Introduction
Dry, oligotrophic, open-land habitats, such as calcareous grassland, sand grassland, steppe habitats, heathland and inland dunes are hotspots of biodiversity in Central and northern Europe (Muller et al., Reference Muller, Dutoit, Alard and Grévilliot1998; Webb, Reference Webb1998; Pywell et al., Reference Pywell, Bullock, Hopkins, Walker, Sparks, Burke and Peel2002; Bieringer & Zulka, Reference Bieringer and Zulka2003; Maes & Bonte, Reference Maes and Bonte2006; Riksen et al., Reference Riksen, Ketner-Oostra, van Turnhout, Nijssen, Goossens, Jungerius and Spaan2006). They support a rich flora and invertebrate fauna, including many threatened species. Since the end of the 19th century, these xeric habitats have been destroyed, degraded and fragmented on a large scale as a result of changes in land use, agricultural intensification, abandonment and plantation of coniferous forest on low yield sites (Muller et al., Reference Muller, Dutoit, Alard and Grévilliot1998). In addition, eutrophication due to increased nitrogen deposition promotes succession on sparsely vegetated sites (Bakker & Berendse, Reference Bakker and Berendse1999). The decline of dry oligotrophic habitats is accompanied by local extinction of many xerophilous species.
While the conservation and management of xeric open-land habitats has received much attention, their boundaries with other habitat types (ecotones) are often disregarded (Ries et al., Reference Ries, Fletcher, Battin and Sisk2004). One natural ecotone associated with xeric habitats is the transition zone between these habitats and forests. Shading by adjacent forest is sometimes seen as a negative factor for the biodiversity within open habitats (e.g. Kotze & Samways, Reference Kotze and Samways2001; Bieringer & Zulka, Reference Bieringer and Zulka2003; Hochkirch et al., Reference Hochkirch, Witzenberger, Teerling and Niemeyer2007a), and edges are also often considered as low-quality sites for forest species (e.g. Mills, Reference Mills1995; Watson et al., Reference Watson, Whittaker and Dawson2004; Fletcher, Reference Fletcher2005; Guirado et al., Reference Guirado, Pino and Roda2006). Moreover, forest boundaries are believed to promote invasive species (e.g. Honnay et al., Reference Honnay, Verheyen and Hermy2002; Guirado et al., Reference Guirado, Pino and Roda2006) and are, therefore, sometimes seen as undesirable landscape features (Ries et al., Reference Ries, Fletcher, Battin and Sisk2004). For these reasons, the development of natural forest boundaries is often disregarded in nature conservation. Nevertheless, many studies show that diversity peaks in this zone (e.g. Lay, Reference Lay1938; Bedford & Usher, Reference Bedford and Usher1994; Downie et al., Reference Downie, Coulson and Butterfield1996; Magura et al., Reference Magura, Tóthmérész and Molnár2001; Theuerkauf & Rouys, Reference Theuerkauf and Rouys2006). Some endangered species are associated with pine forest edges in parts of Europe, such as the woodlark, Lullula arborea (Bowden, Reference Bowden1990) or the Scotch argus, Erebia aethiops (Weidemann, Reference Weidemann1995). The transition zone between pine forest and xeric sand habitats is naturally sparsely vegetated and characterized by a high frequency of bare patches. Hence, it differs substantially from clear-cut canopy dripline edges of modern pine plantations. The structural changes in these habitats due to land-use changes impose a considerable threat to species adapted to natural ecotones. Current forestry practices in Germany are focused on the conversion of single-species coniferous plantations to more natural deciduous forests with a rich understorey (Zerbe, Reference Zerbe2002). This process might impose a severe threat to species confined to pine forest ecotones, since such habitats are difficult to protect without administrative support (Rauh, Reference Rauh, Schlumprecht and Waeber2003).
