INTRODUCTION
Global climate change is driven by the anthropogenic increase of atmospheric greenhouse gases such as CO2 (IPCC, Reference Pachauri and Meyer2014). The second largest anthropogenic source of CO2 emissions is deforestation (van der Werf et al., Reference van der Werf, Morton, DeFries, Olivier, Kasibhatla, Jackson, Collatz and Randerson2009). The share of deforestation and forest degradation in total anthropogenic CO2 emissions is estimated to be approximately 20% (van der Werf et al., Reference van der Werf, Morton, DeFries, Olivier, Kasibhatla, Jackson, Collatz and Randerson2009). Half of the global CO2 emissions from deforestation are related to the clearing of tropical forests in Latin America (Harris et al., Reference Harris, Brown, Hagen, Saatchi, Petrova, Salas, Hansen, Potapov and Lotsch2012). For instance, in Bolivia, the gross forest cover loss due slash-and-burn practices from 2000 to 2005 is estimated to 1290 km2 year−1, which corresponds to an annual carbon (C) loss of 11 Tg C year−1 (Harris et al., Reference Harris, Brown, Hagen, Saatchi, Petrova, Salas, Hansen, Potapov and Lotsch2012). Therefore, the loss of tropical and subtropical forests plays a prominent role in climate change.
Reducing CO2 emissions by limiting deforestation, and increasing ecosystem C stocks by afforestation and reforestation are possible ways to decrease the quantity of greenhouse gases in the atmosphere. However, another key to preserving C stocks in tropical lowlands is the implementation of sustainable land management alternatives (Albrecht and Kandji, Reference Albrecht and Kandji2003), such as agroforestry systems (AFS). AFS also mitigate losses of biodiversity, prevent soil degradation and maintain air and water quality (Mortimer et al., Reference Mortimer, Saj and David2017; Tscharntke et al., Reference Tscharntke, Clough, Bhagwat, Buchori, Faust, Hertel, Hölscher, Juhrbandt, Kessler, Perfecto, Scherber, Schroth, Verldkamp and Wanger2011). Sufficiently complex AFS, composed of perennial plants, trees and shrubs, and combined with various annual crops, can increase the diversity of plant species with different physiological properties, allowing for a more efficient use of soil resources (Jose, Reference Jose2009). Various AFS trees and other plants have a positive influence on the quality and quantity of soil organic matter (SOM) through input of crop and pruning residues, litterfall and root turnover. Moreover, through these pathways, organic matter and nutrient inputs stimulate the soil fauna and microbial matter transformations (Nair et al., Reference Nair, Kumar and Nair2009).
Further advantages of AFS are obtained by (i) including fast-growing and nitrogen (N)-fixing leguminous trees, e.g., Erythrina spp. and Inga spp. (Albrecht and Kandji, Reference Albrecht and Kandji2003; Beer et al., Reference Beer, Bonnemann, Chavez, Fassbender, Imbach and Martel1990), since these trees increase the N supply for all AFS plants by incorporating N into their own biomass and contributing it to soil N stocks through litterfall; (ii) the function of deep-rooting trees as nutrient pumps, as they extract nutrients from sub-soils and return them to top-soils through litterfall (Beer, Reference Beer1988); (iii) reduced N leaching, since AFS, compared to monoculture systems (MCS), are characterised by increased biomass production and thus enhanced uptake of nitrate, and deep-rooting AFS trees may still reach nitrate that has been leached to a depth where other plants cannot reach it anymore (Jose, Reference Jose2009). Due to these effects, AFS allow for a substantial reduction of external inputs of inorganic fertilisers, compared to MCS. In this way, the economic dependency of farmers on the agrochemical market can be reduced (Johns, Reference Johns1999). This effect is well in-line with the intention of organic agriculture that also aims at avoiding chemical inputs and promotes production systems that are adapted to local conditions. Some advantages of organic agriculture include (i) the preservation of biodiversity and soil fertility (Kilcher, Reference Kilcher2007), (ii) reduced production costs due to the omission of industrial-chemical inputs and (iii) a wide range of products and premium prices for organic cash crops that may enhance the economic living conditions of the farmers (Armengot et al., Reference Armengot, Barbieri, Andres, Milz and Schneider2016). Thus, the practices of AFS are compatible with the principles and perspectives of organic agriculture, mainly for small-scale farmers.
Cacao (Theobroma cacao L.) is a shade-tolerant plant and an important cash crop in tropical countries. Cacao is mostly grown by small-scale farmers, who produce 80–90% of the cacao worldwide (WCF, 2014). In 2016/17, a total of 4.7 million tons of cocoa beans were produced on approximately 10 million ha (ICCO, 2017). Organic production makes up less than 2.5% of total cacao production. Cacao production is predominantly located in South America, where more than 80% of the world's organic cacao is produced (Lernoud and Willer Reference Lernoud and Willer2016). In 2014, organic farming was practised on an estimated area of 6.8 million ha in Latin America (1.1% of the agricultural area). In Bolivia, the percentage of agricultural area that is farmed organically has reached only 0.3% (114 306 ha). A total of 12 114 producers practised organic agriculture in 2014 (Lernoud et al., Reference Lernoud, Willer and Schlatter2016). These data demonstrate that the potential for organic agriculture in Latin America, and particularly in Bolivia, is by far not yet realised. However, the above-mentioned benefits must be quantified and evaluated for each region and then disseminated to farmers in order to effectively promote organic agriculture.
Organically managed cacao AFS, in addition to their beneficial effects on farmers’ income and on ecosystem services, offer a high capacity for C sequestration (Saj et al., Reference Saj, Jagoret and Ngogue2013). In the past, this aspect of C sequestration was not as much in the focus of research as the economic and ecological aspects of AFS, which have been extensively analysed and compared to those of MCS's shifting cultivation (Steffan-Dewenter et al., Reference Steffan-Dewenter, Kessler, Barkmann, Bos, Buchori, Erasmi, Faust, Gerold, Glenk, Gradstein, Guhardja, Harteveld, Hertel, Höhn, Kappas, Köhler, Leuschner, Maertens, Marggraf, Migge-Kleian, Mogea, Pitopang, Schaefer, Schwarze, Sporn, Steingrebe, Tjitrosoedirdjo, Tjitrosoemito, Twele, Weber, Woltmann, Zeller and Tscharntke2007). Only in the past 20 years has the potential of AFS for C sequestration received increasing attention (Jose, Reference Jose2009; Nair et al., Reference Nair, Kumar and Nair2009). Current studies show that cacao AFS may store up to 140 Mg C ha−1 in aboveground biomass alone (Saj et al., Reference Saj, Durot, Mvondo Sakouma, Tayo Gamo and Avana-Tientcheu2017). The amounts of stored C mainly depend upon the composition of the tree species, tree density and the age of the AFS (Abou Rajab et al., Reference Abou Rajab, Leuschner, Barus, Tjoa and Hertel2016; Beer, Reference Beer1988; Nair et al., Reference Nair, Kumar and Nair2009). However, management practices and cacao tree age can vary widely within a region, which makes comparisons difficult. Therefore, the FiBL (Research Institute of Organic Agriculture, Switzerland) established a systematic long-term field trial in the eastern Andean foothills of Bolivia, where the most common cacao MCS and AFS under organic and conventional management are compared (http://www.systems-comparisons.fibl.org.).
