barratt Simplified Measure of Social Status; SDMT=Symbol Digit Modalities Test - oral version (Smith, Reference Smith2002), total raw score used; TMT-B=Trail Making Test - Part B (Reitan, Reference Reitan1959), Z-score used.
DISCUSSION
This is the first longitudinal study to examine changes in cognitive maturation in a cohort of pediatric-onset MS patients as a function of age at disease onset and cognitive reserve factors. The TMT-B and SDMT, measures that have been identified to be particularly sensitive to decline in children with MS (Amato et al., Reference Amato, Goretti, Ghezzi, Lori, Zipoli, Portaccio and Trojano2008; Portaccio et al., Reference Portaccio, Goretti, Lori, Zipoli, Centorrino, Ghezzi and Amato2009; Till et al., Reference Till, Ho, Dudani, Garcia-Lorenzo, Collins and Banwell2012), were used to assess information processing, visual scanning, perceptual/motor speed, and working memory. Several conclusions can be made about changes on these cognitive skills from the current study. First, results showed that growth curves varied considerably across individuals. Second, age at disease onset predicted the slope of the TMT-B and SDMT trajectories. As predicted, younger age of disease onset was associated with less developmental gain on the TMT-B and SDMT. Finally, proxies of cognitive reserve, including baseline Full Scale IQ and parental social status, did not predict cognitive outcome trajectories. However, this finding should be interpreted with caution because the use of IQ as a proxy of cognitive reserve capacity may have been influenced by the disease or, in the case of social status, limited in its range of values.
Despite varying from 4.6 to 16.7 years at age at first attack, patients did not differ on the TMT-B and SDMT at the baseline assessment, as compared to normative data (age at actual first assessment between 9.4 and 18.7 years). This finding is important because it demonstrates that the decline in functioning among younger disease-onset patients is not related to their initial level of functioning, and suggests that cognition is not measurably impacted by the presumed subclinical pathobiological processes that likely occur before the first clinical attack.
Given that MS is a chronic disease, it is perhaps not surprising that longitudinal analyses allow for a better understanding of cognitive sequelae associated with the disease as compared with cross-sectional studies in pediatric-onset MS. Cross-sectional studies cannot address the key issue of whether patients who are young at onset start at a lower level or grow into deficit. Even “growing into a deficit” requires further consideration; is the patient experiencing progressive loss of function, or have they experienced a relatively static insult to a cognitive trajectory that was impacted early in its maturation, but not necessarily in a progressively deteriorating manner? Further to this point, it is important to note that the negative SDMT trajectory in the young-onset patients does not merely indicate a loss of developmental gain, which would be reflected by participants achieving the same raw score at each evaluation, but rather suggests an actual loss of ability over the course of childhood and adolescence. The current results thus provide compelling evidence of a greater vulnerability, rather than a greater resiliency, of the immature brain. This finding is consistent with prior cross sectional studies showing an association between younger age at disease onset and cognitive impairment (Banwell & Anderson, Reference Banwell and Anderson2005), lower IQ (Amato et al., Reference Amato, Goretti, Ghezzi, Lori, Zipoli, Portaccio and Trojano2008), and executive dysfunction (Till et al., Reference Till, Ho, Dudani, Garcia-Lorenzo, Collins and Banwell2012), but not another study showing no relation between age at disease onset and executive function performance (Holland et al., Reference Holland, Graves, Greenberg and Harder2014). It is possible that the patients in this latter study had yet to manifest executive dysfunction given the relatively short disease duration of the group (14 months), or, the discrepancy may reflect differences with respect to how executive functioning was assessed across studies.
There were no significant moderating effects of Full Scale IQ and parental social status on the cognitive trajectories. It was hypothesized that individuals with higher cognitive reserve would be better able to cope with brain insult and keep pace cognitively with healthy children by relying upon established and more efficient cognitive resources. One reason why IQ did not moderate the trajectories in the current study may be that we know little about the evolution of IQ outcome as no studies have included longitudinal assessment of IQ from time of MS disease onset. In the current study, baseline IQ was estimated on average 3.2 years post-disease onset, which is still considered an early time point in the disease. Nonetheless, we cannot exclude the possibility that the IQ metrics measured at this point may have already been negatively impacted by the disease, with the extent of impact possibly interacting with age of disease onset as suggested by a previous pediatric MS study (e.g., Amato et al., Reference Amato, Goretti, Ghezzi, Lori, Zipoli, Portaccio and Trojano2008) whereby early disease onset was associated with lower IQ. In the current study, baseline IQ was approximately 1/3 of a standard deviation lower among the 14 patients who were 10 years or younger at disease onset as compared with the 21 patients who were 11 years and older at disease onset, consistent with an early-onset vulnerability hypothesis; however, the finding failed to reach significance, perhaps owing to the small sample sizes. Given the limitations associated with IQ in this population, future studies should consider other proxies for estimating cognitive reserve that might be less prone to the effects of the disease, such as intellectual and environmental enrichment.