One species confined to the transition zone between pine forests and xeric habitats, such as inland dunes, throughout much of its European range is the heath grasshopper, Chorthippus vagans (Eversmann, 1848). This insect has a fragmented distribution in Europe and is red listed in Germany (Ingrisch & Köhler, Reference Ingrisch, Köhler, Binot, Bless, Boye, Gruttke and Pretscher1998), UK (Marshall & Haes, Reference Marshall and Haes1990), Switzerland (Thorens & Nadig, Reference Thorens and Nadig1997), the Netherlands (Kleukers et al., Reference Kleukers, v. Nieukerken, Odé, Willemse and v. Wingerden1997), Belgium (Decleer et al., Reference Decleer, Devriese, Hofmans, Lock, Barenbrug and Maes2000) and Luxembourg (Proess & Meyer, Reference Proess and Meyer2003). The decline of C. vagans is particularly well documented in Lower Saxony (Germany), where it has lost 50% of its populations during the last two decades (Brandt & Schäfer, Reference Brandt and Schäfer2004; S. Krause, 2006 unpublished data) and is listed as ‘endangered’ (Grein, Reference Grein2005). One of the largest populations of C. vagans in northern Germany was rediscovered in 2002 on a forested inland dune near lake ‘Steinhuder Meer’ near Hanover (Brandt, Reference Brandt2003; Brandt & Schäfer, Reference Brandt and Schäfer2004). In winter 2003/04, conservation measures were implemented in this habitat to improve the conditions for this threatened species. The forest was thinned out by logging larger deciduous trees and coppice at the southern slopes of the dune. Moreover, litter was removed in order to enhance the number of bare patches, and some taller Douglas-firs (Pseudotsuga menziesii) were logged.
Here, we evaluate the success of these management measures for the conservation of C. vagans. We studied changes in population size and distribution of this species within the managed area and examined dispersal distances and interchange between two subpopulations. In addition, we analyzed the microhabitat preferences of the species, to assess key factors influencing local distribution.
Methods
Study species and study site
Chorthippus vagans is a univoltine, polyphagous grasshopper species. Nymphs hatch in May and become adult in July and August (Kleukers et al., Reference Kleukers, v. Nieukerken, Odé, Willemse and v. Wingerden1997). Egg pods are laid 0.5–1.5 cm below the soil surface (Treiber, Reference Treiber and Detzel1998) and have the highest drought resistance among central European grasshoppers (Ingrisch, Reference Ingrisch1983). Chorthippus vagans occurs in xerothermic habitats with high radiation and little nocturnal cooling (Treiber, Reference Treiber and Detzel1998). It is mainly found at southern forest edges with adjacent sparsely vegetated sites, such as sand dunes. In addition, it seems to benefit from southern inclination of its habitat (Treiber, Reference Treiber and Detzel1998). Main threats to C. vagans are plantation of dense coniferous or mixed deciduous forests and succession promoted by eutrophication or abandonment, resulting in the establishment of trees, bushes, brambles or nettles degrading its habitat (Rauh, Reference Rauh, Schlumprecht and Waeber2003; Brandt & Schäfer, Reference Brandt and Schäfer2004).
The study site is a forested inland dune, situated ca. 30 km NW of Hanover at the northwestern edge of lake ‘Steinhuder Meer’ (Lower Saxony, Germany, fig. 1). It is forested with pines (Pinus sylvestris) and oaks (Quercus robur) and sustains two subpopulations of C. vagans. One is found at the eastern edge of the dune in the transition zone between the forest and an open sand dune with growth of Carex arenaria (‘sand dune’: SD). A second subpopulation inhabits the centre of the dune (‘grid’: G), which is more densely forested and partly shaded by an adjacent plantation of introduced Douglas-fir south of the site. In winter 2003/04, almost all larger deciduous trees and coppice (mainly Sorbus aucuparia and Frangula alnus) were removed from G, so that only larger pines (ca. 18 m) and some small oaks remained. The leaf litter was removed in large parts of the managed area to enlarge the amount of bare ground.

Fig. 1. Location of the study site in northwestern Germany (Lower Saxony).
Data collection
A 1500 m2 grid containing 60 grid cells (5×5 m) was established in July 2004, enclosing the complete G subpopulation (2003) and the managed area. The area was marked out by placing numbered wooden pegs at 5 m intervals throughout. To assess habitat characteristics, we calculated the abundance of several vegetation descriptors in each square in the summer of 2004. For each grid cell, we measured the size of homogeneous vegetation patches and estimated the cover of the following variables within each patch: bare ground, litter, grasses (Festuca ovina, Carex arenaria, Corynephorus canescens, Deschampsia flexuosa, Agrostis stolonifera), forbs and shrubs (Rumex acetosella, Rubus fruticosus agg.), mosses (Dicranum scoparium, Pseudoscleropodium purum, Pleurozium schreberi, Polytrichum formosum), conifers (Pinus sylvestris) and deciduous trees (Quercus robur, Frangula alnus, Sorbus aucuparia). From these values, we calculated the vegetation cover for each grid cell. Since shading is known to affect Orthoptera substantially (Bieringer & Zulka, Reference Bieringer and Zulka2003), we also estimated the shaded area in each grid cell at 03 August 2004 and 05 September 2005. We estimated the size of the shaded area in each grid cell every two hours between 12:00 and 16:00, which is the main period of activity of C. vagans (Brose et al., Reference Brose, Peschel and Klatt1999) and calculated the average of the three measures.
