Introduction
Members of the genus Drepanocephalus Dietz, 1909 (Digenea: Echinostomatidae) use fish-eating birds of the genera Nannopterum Brisson and Sula Brisson as definitive hosts (Fedynich et al., Reference Fedynich, Pence and Bergan1997; Kostadinova et al., Reference Kostadinova, Voucher and Gibson2002; Flowers et al., Reference Flowers, Poore, Mullen and Levy2004; Robinson et al., Reference Robinson, Forbes, Hebert and McLaughlin2008; Monteiro et al., Reference Monteiro, Amato and Amato2011; Violante-González et al., Reference Violante-González, Monks, Gil-Guerrero, Rojas-Herrera, Flores-Garza and Larumbe-Morán2011; Griffin et al., Reference Griffin, Khoo, Steadman, Ware, Quinou, Mischke, Greenway and Wise2014). Freshwater fish such as siluriforms (Ictalurus punctatus Rafinesque) and cichlids (e.g. Astatheros robertsoni Allen, Paratheraps bifasciatus Steindachner, P. fenestratus Günther, Paraneetroplus synspilus Hubbs, Thorhichthys helleri Steindachner, Cichlasoma pearsei Hubbs, Mayaheros urophthalmus Günther and Oreochromis niloticus L.) are used as second intermediate hosts (Jímenez-García, Reference Jímenez-García1993; Vidal-Martínez et al., Reference Vidal-Martínez, Aguirre-Macedo, Scholz, González-Solís and Mendoza Franco2002; Griffin et al., Reference Griffin, Khoo, Quiniou, O'Hear, Pote, Greenway and Wise2012; Pinto et al., Reference Pinto, Mati and Melo2014). Planorbid snails (Planorbella trivolvis Say and Biomphalaria straminea Dunker) are used as the first intermediate hosts (Griffin et al., Reference Griffin, Khoo, Quiniou, O'Hear, Pote, Greenway and Wise2012).
Currently, Drepanocephalus comprises three species, i.e. D. spathans Dietz, 1909 (type species), D. olivaceus Nasir & Marval, 1968 and D. auritus Kudlai, Kostadinova, Pulis & Tkach, 2015, all distributed across the Americas (Dietz, Reference Dietz1910; Nasir & Marval, Reference Nasir and Marval1968; Kostadinova et al., Reference Kostadinova, Voucher and Gibson2002; Kostadinova, Reference Kostadinova, Jones, Bray and Gibson2005; Kudlai et al., Reference Kudlai, Kostadinova, Pulis and Tkach2015a). However, the taxonomic status of some species has been controversial and, in the first taxonomic revision of the genus, two species, D. parvicephalus Rietschel & Werding, 1978 and D. mexicanus Lamothe-Argumedo & Pérez Ponce de León, 1989, were transferred to the genus Paryphostomum Dietz 1909 by Kostadinova et al. (Reference Kostadinova, Voucher and Gibson2002), and more recently Tkach et al. (Reference Tkach, Kudlai and Kostadinova2016) transferred these species from the genus Paryphostomum to Petasiger Dietz, 1909, using a phylogenetic framework, inferred with partial sequences from the large subunit of the ribosomal DNA.
Over the past several years, two species of cormorants (Nannopterum brasilianus Gmelin and Nannopterum auritus Lesson), as well as one species of water turkey (Anhinga anhinga L.) have been sampled in several localities across Mexico. Gravid echinostomatids belonging to the genera Drepanocephalus, Petasiger and Euparyphium Dietz, 1909 were collected from the intestines of these fish-eating birds. Fresh samples of these trematodes allowed us to analyse their morphology using light and scanning electron microscopy, and to obtain DNA sequences; host details and geographic distribution of Drepanocephalus species across the Americas were also retrieved from several bibliographical sources. In some cases, the literature indicates confusion about the taxonomic status of D. spathans / D. auritus (see Drago et al., Reference Drago, Lunaschi and Schenone2011; Sheehan et al., Reference Sheehan, Hanson-Dorr, Dorr, Yarrow and Johnson2016). In this study, we conducted a multilocus phylogenetic analysis of species of Drepanocephalus and Petasiger, using newly generated as well as available sequences from GenBank to assess their evolutionary relationships within the Echinostomatidae. Our phylogenetic and morphological analyses clearly suggested that the species composition of the genus Drepanocephalus should be re-evaluated.
Materials and methods
Specimen collection
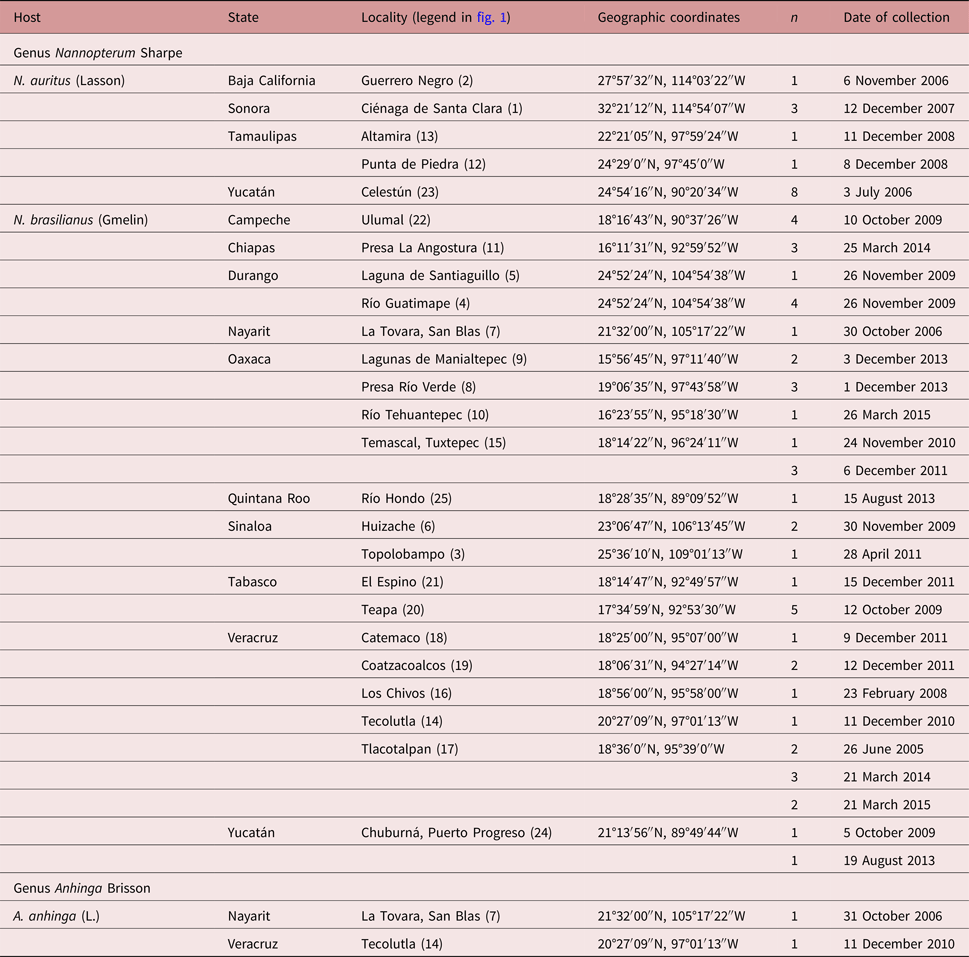
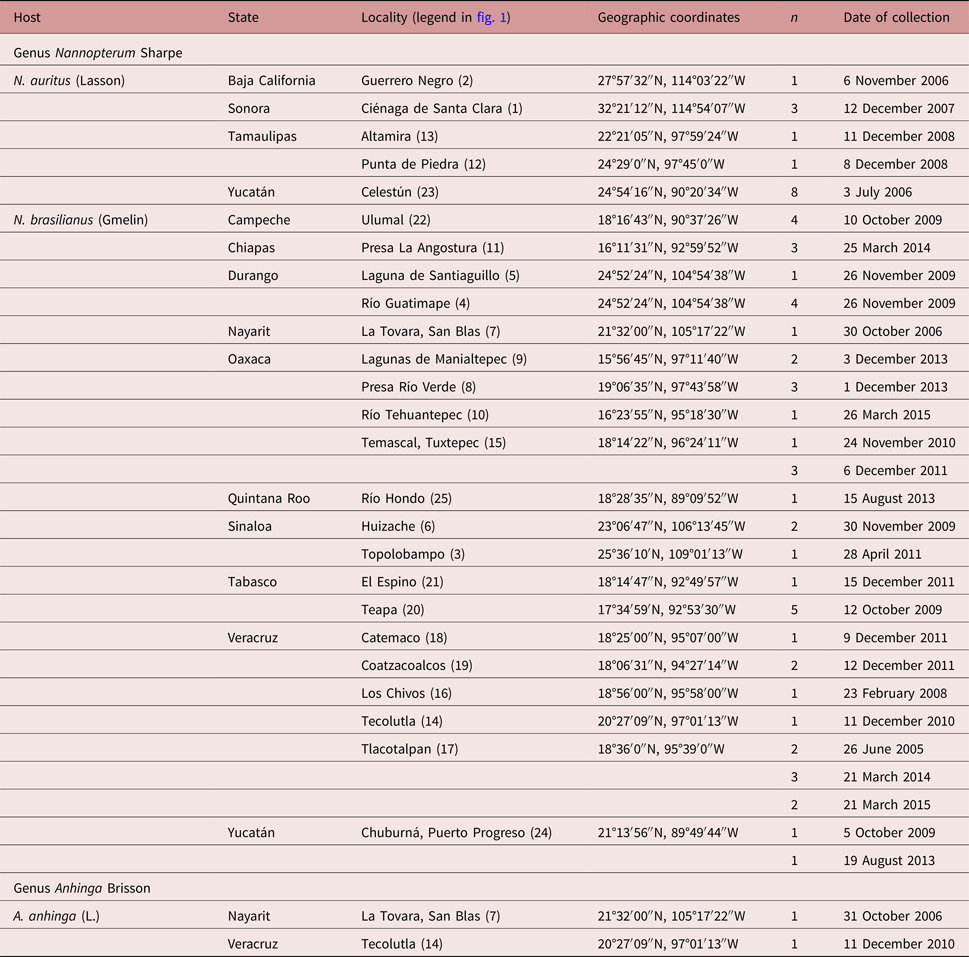
A total of 63 birds including 47 Neotropical cormorants (N. brasilianus), 14 double-crested cormorants (N. auritus) and 2 water turkeys (A. anhinga) were collected between June 2005 and March 2015 in 25 localities across Mexico (table 1, fig. 1). Bird nomenclature follows Kennedy & Spencer (Reference Kennedy and Spencer2014). Birds were dissected within 2 h after capture; their viscera were placed in separate Petri dishes containing a 0.75% saline solution and examined under a dissecting microscope. Trematodes were washed in 0.75% saline solution and fixed with hot 4% formalin for morphological studies and in 100% ethanol for DNA analyses.
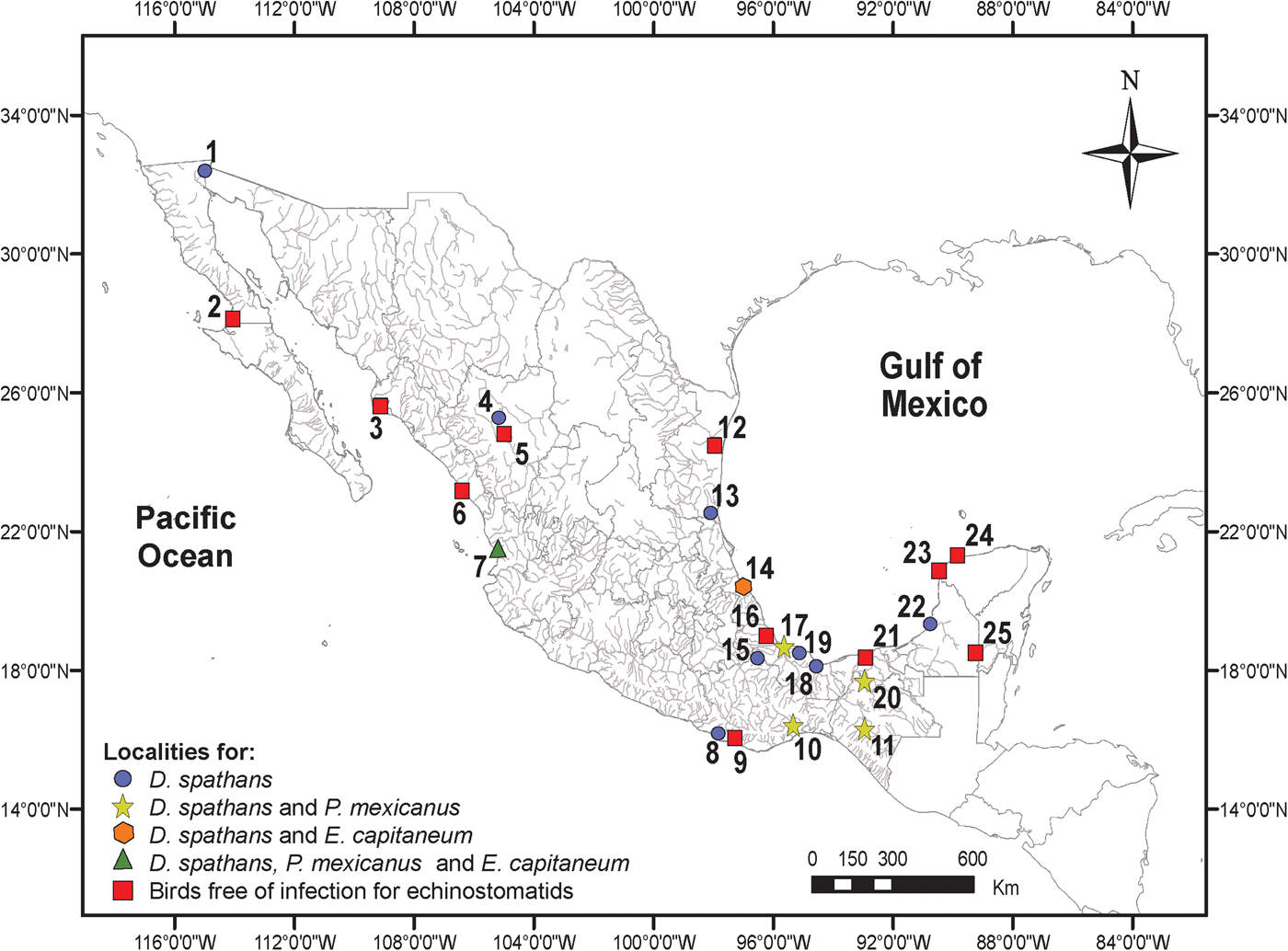
Fig. 1. Map of Mexico showing sampling sites for cormorants and water turkeys. Legends for localities are indicated in table 1.
