Introduction
Somatic cell nuclear transfer has been used extensively as a valuable tool for the production of animal clones from adult somatic cells and for the generation of transgenic animals for agricultural and biomedical research. However, the frequency of early postimplantation developmental arrest and abortion is high, especially in bovine (Hill et al., Reference Hill, Burghardt, Jones, Long, Looney, Shin, Spencer, Thompson, Winger and Westhusin2000). Biological and technical aspects related to somatic cell nuclear transfer may account for the limited success of the technique; oocyte enucleation is one of the crucial technical steps that need to be improved. Successful reprogramming of the donor nuclei depends on the sufficient supply of factors in the recipient oocyte. In this respect, any cytoplasmic alterations such as changes in nuclear maturation status, microtubule organization and microfilaments, or biochemical and physiological changes can potentially affect nuclear reprogramming and result in cloning failure (Li et al., Reference Li, White, Aston, Bunch, Hicks, Liu and Sessions2009).
The standard method of oocyte enucleation is time consuming, technically demanding, invasive and damaging to cytoplast spatial organization, including the loss of molecular factors that play a key role in subsequent oocyte activation, reprogramming of the transferred nucleus, mitotic spindle assembly, and early embryo development (Simerly et al., Reference Simerly, Dominko, Navara, Payne, Capuano, Gosman, Chong, Takahashi, Chace, Compton, Hewitson and Schatten2003; Miyara et al., Reference Miyara, Han, Gao, Vassena and Latham2006; Van Thuan et al., Reference Van Thuan, Wakayama, Kishigami and Wakayama2006). Furthermore, although nuclear DNA is removed during oocyte enucleation, there is the possibility of damage to mitochondrial DNA (Fischer-Russel et al., Reference Fischer-Russel, Ibáñez, Albertini and Overstrom2005). Therefore, simplification of this step is constantly a center of interest for scientists involved in the production of clones (Fulka et al., Reference Fulka, Loi, Fulka, Ptak and Nagai2004).
Chemical enucleation using microtubule-depolymerizing drugs is an attractive procedure that has been used to simplify conventional enucleation methods. In chemically assisted enucleation (Yin et al., Reference Yin, Kato and Tsunoda2002a, Reference Yin, Tani, Yonemura, Kawakami, Miyamoto, Hasegawa, Kato and Tsunodab), metaphase II oocytes are exposed to an antimitotic drug and spindle depolymerization leads to the formation of a cortical protrusion on the surface of the oocyte that contains all oocyte chromosomes. This protrusion can be easily removed by micromanipulation techniques (Yin et al., Reference Yin, Kato and Tsunoda2002a) or by oocyte bisection (handmade cloning; Vajta et al., Reference Vajta, Lewis, Trounson, Purup, Maddox-Hyttel, Schmidt, Pedersen, Greve and Callesen2003). Anti-mitotic drugs have also been applied to pre-activated oocytes, resulting in the extrusion of a second polar body (PB) that contains all oocyte chromosomes. This technique is called chemically induced enucleation (IE).
In chemically IE, the treatment of pre-activated oocytes with high concentrations of demecolcine (0.4 to 0.5 μg/ml), a microtubule-depolymerizing drug, has been proven to be effective for oocyte enucleation in mice (Baguisi & Overström, Reference Baguisi and Overström2000; Gasparrini et al., Reference Gasparrini, Gao, Ainslie, Fletcher, Mc Garry, Rithie, Springbett, Overström, Wilmut and de Sousa2003; Ibáñez et al., Reference Ibáñez, Albertini and Overström2003), goats (Ibáñez et al., Reference Ibáñez, Sanfins, Combelles, Albertini and Overström2002), and cattle (Fischer-Russel et al., Reference Fischer-Russel, Ibáñez, Albertini and Overstrom2005). Although mouse offspring were produced by nuclear transfer using this protocol (Baguisi & Overström, Reference Baguisi and Overström2000; Gasparrini et al., Reference Gasparrini, Gao, Ainslie, Fletcher, Mc Garry, Rithie, Springbett, Overström, Wilmut and de Sousa2003), the percentage of embryos that developed to blastocyst stage was much lower among chemically enucleated nuclear transfer embryos than in those enucleated by conventional mechanical methods (Gasparrini et al., Reference Gasparrini, Gao, Ainslie, Fletcher, Mc Garry, Rithie, Springbett, Overström, Wilmut and de Sousa2003).
To our knowledge, there has been to date only one report using chemically IE in cattle, and in which the authors employed intracytoplasmic injection for embryo reconstitution (Fischer-Russel et al., Reference Fischer-Russel, Ibáñez, Albertini and Overstrom2005). An understanding of the mechanism of chemically IE and modifications of the current protocol would lead to an increase in the frequency of induced enucleation and thus minimize the invasiveness of currently employed enucleation procedures. As further data are needed to fine tune the dose and duration of exposure to demecolcine in combination with modifications of the oocyte activation protocol (Fischer-Russel et al., Reference Fischer-Russel, Ibáñez, Albertini and Overstrom2005), the objective of the present study was to optimize the culture conditions of bovine oocytes exposed to demecolcine after oocyte activation in an attempt to improve the efficiency of chemically IE, and to evaluate tubulin and chromatin organization and the viability of cytoplasts produced by this technique.