In 2004 and 2005, the abundance and distribution of C. vagans was regularly mapped on both sites (G, SD; 14 visits in 2004 and 15 in 2005; fig. 2). Since grasshoppers are difficult to detect during clouded conditions, the visits were mainly undertaken on sunny days. Each adult was marked on the pronotum with a non-toxic paint marker (Edding 780). Specimens from SD were marked with a different colour to allow a faster assessment of between-site dispersal. In 2004, each specimen was marked individually using the 1-2-4-7 system (Buchweitz & Walter, Reference Buchweitz and Walter1992). Sex and location were noted and the individual was released at the point of capture. Since it was not possible to establish a grid at site SD, the approximate location of the individuals was noted in five sections. In a preceding study (2003), the number of specimens was noted during three (SD) and four (G) visits, but no individual was marked (Brandt & Schäfer, Reference Brandt and Schäfer2004).

Fig. 2. Cumulative number of marked C. vagans adults in the grid (□, 2004; ●, 2005).
For the analysis of microhabitat preferences, we followed the method described by Gröning et al. (Reference Gröning, Krause and Hochkirch2007a). Data were obtained in 2004 and 2005 at the exact location of undisturbed individuals during their activity period (from 10:00 to 17:30). Recorded data included date, site, time, weather, sex and behaviour. Radiation was measured using a luxmeter, Elvos LM 1010, aligned horizontally above the insect. Temperature measurements were made with a digital infrared thermometer (Raytek MiniTempTM). Relative humidity was measured with a digital thermohygrometer (Lutron HAT 3004) one centimetre above the ground. The vegetation structure was estimated in a circle of 30 cm diameter surrounding the insect, including the cover of bare ground, fresh grasses, dry grasses, litter, forbs, mosses and trees, as well as the maximum vegetation height. In addition to the individual data, a corresponding control sample of all factors was measured at one metre distance from the location (in a random direction). This distance is easily attainable for the insects, but changes in vegetation structure and microclimate often occur on this micro-scale, allowing us to test for microhabitat preferences (Gröning et al., Reference Gröning, Krause and Hochkirch2007a; Hochkirch et al., Reference Hochkirch, Gröning and Krause2007b).
Data analysis
The correlation of vegetation parameters in the grid was analyzed using non-metric multidimensional scaling (NMDS). We employed the function ‘metaMDS’, as implemented in the community ecology package vegan 1.6–10 for R (Oksanen et al., Reference Oksanen, Kindt and O'Hara2005). This function first transforms the data by Wisconsin double standardization and applies Bray-Curtis dissimilarities as a measure of ecological distance. We plotted the distribution of C. vagans at each census date onto the NMDS plots to assess the most important variables for the species, using the function ‘envfit’ with 10,000 random permutations of the data (Oksanen et al., Reference Oksanen, Kindt and O'Hara2005). This procedure also generates an R 2 measure and significance values based on the probability that random permutations would yield a higher degree of fit than the true data (see also Gröning et al., Reference Gröning, Lücke, Finger and Hochkirch2007b). The relationship between frequency of C. vagans in each square and the degree of shading was examined with Spearman rank correlations separately for each count.
To obtain dispersal distances for 2005, we followed the procedure suggested by Altmoos (Reference Altmoos2000). The centre of the square in which each capture had occurred was taken to be the point of capture. The cumulative dispersal distance (cdd) is the sum of all measured distances obtained for an individual throughout the research period. The total dispersal distance (tdd) is the maximum distance between any two records of an individual. Since site SD was not covered with a grid, only estimates of dispersal distances can be provided for specimens moving between both sites. We tested the dispersal distances for sexual differences with an ANOVA. We applied Box-Cox-transformation using Venables and Ripley's MASS library for R (Venables & Ripley, Reference Venables and Ripley2002), which reveals the optimal power transformation (λ) to fit the data to meet the model assumptions. The emigration rate was computed according to the procedure described by Kuusaari et al. (Reference Kuusaari, Nieminen and Hanski1996). Differences in emigration rates were analyzed by χ2 two-sample tests for equality of proportions with continuity correction (Crawley, Reference Crawley2005). For estimating population sizes, we used the Jolly-Seber method, which corrects for bias in small samples (Seber, Reference Seber1982).