Table 1. Sampling details of the fish-eating birds examined for the presence of echinostomatids in Mexico.
Morphological study
Unflattened digeneans preserved in formalin were stained with Mayer's paracarmine or with iron acetocarmine, dehydrated in a graded ethanol series, cleared with methyl salicylate and mounted as permanent slides in Canada balsam. Drawings were made with the aid of a drawing tube. Measurements are given in micrometres (μm) followed by the range.
Abbreviations were used to refer to each morphological character, following the terminology of Kudlai et al. (Reference Kudlai, Kostadinova, Pulis and Tkach2015a): BL, body length; BWVS, body width at level of ventral sucker; BWAT, body width at level of anterior testis; CL, collar length; CW, collar width; OSL, oral sucker length; OSW, oral sucker width; PL, prepharynx length; PHL, pharynx length; PHW, pharynx width; OL, oesophagus length; CSL, cirrus sac length; CSW, maximum cirrus sac width; SV1L, length of anterior portion of seminal vesicle; SV1W, width of anterior portion of seminal vesicle; SV2L, length of posterior portion of seminal vesicle; SV2W, width of posterior portion of seminal vesicle; VSL, ventral sucker length; VSW, ventral sucker width; OVL, ovary length; OVW, ovary width; MEL, Mehlis’ gland length; MEW, Mehlis’ gland width; ATL, anterior testis length; ATW, anterior testis width; PTL, posterior testis length; PTW, posterior testis width; EL, egg length; EW, egg width; FORE, forebody length; OVAR, distance between ovary and posterior margin of ventral sucker; TEND, distance between posterior margin of posterior testis and posterior extremity of body. In addition to the standard measurements, the following relative proportions were calculated according to Kostadinova (Reference Kostadinova, Jones, Bray and Gibson2005): BW (%), maximum body width as a proportion of body length; FO (%), length of forebody as a proportion of body length; U (%), length of uterine field posterior to ventral sucker (used as an approximation for uterine length) as a proportion of body length; T (%), length of post-testicular field as a proportion of body length.
Voucher specimens (paragenophore) were deposited in the Colección Nacional de Helmintos (CNHE), Instituto de Biología, Universidad Nacional Autónoma de México (UNAM), Ciudad de México, Mexico. The species identification was conducted following the most recent revision of the genus Drepanocephalus by Kostadinova et al. (Reference Kostadinova, Voucher and Gibson2002) and from original descriptions (Lamothe-Argumedo & Pérez-Ponce de León, Reference Lamothe-Argumedo and Pérez-Ponce de León1989; Kudlai et al., Reference Kudlai, Kostadinova, Pulis and Tkach2015a). Newly collected specimens (paragenophore) were compared with type and voucher material of Drepanocephalus and Petasiger, deposited in the CNHE: P. mexicanus (syn. D. mexicanus), holotype (CNHE 1279), three paratypes (CNHE 1280.2–4); D. olivaceus (syn. D. spathans), seven vouchers (CHNE 1411, three slides; CNHE 4998–9, two slides; CNHE 7577–8, two slides).
Six specimens, three of D. spanthans and three of P. mexicanus, fixed in 4% hot formalin were examined using scanning electron microscopy (SEM). Specimens were dehydrated through an ethanol series, mounted on a metal stub with carbon adhesive tabs, then gold coated, and examined at 15 kV in a Hitachi Stereoscan Model SU1510 scanning electron microscope (Hitachi Ltd, Tokyo, Japan) at the Instituto de Biología, UNAM, Mexico.
Specimens and DNA isolation
A total of 60 specimens, 38 identified as D. spathans, 20 as P. mexicanus and 2 as Euparyphium capitaneum Dietz, 1909 (see table 2 for details), were placed individually in tubes and digested overnight at 56°C in a solution containing 10 mm Tris-HCl (pH 7.6), 20 mm NaCl, 100 mm Na2EDTA (pH 8.0), 1% Sarkosyl, and 0.1 mg/ml proteinase K. Following digestion, DNA was extracted from the supernatant using the DNAzol reagent (Molecular Research Center, Cincinnati, Ohio, USA) according to the manufacturer's instructions.
Table 2. Sequences used in the phylogenetic analyses. LSU, large subunit rDNA; SSU, small subunit rDNA; ITS, internal transcribed spacer; nad1, nicotinamide adenine dinucleotide dehydrogenase subunit 1 gene; cox1, cytochrome c oxidase subunit I gene.
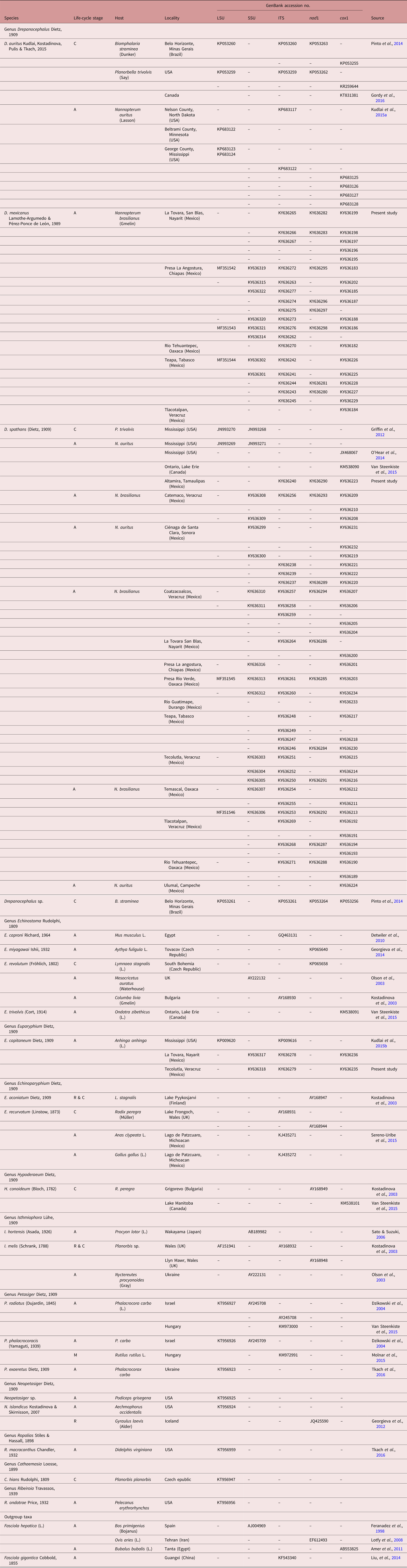
Life-cycle stage: adult (A), metacercariae (M), cercariae (C), rediae (R).
Amplification and sequencing of DNA
Three regions of nuclear ribosomal DNA (rDNA) were amplified using the polymerase chain reaction (PCR). The D1–D3 domains of the large subunit (LSU) ribosomal DNA (rDNA) were amplified using the forward primer 5′-GAA CAT CGA CAT CTT GAA CG-3′ (Hernández-Mena et al., Reference Hernández-Mena, García-Prieto and García-Varela2014) and reverse primer 5′-CAG CTA TCC TGA GGG AAA C-3′ (García-Varela & Nadler, Reference García-Varela and Nadler2005). The near-complete small subunit (SSU) rDNA (~1800 bp) was amplified in two overlapping PCR fragments of 1000–1200 bp. Primers used for SSU amplicon 1 were forward primer 5′-GGC GAT CGA AAA GAT TAA GCC ATGC A-3′ and reverse primer 5′-CGA AGT CCT ATT CCA TTA TTC-3′; amplicon 2, forward 5′-GCA GCC GCG GTA ATT CCA GCT C-3′ and reverse 5′-CGA AGT CCT ATT CCA TTA TTC-3′ (García-Varela & Nadler, Reference García-Varela and Nadler2005). The internal transcribed spacer (ITS) region, including ITS1, 5.8S and ITS2, was amplified using the forward primer 5′-GTC GTA ACA AGG TTT CCG TA-3′ and the reverse primer 5′-ATC TAG ACC GGA CTA GGC TGT G-3′ (Bowles & McManus, Reference Bowles and McManus1993). Two partial regions of the mitochondrial DNA were also amplified. Primers for the nicotinamide adenine dinucleotide dehydrogenase subunit 1 (nad1) were forward 5′-AGA TTC GTA AGG GGC CTA ATA-3′ (Morgan & Blair, Reference Morgan and Blair1998) and reverse 5′-CTT CAG CCT CAG CAT AAT-3′ (Kostadinova et al., Reference Kostadinova, Herniou, Barrett and Littlewood2003) and, finally, to amplify the gene cytochrome c oxidase subunit I (cox1) the primers were forward 5′-TCT TTR GAT CAT AAG CG-3′ and reverse 5′-CCA AAA AAC CAA AAC ATA TGC TG-3′ (Kudlai et al., Reference Kudlai, Kostadinova, Pulis and Tkach2015a).
PCR reactions (25 μl) consisted of 10 μm of each primer, 2.5 μl of 10× buffer, 2 mm MgCl2 (1.5 μl), 10 μm of deoxynucleoside triphosphates (dNTPs) (0.5 μl), 2 μl of the genomic DNA and 1 U of Taq DNA polymerase (0.125 μl) (Platinum Taq, Invitrogen Corporation, São Paulo, Brazil). PCR cycling parameters for amplifications included denaturation at 94°C for 3 min; followed by 35 cycles of 94°C for 1 min, annealing at 45°C for cox1 and nad1 and 50°C for LSU, SSU and ITS for 1 min, and extension at 72°C for 1 min; followed by a post-amplification incubation at 72°C for 10 min. Sequencing reactions were performed using ABI Big Dye (Applied Biosystems, Boston, Massachusetts, USA) terminator sequencing chemistry, and reaction products were separated and detected using an ABI 3730 capillary DNA sequencer. Contigs were assembled and base-calling differences resolved using Codoncode Aligner version 5.0.2 (Codoncode Corporation, Dedham, Massachusetts, USA). Sequences were deposited in the GenBank dataset (table 2). All sequences were aligned using the software Clustal W (Thompson et al., Reference Thompson, Gibson, Plewniak and Jeanmougin1997). Maximum likelihood (ML) and Bayesian inference analyses (BI) were performed for each dataset. The ML tree was inferred using RAxML 7.0.4. (Stamatakis, Reference Stamatakis2006). jModelTest v0.1.1 was used to get the best model of nucleotide substitution (Posada, Reference Posada2008). Tree searches were performed using 1000 (ML) random taxon addition heuristic searches. Clade support was assessed by bootstrap resampling with 10,000 replicates. Bayesian analyses were performed with MrBayes version 3.2.2 (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). Settings were two simultaneous runs of the Markov chain (MCMC) for 10 million generations, sampling every 1000 generations, a heating parameter value of 0.2 and a ‘burn-in’ of 25%. Trees were drawn using FigTree version 1.3.1 (Rambaut, Reference Rambaut2012). The genetic divergence among taxa was estimated using uncorrected ‘p’ distances with the program MEGA version 6 (Tamura et al., Reference Tamura, Stecher, Peterson, Filipski and Kumar2013).
Phylogenetic analyses
Our strategy was to obtain sequence data of the same molecular markers (LSU, SSU, ITS, cox 1 and nad 1) as those available for other species of Drepanocephalus (e.g. Griffin et al., Reference Griffin, Khoo, Quiniou, O'Hear, Pote, Greenway and Wise2012; O'Hear et al., Reference O'Hear, Pote, Yost, Doffitt, King and Panuska2014; Kudlai et al., Reference Kudlai, Kostadinova, Pulis and Tkach2015a; Van Steenkiste et al., Reference Van Steenkiste, Locke, Castelin, Marcogliese and Abbott2015; Pinto et al., Reference Pinto, Griffin, Quiniou, Ware and Melo2016), as well as other echinostomatids, and Fasciola hepatica was used as outgroup to root the trees. We constructed five alignments, one for each of the three nuclear and the two mitochondrial genes.