Material and methods
Chemicals
Chemicals and media were purchased from Sigma Chemical Co. (St. Louis, MO, USA), unless otherwise stated.
Experimental design
Experiment I: Evaluation of different culture conditions with demecolcine for chemically induced enucleation
First, different experiments were conducted to standardize the chemically IE technique in bovine. Oocytes were treated with demecolcine at the beginning (Experiment Ia) or after the onset (Experiment Ib) of the activation process.
Experiment Ia: Exposure to demecolcine at the beginning of parthenogenetic activation
Oocytes matured in vitro for 26 h were denuded and activated artificially with ionomycin (5 μM for 5 min) and cycloheximide (10 μg/ml for up to 5 h). Three groups were established: (1) a control (not activated and not treated with demecolcine); (2) an activated (exposed only to the oocyte-activating agents); and (3) a treated group (DEME; exposed to the oocyte-activating agents and 0.05 μg/ml demecolcine). Treatment with demecolcine was initiated at the beginning of oocyte activation and samples were collected in quintuplicate from the three groups after 0, 4 or 5 h of treatment. The oocytes were stained with 10 μg/ml Hoechst 33342 for 15 min and the percentage of metaphase II oocytes and activation and enucleation rates was determined under an epifluorescence microscope (330–385 nm).
Experiment Ib: Exposure to demecolcine after the onset of activation
After 26 h of in vitro maturation, oocytes were denuded and activated as described in Experiment Ia for up to 4 h. Control, activated and demecolcine-treated (DEME) groups were also defined. However, in the DEME group the activation lasted for up to 4 h and treatment with 0.05 μg/ml demecolcine was started 0, 0.5, 1.0, 1.5, or 2.0 h after the onset of oocyte activation. Therefore, the following subgroups were established: (1) DEME 0–2 h (exposure to demecolcine between 0 and 2 h of activation); (2) DEME 0–4 h; (3) DEME 0.5–2.5 h; (4) DEME 0.5–4 h; (5) DEME 1–3 h; (6) DEME 1–4 h; (7) DEME 1.5–3.5 h; (8) DEME 1.5–4 h, and (9) DEME 2–4 h. Quintuplicate samples were obtained. The oocytes were stained with 10 μg/ml Hoechst 33342 for 15 min and activation and enucleation rates were determined under an epifluorescence microscope (330–385 nm).
Experiment II: Tubulin and chromatin organization in parthenogenetically activated oocytes submitted to chemically induced enucleation
In the third experiment, we evaluated the effects of demecolcine on nuclear and microtubule organization of activated oocytes submitted to chemically IE. For this purpose, three groups were defined as described before (control, activated, and treated with demecolcine–DEME). In the DEME group, activated oocytes were exposed to the drug in the last 2 h of activation (DEME 2–4 h group). The samples were fixed and the configuration of chromatin and meiotic spindle (microtubules) was evaluated in triplicate at 0, 0.5, 1.0, 2.0, 2.5, 3.0, 4.0, or 10 h after the onset of activation.
Experiment III: Early development of embryos reconstituted by nuclear transfer from chemically enucleated cytoplasts
In the final experiment, after chemical or conventional enucleation, part of the cytoplasts were fixed in 4% paraformaldehyde for 1 h at room temperature and stored in phosphate-buffered saline (PBS) + 1% PVP for evaluation of the efficiency of enucleation by staining with Hoechst 33342 for 15 min and observation under an epifluorescence microscope (330–385 nm). The remaining cytoplasts were reconstituted by nuclear transfer (NT) and presumptive zygotes were cultured in vitro. The rates of embryo development were determined after 48 h (cleavage) and on day 7 of culture (blastocysts).
Oocyte recovery and in vitro maturation
Bovine oocytes were obtained by follicular aspiration from ovaries obtained at a local slaughterhouse. The ovaries were transported to the laboratory in 0.9% saline solution at 30–35°C. Follicles with diameters between 3 and 8 mm were aspirated using an 18-gauge needle attached to a 20-ml syringe and cumulus oocyte complexes (COCs) with at least three layers of cumulus cells and homogenous cytoplasm were selected. The COCs were washed in HEPES-buffered tissue culture medium-199 (TCM-199; Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal calf serum (FCS), 0.20 mM sodium pyruvate, and 83.4 μg/ml amikacin (Instituto Biochimico, Rio de Janeiro, Brazil). Groups of 15 COCs were matured in droplets (100 μL) of TCM-199 supplemented with 10% FCS, 1.0 μg/ml FSH, 50 μg/ml hCG (Profasi™, Serono, São Paulo, Brazil), 1.0 μg/ml estradiol, 0.20 mM sodium pyruvate, and 83.4 μg/ml amikacin under mineral oil (Dow Corning Co., Midland, MI, USA) for up to 26 h at 38.5°C in an atmosphere of 5% CO2 in air and maximum humidity.