To analyze microhabitat preferences of C. vagans, we first performed two-way ANOVAs including site and sex as explanatory variables and the vegetation data as response variables. We did not include microclimatic parameters in these models, as they are dependent on time and weather conditions. Model simplification was achieved using Akaike's Information Criterion (AIC). We simplified the models further by removing stepwise non-significant interactions (Crawley, Reference Crawley2005). All data were Box-Cox-transformed prior to statistical treatment to comply with the model assumptions. We conducted paired t-tests for all abiotic and biotic variables to compare the occupied microhabitats with the surrounding environment. All statistical analyses were carried out with ‘R 2.4.0’ (R Development Core Team, 2006). Standard errors are provided in parentheses.
Results
Population size and distribution
The total number of marked C. vagans at site G increased from 135 (2004) to 209 (2005; fig. 2). Jolly-Seber estimates (JSE) for the population size were 167 for 2004 and 230 for 2005. The maximum count for one visit was ca. 50 for 2003 (31 August), 57 for 2004 (02 September) and 94 for 2005 (23 August). The number of occupied grid cells increased from 24 in 2004 (40%) to 32 in 2005 (53.3%; fig. 3). Several grid cells in the east of the managed area were colonized in 2005 (around square J7). The high recapture rate in 2005 (84%) indicates an accurate estimation of the population size. On average, each specimen was recaptured 3.2 (±0.17) times. The SD subpopulation increased from 63 marked individuals (JSE: 68) in 2004 to 208 specimens in 2005 (JSE: 218). The recapture rate in 2005 was 85% and each specimen was recaptured 4.4 (±0.19) times on average. The maximum count for one visit was ca. 25 for 2003 (31 August), 29 for 2004 (04 August) and 122 for 2005 (02 August).

Fig. 3. Maximum number of C. vagans counts in each grid cell for 2004 and 2005. Each square has an edge length of 5 m.
The distribution of C. vagans in the grid was significantly correlated to the vegetation matrix revealed by the NMDS (fig. 4). This was true for the mean and the maximum abundance of grasshoppers in each grid cell, but not for each single count. Until mid-August (2004, 09 August; 2005, 11 August), the distribution correlated significantly with the NMDS matrix (R2>0.14, P<0.05 for all visits except for 26 July 2004: R2=0.13, P=0.073). Figure 4 illustrates that, until mid-August, the occurrence of C. vagans mainly correlated with cover of bare ground and grasses, whereas cover of trees, litter and mosses were negatively correlated with the grasshoppers' abundance. Later in the season, the distribution was not significantly correlated to the vegetation matrix (all R2<0.13, P>0.05), reflecting a shift of the population to the upper slopes (i.e. northern parts) of the grid. Instead, a strong negative correlation with the shaded area (measured on 05 September 2005) was revealed for the five counts from 23 August to 20 September 2005 (Spearman rank correlation, all R<−0.34; all P<0.03, N=42).

Fig. 4. Plot of the first two axes of a non-metric multidimensional scaling for the vegetation matrix (NMDS). ○, grid cells; ▲, loadings of the vegetation descriptors. The daily distributions of C. vagans are fitted as vectors onto the ordination. Only data with strong correlations (R>0.14, P<0.05) are plotted (i.e. distributional data until mid-August). It is obvious that the distribution of the grasshoppers was strongly correlated with the parameters ‘bare ground’ and ‘grasses’ during all counts.