Results
Morphological identification
Following the original description of Lamothe-Argumedo & Pérez-Ponce de León (Reference Lamothe-Argumedo and Pérez-Ponce de León1989) and Kostadinova et al. (Reference Kostadinova, Voucher and Gibson2002), our specimens collected in cormorants from Mexico were identified as P. mexicanus and D. spathans; however, wide morphological variation between testes was found and therefore some specimens were similar to D. auritus (see Kudlai et al., Reference Kudlai, Kostadinova, Pulis and Tkach2015a).
Nuclear genes
The LSU dataset included 1230 characters and the best evolution model obtained was TIM3 + I + G. The alignment includes four sequences of D. spathans, five of D. auritus, a sample identified as Drepanocephalus sp. from Brazil, and three specimens of P. mexicanus (including a specimen from the type host and type locality), and two other species of the genus Neopetasiger, plus E. capitaneum and other echinostomids (see table 2). The phylogenetic analysis inferred with ML and BI shows that the sequences of P. mexicanus and a single sequence of a cercaria identified as Drepanocephalus sp. (KP053261) from B. straminea from Brazil formed a clade, which is sister species to a clade containing sequences of D. spathans and D. auritus. Our analysis revealed that the new sequences of P. mexicanus are not nested with other species of the genus Petasiger (fig. 2a). The SSU dataset included 1783 characters and the best evolution model obtained was TIM3 + I + G. This alignment includes 16 sequences of D. spathans, and eight of P. mexicanus (including specimens from the type host and type locality), plus two species of the genus Petasiger and two specimens of E. capitaneum, other species of echinostomatids, and F. hepatica (outgroup) (see table 2). The phylogenetic analysis inferred with ML and BI also showed that P. mexicanus is not nested with the other members of the genus Petasiger. In addition, the eight sequences of P. mexicanus plus 16 sequences of D. spathans are nested in a clade forming a basal polytomy (see fig. 2b). The topologies inferred with LSU and SSU datasets are similar to the phylogenetic tree inferred with ITS, although the latter includes a larger number of terminals (a larger number of newly generated sequences, and other species of echinostomatids for which ITS sequences are available). The ITS dataset consists of 1146 characters, with 59 terminals and the best evolution model for this dataset was TVM + G. The ITS tree also showed that the species P. mexicanus is nested within the genus Drepanocephalus. In addition, four sequences identified as D. auritus, three of them from either adults or cercariae in the USA, and one from Brazil (see table 2), plus 24 newly generated sequences of D. spathans, formed a clade with relatively high bootstrap and posterior probability support values (see fig. 2c). Interrelationships among species of Drepanocephalus are not well resolved with this nuclear gene.
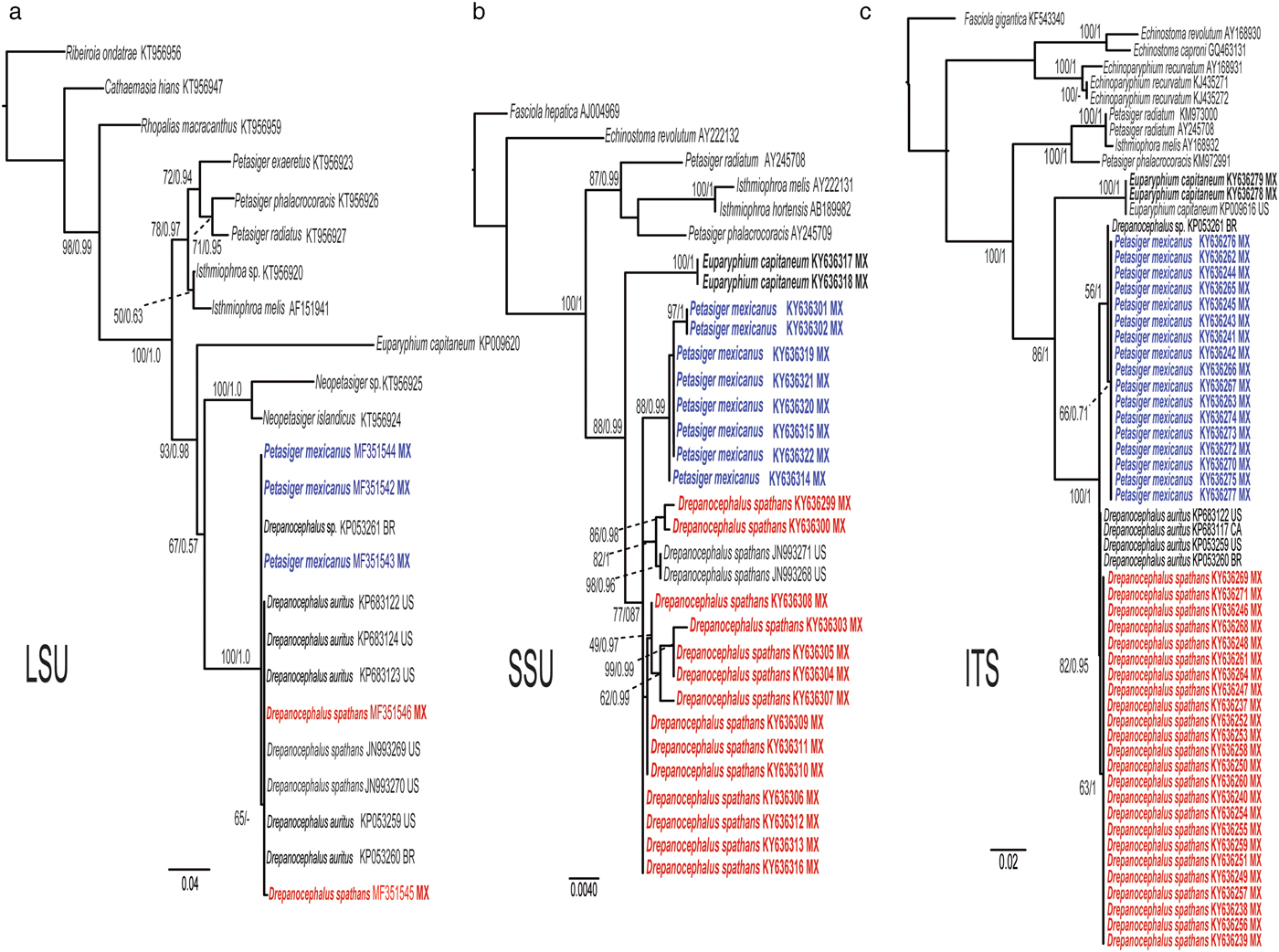
Fig. 2. Maximum likelihood tree and consensus Bayesian inference trees inferred for nuclear ribosomal DNA markers. (a) LSU dataset; (b) SSU dataset; (c) ITS dataset. Numbers near the internal nodes show ML bootstrap clade frequencies and posterior probabilities (BI). Abbreviations of each country: MX, Mexico; US, USA; CA, Canada; BR, Brazil.
Mitochondrial genes
The cox1 dataset included 658 characters with 68 terminals and the best selected model was GTR + I + G. This tree supported the monophyly of Drepanocephalus with very high bootstrap and posterior probability support values. The samples of 18 specimens of P. mexicanus and a single sequence for a cercaria identified as Drepanocephalus sp. from B. straminea from Brazil (Pinto et al., Reference Pinto, Griffin, Quiniou, Ware and Melo2016), formed a monophyletic clade that is nested within the genus Drepanocephalus. cox1 sequences from specimens identified by us as D. spathans from different localities across Mexico, as well as those available in the GenBank dataset from Mississippi, USA, and Canada (O'Hear et al., Reference O'Hear, Pote, Yost, Doffitt, King and Panuska2014; Gordy et al., Reference Gordy, Kish, Tarrabain and Hanington2016) are nested within a monophyletic clade with those identified as D. auritus from Brazil, Canada and the USA (Kudlai et al., Reference Kudlai, Kostadinova, Pulis and Tkach2015a; Gordy et al., Reference Gordy, Kish, Tarrabain and Hanington2016; Pinto et al., Reference Pinto, Griffin, Quiniou, Ware and Melo2016) (see fig. 3a, table 2). Finally, the nad1 dataset included 510 characters with 30 terminals. The best model for this dataset was GTR + G. This tree showed similar topology to that of the cox1 tree, with the genus Drepanocephalus as a monophyletic assemblage with very high nodal support values. For this molecular marker, a basal polytomy is also formed, although two well-defined clades are recovered, one containing nad1 sequences of eight specimens identified as P. mexicanus plus a single specimen identified as Drepanocephalus sp. from Brazil (KP053264) (Pinto et al., Reference Pinto, Griffin, Quiniou, Ware and Melo2016). Another clade includes 11 specimens identified as D. spathans, whose sequences were generated in this study, plus a specimen identified as D. auritus from Brazil (KP053263) (Pinto et al., Reference Pinto, Griffin, Quiniou, Ware and Melo2016). Interestingly, a single sequence of D. auritus from USA (KP053262) is not nested within this clade and forms a basal polytomy (fig. 3b).
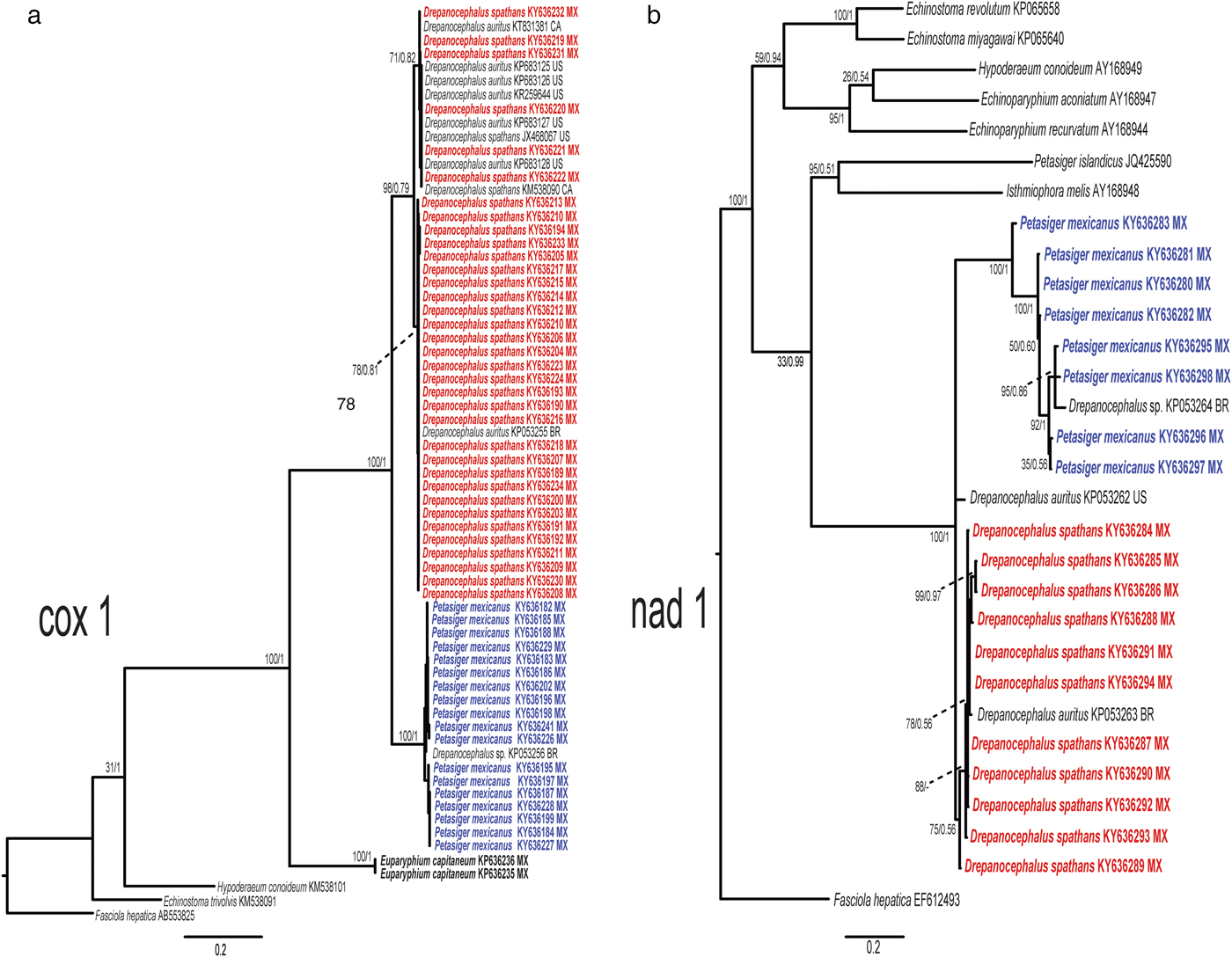
Fig. 3. Maximum likelihood tree and consensus Bayesian inference trees inferred for mitochondrial DNA markers. (a) cox1 dataset; (b) nad1 dataset. Numbers near internal nodes show ML bootstrap clade frequencies and posterior probabilities (BI). Abbreviations of each country: MX, Mexico; US, USA; CA, Canada; BR, Brazil.