Isolation of nucleus donor cells
Blastomeres were used as donor nucleus cells in the NT procedures. Cells were obtained from embryos produced in vitro with female and male sexed semen. The in vitro fertilization procedure was performed 24 h after in vitro maturation. For this purpose, groups of 15 oocytes were washed twice and transferred to 30 μl drops of TALP-IVF medium supplemented with 0.6% bovine serum albumin (BSA), 10 μg/ml heparin, 18 μM penicillamine, 10 μM hypotaurine and 1.8 μM epinephrine, and covered with sterile mineral oil. Frozen straws of semen from the same bull sexed by flow cytometry (Lagoa da Serra, Sertãozinho, Brazil) were used. Each straw containing approximately 2 million spermatozoa was centrifuged separately on a discontinuous 45/90 Percoll gradient for 7 min at 3600 g. The pellet was resuspended in 500 μl TALP-IVF medium and again centrifuged for 5 min at 520 g. After centrifugation, 100 μl of the medium containing the pellet was collected from the bottom of the tube and homogenized in a conic tube. The final suspension was divided among 10 oocyte-containing drops, at a final concentration of approximately 104 spermatozoa per oocyte. The plates were incubated for 18–20 h at 38.5°C in an atmosphere of 5% CO2 under saturated humidity. After this period, embryos were cultured in synthetic oviductal fluid (SOF) supplemented with 2.5% FCS and 5 mg/ml BSA at 38.5°C in a humidified atmosphere of 5% CO2 in air. Groups of 10–20 1-cell embryos were cultured in 100 μl microdrops of culture medium for 5 days. The medium was renewed on the third day of embryo culture.
For the isolation of blastomeres used for reconstitution by NT from cytoplasts enucleated by the conventional technique, day-5 embryos were cultured in SOF medium containing 0.4 μg/ml demecolcine for 12 h to synchronize cells in the metaphase stage. Approximately 3 h before the beginning of micromanipulation, the embryos were washed several times and cultured in demecolcine-free SOF medium so that at the time of use the cells were in G1, a stage compatible with oocytes exhibiting high activity of maturation-promoting factor (MPF). For the chemical enucleation technique, in which activated oocytes (low MPF activity) are used, the embryos were not synchronized as about 80% of the cells are naturally in the S phase of the cell cycle (Campbell et al., Reference Campbell, Loi, Otaegui and Wilmut1996).
At the time of use, the zona pellucida was removed from the embryos by incubation in 0.5% pronase for 30 s. Next, the embryos were transferred to microdroplets of Ca2+- and Mg2+-free PBS containing 0.3% PVA and disaggregated into single blastomeres with a pipette. Blastomeres derived from early and compact morulae were used. The blastomeres were incubated for 30 min in 7.5 μg/ml cytochalasin B and then transferred to droplets for micromanipulation and used as donor nucleus cells.
Nuclear transfer procedure
Oocyte enucleation techniques
For conventional oocyte enucleation, oocytes matured in vitro for 18 to 20 h were used. After denuding with 2 mg/ml hyaluronidase, the structures were incubated for 30 min at 38.5°C in SOF medium with HEPES buffer (HSOF) supplemented with 10% FCS, 7.5 μg/ml cytochalasin B, and 7.5 μg/ml Hoechst 33342. Enucleation was performed under an inverted light microscope (Olympus IX-70), removing the first PB and part of the adjacent cytoplasm. Enucleation was confirmed by examining the oocyte under ultraviolet light to determine the absence of the metaphasic plate.
For chemically IE, oocytes matured in vitro for 26 h were denuded and activated artificially (5 μM ionomycin for 5 min and 10 μg/ml cycloheximide for 5 h). After 1.5 h of activation, the oocytes were treated with demecolcine (0.05 μg/ml) for complete extrusion of the nuclear material of the oocyte together with the second PB. A the end of treatment, the second PB and a small part of the adjacent cytoplasm were removed by micromanipulation and the cytoplasts were subsequently used for NT.
Embryo reconstitution
Embryonic cells were individually transferred to the perivitelline space of each recipient cytoplast and cytoplast–cell complexes were electrofused in 0.3 M mannitol solution containing 0.05 mM CaCl2, 0.1 mM MgSO4 and 0.1% PVA. Electrofusion was induced by a direct pulse of 1.5 kV/cm for 70 μs (Bordignon et al., Reference Bordignon, Clarke and Smith1999) and successfully reconstituted structures were incubated in SOF + 10% FCS at 38.5°C in a humidified atmosphere of 5% CO2 in air.
Parthenogenetic activation
Only reconstituted oocytes obtained by the conventional technique were activated at the end of the procedure, whereas in chemically IE oocytes were activated at the beginning of the protocol. The oocytes were chemically activated with 5 μM ionomycin in HSOF supplemented with 10% FCS for 5 min and with 10 μg/ml cycloheximide + 10 μg/ml cytochalasin B in SOF supplemented with 10% FCS for 5 h at 38.5°C in a humidified atmosphere of 5% CO2 in air. Next, embryos were cultured until day 7.