Due to the widespread cultivation of cacao as a cash crop, an assessment of the potential impacts of different cacao production systems on C sequestration and N supply is of particular relevance. Therefore, in the present study, we compared different cacao production systems with respect to C stocks in the plant biomass, litter and pruning residues, and to temporal changes in C stocks. Thereby, we focussed on the following research questions: (i) how much more C can be stored in organic cacao production systems compared to conventional production systems in both AFS and MCS? and (ii) to what extent do the amounts of litterfall and pruning residues in AFS exceed those of MCS, and how much increase in N supply is achieved by including N-fixing trees in AFS?
MATERIALS AND METHODS
Study site and trial description
The FiBL long-term trial in ‘Sara Ana’ (15°27′S, 67°28′W) was started in October 2007. It is located in the lowlands of the Alto Beni region in the north-eastern foothills of the Bolivian Andes. The valley of the river Alto Beni was state colonised in the 1960s and is now the main cacao production area in Bolivia. The cacao trees of the long-term experiment were planted in October 2008 and the following cacao production systems were compared: (i) MCS (under full sun) with conventional management (with synthetic fertiliser, pesticides, herbicides, manual weeding) = MonoConv, (ii) MCS (under full sun) with organic management (compost, legumes, cover crops, bio-control, manual weeding) = MonoOrg, (iii) AFS (including diverse trees) with conventional management (synthetic fertiliser (50% compared to monoculture), leguminous trees, cover crops and shade trees, pesticides, herbicides) = AFConv and (iv) AFS (including diverse trees) with organic management (compost (50% compared to monoculture), leguminous trees, cover crops and shade trees, bio-control, manual weeding) = AFOrg (Schneider et al., Reference Schneider, Andres, Trujillo, Alcon, Amurrio, Perez, Weibel and Milz2016).
The valley bottom of the Alto Beni river is at 350 m to 490 m a.s.l. and it is surrounded by the mountain chains of the eastern Andes. The climate is humid tropical with a dry season from April to September and two arid months (June and July). The mean annual precipitation is 1540 mm, and the average monthly air temperature ranges between 22 °C in July and 27 °C in December (Elbers, Reference Elbers2002). The natural vegetation of the Alto Beni region is composed of nearly evergreen rainforest (Seidel and Vargas, Reference Seidel and Vargas1994). The research site is at approximately 380 m a.s.l., situated on a flat subrecent river-terrace of the Alto Beni river. The soils are Luvisols and Lixisols with loamy to clayey–loamy texture, having clay contents of 17 to 35%. Below 50 cm depth, the soils are strongly clayey and have bulk densities up to 1.9 g cm−2. The argic horizons often show a stagnic colour pattern and are saturated with water during the rainy season. Soil pH (in water) ranges from 5 to 8. At the time of establishment of the trial in 2008, the average soil organic carbon (SOC) content (1.5%) and soil N content (0.16%) in the upper 25 cm of the soils did not differ between the production systems. From 2008 to 2014, both SOC and soil N contents increased, and differences between organic (1.7% C; 0.19% N) and conventional (1.5% C; 0.16% N) management were detected.
The long-term experiment is arranged in a randomised complete block design with four replicates (Schneider et al., Reference Schneider, Andres, Trujillo, Alcon, Amurrio, Perez, Weibel and Milz2016). The factors are ‘crop diversity’ (MCS vs. AFS) and ‘management practice’ (conventional vs. organic). Gross trial plots are 48 × 48 m, corresponding to 2304 m², while net plots are 24 × 24 m, corresponding to 576 m2 (Supplementary Figure S1, available online at https://doi.org/10.1017/S001447971800011X). Thirty-six cacao trees were planted per net plot, using 12 different national and international clones (grafted) and hybrids (seeded), spaced at 4 × 4 m (625 trees ha−1), which is a common spacing in the study area (Schneider et al., Reference Schneider, Andres, Trujillo, Alcon, Amurrio, Perez, Weibel and Milz2016). After the cacao was planted, perennial soybean (Neonotonia wightii (Wight and Arn.) J.A. Lackey) was sown as a ground cover crop for weed control in the organically managed systems. Weed control in the conventionally managed systems was done by herbicide application. Cooking bananas (Musa × paradisiaca (L.)) were planted to ensure initial shading of young cacao plants in both AFS and MCS at the same spacing of 4 × 4 m. The cooking bananas were removed at the end of 2011. They were replaced by dessert bananas but only in the AFS (Schneider et al., Reference Schneider, Andres, Trujillo, Alcon, Amurrio, Perez, Weibel and Milz2016). In addition, 13 AFS trees were planted in each AFS net plot, including fruit and timber trees and palms (i.e., Erythrina spp. (L.), Euterpe precatoria (Mart.), Garcinia gardneriana ((Planch. and Triana) Zappi), Inga spp. (Mill.), Myroxylon balsamum ((L.) Harms), Nephelium lappaceum (L.), Persea americana (Mill.), Theobroma grandiflorum ((Willd. ex Spreng.) K. Schum.)). These trees were planted in between the cacao trees in the first year in a uniform pattern with a distance of 8 m between the cacao trees, corresponding to 225 trees ha−1 (Figure S1). Erythrina, a fast-growing leguminous tree was the predominant tree species in the net plots, making up 6 out of 13 trees.