In summary, all phylogenetic analyses are congruent in that P. mexicanus is nested within Drepanocephalus and, as a result, it should be transferred to Drepanocephalus, i.e. to the genus in which the species was placed when it was described by Lamothe-Argumedo & Pérez-Ponce de León (Reference Lamothe-Argumedo and Pérez-Ponce de León1989) as D. mexicanus. Sequences identified as Drepanocephalus sp., from cercariae collected from snails in Brazil, correspond with this species. Additionally, the synonymy between D. auritus and D. spathans is confirmed based on the multilocus phylogenetic analyses. These nomenclatural acts require the re-description of the two species that we are validating in this study, D. spathans and D. mexicanus, as follows.
Drepanocephalus spathans Dietz, 1909
Taxonomic summary
Synonym. Drepanocephalus auritus Kudlai, Kostadinova, Pulis & Tkach, 2015.
Type host. Nannopterum brasilianus Gmelin (Pelecaniformes: Phalacrocoracidae).
Other hosts. Nannopterum auritus Lasson (Pelecaniformes: Phalacrocoracidae), Sula leucogaster Brisson (Pelecaniformes: Sulidae).
Type-locality. Brazil.
Localities in Mexico. Ulumal (18°16′43″N, 90°37′26″W), Campeche; Presa La Angostura (16°11′31″N, 92°59′52″'W), Chiapas; Río Guatimape (24°52′24″'N, 104°54′38″W) Río Guatimape (24°52′24″N, 104°54′38″W), Durango; La Tovara, San Blas (21°32′00″N, 105°17′22″W), Nayarit; Presa Río Verde (19°06′35″N, 97°43′58″W), Río Tehuantepec (16°23′55″N, 95°18′30″W), Temascal (18°14′22″N, 96°24′11″W), Oaxaca; Ciénaga de Santa Clara (32°21′12″N, 114°54′07″W), Sonora; Teapa (17°34′59″N, 92°53′30″W), Tabasco; Altamira (22°21′05″N, 97°59′24″W), Tamaulipas; Catemaco (18°25′00″N, 95°07′00″W), Coatzacoalcos (18°06′31″N, 94°27′14″W), Tecolutla (20°27′09″N, 97°01′13″W), Tlacotalpan (18°36′0″N, 95°39′0″W), Veracruz.
Localities in other countries. Colombia (Rietschel & Werding, Reference Rietschel and Werding1978), Venezuela (Lutz, Reference Lutz1928; Caballero Caballero & Díaz Ungria, Reference Caballero Caballero and Díaz Ungria1958), Argentina (Ostrowski de Núñez, Reference Ostrowski de Núñez1968; Drago et al., Reference Drago, Lunaschi and Schenone2011), USA (Threlfall, Reference Threlfall1982; Fedynich et al., Reference Fedynich, Pence and Bergan1997; Flowers et al., Reference Flowers, Poore, Mullen and Levy2004; Griffin et al., Reference Griffin, Khoo, Steadman, Ware, Quinou, Mischke, Greenway and Wise2014; O'Hear et al., Reference O'Hear, Pote, Yost, Doffitt, King and Panuska2014; Sheehan et al., Reference Sheehan, Hanson-Dorr, Dorr, Yarrow and Johnson2016), Paraguay (Kostadinova et al., Reference Kostadinova, Voucher and Gibson2002) and Canada (Robinson et al., Reference Robinson, Forbes, Hebert and Mclauglin2010; Wagner et al., Reference Wagner, Hoberg, Somer, Soos, Fenton and Jenkins2012) (see table 2).
Site in host. Intestine.
Voucher specimens. CNHE 10310–10318.
Description
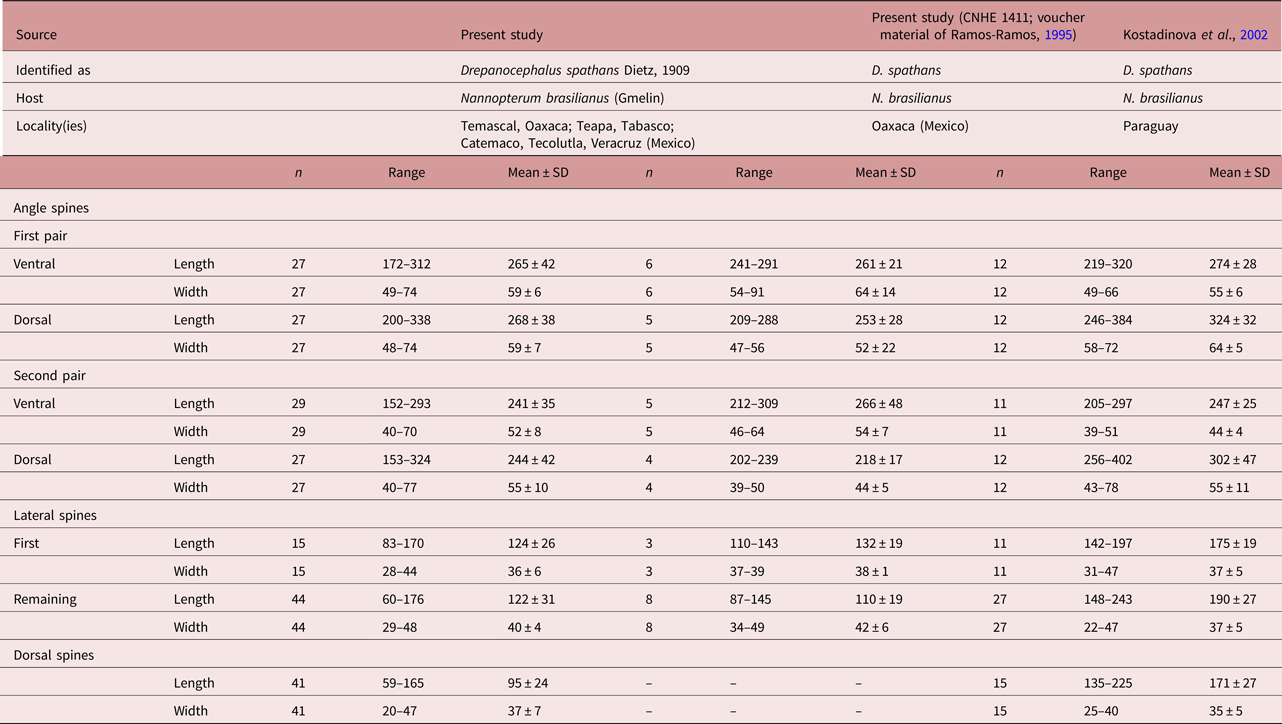
Based on 15 gravid specimens from N. brasilianus. Body (figs 4a–c, 5) elongate, with maximum width at level of head collar (fig. 5a). Body 4459–9181 long, 917–1439 wide at level of ventral sucker and 734–1,512 at level of anterior testis. BW = 3.6–8.5%. Forebody 1031–1627 long (FO = 3.4–6.7%); lateral area between head collar and ventral sucker covered with small spines (fig. 4a–c). Head collar falciform, strongly muscular, 646–995 long, 1044–1794 wide; ventral lappets possessing two invaginations. Collar spines 27, large, in single row. Eight angle spines, four on each edge of ventral lappet (pair 1: ventral 172–312 × 49–74, dorsal 200–338 × 48–74; pair 2: ventral 152–293 × 40–70, dorsal 153–324 × 40–77). Four lateral spines on each lappet, in single row, first 83–170 × 28–44 and remaining 60–176 × 29–48. Dorsal spines 11 in single row, smaller than lateral, diminishing in size towards the middle of the collar (59–165 × 20–47). Oral sucker subterminal, subspherical, 232–362 long, 256–403 wide. Ventral sucker muscular, cup-shaped, located in anterior half of body, 814–1118 long, 611–1066 wide. Prepharynx visible, 33–74 long. Pharynx muscular, elongate, 206–363 long, 149–240 wide (fig. 5a). Oesophagus 522–742 long, leading to an intestinal bifurcation anterior to ventral sucker. Caeca extended laterally to the posterior end of body. Testes in posterior half of body, deeply lobated, contiguous or widely separated, in tandem; anterior testis with lobes, 396–626 long, 425–911 wide; posterior testis with 4–7 lobes, 385–717 wide, 425–894 long (fig. 5b, c) . Distance between posterior testis and posterior margin of body 1178–3751. Cirrus sac relatively small, oval, 646–995 long, 1044–1794 wide, antero-dorsal to ventral sucker. Cirrus tubular, coiled, unspined. Internal seminal vesicle bipartite. Anterior portion smaller than posterior portion. Pars prostatica weakly developed; prostatic cells surround pars prostatica. Genital pore median, between ventral sucker and intestinal bifurcation (fig. 5d). Ovary small, slightly dextral, entire, oval, 176–308 long, 182–270 wide, anterior to Mehlis' gland. Mehlis’ gland median. Laurer's canal not observed. Metraterm muscular, slightly larger than cirrus sac (fig. 5d). Eggs numerous, 65–91 long, 37–61 wide. Vitellarium in two lateral fields consisting of small follicles, between posterior margin of ventral sucker and level of the end of caeca, confluent in the post-testicular region; excretory pore terminal.
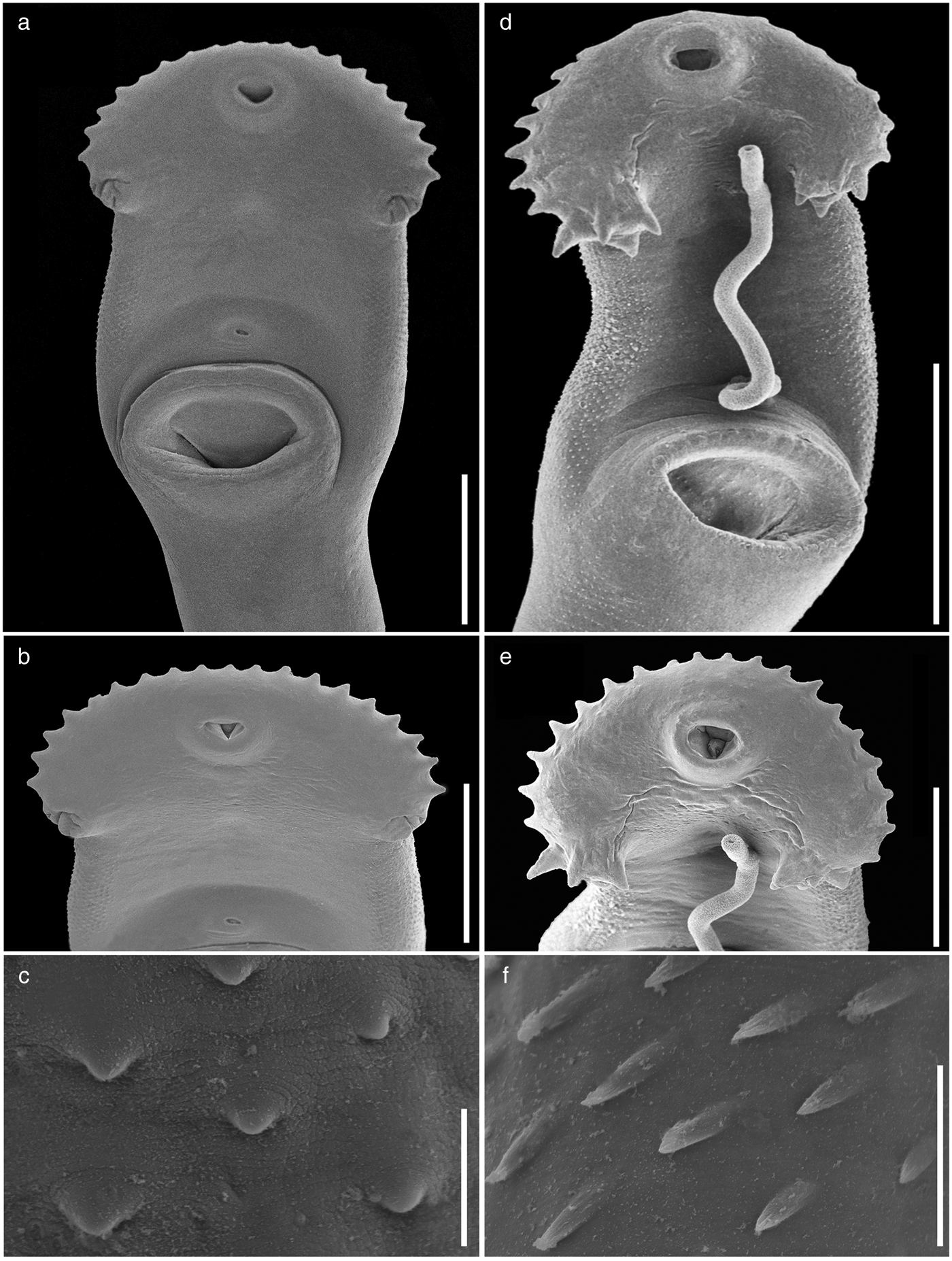
Fig. 4. Scanning electron micrographs of the forebody of adults of Drepanocephalus spp. from Mexico. Drepanocephalus spathans from N. brasilianus: (a) forebody; (b) head collar; (c) tegumental spines. Drepanocephalus mexicanus from N. brasilianus: (d) forebody; (e) head collar; (f) tegumental spines. Scale bars: (a, b, d) 500 μm; (c, f) 20 μm; (e) 300 μm.