Immunofluorescence analysis of tubulin and chromatin
The protocol described by Liu et al. (Reference Liu, Ju and Yang1998) was used. Briefly, oocytes were incubated for 30 min in microtubule-stabilizing buffer (0.1 M PIPES, pH 6.9, 5 mM MgCl2.6H2O (Merck, Darmstadt, Germany), 2.5 mM EGTA) containing 2% formaldehyde, 0.1% Triton X-100, 1 μM Taxol, 0.01% aprotinin, 1 mM dithiothreitol, and 50% deuterium oxide (Aldrich Chem. Co., Milwaukee, WI, USA). Next, oocytes were kept overnight at 4°C in washing medium (WM; PBS with 0.02% NaN3, 0.01% Triton X-100, 0.2% non-fat dry milk, 2% FCS, 2% BSA, and 0.1 M glycine) and were then incubated in WM containing anti-α-tubulin mouse monoclonal antibody (1:50) conjugated with fluorescein isothiocyanate (FITC) for 2 h at 38°C. After washing in WM, oocytes were stained with 10 μg/ml Hoechst 33342 in WM for 10 min, mounted on slides with 90% glycerol in PBS, and examined under an epifluorescence microscope at 330–385 and 420–490 nm. For the evaluation of nuclear kinetics, the oocytes were classified as immature, metaphase II (MII) or activated (AII/TII with extrusion of the 2nd PB or pronucleus stage) (Fig. 1). In addition, the oocytes were classified according to microtubule organization (present, reduced, or absent) as described in Fig. 2.
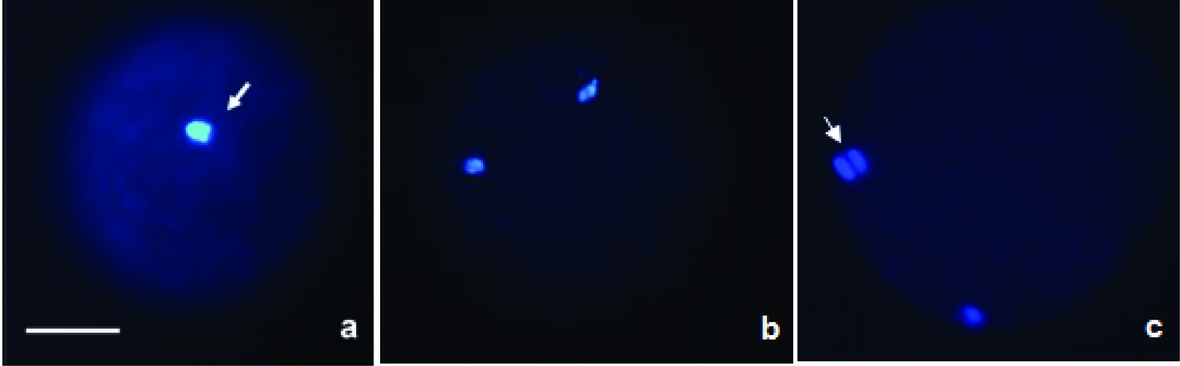
Figure 1 Evaluation of nuclear kinetics. Photomicrographs of activated bovine oocytes treated with demecolcine. Categories of oocytes observed: (a) metaphase I (MI) oocyte; (b) metaphase II (MII) oocyte; (c) anaphase/telophase II (AII/TII) oocyte. Oocytes were stained with Hoechst 33342 and observed under an epifluorescence microscope. The arrows indicate nuclear material (blue). Bar = 50 μm.
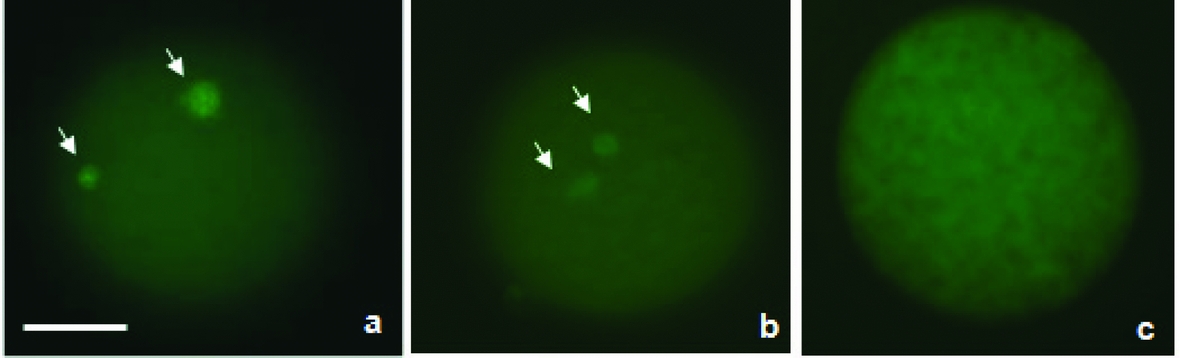
Figure 2 Evaluation of microtubule configuration. Photomicrographs of activated bovine oocytes treated with demecolcine. Categories of oocytes observed: (a) oocyte with microtubules (MTs); (b) oocyte with reduced MTs; (c) oocyte without MTs. Oocytes were stained with FITC-conjugated anti-α tubulin antibody (1:50) and observed under an epifluorescence microscope. The arrows indicate MTs (green). Bar = 50 μm.