The cacao trees and bananas were pruned two to three times per year between February and August. The AFS tree pruning was performed once a year at the end of the rainy season (August/September) (Schneider et al., Reference Schneider, Andres, Trujillo, Alcon, Amurrio, Perez, Weibel and Milz2016). The pruning residues of the cacao, banana and AFS trees were roughly chopped and left in the plots in all production systems. In the MonoConv systems, the cacao trees were fertilised with 150 kg ha−1 year−1 Blaukorn (BASF, Germany, 12–8–16–3 N–P2O5–K2O–MgO). In the MonoOrg systems, locally prepared compost was applied (8 Mg ha−1, 24–17–20–18 kg ha−1 N–P–K–Mg). The AFS received half of the amount of fertiliser used in the MCS. Mineral fertiliser was applied twice a year at the beginning and end of the rainy season, while compost was applied once a year at the onset of the rainy season (Schneider et al., Reference Schneider, Andres, Trujillo, Alcon, Amurrio, Perez, Weibel and Milz2016).
Aboveground plant biomass (AGB) and root biomass (RB)
The aboveground plant biomass (AGB) was estimated in 2011 and in 2015, before pruning was carried out at the end of the dry season. The inventory method mainly followed the recommendations of Pearson et al. (Reference Pearson, Walker and Brown2005). The AGB cacao trees, AFS trees and palms, bananas, herbal and shrub layer, litter layer and deadwood were quantified in the net plots. The AGB (kg tree−1) of each single tree was estimated non-destructively by allometric equations according to Andrade et al. (Reference Andrade, Segura, Somarriba and Villalobos2008). Cacao tree diameter (d, in cm) was measured at a height of 30 cm, and AGB was estimated from the allometric equation developed by Andrade et al. (Reference Andrade, Segura, Somarriba and Villalobos2008) (Eq. 1). For plants with stems that ramified below 30 cm, the diameters of all ramifications were measured, and a generalised stem diameter was calculated using Eq. (2) after MacDicken et al. (Reference MacDicken, Wolf and Briscoe1991). In the AFS, the tree diameters at breast height (dbh, 130 cm) and the heights (h, in m) of all AFS trees and palms were measured. The AGB of Inga spp. was calculated using Eq. (3), whereas Eq. (4) was applied to all other AFS trees (Segura et al., Reference Segura, Kanninen and Suárez2006). The AGB of the palm Euterpe precatoria (Mart.) was calculated by the use of Eq. (5) for asai palms, after Pearson et al. (Reference Pearson, Walker and Brown2005). The biomass of bananas was estimated using Eq. (6) after van Noordwijk et al. (Reference van Noordwijk, Rahayu, Hairiah, Wulan, Farida and Verbist2002). The AGB estimates of the single trees per net plot were summed and the AGB in (Mg ha−1) was calculated for each component (i.e., cacao trees, AFS trees, bananas and palms).
In addition, within each net plot, destructive sampling of the herb, shrub and litter layers was carried out at four points along a diagonal transect (33.9 m) through the net plot. Litter (dead plant material above the mineral soil with a diameter <10 cm), and herbs and other living plants with a stem diameter <10 cm were collected separately inside a 50 × 50 cm square frame. All collected plant material was weighed, oven dried at 70 °C until constant weight and weighed again for the dry weight. The data from the four points along this transect were averaged. Additionally, all deadwood with a diameter >10 cm that occurred along the transect was measured (Pearson et al., Reference Pearson, Walker and Brown2005).
The root biomass (RB) of the cacao trees was indirectly estimated based on the AGB. Norgrove and Hauser (Reference Norgrove and Hauser2013) reported that AGB of cacao trees contributed 87% to the total plant biomass. The contribution of roots to the total plant biomass included 5% from the taproot and 8% from other roots. These proportions were obtained using a biomass partitioning model for cacao developed by Zuidema et al. (Reference Zuidema, Leffelaar, Gerritsma, Mommer and Anten2005). The RB of the AFS trees was estimated using Eq. (7) after Cairns et al. (Reference Cairns, Brown, Helmer and Baumgardner1997).
AGB and RB were converted into aboveground carbon (AGC) and root carbon (RC) stocks through multiplication by 0.5 (Pearson et al., Reference Pearson, Walker and Brown2005). The same factor was used for converting the AGB of the herbs, shrubs and the litter layer into C stocks.
Litterfall and pruning residues
The amounts of litterfall and pruning residues differ between cacao varieties (Daymond et al., Reference Daymond, Tricker and Hadley2011) and AFS tree species. As a complete survey of all trees was not feasible within this study, one cacao variety, the Bolivian clone IIa-22, was selected. This local cultivar performs well in both MCS and AFS (Schneider et al., Reference Schneider, Andres, Trujillo, Alcon, Amurrio, Perez, Weibel and Milz2016). In addition, the amounts of litterfall and pruning residues were quantified for Erythrina, as it was the predominant AFS tree species in the net plots, even though other AFS trees were also pruned. Erythrina trees can be significantly pruned and will still regrow very quickly over the rainy season. The cacao trees in the AFS were randomly selected, whereby only cacao trees within the area of influence of Erythrina trees were included.
Circular litter traps with an area of 0.25 m2 were installed at a distance of 0.5 m from stems and 0.5 m above the soil surface. In the MCS, the traps were placed under three cacao trees per net plot, while in the AFS, two traps were placed under cacao trees and two under Erythrina trees. Litter was collected monthly from October 2014 until September 2015. The litter of each trap was dried separately for 72 h at 70 °C and weighed. Annual litterfall per hectare was calculated based on the degree of canopy coverage in the different production systems. Canopy coverage was calculated by analysing 24 hemispherical photographs per plot, which were taken before (July) and after the main pruning of the cacao and AFS trees (October) in 2014 and 2015. Photographs were taken at 1.3 m aboveground along a V-shaped transect within each net plot. Data were computed for three degrees of coverage occurring over the course of a year, i.e., maximum coverage (before pruning), minimum coverage (after pruning) and an intermediate stage of coverage. Litterfall in the AFS was not separated into cacao and AFS tree litter. Litter biomass was converted into amounts of C through multiplication by a factor of 0.5.
The pruning residues of cacao and AFS trees were recorded separately during the main pruning event before the start of the rainy season. For this purpose, the pruning residues of two randomly selected cacao trees (clone IIa-22) per net plot were collected. In addition, the pruning residues of two Erythrina trees were collected in each AFS net plot. The pruning residues were divided into leaves and branches, the fresh biomass of which was determined. A subsample of each fraction was dried for 72 h at 70 °C to estimate the dry mass of the pruning residues. The amount of AFS tree pruning residues per hectare was calculated based on 13 trees per net plot, using the amounts of pruning residues per tree obtained for Erythrina. This approach includes a simplification of the production system, as only 6 out of 13 AFS trees were Erythrina trees. The C and N contents of the pruning residues were analysed by use of a CHN analyser (PerkinElmer). The amounts of pruning residues were converted into amounts of C by multiplying their biomass by their measured C content.