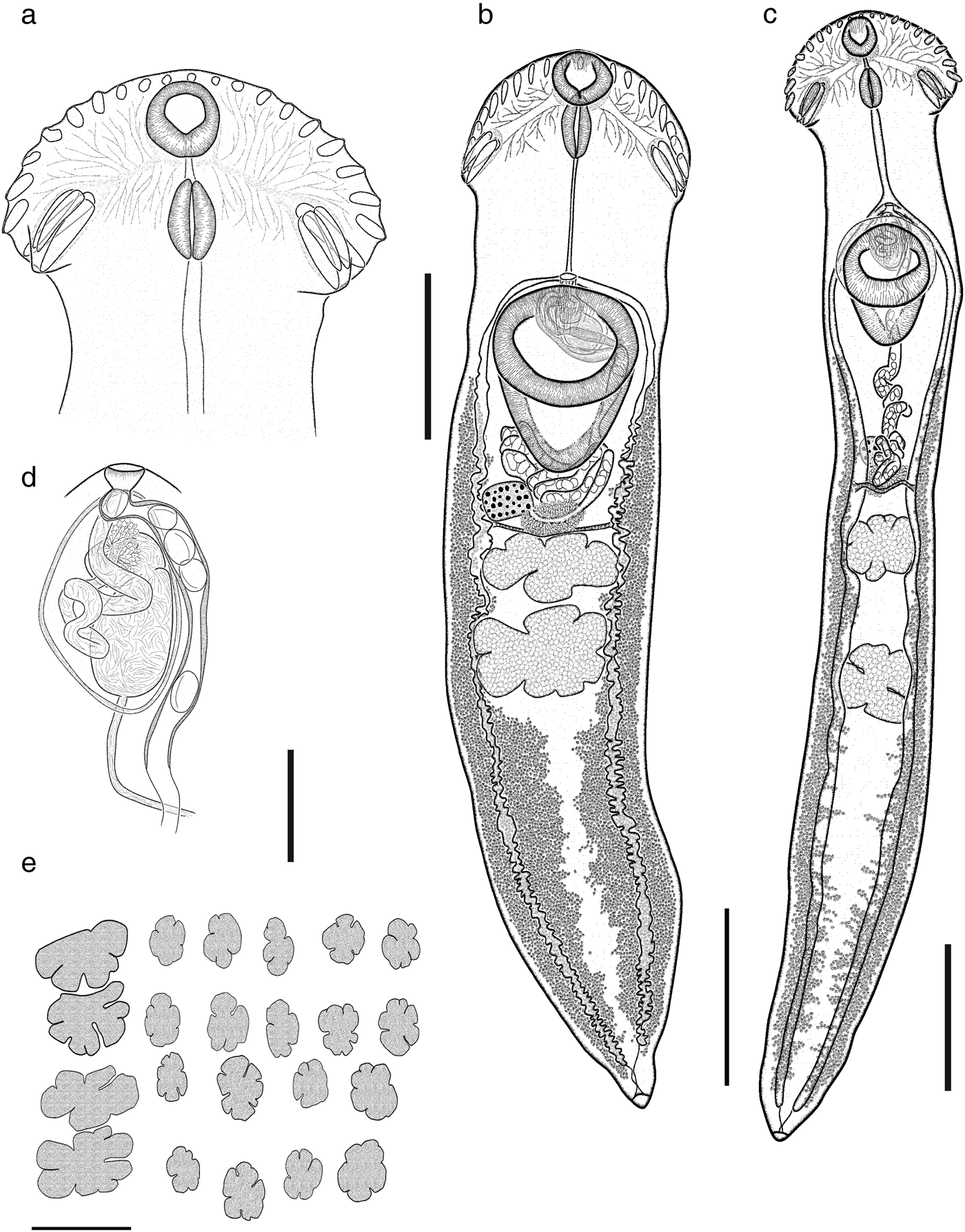
Fig. 5. Drepanocephalus spathans from N. brasilianus: (a) head collar; (b, c) whole worm with contiguous and widely separated testes, respectively; (d) terminal genitalia; (e) variation in shape of testes. Scale bars: (a) 500 μm; (b, c) 1000 μm; (d) 200 μm; (e) 1000 μm.
Remarks
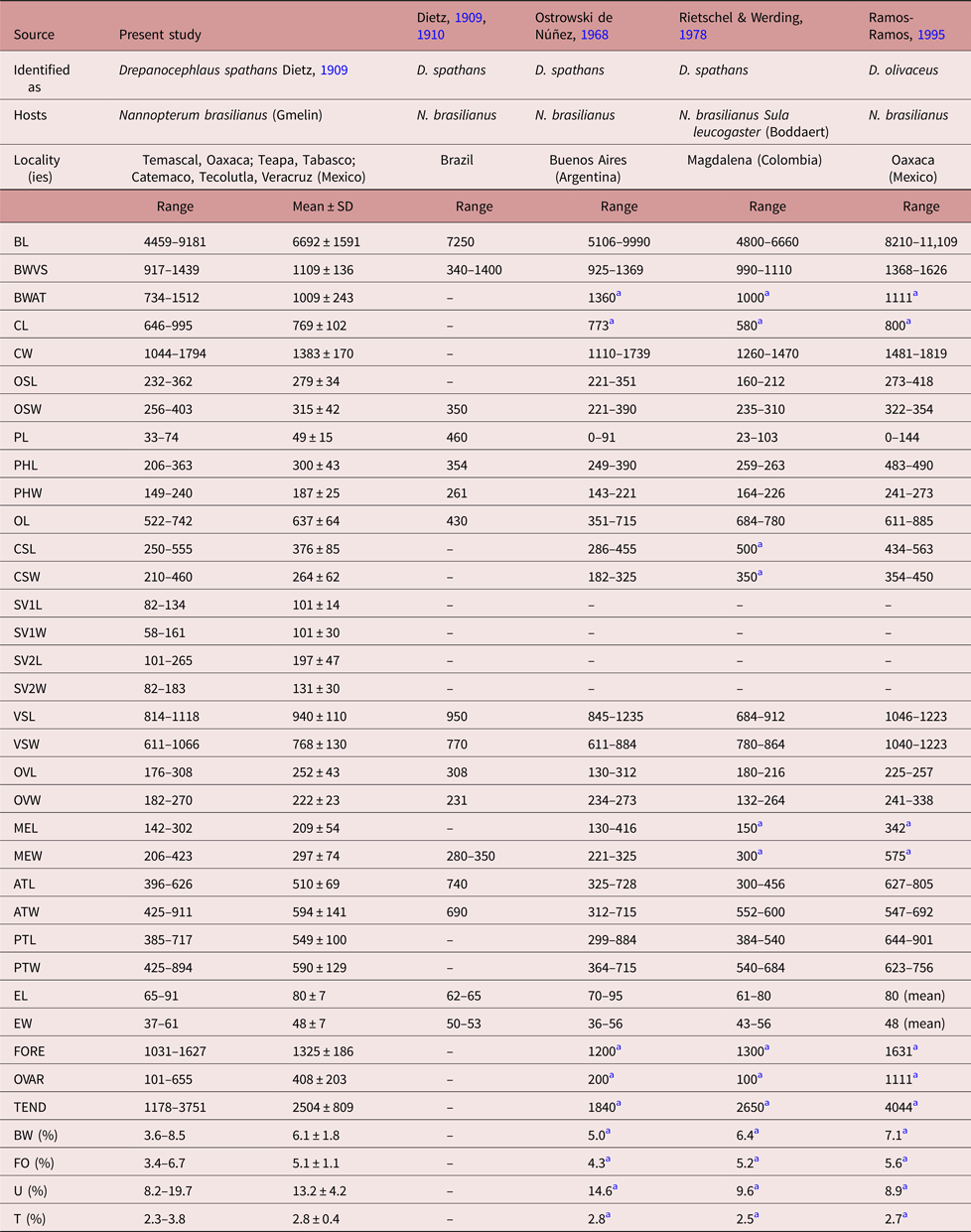
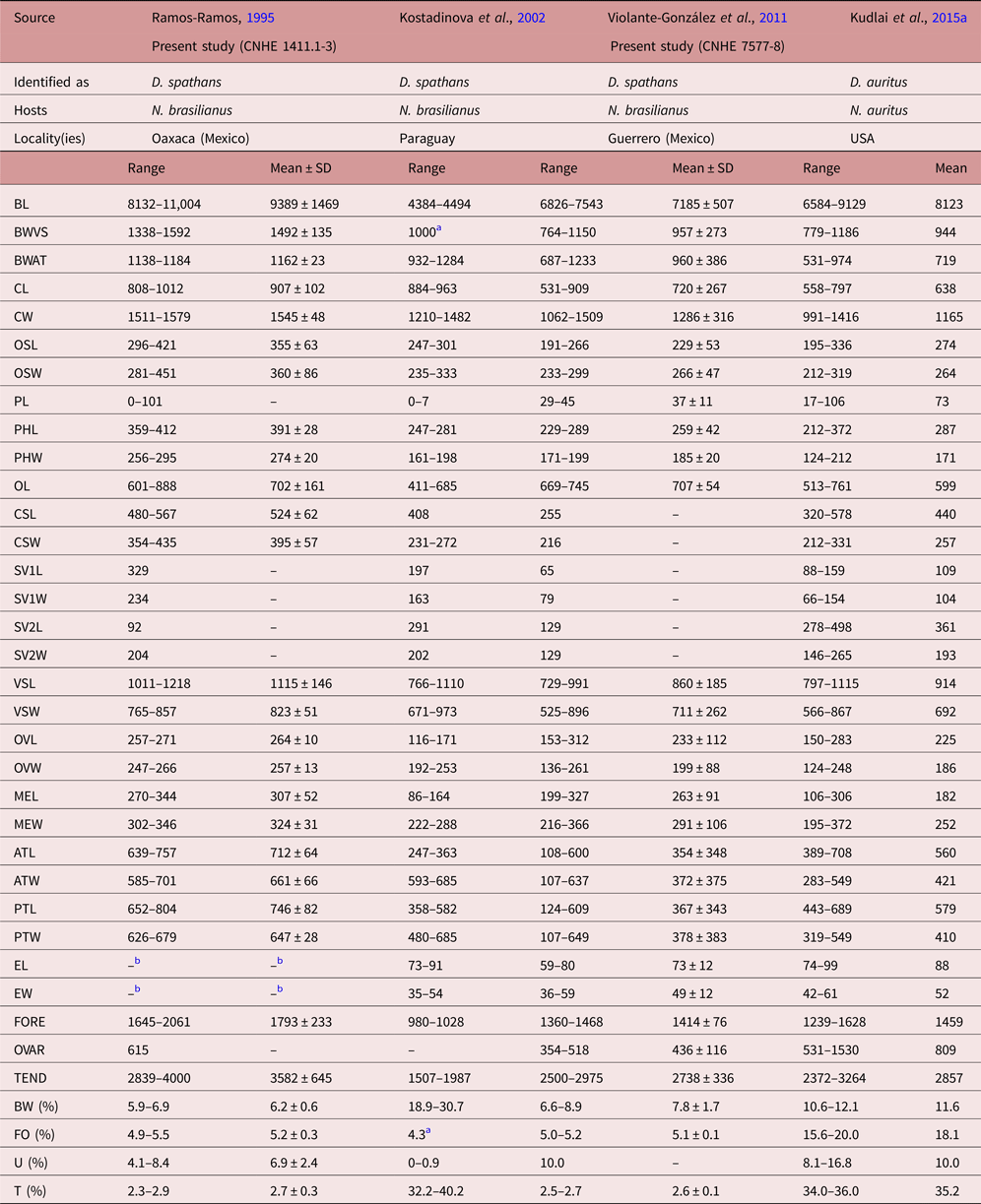
Specimens collected in 14 localities of Mexico, from two species of cormorants, N. brasilianus and N. auritus, possess features that are consistent with the diagnosis of the genus Drepanocephalus: an elongate body, forebody short, tegument armed with small spines, head collar wide with 27 spines in a single row, angle spines 2 × 4, oral sucker spherical, ventral sucker cup-shaped, prepharynx short, pharynx similar in size to oral sucker, oesophagus short, intestinal bifurcation just anterior or dorsal to ventral sucker, testes in tandem, contiguous and widely separated, lobed; cirrus sac elongated, ovary small, dextral, pre-equatorial; uterus short, excretory vesicle Y-shaped and excretory pore subterminal (Kostadinova et al., Reference Kostadinova, Voucher and Gibson2002; Kostadinova, Reference Kostadinova, Jones, Bray and Gibson2005; Kudlai et al., Reference Kudlai, Kostadinova, Pulis and Tkach2015a). In particular, our specimens were identified as D. spathans by possessing a falciform head collar, with the maximum width at head-collar level, and tegument with small and wide spines laterally placed between head collar and ventral sucker (see fig. 4a–c). Our specimens identified as D. spathans showed some level of morphological intraspecific variation (see fig. 5b, c, e). For instance, some meristic data of newly collected material with respect to previous descriptions possess lower limits for the following characters: CW (1044–1794), PHL (206–363), CSL (250–555), SV1W (58–161), SV2W (82–183), MEW (206–423), TEND (1178–3751), BW (%) (3.6–8.5), FO (%) (3.4–6.7) and T (%) (2.3–3.8). Likewise, newly collected material provides higher limits for the following characters BWAT (734–1512), CSW (210–460), ATW (425–911), PTW (425–894), EW (37–61), OVAR (101–655), FO (%) (3.4–6.7), U (%) (8.2–19.7) (see table 3). Our morphological observations and measurements of D. spathans (table 3), indicate some level of intraspecific morphological variability and, apparently, the morphological characters used by Kudlai et al. (Reference Kudlai, Kostadinova, Pulis and Tkach2015a) to distinguish D. auritus from D. spathans, i.e. shape of the testes, position and distance between the testes (contiguous and widely separated see fig. 5e), position of the ovary and Mehlis’ gland, size of head collar, prepharynx, pharynx and seminal vesicle, fall into the range variation of D. spathans, and these observations are corroborated by molecular data, since sequences are nested together in a monophyletic clade. Morphological and molecular data obtained in the present study then suggest that these species are indistinguishable from each other. Therefore we consider D. auritus as a synonym of D. spathans.