Statistical analysis
The rates of activation, enucleation and embryo development were analyzed by chi-squared (χ2) test or Fisher's exact test, when appropriate. The percentages obtained in Experiment II were submitted to analysis of variance (ANOVA) and means were compared by the Tukey test. All analyses were performed using Statistical Analysis System (SAS) 9.1 software, adopting a level of significance of 5%.
Results
Addition of demecolcine at the beginning of parthenogenetic activation
At the beginning of assessment (0 h), most oocytes were at the MII stage (133/163, 81.6%). A significant increase in the percentage of oocytes with extrusion of the second PB was observed in the groups activated for 4 or 5 h. These groups did not differ from one another and the percentage of oocytes was higher than in the groups treated with demecolcine (Table 1).
Table 1 Percentage of metaphase II (MII), activated, and enucleated (EN) oocytes in the control group (CON), group activated with 5 μM ionomycin for 5 min and 10 μg/ml cycloheximide (ACTIV), and group activated and treated with 0.05 μg/ml demecolcine (DEME) for 0, 4 and 5 h

a ,b,c Values with different superscript letters within the same column differ (P < 0.05, chi-squared test).
Enucleation rates were higher in the DEME group (P < 0.05) than in the control and activated groups after 4 and 5 h of activation (Table 1), which also differed from one another. However, demecolcine probably interfered with oocyte activation, resulting in low enucleation rates (39.4–41.8%). No significant difference was observed between groups treated with demecolcine for 4 and 5 h (Table 1).
Addition of demecolcine after the onset of parthenogenetic activation
Demecolcine exerted no damaging effects on oocyte activation when added to the medium 1.5 h after the beginning of the process, with no significant differences (P > 0.05) between the DEME 1.5–3.5 h, DEME 1.5–4 h or DEME 2–4 h groups and the group submitted to activation only (mean of 62.7–77.0%) (Table 2).
Table 2 Percentage of activated oocytes after incubation in medium containing 5 μM ionomycin for 5 min and 10 μg/ml cycloheximide for 4 h and different times of treatment with demecolcine: DEME 0–2 h (exposure to demecolcine between 0 and 2 h of activation); DEME 0–4 h; DEME 0.5–2.5 h; DEME 0.5–4 h; DEME 1–3 h; DEME 1–4 h; DEME 1.5–3.5 h; DEME 1.5–4 h; DEME 2–4 h

*The asterisk indicates difference in relation to activated group.
a ,b Values followed by the same letter between rows did not differ significantly (P > 0.05, chi-squared test).
ACTIV: activated group; CON: control group.
Enucleation rates differed significantly between all groups treated with demecolcine and the control (0/114) and activated (0/130) groups (Fig. 3). The DEME 1.5–4 h (42.2%) and DEME 2–4 h (54.0%) groups presented higher enucleation rates (P < 0.05) than all other groups (26.8–36.3%), but there was no difference between these two groups. As the percentage of activated and enucleated oocytes was higher in the DEME 2–4 h group, this treatment was chosen for the following experiments.

Figure 3 Graphic representation of the percentage of enucleated oocytes (EN) after activation with 5 μM ionomycin for 5 min and 10 μg/ml cycloheximide for up to 4 h and different treatments with demecolcine: group 1 (DEME 0–2 h); group 2 (DEME 0–4 h); group 3 (DEME 0.5–2.5 h); group 4 (DEME 0.5–4 h); group 5 (DEME 1–3 h); group 6 (DEME 1–4 h); group 7 (DEME 1.5–3.5 h); group 8 (DEME 1.5–4 h); group 9 (DEME 2–4 h). a–dColumns followed by different superscript letters differ from one another (P < 0.05, chi-squared test). ACTIV: activated group; CON: control group.
Evaluation of nuclear and microtubule kinetics in pre-activated oocytes treated with demecolcine
The frequency of immature oocytes was similar in the three groups (control, activated and DEME) throughout the period of evaluation (Fig. 4). Effects of activation were detected after 2 h, when the mean percentage of activated oocytes was higher in the activated and DEME groups in comparison to the control group (Fig. 4).

Figure 4 Graphic representation of nuclear kinetics in activated bovine oocytes treated with demecolcine and evaluated at intervals of 0.5 and 1.0 h between 0 and 10 h after the onset of activation. Control (not activated or treated with demecolcine); Activated (exposed only to the oocyte-activating agents); DEME (activated and treated with demecolcine for 2 h). (A) Immature oocytes; (B) mature oocytes; (C) activated oocytes. The asterisk indicates exposure to demecolcine.
The percentage of oocytes with microtubules was significantly reduced (P < 0.05) in the DEME group when compared with the activated group 2.5 h after the onset of activation (0.5 h of exposure to demecolcine). After 10 h, lower mean percentages of oocytes with microtubules were observed in the activated and DEME groups compared to control and this percentage also differed between the first two groups (Fig. 5). In parallel, there was a significant increase (P < 0.05) of oocytes with reduced microtubules in the DEME group 2.5 h after the onset of activation. This higher mean percentage was observed up to 4 h after the onset of activation when compared to the control and activated groups (Fig. 5).