Dispersal ability
In 2005, the proportion of movements between grid cells among all recaptures (emigration rate) was 64.1%. Only in three grid cells was the emigration rate lower than 50% (G3: 45.6%; E5: 40.9%; J7: 36.4%). Square E5 received by far the most immigration events (108=25.8% of all movements). The number of recaptures decreased with increasing distance from the point of first capture, but a second (smaller) peak was found at ca. 90 m caused by dispersal between the two sites (fig. 5). Eighteen grasshoppers marked on site G emigrated to site SD (8.6%), but only one male returned to the grid and covered a cumulative dispersal distance of ca. 180 m (the maximum recorded). The movement rate in the opposite direction was significantly lower (χ2 two-sample test, df=1, χ2=8.77, P=0.003): Only three individuals marked on site SD emigrated to site G (1.4%). No intersexual difference was found in the proportion of emigrating grasshoppers (χ2 two-sample test, df=1, χ2=0.1) or dispersal distance (ANOVA, λ=0.2, Ftdd,1,205=0.06, Fcdd,1,204=0.26). While the maximum population growth occurred at the end of July, emigration to site SD mainly took place between 11 and 23 August.

Fig. 5. The relative number of movements over each distance, split into ten-metre distance intervals (n=210). ■, cumulative dispersal distances; □, total dispersal distances. Only specimens marked in the grid were considered.
Microhabitat preferences
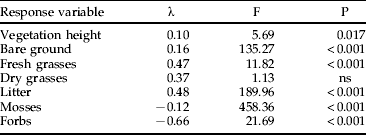
A total of 514 individual microhabitat records was obtained. The two-way ANOVA neither revealed significant effects of sex nor interactions between site and sex, but strong differences between the two sites (table 1). The occupied locations at SD were characterized by a greater maximum vegetation height, a greater cover of bare ground and fresh grasses, whereas the cover of litter and forbs were greater in the grid. Mosses were only present at site G. Based on these differences, we analyzed the subsets for each site separately for differences between the locations of the insects and the associated control samples (table 2). In the grid, grasshoppers occupied locations with a greater cover of fresh and dried grasses, a greater vegetation height and irradiation, but a smaller amount of litter. At site SD, grasshoppers choose cooler locations with greater humidity and higher vegetation compared to the control. The cover of fresh and dry grasses was also greater than at the corresponding control samples, but the amount of bare ground was less (table 2).
Table 1. Results of the two-way ANOVA for the biotic response variables and the explanatory variable ‘site’ (df=1, 512; ns=not significant).
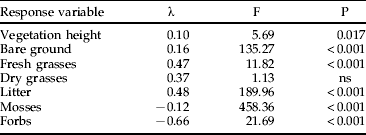
No significant effects were found for ‘sex’ and no interactions occurred.
Table 2. Results of the paired t-tests between the location of Chorthippus vagans and the corresponding control sample in one metre distance for both study sites.

Units: radiation, lux 1000−1; temperature, °C; relative humidity, %; vegetation height, cm; vegetation cover, %; dfG=296, dfSD=216.
Discussion
Evaluation of the management measures
Our data show that the abundance of C. vagans increased significantly in the grid during the research period and reached at least 209 individuals in 2005. This increase also resulted in a wider distribution of the grasshopper. The colonization of the eastern part of the grid will possibly increase the rate of interchange with the SD subpopulation in the future, but in 2004 and 2005 emigration was mainly observed from the central part of the grid (area from D5 to H3). Both subpopulations combined contained a minimum of 417 individuals, which is a comparatively large number for this species. Compared to other grasshoppers, populations of C. vagans are usually very small and rarely exceed 50 individuals (Meineke et al., Reference Meineke, Menge and Grein1994; Treiber, Reference Treiber and Detzel1998; Rauh, Reference Rauh, Schlumprecht and Waeber2003). Unfortunately, only sparse data exist from the period before the onset of management measures. A maximum of ca. 50 specimens was counted at the end of August 2003 (Brandt & Schäfer, Reference Brandt and Schäfer2004), which is the period of maximum adult abundance. In 2004 and 2005, the ratio of minimum population size to maximum abundance in the grid was rather constant (2.3±0.07), and so it is possible to estimate a minimum population size of 115 (±3.6) for 2003.
Although the increase in population size immediately followed the management measures, there are alternative explanations for this pattern. Stochastic fluctuations are common among insect populations (Dennis et al., Reference Dennis, Desharnais, Cushing, Henson and Constantino2001), but the low population sizes documented for C. vagans suggest that it is not prone to such strong variation. Climatic fluctuations could also have dramatic effects on Orthoptera (Gyllenberg, Reference Gyllenberg1974; Gottschalk et al., Reference Gottschalk, Griebeler, Waltert and Mühlenberg2003), but the weather conditions in 2004 were not unusual and other populations of C. vagans in Lower Saxony did not experience such a substantial growth (Krause, unpublished data). Hence, the population increase could have been caused by the management measures. This is also supported by the negative correlation of the grasshoppers' distribution with litter and deciduous trees.