Table 3a. Comparative measurements of Drepanocephalus spathans Dietz, Reference Dietz1909.
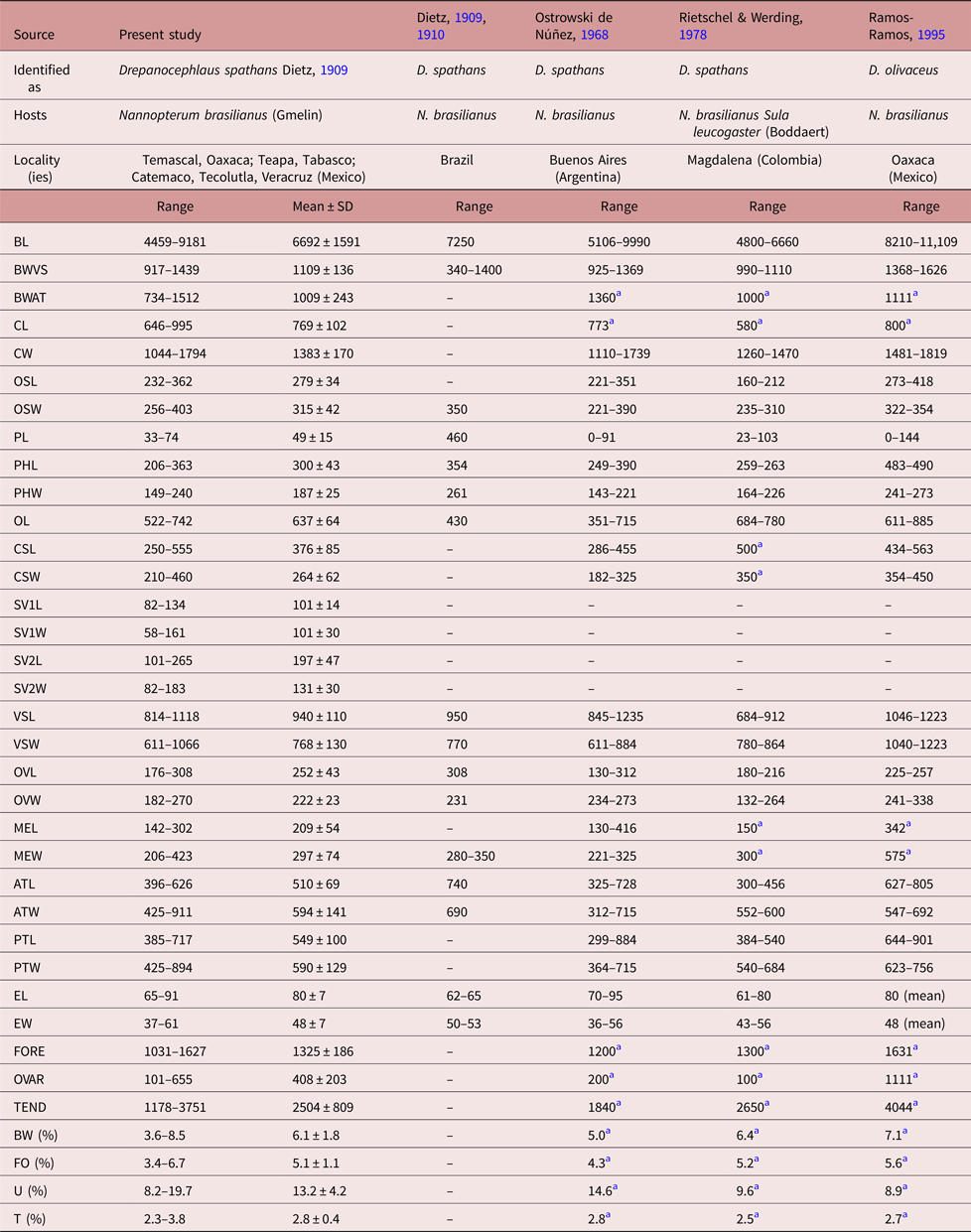
a Measurements taken from the published figure according to the scale presented in the figure.
Table 3b. Comparative measurements of Drepanocephalus spathans Dietz, Reference Dietz1909, continued.
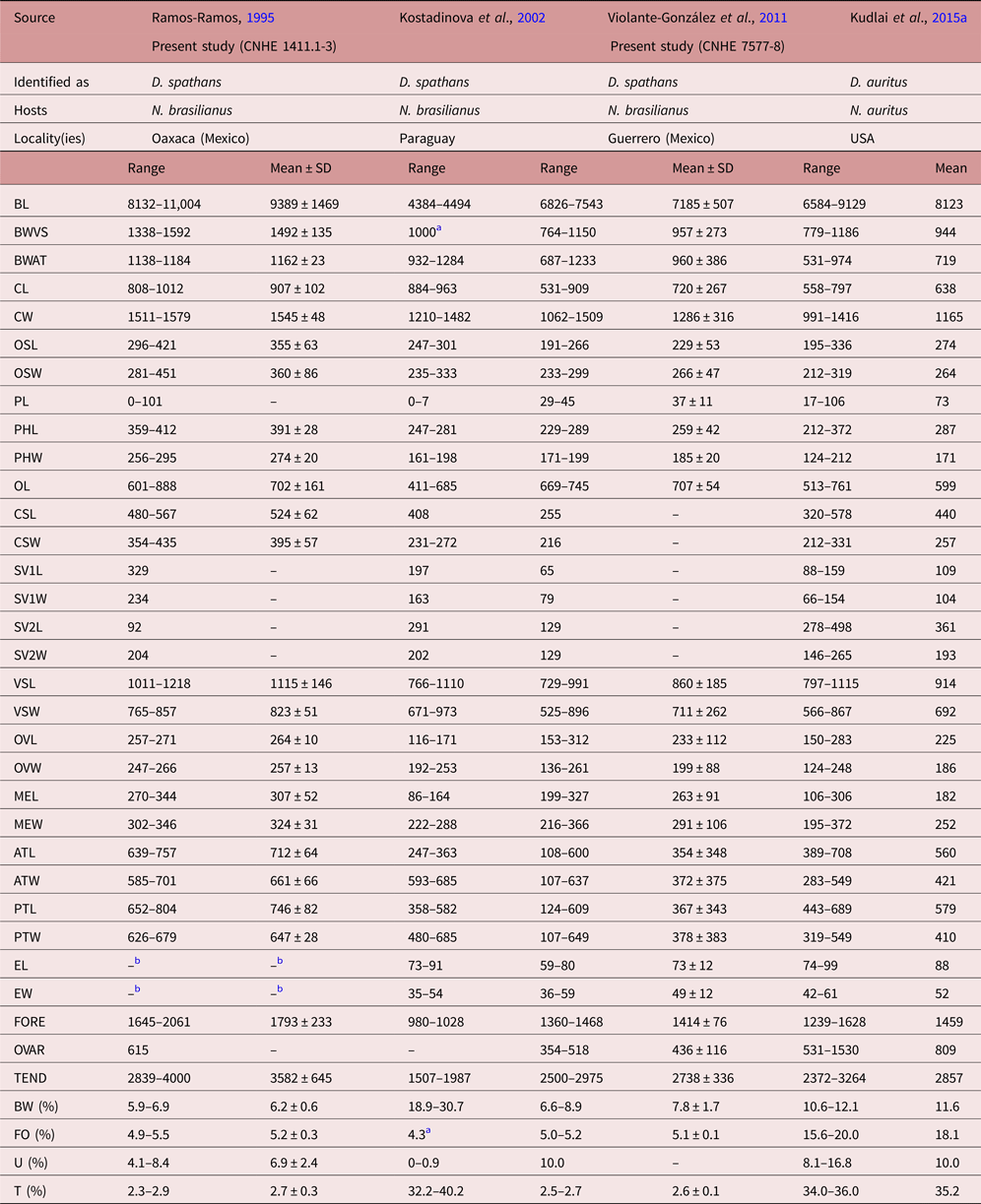
a Measurements taken from the published figure.
b Eggs hydrated in all specimens examined.
Drepanocephalus mexicanus Lamothe-Argumedo & Pérez-Ponce de León, 1989
Taxonomic summary
Synonyms. Paryphostomum mexicanum (Kostadinova et al., 2002); Patasiger mexicanus (Tkach et al., 2016).
Type host. Nannopterum brasilianus Gmelin (Pelecaniformes: Phalacrocoracidae).
Type locality. Teapa (17°34′59′′N, 92°53′30′′W), Tabasco.
Other localities. Presa La Angostura (16°11′31″N, 92°59′52″W), Chiapas; La Tovara, San Blas (21°32′00″N, 105°17′22″W), Nayarit; Río Tehuantepec (16°23′55″N, 95°18′30″W), Oaxaca; Tlacotalpan (18°36′0″N, 95°39′0″W), Veracruz.
Localities in other countries. Brazil (Pinto et al., Reference Pinto, Griffin, Quiniou, Ware and Melo2016, as Drepanocephalus sp., cercariae from Biomphalaria straminea).
Site in host. Intestine.
Voucher specimens. CNHE 10307–10309.