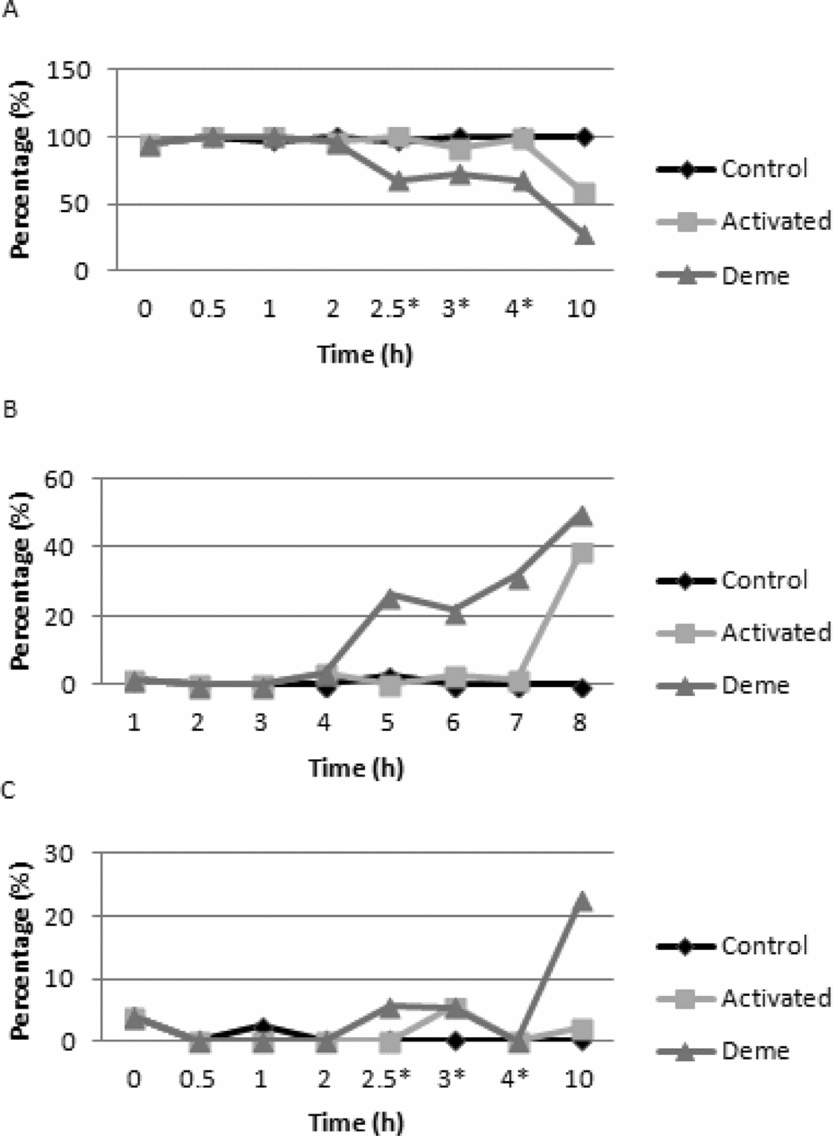
Figure 5 Graphic representation of microtubule kinetics in activated bovine oocytes treated with demecolcine and evaluated at intervals of 0.5 and 1.0 h between 0 and 10 h after the onset of activation. Control (not activated or treated with demecolcine); Activated (exposed only to the oocyte-activating agents); DEME (activated and treated with demecolcine for 2 h). (A) oocytes with microtubules; (B) oocytes with reduced microtubules; (C) oocytes without microtubules. The asterisk indicates exposure to demecolcine.
Microtubule reduction was observed in approximately 50% of oocytes of the DEME group 10 h after the onset of activation. However, no significant difference was observed between the treated and activated groups, only when compared with the control group. In addition, 23% of oocytes displayed absent microtubules in the DEME group, a mean value higher than that observed in the control and activated groups (P < 0.05) (Fig. 5).
Early development of embryos reconstituted by nuclear transfer from cytoplasts produced by chemically induced enucleation
In relation to cleavage and blastocyst rates, no significant differences were observed between groups (P > 0.05) despite a tendency towards lower embryo production in the chemically enucleated group. As no significant difference was observed between sexes, the results of female and male embryos were pooled for each technique (Table 3).
Table 3 Number and percentage of cleaved embryos and blastocysts produced by nuclear transfer from donor embryo cells after conventional or chemically induced enucleation in bovine

No significant differences were observed between groups (P > 0.05, chi-squared test).