Data analysis
All statistical analyses were performed by the use of R (3.2.3) and RStudio (0.99.486) software (R Core Team 2015) using the package ‘lme4’ (Bates et al., Reference Bates, Mächler, Bolker and Walker2015) and ‘lmerTest’ (Kuznetsova et al., Reference Kuznetsova, Brockhoff and Christensen2015). All datasets were tested for a normal distribution using the Shapiro–Wilk test. In case of a non-normal distribution, the skew of the respective dataset was calculated, and the dataset was transformed as recommended by Webster (Reference Webster2001). Linear mixed-effects models were applied for AGC stocks, litterfall and pruning. Crop diversity and management practices entered the model as fixed factors, whereas the repetition blocks were considered as random factors. Differences between the years were determined by including the factor year as a fixed factor in the linear mixed-effects models. An ANOVA was carried out for each model. Datasets showing differences between management practices or crop diversity were subjected to pairwise comparisons of least square means (LSMeans) using the ‘lsmeans’ function of the ‘lsmeans’ package (Lenth and Hervé, Reference Lenth and Hervé2015). The significance level was set to α = 0.05. The R package ‘ggplot2’ was used for producing graphs (Wickham, Reference Wickham2009).
RESULTS
Aboveground carbon (AGC) and root carbon (RC) stocks
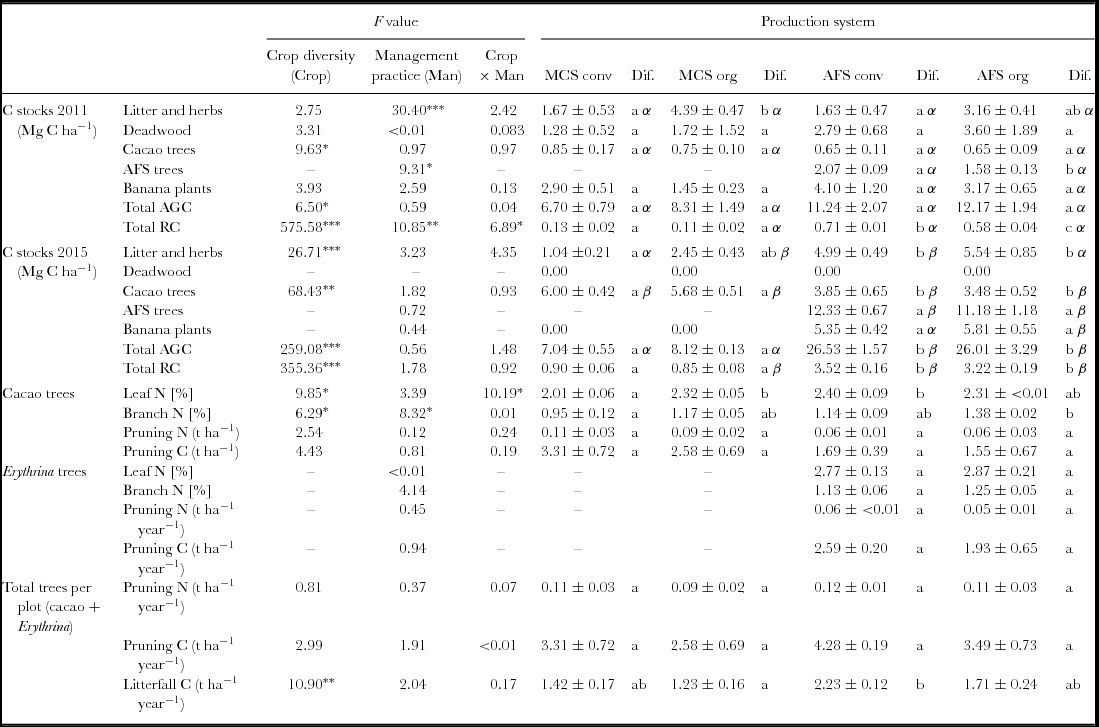
In 2011, 3 years after the cacao trees were planted, the AGC in the MCS ranged from 6.7 to 8.3 Mg C ha−1, while the total AGC (of all plants) in the AFS was more than 11 Mg C ha−1 irrespective of the management style (Table 1). However, AGC of cacao trees in the MCS exceeded that of cacao trees in the AFS, where the AGC stocks of cacao trees contributed only 5–12% to the total AGC. Another four years later, in 2015, the amounts of total AGC in the AFS were generally greater than in 2011 (P < 0.001). The overall trends showed relative differences between the management variants similar to those in 2011, which had greater amounts of AGC in the AFS (26 Mg C ha−1) than in the MCS (7–8 Mg C ha−1) (Table 1). The AGC stocks of the AFS trees showed a six-fold increase from 2011 to 2015 both under conventional and organic management (P < 0.001). The AGC contribution of AFS trees to total AGC in 2015 was 11 Mg C ha−1 (43%) in the organic and 12 Mg C ha−1 (47%) in the conventional AFS regimes (Figure 1). In MCS, cacao trees in 2015 contributed 85% to the total AGC under conventional management and 70% to total AGC under organic management. In the AFS, cacao trees contributed only 14% of the total AGC. The C stocks of cacao trees in MCS (~6 Mg C ha−1) exceeded those of cacao trees in AFS (3–4 Mg C ha−1). The total AGC in MCS did not change significantly between 2011 and 2015, while total AGC in AFS increased. No significant differences between organic and conventional management with respect to total AGC were observed. In 2011, the cacao tree RC ranged between 0.13 Mg C ha−1 (in conventional MCS) and 0.10 Mg C ha−1 (in organic AFS), not yet showing a significant difference. However, between 2011 and 2015, the amounts of cacao tree RC increased up to eight times in MCS and up to six times in AFS. The RC of all trees in the AFS increased likewise (Table 1).
Table 1. Results of the linear mixed models and mean ± SE for aboveground (AGC) and root (RC) carbon stocks (years 2011 and 2015), Carbon and Nitrogen in pruning residues (2015), and litterfall for cacao and erythrina trees (2015) for four different cacao production systems.