Description
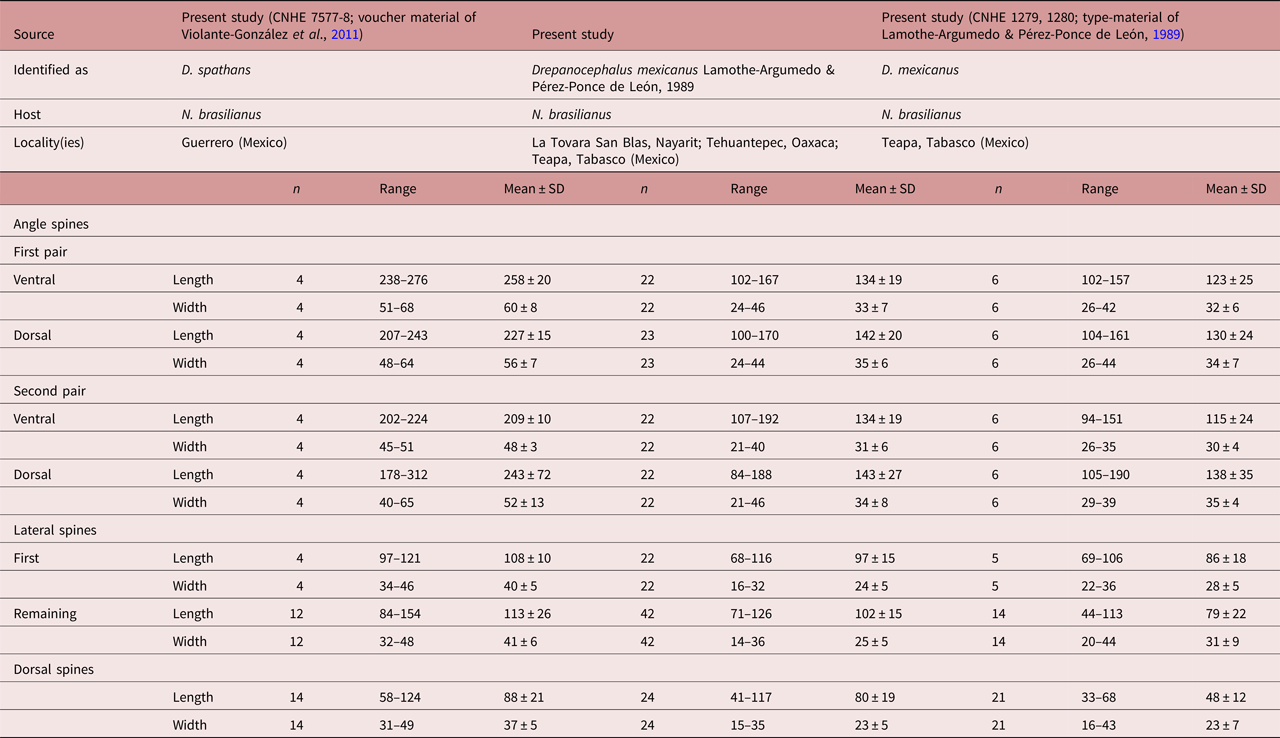
Based on 21 gravid specimens. Body (figs 4d–f, 6) elongate (BW = 5.2–7.9%) with maximum width (598–946) at level of ventral sucker. Body length 3060–6450; width at level of anterior testis 390–826. Forebody 569–1342 long (FO = 4.1–6.9%). Tegument with small spines distributed laterally, from head collar, to reach the posterior end of ventral sucker (fig. 4d–f). Head collar reniform, strongly muscular, 282–560 long, 402–836 wide, ventral lappets well developed, inclined towards the ventral region. Collar spines 27, large, in single row. Four angle spines in two pairs with ventral and dorsal spines (pair 1: ventral 102–157 long, 24–46 wide; dorsal 104–161 long, 26–44 wide; pair 2: ventral 94–151 long, 26–35 wide; dorsal 105–190 long, 29–39 wide). Four lateral spines on each lappet, in single row, first 69–106 long, 22–36 wide and remaining 44–113 long, 20–44 wide. Dorsal spines 11 in single row, of the same size (33–68 long, 16–43 wide). Oral sucker subterminal, subspherical, 106–219 long, 123–233 wide. Ventral sucker strongly muscular, cup-shaped, located in first quarter of body, 480–835 long, 363–657 wide. Prepharynx visible, 34–200 long. Pharynx muscular, elongate, 127–228 long, 97–209 wide (fig. 6a). Oesophagus 218–637 long, leading to an intestinal bifurcation anterior to ventral sucker. Caeca reach close to posterior extremity of body. Testes tandem, lobed, widely separated; anterior testis just post-equatorial, with 3–6 lobes, 300–602 long, 180–534 wide; posterior testis with 3–7 lobes, 248–655 long, 199–546 wide. Post-testicular region 1168–2120 long (fig. 6b). Cirrus sac small, oval 172–377 long, 111–253 wide, antero-dorsal to ventral sucker. Cirrus tubular, coiled, unspined. Internal seminal vesicle bipartite. Anterior portion smaller than posterior portion. Pars prostatica weakly developed; prostatic cells surround pars prostatic. Genital pore median just post-bifurcal (fig. 6c). Ovary small, dextral, entire, rounded, 122–248 long 113–201 wide anterior to Mehlis’ gland. Distance from ventral sucker to ovary (U = 5.6–33.4%). Mehlis’ gland median. Uterus relatively long, metraterm muscular, slightly larger than cirrus sac (fig. 6c). Eggs 62–86 long, 33–57 wide. Vitellarium in two lateral fields of small follicles, between posterior margin of ventral sucker and posterior extremity. Follicles tend to overlap in the post-testicular space (fig. 6b).
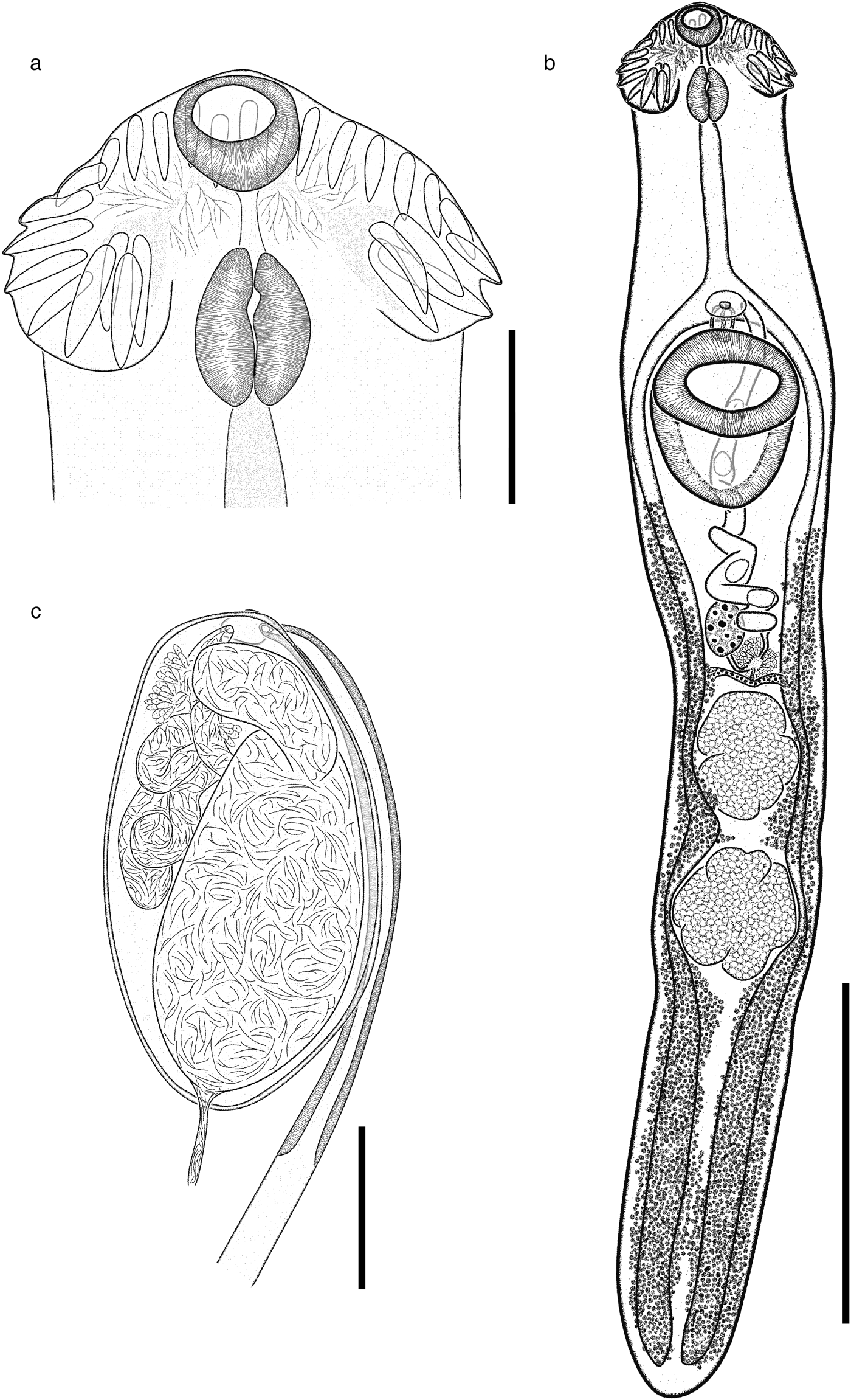
Fig. 6. Drepanocephalus mexicanus from N. brasilianus: (a) head collar; (b) whole worm; (c) terminal genitalia. Scale bars: (a) 200 μm; (b) 1000 μm; (c) 100 μm.
Remarks
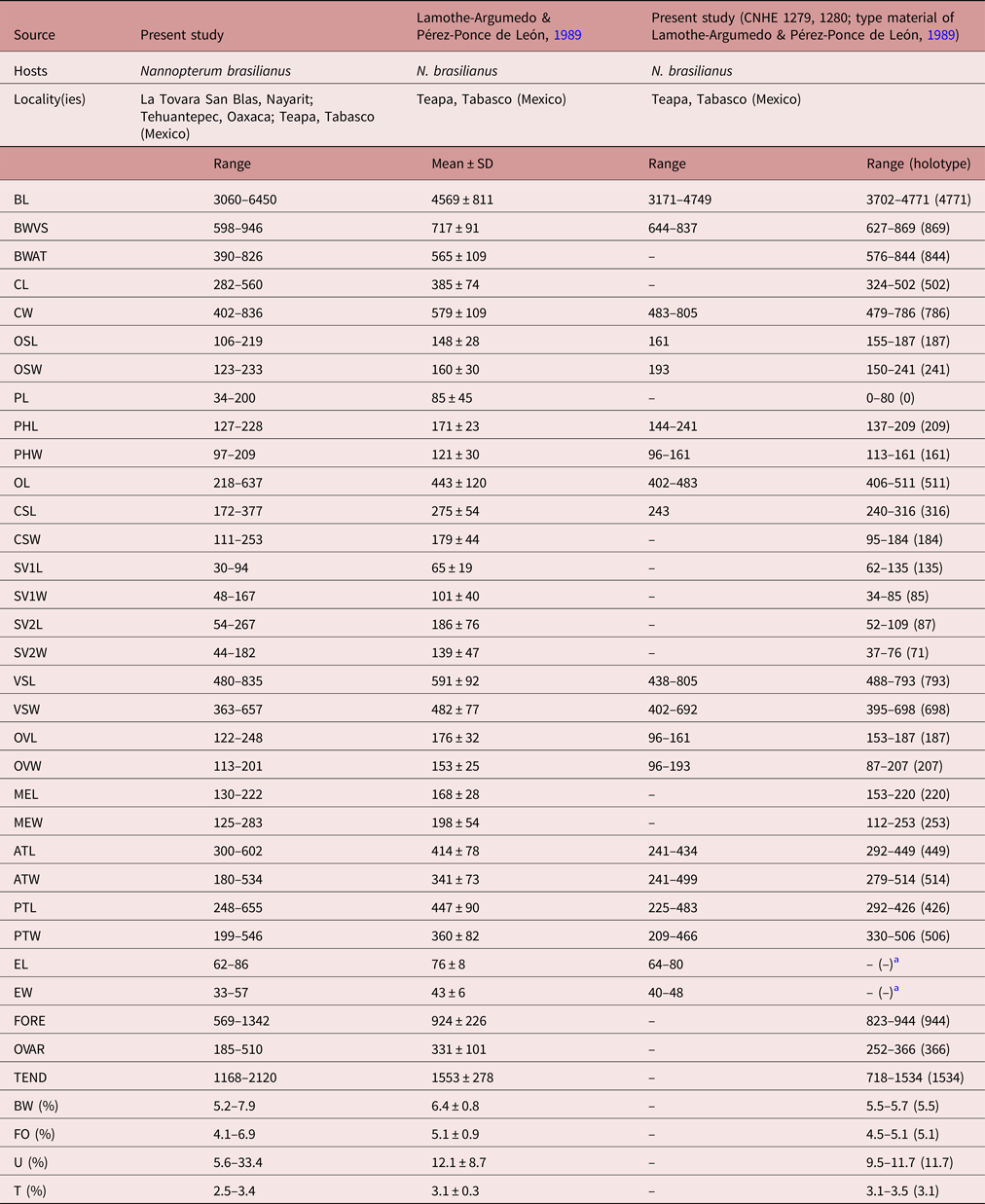
Specimens of this species were collected in four localities of Mexico as parasites of N. brasilianus. In particular, our specimens were identified as D. mexicanus by having a reniform head collar, tegument with long and thin spines placed laterally between head collar and posterior part of ventral sucker (see fig. 4d–f). Our specimens of D. mexicanus showed some level of morphological variability. For instance, meristic data of newly collected material provide lower limits for the following characters: BL (3060–6450), BWVS (598–946), BWAT (390–826), CL (282–560), CW (402–836), OSL (106–219), OSW (123–233), PHL (127–228), PHW (97–209), OL (218–637), CSL (172–377), SV1L (30–94), VSW (363–657), MEL (130–222), ATW (180–534), PTW (199–546), EL (62–86), EW (33–57), FORE (569–1342), OVAR (185–510), BW (%) (5.2–7.9), FO (%) (4.1–6.9), U (%) (5.6–33.4) and T (%) (2.5–3.4) (table 4). Likewise, newly collected material provides higher upper limits for the size of the body, head collar, cirrus sac, posterior portion of seminal vesicle, Mehlis’ gland, testes and eggs (table 4). Additionally, our study reveals higher limits for the length of the oral and ventral suckers, prepharynx, oesophagus, ovary, forebody and uterus, and for the width of the pharynx and the anterior portion of the seminal vesicle (table 4).
Table 4. Comparative measurements of Drepanocephalus mexicanus Lamothe-Argumedo & Pérez-Ponce de León, 1989.