Discussion
First, oocytes were exposed to demecolcine at the start of activation. However, as the enucleation rates were relatively low (39.45–42.38%), a second experiment was conducted in which demecolcine was added after the onset of oocyte activation and higher enucleation rates were obtained. No damaging effect of demecolcine on oocyte activation was observed when treatment was started 1.5 h after the exposure of oocytes to the activating agents, with a mean enucleation rate of 54%. Similar findings have been reported by Ibáñez et al. (Reference Ibáñez, Albertini and Overström2003) and Fischer-Russel et al. (Reference Fischer-Russel, Ibáñez, Albertini and Overstrom2005) for mouse and bovine oocytes, respectively, and can be explained by the fact that demecolcine impairs spindle rotation in activated oocytes, an event that normally precedes formation of the PB. Thus, the beginning of demecolcine treatment in relation to oocyte activation is the key point for successful enucleation. The present results agree with previous studies showing that an intact spindle is required for the degradation of cyclin B and the consequent inactivation of MPF and exit from the M phase (Kubiak et al., Reference Kubiak, Weber, De Pennart, Winston and Maro1993).
The enucleation rates obtained in the present study were lower than those reported by Fischer-Russel et al. (Reference Fischer-Russel, Ibáñez, Albertini and Overstrom2005) for activated bovine oocytes treated with demecolcine (up to 91.7%). However, the high rates reported by these authors were obtained 5 h after the onset of oocyte activation. A new assessment performed 17 h after the onset of activation showed a decline in enucleation, with rates ranging from 3.4–46.1%. These findings indicate that the chromosomes were reintegrated into the oocyte after incomplete extrusion of the second PB, demonstrating the inefficiency of the enucleation process in the absence of micromanipulation of these structures.
In this study, several oocytes contained subcortical nuclear material which was connected to the chromosomes present in the PB by a spindle remnant, a feature also observed in activated mouse oocytes (Ibáñez et al., Reference Ibáñez, Albertini and Overström2003). These oocytes were considered to be non-enucleated. Incomplete release of the PB may have occurred in these treated oocytes as a consequence of the lack of intact microtubules for the formation of contractile rings, which are responsible for cytokinesis (Larkin & Danilchik, Reference Larkin and Danilchik1999). However, since this material is juxtaposed to the PB, the latter can be easily removed by micromanipulation, facilitating the enucleation procedure and promoting an increase of oocyte enucleation rates. In addition, we found that exposure to demecolcine can be interrupted after 1 h of treatment, contributing to the optimization of the current protocol, which recommends treatment for 2 h (Fischer-Russel et al., Reference Fischer-Russel, Ibáñez, Albertini and Overstrom2005), reducing the time of exposure to the drug.
After optimization of the chemical enucleation protocol, we analyzed chromatin and microtubule kinetics in these activated oocytes treated with demecolcine. Previously our group observed that in MII bovine oocytes, the microtubule-depolymerizing action of demecolcine occurred after only 0.5 h of treatment, with the complete absence of these structures in most of the oocytes analyzed (Saraiva et al., Reference Saraiva, Perecin, Méo, Ferreira, Tetzner and Garcia2009). Different results were obtained in the present study for pre-activated oocytes. Although the density of microtubules was reduced in these oocytes, complete disappearance of these structures was not seen in most of them. Similar results have been reported for mouse oocytes in which microtubules also had not disappeared completely even after 2 h of treatment with demecolcine (Ibáñez et al., Reference Ibáñez, Albertini and Overström2003).
The rapid effect of demecolcine on metaphase II oocytes (Saraiva et al., Reference Saraiva, Perecin, Méo, Ferreira, Tetzner and Garcia2009), in which microtubules disappeared completely within 0.5 h of treatment, is likely to reflect differences in the sensitivity of the meiotic spindle of these structures, with the spindle of metaphase II oocytes being a more sensitive target for demecolcine than the spindle of activated oocytes. The presence of these spindle remnants may indicate differences in the stability of some microtubules in the spindle, which probably correspond to interpolar microtubules (Combelles & Albertini, Reference Combelles and Albertini2001).
In the present study, reduction of microtubules was observed in approximately 50% of treated oocytes at the end of assessment and complete disappearance of these structures in 23%, with visible microtubules being seen in only 27% of oocytes. These findings indicate the absence of immediate repolymerization of microtubules after culture of pre-activated oocytes in demecolcine-free medium, in contrast to metaphase II oocytes, as evaluated previously by our group (Saraiva et al., Reference Saraiva, Perecin, Méo, Ferreira, Tetzner and Garcia2009). This fact may have some negative consequences for subsequent embryonic development as microtubules are important during the first cleavages. However, a reduction of microtubule density was also observed in oocytes of activated group 10 h after the onset of the process, suggesting that this reduction is a ‘physiological’ process.
It is important that future research also assesses the behaviour of microfilaments during treatment with demecolcine, especially in cases of pre-activated oocytes. Demecolcine-induced enucleation requires the artificial activation of the oocyte prior to its exposure to the microtubule-destabilizing drug. Parthenogenetic activation of bovine oocytes can be achieved by using compounds that increase intracellular calcium, such as ionomycin (calcium ionophore), followed by treatment for several hours with the protein synthesis inhibitor CHX, which induces the resumption of meiosis by inactivation of MPF and mitogen-activated protein kinase (MAPK) (Fischer-Russel et al., Reference Fischer-Russel, Ibáñez, Albertini and Overstrom2005). Recently, Vasilev et al. (Reference Vasilev, Chun, Gragnaniello, Garante and Santella2012) observed that artificial elevation of intracellular Ca2+ levels with ionomycin immediately led to drastic alteration of the actin cytoskeleton in the immature oocytes, which exhibited depolymerization of the actin meshwork in the subplasmalemmal regions along with highly enhanced polymerization and bundling of the actin filaments in the inner cytoplasm.