We note that our measure of parental social status, which takes into account years of education and type of occupation in the parents, may not be as strongly correlated with a child’s cognitive reserve because of inherent challenges in the measurement of social status. Moreover, it might be necessary to include a broader range of social status in the patient population to detect a relationship with cognitive outcomes. In the current sample, 80% had a BSMSS score that was 40 or above, signifying middle-class social status or greater, consistent with the established higher risk of MS among individuals with a higher socioeconomic status (Marrie et al., Reference Marrie, Horwitz, Cutter, Tyry, Campagnolo and Vollmer2008).
One limitation of this study relates to the small sample size and relatively short overall follow-up interval. Even though one-third of the sample had data spanning 5 or more years, assessment of more individuals and over a longer period of time would allow greater precision in determining how cognition is affected over the course of development.
Another limitation is that the current study only examined performance on two measures that overlap in terms of the cognitive mechanisms required to perform each test. It is possible that information processing and visual scanning speed are more vulnerable to the effects of the disease given that weaknesses in these skills are commonly reported in cross-sectional studies of pediatric-onset MS (Amato et al., Reference Amato, Goretti, Ghezzi, Lori, Zipoli, Portaccio and Trojano2008; MacAllister et al., Reference MacAllister, Belman and Milazzo2005; Till et al., Reference Till, Ho, Dudani, Garcia-Lorenzo, Collins and Banwell2012). Given the wide range of cognitive abilities related to the TMT, performance on the TMT-A was statistically controlled to isolate a more pure measure of the executive component of this test and thus differentiate it from the SDMT. Further research will be required to examine whether the results of the current study—with respect to both rate and predictors of change—generalize to other cognitive domains (e.g., language, memory). Other potential patient-related moderators, such as mood and the use of disease-modifying treatments, should also be explored.
An additional limitation of this study is that it did not include healthy controls. The incorporation of age-matched healthy controls could control for potential practice effects and would provide a measure of normal developmental changes that occur in cognitive abilities. Performance gains may occur due to familiarity associated with repeated test administration of the SDMT and TMT-B, even when assessments are separated by a minimum of one year. If anything, such a concern would tend to reduce our ability to detect a negative trajectory over time, as the observed trajectories would be inclined toward a more positive direction. In addition, the child and adult version of the TMT-B were used to assess working memory. The use of different test versions prevented an investigation of how reaction time changed over time and required the use of Z-scores instead of reaction time scores. Moreover, it is possible that the interaction between TMT-B and age at disease onset could potentially be related to using two test versions with different normative data for younger and older pediatric patients. The use of standardized values is a limitation in longitudinal research because the derivation of the Z-score is dependent on the quality of the normative data, which in turn introduces more variability.
In summary, our data demonstrate that younger age at MS onset places children and adolescents at greater risk of decline in information processing, visual scanning, perceptual/motor speed, and working memory. These findings refute the idea that childhood is a period in which brain plasticity may offset the potential consequences of central nervous system damage. Considering that age at disease onset emerged as the strongest predictor of long-term outcome, young patients with MS should be advised to seek follow-up cognitive evaluation as the demands at school grow and new challenges may be presented. It is now imperative to determine whether the negative prognosis for cognitive functioning on the basis of age at disease onset is influenced by sex, psychosocial factors, use of neuroprotective strategies, and whether MRI metrics such as the rate of brain atrophy or accrual of T2 lesions correlate with cognitive trajectories. Such correlations will aid in identification of patients in greatest need for cognitive intervention.
Acknowledgments
This study was supported in part by the Canadian Institutes of Health Research, the Multiple Sclerosis Society of Canada, and the Scottish Rite Charitable Foundation. We thank Emily Ursell, Courtney Fairbrother, Julie Coleman and Austin Sye for assistance with recruitment and testing of research participants. We also thank the children and families who generously contributed their time to this research. The authors of this study declare no conflicts of interest.