MCS conv = Monoculture systems under conventional management, MCS org = Monoculture systems under organic management, AFS conv = Agroforestry systems under conventional management, AFS org = Agroforestry systems under organic management. Dif. = Different lower case Latin letters within lines indicate significant differences (P ≤ 0.05) between the production systems; lower case Greek letters, within columns, indicate significant differences (P ≤ 0.05) between the year 2011 and 2015 within a production system; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
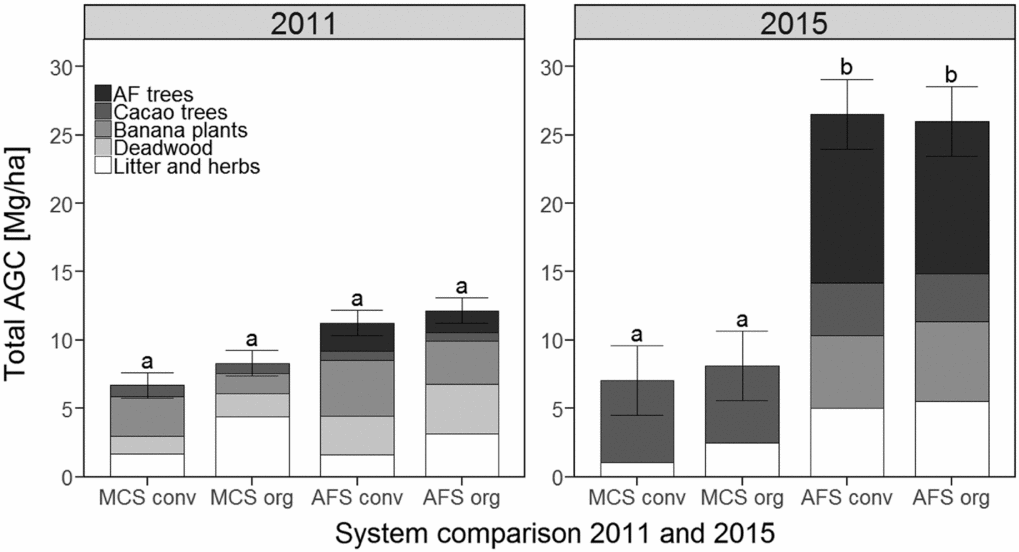
Figure 1. Aboveground carbon (AGC) of the four different cacao production systems for 2011 and 2015. Cumulative bars represent the mean total AGC for the different summed carbon stocks (litter and herbs, deadwood, banana plants, cacao trees, AFS trees). MonoConv = Monoculture systems under conventional management, MonoOrg = Monoculture systems under organic management, AFConv = Agroforestry systems under conventional management, AFOrg = Agroforestry systems under organic management. Means and standard errors presented are non-transformed data. Lower case latin letters indicate significant differences (P ≤ 0.05) between the production systems.
Annual litterfall
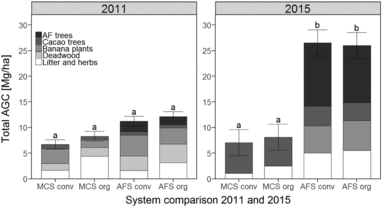
The temporal pattern of litterfall over the year was similar in all production systems. The monthly maximum litterfall was at the end of the dry season in August, while the smallest amounts of litter were recorded during the rainy season in December and January. The total annual litterfall of the AFS significantly exceeded that of the MCS (P = 0.01) (Figure 2a). Conventional and organic management had no effect on the total annual litterfall (P = 0.2). However, the recorded litterfall did not include chopped branches, twigs and husks produced during routine cacao tree phytosanitary treatment, as these were distributed around the trees and not caught in the litter traps. Thus, these components were recorded as constituents of the litter layer but not considered in the annual litterfall.
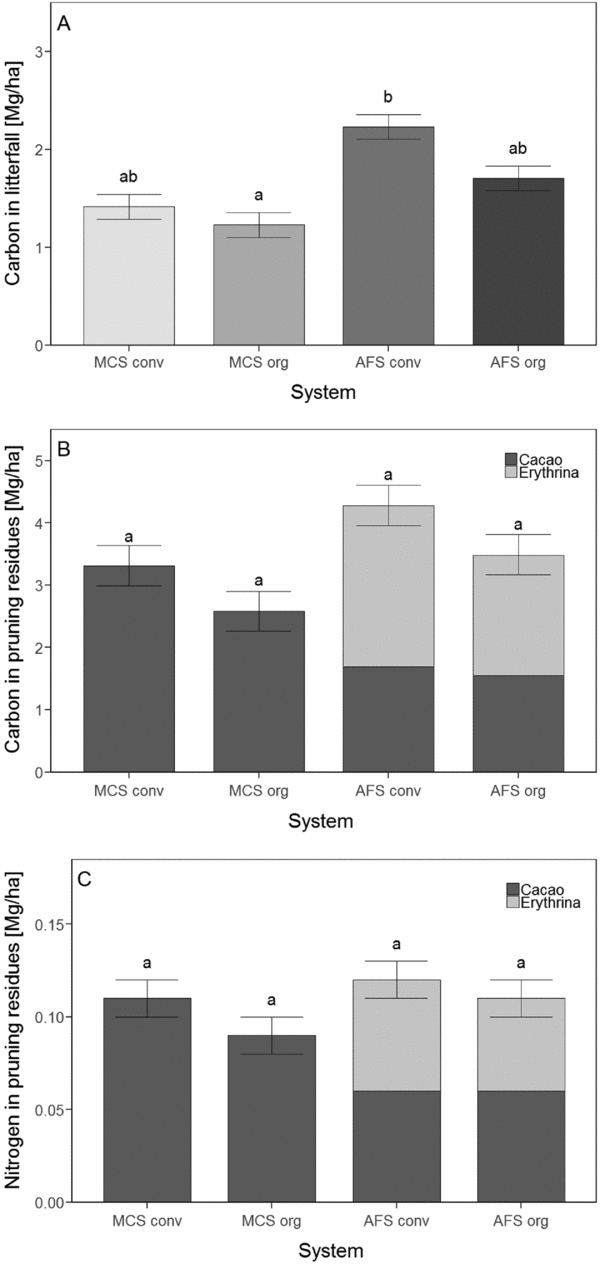
Figure 2. (a) Carbon contents in total annual litterfall, (b) carbon and (c) nitrogen contents in pruning residues of four different cacao production systems. Bars show the summed pruning residues of cacao and Erythrina trees. MonoConv = Monoculture systems under conventional management, MonoOrg = Monoculture systems under organic management, AFConv = Agroforestry systems under conventional management, AFOrg = Agroforestry systems under organic management. Means and standard errors presented are non-transformed data. Lower case latin letters indicate significant differences (P ≤ 0.05) between the production systems.