As demonstrated in the sea urchin, zygote cell cleavage depends heavily on the regulation of the actin cytoskeleton (Dales & De Santis, Reference Dales and De Santis1981). Hence, actin is intimately implicated in fertilization and in the early stages of development. These alterations led to difficulties in cell cleavage in the monospermic zygotes and eventually to a higher rate of abnormal development. Then, further studies are needed to better characterize the mechanism of demecolcine-induced enucleation of preactivated oocytes, considering the involvement of microfilaments on the subsequent embryonic development. In this context, Meng et al. (Reference Meng, Wu, Bunch, White, Sessions, Davies, Rickords and Li2011) observed that in bovine MII oocytes, the microtubules were concentrated to form the normal spindle structure with microfilaments distributed uniformly in the cytoplasm, while in the demecolcine-treated oocytes, the spindle microtubules were nearly depleted by demecolcine, but the microfilaments were hardly affected. The demecolcine treatment seemed to have altered the balance between the interaction of microtubules and microfilaments in the treated oocytes, which appears to be the result of the cytoplasmic protrusion. Without the aid of the microtubules, the chromosomes were compressed by the microfilaments to form a protrusion on the oocyte membrane.
Evaluation of embryonic development showed cleavage rates ranging from 63.6–70.0% in the groups reconstituted by the different techniques. Blastocyst rates ranged from 15.5–24.2%. Although embryo production tended to be lower for oocytes obtained by chemically IE, no significant differences were observed when compared with the conventional procedure.
There are various factors that justify the use of demecolcine for NT. According to Simerly et al. (Reference Simerly, Dominko, Navara, Payne, Capuano, Gosman, Chong, Takahashi, Chace, Compton, Hewitson and Schatten2003, Reference Simerly, Navara, Hyun, Lee, Kang, Capuano, Gosman, Dominko, Chong, Compton, Hwang and Schatten2004), removal of the meiotic spindle during conventional enucleation seems to be responsible for the low development of embryos reconstituted from somatic cells in primates, since the centrosome and some motor proteins, such as NuMA and KIFC1, which are fundamental for the formation of the spindle poles during mitosis, are depleted from the ooplasm, resulting in spindle dysfunction and aneuploid embryos. In addition, traditional mechanical enucleation has been shown to reduce the amount of spindle-associated γ-tubulin in mouse oocytes (Van Thuan et al., Reference Van Thuan, Wakayama, Kishigami and Wakayama2006). Within this context, alternative methods of oocyte enucleation may permit the preservation of spindle-associated factors in enucleated oocytes and consequently improve embryo quality.
Cloned mammalian species have been produced from either demecolcine-assisted (Yin et al., Reference Yin, Kato and Tsunoda2002a; Kawakami et al., Reference Kawakami, Tani, Yabuuchi, Kobayashi, Murakami, Fujimura, Kato and Tsunoda2003; Vajta et al., Reference Vajta, Lewis, Trounson, Purup, Maddox-Hyttel, Schmidt, Pedersen, Greve and Callesen2003; Tani et al., Reference Tani, Shimada, Kato and Tsunoda2006) or demecolcine-induced enucleated oocytes (Baguisi & Overström, Reference Baguisi and Overström2000; Gasparrini et al., Reference Gasparrini, Gao, Ainslie, Fletcher, Mc Garry, Rithie, Springbett, Overström, Wilmut and de Sousa2003) reconstituted with somatic or embryonic stem cells. The only report of demecolcine-induced enucleation in bovine (Fischer-Russel et al., Reference Fischer-Russel, Ibáñez, Albertini and Overstrom2005) showed that intracytoplasmic injection of the donor nucleus prior to oocyte activation and treatment with demecolcine or after 1.5 and 2.0 h post-activation yielded 19 and 15.7% of blastocysts, respectively. For both treatments, cleavage and blastocyst rates were similar to those observed for the control group (26.1%). These data agree with the present results, indicating that demecolcine-induced enucleation does not compromise embryo development in cattle.
In summary, we optimized the chemically IE protocol and showed that the procedure may be shortened. However, demecolcine impaired meiotic progression when it was added to the medium during the onset of the activation process. Furthermore, demecolcine reduced microtubule density in pre-activated oocytes, but these structures did not disappear completely in most oocytes, and apparently there was no immediate repolymerization of microtubules after culture in demecolcine-free medium. Nevertheless, the chemically IE technique permitted to obtain viable cytoplasts for NT, providing results similar to those observed for the conventional method. Studies evaluating the ability to establish pregnancy and to produce live offspring are needed to confirm the use of demecolcine-induced enucleation in bovine.
Acknowledgements
This study was supported by the São Paulo Research Foundation (FAPESP), Brazil.