Insect movements are often seen as indicators of habitat quality, with a high emigration rate suggesting low habitat quality (e.g. Matter & Roland, Reference Matter and Roland2002). However, movements between grid cells are also likely to occur as a result of the grasshoppers' normal activity, and the overall ‘emigration rate’ is influenced by individuals inhabiting the edge of a square. An obvious pattern revealed by our analysis in both years was an upward shift of the population during mid-August. This pattern was underlined by several aspects of our data: (i) the distribution of C. vagans in the grid correlated with the vegetation matrix only until 11 August; (ii) more than 80% of the emigration events from G to SD occurred after 11 August; (iii) from 23 August to 20 September the distribution was negatively correlated with shade; (iv) at the beginning of the season, the maximum abundance was found in the south, while later most specimens occurred on the upper slopes (particularly in E5 and J7). These results suggest that, in the southern part of the grid, habitat quality decreased in August, due to the lower position of the sun and subsequent shading by the adjacent Douglas-fir plantation. The removal of this plantation would probably increase the quality of the habitat.
Habitat preferences and conservation of the heath grasshopper
The multivariate analysis revealed that high quality habitats for C. vagans are characterized by sparse vegetation dominated by grasses and patches of bare ground. Although the species typically occurs in habitats with trees, too much shade affects the population adversely, confirming statements of other authors (Meineke et al., Reference Meineke, Menge and Grein1994; Treiber, Reference Treiber and Detzel1998; Rauh, Reference Rauh, Schlumprecht and Waeber2003; Baur et al., Reference Baur, Baur, Roesti and Roesti2006). A sparsely vegetated pine forest boundary seems to be a suitable habitat for C. vagans. The two sites studied differed significantly in vegetation structure with substantial consequences for microhabitat utilization. While, on the open sand dune, individuals chose cooler and more humid locations with denser vegetation compared to the control measures, specimens in the grid preferred places with greater radiation, less litter and more grasses. Hence, the grasshoppers seem to avoid the strong heat and drought of the sand dune as well as the shaded places with greatest litter cover in the grid. These results illustrate the dilemma an ecotone species faces; the adjacent habitat types alone are not suitable for its survival. Compared to other ecotone species, C. vagans does not utilize resources from both adjacent habitats (Ries et al., Reference Ries, Fletcher, Battin and Sisk2004) but occurs exclusively in the transition zone. Habitat edges are usually intermediate between adjacent ecosystems in environmental characteristics (Ries et al., Reference Ries, Fletcher, Battin and Sisk2004), and species adapted to these conditions will not profit from conservation measures defined by conditions at the centres of the two adjacent habitats. In modern, densely populated landscapes of industrialized countries, sharp transitions between habitat types dominate, restricting ecotone species to narrow zones of appropriate habitat. Since population survival is correlated with habitat size (MacArthur & Wilson, Reference MacArthur and Wilson1967), extinction is a logical consequence of the general decrease in ecotone breadth. The rapid decline of C. vagans populations in northern Germany is mainly caused by habitat loss. Sparsely vegetated forest edges have been replaced by ‘hard edges’, and adjacent xeric habitats are threatened by changes in land use or succession promoted by eutrophication and abandonment (Treiber, Reference Treiber and Detzel1998; Brandt & Schäfer, Reference Brandt and Schäfer2004). Hence, it is necessary also to restore deep pine forest edges at other locations. The removal of deciduous trees and litter are useful measures to enhance habitat quality for C. vagans, and possibly for other species associated with this transition zone. Forest management should, therefore, consider the requirements of species confined to the rather special conditions at the boundary to xeric habitats.
Acknowledgements
We are grateful to the Division of Ecology at the University of Osnabrück for providing research facilities and financial support. Access to the study sites was kindly permitted by the Forstinteressengemeinschaft Mardorf. The management measures were financially supported by the ‘Region Hannover’. Anselm Kratochwil, Julia Gröning and Kathrin A. Witzenberger gave valuable comments on a previous version of the manuscript. We also would like to thank Till Eggers for statistical advice.