Pruning residues
The C in the pruning residues of the AFS contained 3.5 Mg C ha−1 under organic management and 4.3 Mg C ha−1 under conventional management. Pruning residues in the MCS comprised only 2.6 Mg C ha−1 under organic management and 3.3 Mg C ha−1 under conventional management (Figure 2b; Table 1). The increased C stocks in pruning residues of AFS primarily resulted from the abundant pruning residues of the Erythrina trees (~60%). The C stocks of the pruning residues of cacao plants were slightly lower in the AFS than in the MCS (Table 1).
Cacao leaves showed higher N contents in AFS than in MCS (P = 0.01). The significant interaction between crop diversity and management showed that there were no differences in the N content between AFS and MCS under organic management, but N content was higher in the AFS compared with the MCS when conventionally managed (Table 1). N contents in cacao branches were higher under organic than under conventional management (P < 0.02), and cacao branches in AFS had higher N contents than cacao branches in MCS (P < 0.04). Amounts of N in cacao pruning residues were 0.06 Mg N ha−1 in the AFS and approximately 0.10 Mg N ha−1 in the MCS, but this difference was not significant (P = 0.11). The amounts of N in pruning residues of Erythrina trees (~0.06 Mg N ha−1) did not differ between conventional and organic management (Table 1). Similarly, the amount of N in the total AFS pruning residues did not differ between conventional and organic management (~0.1 Mg N ha−1, Figure 2c).
DISCUSSION
Aboveground carbon (AGC) in cacao monoculture systems (MCS) and agroforestry systems (AFS)
Seven years after establishing the FiBL long-term experiment, the cacao AFS comprised significantly more AGC (21 Mg C ha−1) than the cacao MCS (Table 1; Figure 1). This difference is mainly due to the amounts of C stored in the AFS trees. However, the AGC of the AFS was still only one-third of the tree-AGC of trees in the surrounding natural forests (~ 65 Mg C ha−1; Yaffar, Reference Yaffar2014). Nevertheless, it had only been 7 years since the establishment of the experimental plots, and a further increase in AGC can be expected, as total amounts of AGC up to 50 Mg C ha−1 have been reported from fully developed AFS in the same region (Jacobi et al., Reference Jacobi, Andres, Schneider, Pillco, Calizaya and Rist2014).
The contribution of the AFS trees to the total AGC was comparable to that reported from 10-year-old AFS with E. poeppigiana (contributing 18.95 Mg C ha−1, corresponding to 43% of total AGC) in Costa Rica (Beer et al., Reference Beer, Bonnemann, Chavez, Fassbender, Imbach and Martel1990). In contrast, much greater contributions of AFS trees to AGC have been reported for trees in multi-shade cacao systems in Indonesia (39 Mg C ha−1, 81% of total AGC) (Abou Rajab et al., Reference Abou Rajab, Leuschner, Barus, Tjoa and Hertel2016), and even greater contributions of AFS trees to AGC were observed in mature shaded cacao systems in Cameroon (contributing >90 Mg C ha−1, corresponding to ~90% of total AGC) (Norgrove and Hauser, Reference Norgrove and Hauser2013; Saj et al., Reference Saj, Durot, Mvondo Sakouma, Tayo Gamo and Avana-Tientcheu2017).
In our study in Bolivia, the C stocks of cacao tree biomass increased more rapidly in MCS than in AFS (Figure 1). Three years after the experimental plots were established, the biomass of cacao trees in MCS exceeded that of cacao trees in AFS by up to 25%. After 7 years, the AGC stocks of cacao trees in MCS exceeded those of cacao trees in AFS 1.5 times. Thus, the difference in biomass between the cacao trees in MCS and AFS considerably increased from 2011 to 2015. Enhanced increases in cacao tree biomass under full-sun conditions have also been reported by Tscharntke et al. (Reference Tscharntke, Clough, Bhagwat, Buchori, Faust, Hertel, Hölscher, Juhrbandt, Kessler, Perfecto, Scherber, Schroth, Verldkamp and Wanger2011). Comparatively low growth rates of the cacao trees in the Bolivian trial may explain the relatively lower cacao yield found in the AFS compared to the MCS (Armengot et al., Reference Armengot, Barbieri, Andres, Milz and Schneider2016). However, Abou Rajab et al. (Reference Abou Rajab, Leuschner, Barus, Tjoa and Hertel2016) showed that cacao yields do not necessarily decrease under shading. In our study, the higher tree density and shade level of approximately 50% (Schneider et al., Reference Schneider, Andres, Trujillo, Alcon, Amurrio, Perez, Weibel and Milz2016), in the AFS compared to the MCS, may explain the slower growth rates in biomass of the cacao trees. In detail, the MCS comprised 625 cacao trees ha−1, while the AFS comprised a total of 850 trees per hectare, plus an additional 625 banana plants ha−1. This setting might have reduced cacao tree growth due to limited insolation and inter-species competition for nutrients. The importance of light intensity for cacao tree growth has been emphasised by Wood and Lass (Reference Wood and Lass1985). While moderate shade is important for sensitive young cacao trees, cacao tree biomass production rates show a general increase with light intensity of up to 25% (Abou Rajab et al., Reference Abou Rajab, Leuschner, Barus, Tjoa and Hertel2016; Mortimer et al., Reference Mortimer, Saj and David2017; Triadiati et al., Reference Tjitrosemito, Guhardja, Qayim and Leuschner2007). Since it is known that cacao trees have a low light saturation level of approximately 400 μmol m−2 s−1 (Daymond et al., Reference Daymond, Tricker and Hadley2011), shading between 40 and 70% is considered optimal (Beer et al., Reference Beer, Muschler, Kass and Somarriba1998). Increased biomass of 8-year-old cacao trees under moderate shade (30 AFS trees ha−1) relative to that of trees in MCS was reported by Isaac et al. (Reference Isaac, Timmer and Quashie-Sam2007). To provide sufficient insolation for optimal cocoa tree growth, it is therefore advisable to establish a pruning management regime that is adapted to local climatic conditions as well as to the flowering and growth phases of the cacao trees. AFS comprise diverse kinds of trees, including easy-to-prune and quickly growing trees, enabling the most flexible adaptability to a wide range of situations (Schroth et al., Reference Schroth, Lehmann, Rodrigues, Barros and Macêdo2001; Tscharntke et al., Reference Tscharntke, Clough, Bhagwat, Buchori, Faust, Hertel, Hölscher, Juhrbandt, Kessler, Perfecto, Scherber, Schroth, Verldkamp and Wanger2011). In addition, when selecting AFS trees, compatibility with respect to nutrient competition must be ensured, which may otherwise limit the growth and productivity of cacao trees. The overall effect of various AFS trees on cacao trees also depends on the general availability of water and soil nutrients, and on competition for light (Beer et al., Reference Beer, Muschler, Kass and Somarriba1998; Mortimer et al., Reference Mortimer, Saj and David2017; Saj et al., Reference Saj, Durot, Mvondo Sakouma, Tayo Gamo and Avana-Tientcheu2017; Tscharntke et al., Reference Tscharntke, Clough, Bhagwat, Buchori, Faust, Hertel, Hölscher, Juhrbandt, Kessler, Perfecto, Scherber, Schroth, Verldkamp and Wanger2011). Furthermore, various studies have shown that AFS trees enhance the overall nutrient cycling in AFS (Isaac et al., Reference Isaac, Timmer and Quashie-Sam2007; Saj et al., Reference Saj, Durot, Mvondo Sakouma, Tayo Gamo and Avana-Tientcheu2017; Tscharntke et al., Reference Tscharntke, Clough, Bhagwat, Buchori, Faust, Hertel, Hölscher, Juhrbandt, Kessler, Perfecto, Scherber, Schroth, Verldkamp and Wanger2011).
In comparable studies, the AGC stocks of cacao trees in AFS ranged from 4 Mg C ha−1 to almost 20 Mg C ha−1 (Abou Rajab et al., Reference Abou Rajab, Leuschner, Barus, Tjoa and Hertel2016; Beer et al., Reference Beer, Bonnemann, Chavez, Fassbender, Imbach and Martel1990; Isaac et al., Reference Isaac, Timmer and Quashie-Sam2007; Saj et al., Reference Saj, Durot, Mvondo Sakouma, Tayo Gamo and Avana-Tientcheu2017). This wide range of reported AGC stocks is due to differences in tree composition, age, stem density and cacao variety. Moreover, the studies were carried out in various regions with different conditions, in terms of climate, soil properties and water and nutrient supply, making direct comparisons difficult. For instance, in Indonesia, the AGC of 14- to 22-year-old cacao MCS with a total tree density of approximately 892 trees ha−1 was 7.7 Mg C ha−1 (Abou Rajab et al., Reference Abou Rajab, Leuschner, Barus, Tjoa and Hertel2016), whereas the AGC in 8-year-old cacao MCS in Ghana was 11.4 Mg C ha−1 (Isaac et al., Reference Isaac, Timmer and Quashie-Sam2007). This amount of AGC is almost twice as large as that of the cacao MCS in the current FiBL experiment in Bolivia, which can be explained by different cacao tree densities (1100 trees ha−1 in the study of Isaac et al. (Reference Isaac, Timmer and Quashie-Sam2007) versus 625 trees ha−1 in the FiBL trial in Bolivia). Considering these differences in tree densities results in similar amounts of AGC stored per cacao tree.
C and N fluxes through litterfall and pruning residues in cacao monoculture systems (MCS) and agroforestry systems (AFS)
The amount of C in the total litterfall of the cacao AFS (organic 1.7 Mg C ha−1 and conventional 2.2 Mg C ha−1) significantly exceeded those of the cacao MCS (organic 1.2 Mg C ha−1 and conventional 1.4 Mg C ha−1) (Table 1; Figure 2a). This remarkable difference can be explained by the higher total tree density (sum of cacao trees and AFS trees). The amount of C in the litter was within the lower range of data reported in the literature for plantations of a similar age (Abou Rajab et al., Reference Abou Rajab, Leuschner, Barus, Tjoa and Hertel2016; Beer et al., Reference Beer, Bonnemann, Chavez, Fassbender, Imbach and Martel1990; Dawoe et al., Reference Dawoe, Isaac and Quashie-Sam2010). The amount of C in litter reported from other studies ranges from 2.4 Mg C ha−1 in Indonesia to 5.2 Mg C ha−1 in 30-year-old AFS in Ghana. Litterfall in AFS in Ghana increased with stand age and reached natural forest levels at an AFS age of 15 years (Dawoe et al., Reference Dawoe, Isaac and Quashie-Sam2010). C in the litter of a natural forest in our study region in Bolivia, over a nine-month period (April to December), amounted to almost 5.5 Mg C ha−1 (Yaffar, Reference Yaffar2014), which is more than twice the amount of C in the litter of the AFS in the trial.
In this study, the amounts of C in Erythrina pruning residues (2.6 to 4.3 Mg C ha−1) were twice as high as those found in the litter (Figure 2b). The pruning residues in this experiment may have been overestimated, since they were calculated based only on Erythrina trees that were pruned much more intensively than other timber and fruit trees that were included in the AFS. Nevertheless, our results match well with data reported for AFS with E. poeppigiana in Costa Rica (Beer et al., Reference Beer, Bonnemann, Chavez, Fassbender, Imbach and Martel1990). The pruning results of our study thus apply to simple AFS, including those with fast-growing legume trees. However, various cacao AFS that are not pruned exist around the world (Nair et al., Reference Nair, Viswanath and Lubina2017). AFS with a more diverse AFS tree composition, in which only some of the tree species are pruned, likely have a greater permanent tree biomass than systems with heavily pruned AFS trees, while C and N fluxes are particularly enhanced in strongly pruned AFS. Therefore, fast-growing, easy-to-prune AFS trees may also be used to improve the nutrient supply for young AFS that are installed on poor soils and cleared forest areas. However, until now little attention has been paid to the role of pruning residues from different AFS trees for C and nutrient fluxes in cacao plantations. In some studies, the pruning residues were either included as part of the litterfall or assumed to be negligible (Abou Rajab et al., Reference Abou Rajab, Leuschner, Barus, Tjoa and Hertel2016) partly because cacao plantations are not always systematically established with shade trees, and if so, this does not necessarily include a purposeful introduction of certain tree species. Many different types of local and indigenous AFS exist, including cultivation of cacao trees under remaining natural forest trees or tree species that cannot be pruned strongly enough to provide optimal light conditions for the cacao trees (Nair et al., Reference Nair, Viswanath and Lubina2017).
The relative proportion of the pruning residues of cacao tree biomass was similar in all systems (45–55%). The greater biomass of the cacao trees in the MCS corresponded to a higher proportion of cacao tree pruning residues compared AFS's tree pruning residues; however, the lower amounts of cacao tree pruning residues in the AFS were compensated by the other AFS trees.
Pruning is important in AFS, for ensuring that sufficient sunlight reaches the cacao trees, for pest and disease control and for the prevention of soil erosion by a protective cover of pruning residues (Johns, Reference Johns1999; Norgrove and Hauser, Reference Norgrove and Hauser2013; Zuidema et al., Reference Zuidema, Leffelaar, Gerritsma, Mommer and Anten2005). Data in the literature indicate that the C in pruning residues and litterfall from leguminous AFS trees varies from 1.7 to 7 Mg C ha−1 a−1. The associated amounts of N range from 60 to 340 kg ha−1 a−1 (Beer, Reference Beer1988). In the FiBL trial in Bolivia, the tree-soil N fluxes via litterfall and pruning residues amounted to 90–120 kg N ha−1 a−1, both in MCS and AFS (Table 1). The N contents of the pruning residues of cacao clone IIa–22 (2.0 to 2.4% N) were similar to those reported from a cacao experiment by Kähkölä et al. (Reference Kähkölä, Nygren, Leblanc, Pennanen and Pietikäinen2012), in which the cacao trees were inoculated with Inga edulis (Mart.). The pruning residues in that experiment contained 2.6% N for inoculated and 2.1% N for non-inoculated cacao trees. Comparably high leaf N contents (2.4 to 3.2% N) were also reported by Daymond et al. (Reference Daymond, Tricker and Hadley2011) for different cacao clones used in a greenhouse experiment, whereby the N contents depended on the clones. Triadiati et al. (Reference Tjitrosemito, Guhardja, Qayim and Leuschner2007) reported lower N contents for cacao leaves in different cacao AFS with varying canopy coverages (1.2 to 1.5% N). They concluded that increased light intensity leads to enhanced biomass production, and that the presence of N-fixing trees leads to increased soil N contents, from which the cacao trees also benefit. In the FiBL trial, the conventionally managed MCS showed the lowest leaf N contents (2.0% N). All the other production systems in which leguminous plants were included exhibited somewhat higher leaf N contents that were very similar to each other (2.3% for both MCS and AFS under organic management, and 2.4% for AFS under conventional management) (Table 1). Branches of cacao clone IIa–22, in both AFS and in organically managed MCS, exhibited higher N contents than those in conventionally managed MCS. These results suggest that under the conditions of this study, N-fixing Erythrina trees in the AFS, and likewise leguminous cover crops in organically managed systems, lead to increased soil N contents and uptake by the cacao trees. Kähkölä et al. (Reference Kähkölä, Nygren, Leblanc, Pennanen and Pietikäinen2012) found that root litter of the leguminous tree Inga edulis (Mart.) represents a more important and more quickly available N source for cacao trees than the leaf litter of the same tree. However, the N contribution of leaf litter and root litter from leguminous plants in organically managed crop production systems to increases in observed soil N needs further investigation. The beneficial effect of N-fixing leguminous trees in AFS is particularly relevant if N fertiliser input is low, and soils are poor in N (Saj et al., Reference Saj, Durot, Mvondo Sakouma, Tayo Gamo and Avana-Tientcheu2017; Schroth et al., Reference Schroth, Lehmann, Rodrigues, Barros and Macêdo2001). However, including leguminous plants in crop production systems can also lead to enhanced acidification of the rhizosphere. Moreover, Rhizobium requires large amounts of P and may thereby lead to a decrease in P availability for cacao trees (Mortimer et al., Reference Mortimer, Saj and David2017; Yan et al., Reference Yan, Schubert and Mengel1996). These decreases in pH and P availability can be mitigated by ensuring a regular return of biomass through pruning (Yan et al., Reference Yan, Schubert and Mengel1996).
In the FiBL long-term field experiment, the total plant–soil N fluxes through pruning residues exceeded the N inputs through N fertilisation up to 10 times. In the cacao MCS, the N returns through pruning residues derived only from the cacao trees, whereas in the AFS, leguminous trees contributed 50% of the plant–soil N flux through pruning residues, hence generating a considerable N gain for the system. Therefore, the system's N cycling is largely influenced by the composition of AFS trees and by the pruning practices (Schroth et al., Reference Schroth, Lehmann, Rodrigues, Barros and Macêdo2001). The increased N availability in AFS from leguminous trees in turn leads to an increase in biomass production (Beer, Reference Beer1988). Moreover, Beer et al. (Reference Beer, Bonnemann, Chavez, Fassbender, Imbach and Martel1990) observed an increase of SOM contents by 21% under pruned Erythrina trees over a 10-year period.
CONCLUSIONS
The systematic study of C sequestration and C fluxes in conventionally and organically managed cacao AFS and MCS of the FiBL long-term field experiment in Bolivia confirmed that cacao AFS have a greater potential for C sequestration compared to cacao MCS. The experimental setup allowed for the quantification of these differences and for the identification of their underlying causes, namely, the greater biomass and higher tree density of AFS, especially if fast-growing leguminous trees that can be heavily pruned are included. Pruning of leguminous AFS trees also enhances C and N cycling in the soil–plant system and ensures long-term accumulation of C and N in AFS. This may in turn also lead to an increase in SOM contents over time. In addition, this study showed that leguminous trees and cover crops in organically managed systems can improve the N availability for all AFS plants within a short period of time, as indicated by increased N contents in cacao leaves and branches. Tree density, pruning practices and corresponding sunlight intensity were identified as important factors for the growth of cacao trees. In this study, no considerable advantages were observed for conventional over organic management with respect to AGB. We concluded that AFS, especially under organic management, may be productive systems, with the additional benefits of increased C sequestration and enhanced N supply compared to cacao MCS.
Acknowledgements
The authors thank the Bolivian colleagues of the research station in Sara Ana and the Instituto de Ecologia of the UMSA in La Paz for support, and Helga Jacobi for her contribution in the frame of her BSc thesis. We are also grateful to the anonymous reviewers for their helpful comments and suggestions. This work was supported through a PhD scholarship of the first author by the German Exchange Service (DAAD), and a start-up funding for postdoctoral researchers by the Georg-August-University Göttingen.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S001447971800011X