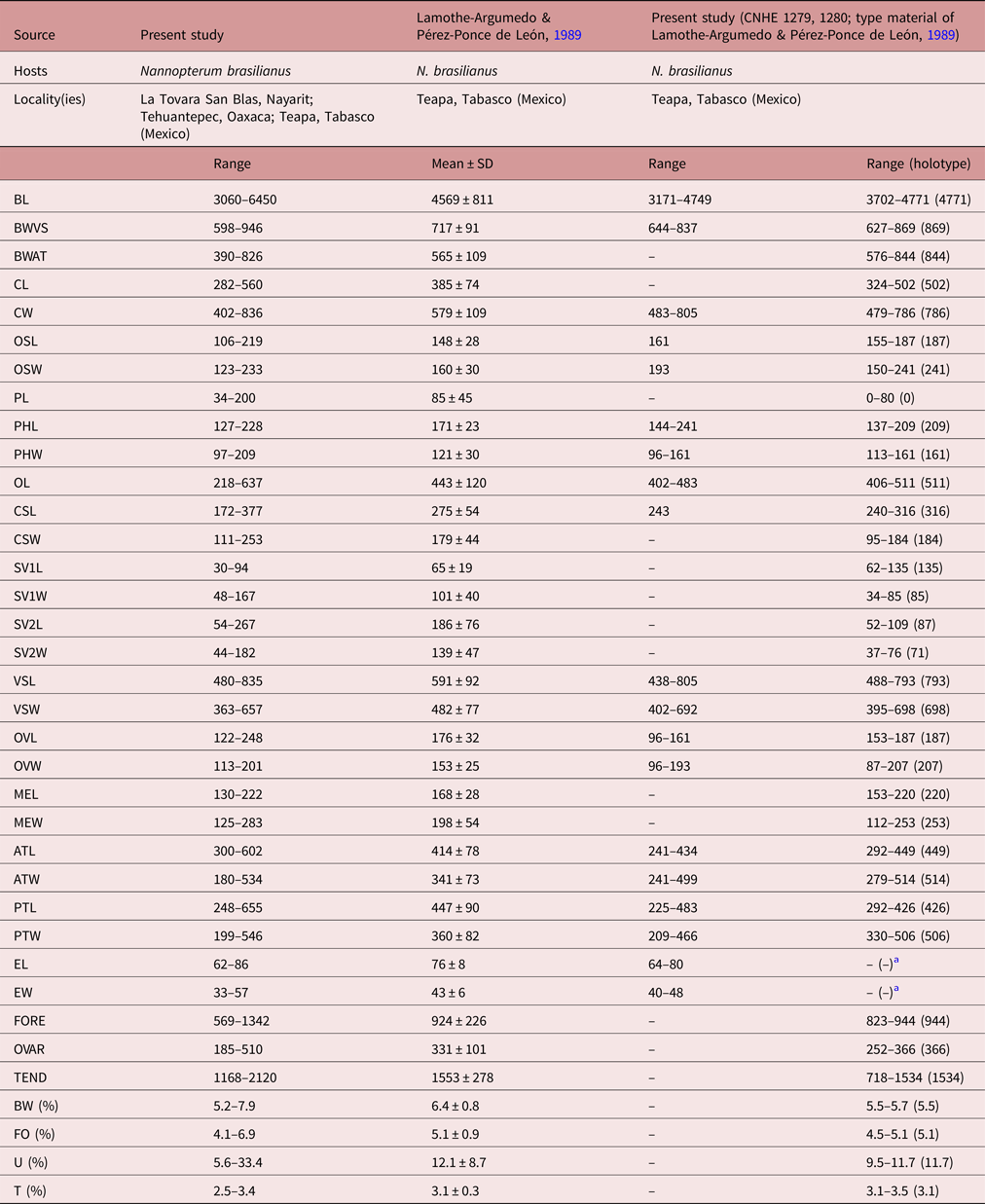
a Eggs hydrated in all specimens examined.
Discussion
The genus Drepanocephalus was erected to include D. spathans, a parasite of the Neotropical cormorant N. brasilianus from Brazil. This species was described from a single specimen (Dietz, Reference Dietz1909). Kostadinova et al. (Reference Kostadinova, Voucher and Gibson2002) re-described the species from specimens collected from the type host in Paraguay. The species composition of the genus has been controversial ever since. Until now, the genus contained three valid species, i.e. D. spathans, D. olivaceus and D. auritus. Drepanocephalus spathans is distributed mostly in the Neotropical region, parasitizing N. brasilianus in localities of Argentina, Brazil, Colombia, Paraguay and Venezuela (Caballero Caballero & Díaz Ungria, Reference Caballero Caballero and Díaz Ungria1958, and references therein; Kostadinova et al., Reference Kostadinova, Voucher and Gibson2002; Drago et al., Reference Drago, Lunaschi and Schenone2011), but also in S. leucogaster in Colombia (Rietschel & Werding, Reference Rietschel and Werding1978). In addition, this species was recorded as a parasite of the double-crested cormorant, N. auritus, in some localities of the USA and Canada (e.g. Flowers et al., Reference Flowers, Poore, Mullen and Levy2004; Wagner et al., Reference Wagner, Hoberg, Somer, Soos, Fenton and Jenkins2012; O'Hear et al., Reference O'Hear, Pote, Yost, Doffitt, King and Panuska2014). The second species, D. olivaceus, is a parasite of N. brasilianus in Venezuela (Nasir & Marval, Reference Nasir and Marval1968), although it was later reported in the same host in Mexico (Ramos-Ramos, Reference Ramos-Ramos1995) and Brazil (Monteiro et al., Reference Monteiro, Amato and Amato2011). The third species, D. auritus, was recently described as a parasite of the double-crested cormorant N. auritus in the USA and, apparently, is also found in Brazil, based on the comparison of sequence DNA data from cercariae obtained from the snail B. straminea (Pinto et al., Reference Pinto, Griffin, Quiniou, Ware and Melo2016). The generation of molecular data for several molecular markers in recent years for individuals of Drepanocephalus, either as larval forms (mostly cercariae from snails) or adults from cormorants, in localities of Brazil, Canada and the USA has resulted in a re-interpretation of distribution range of the aforementioned species. For instance, in the most recent assessment of the taxonomic identity of some species of Drepanocephalus, Pinto et al. (Reference Pinto, Griffin, Quiniou, Ware and Melo2016) concluded that one of the two morphotypes of the cercariae released by B. straminea in Brazil corresponded, according to molecular markers, with D. auritus. Meanwhile, a second cercarial morphotype was smaller and differed significantly from D. auritus for two mitochondrial markers (nad1 and cox1). These authors suggested that this species may be conspecific with D. spathans or, alternatively, with D. olivaceus.
The results obtained in the present study are very useful for re-evaluation of the current species composition of the genus Drepanocephalus. The large number of adult individuals belonging to the genus Drepanocephalus from a wide geographic range, comprising both the Nearctic and Neotropical regions of Mexico, filled a gap in our knowledge of the genetic make-up of species allocated to that genus, because only sequences from Canada, USA and Brazil had been documented (Griffin et al., Reference Griffin, Khoo, Quiniou, O'Hear, Pote, Greenway and Wise2012; O'Hear et al., Reference O'Hear, Pote, Yost, Doffitt, King and Panuska2014; Kudlai et al., Reference Kudlai, Kostadinova, Pulis and Tkach2015a; Pinto et al., Reference Pinto, Griffin, Quiniou, Ware and Melo2016). Based solely on morphology, our specimens were identified as D. spathans, and that included specimens with contiguous and separate testes, a morphological character used by Kudlai et al. (Reference Kudlai, Kostadinova, Pulis and Tkach2015a) to distinguish D. auritus from D. spathans. Our molecular and morphological results revealed the need to conduct some nomenclatural changes, and to revise the current species composition of the genus Drepanocephalus.
First, the ML and Bayesian trees obtained in the current study, inferred with five molecular markers, consistently showed that the species P. mexicanus is not closely related to other members of the genus Petasiger and, in consequence, we propose to re-allocate it to its original denomination as D. mexicanus (Lamothe-Argumedo & Pérez-Ponce de León, Reference Lamothe-Argumedo and Pérez-Ponce de León1989). Interestingly, in our phylogenetic analyses inferred with LSU, ITS, cox1 and nad1, the sequences identified as Drepanocephalus sp. by Pinto et al. (Reference Pinto, Griffin, Quiniou, Ware and Melo2016) from Brazil are nested within all newly generated sequences of D. mexicanus. Accordingly, it seems that the distribution range of this species extends from Mexico to Brazil, infecting the Neotropical cormorant N. brasilianus as the definitive host, and the snail B. straminea as the first intermediate host. According to Thompson (Reference Thompson2011), at least 11 species of Biomphalaria occur in Mexico, although the species B. straminea is restricted to other areas of the Neotropical region. Even though the snail has enlarged its distribution over the past decades and can be considered as the most invasive planorbid species (introduced in the Caribbean area and islands of the Lesser Antilles, and even outside of the neotropics (Pointier et al., Reference Pointier, David and Jarne2005, and references therein)), it has not been introduced into Mexico. However, we can expect to find the cercariae of D. mexicanus parasitizing one or more species of planorbid snails in Mexico, but this needs to be confirmed in future studies using a molecular approach to confirm conspecificity. In the USA, the cercariae of D. spathans have been reported in the planorbid snail P. trivolvis (Griffin et al., Reference Griffin, Khoo, Quiniou, O'Hear, Pote, Greenway and Wise2012). This shows that other species of planorbid snails may act as the first intermediate host of this group of trematodes.
Second, the phylogenetic position of the newly generated sequences with respect to all those available in the GenBank corroborates that D. spathans and D. auritus nest in the same monophyletic lineage. The genetic divergence among specimens was very low for the molecular markers (0% for ITS, SSU, LSU; from 0 to 1.8% for nad1; between 2.65 and 4.41% for cox1 and between 0 and 3% among specimens that showed both morphotypes that shared the same clade for cox1). According to Kudlai et al. (Reference Kudlai, Kostadinova, Pulis and Tkach2015a), the most characteristic morphological traits that differentiate the species D. auritus from D. spathans are the shape of the testes and their position with respect to each other, i.e. the inter-testicular space. Some published reports on the presence of D. spathans from South America (e.g. Drago et al., Reference Drago, Lunaschi and Schenone2011, Fig. 10 on p. 874) clearly show that specimens may possess a wide inter-testicular space. Our observations of the morphology and measurements of D. spathans (table 3), show a wide range of morphological variation in the diagnostic characters used by Kudlai et al. (Reference Kudlai, Kostadinova, Pulis and Tkach2015a) to differentiate D. auritus from D. spathans (fig. 5e). In addition, the morphometric data provided in our study for specimens collected from cormorants across Nearctic and Neotropical areas of Mexico (table 3), show an overlap in the range of the measurements used by Kudlai et al. (Reference Kudlai, Kostadinova, Pulis and Tkach2015a) to further distinguish between D. auritus and D. spathans, including collar length, hindbody width, forebody length, post-testicular space, prepharynx length and length of the posterior part of the seminal vesicle. Overall, our results show that all the specimens distributed between Canada in North America and Argentina in South America are conspecific, corresponding with the species D. spathans. In contrast with the decision taken by Pinto et al. (Reference Pinto, Griffin, Quiniou, Ware and Melo2016) to consider one of the species of Drepanocephalus from Brazil to be conspecific with D. auritus, we follow the principle of priority of the International Code of Zoological Nomenclature (Art. 23) and the valid name for the species should be D. spathans Dietz, 1909, and we propose that the species D. auritus is a synonym of D. spathans. It is our hope that our results contribute to solving the unsettled taxonomic history of this species, and help avoid future confusion about the identification of this species. For instance, considering the current controversy and status of D. auritus, Sheehan et al. (Reference Sheehan, Hanson-Dorr, Dorr, Yarrow and Johnson2016) actually identified adult specimens in cormorants from 11 locations in Alabama, Minnesota, Mississippi and Vermont, USA as D. (auritus) spathans.
Phylogenetic analysis also showed that both D. spathans and D. mexicanus form well-supported monophyletic clades, particularly when mtDNA genes are used for phylogenetic inference. They are sister species, although no sequences of the other allegedly valid species in the genus are yet available. The genetic divergence between D. mexicanus and D. spathans was relatively high, particularly for the two mtDNA genes (13.45–15.90% for nad1, and 12.40–14.40% for cox1). These high levels of genetic divergence of mitochondrial genes among species are similar to those exhibited between species pairs of echinostomatids. For instance, the genetic divergence among species of Echinostoma Rudolphi, 1809 varied from 9.6 to 30.8% for nad1 (Bowles & McManus, Reference Bowles and McManus1993). In a study aimed at comparing between mitochondrial genes and ribosomal internal transcribed regions in prospecting for cryptic species of platyhelminths, Vilas et al. (Reference Vilas, Criscione and Blouin2005) found genetic divergence levels between 11.9 and 16.6% for nad1 between four species pairs of echinostomatids. More recently, divergence levels from 16 to 32% for cox1 were reported between two species of Echinostoma (E. revolutum Fröhlich, 1802 and E. malayanum Leiper, 1911) (Saijuntha et al., Reference Saijuntha, Sithithaworn, Duenngai, Kiatsopit, Andrews and Petney2011).
Morphologically, D. mexicanus differs from D. spathans in: (1) the head-collar shape (reniform vs. falciform, see fig. 4); (2) a shorter first pair of ventral (102–167 vs. 172–320) and dorsal (100–171 vs. 200–384) angle spines (see table 5); (3) well-developed ventral lappets vs. head collar with an invagination formed by the two lappets (fig. 4b, e); (4) tegumental spines distributed between head collar and posterior margin of ventral sucker vs. spines distributed between head collar and anterior margin of ventral sucker (fig. 4a, d); and (5) shape and size of the tegumental spines (large–sharp (11.6–16.1 × 2.9–7.) vs. short–wider (4.2–12.9 × 3.3–13.9)) (see fig. 4c, f).
Table 5a. Measurements of head-collar spines of Drepanocephalus spathans Dietz, 1909 and D. mexicanus Lamothe-Argumedo & Pérez-Ponce de León, 1989.
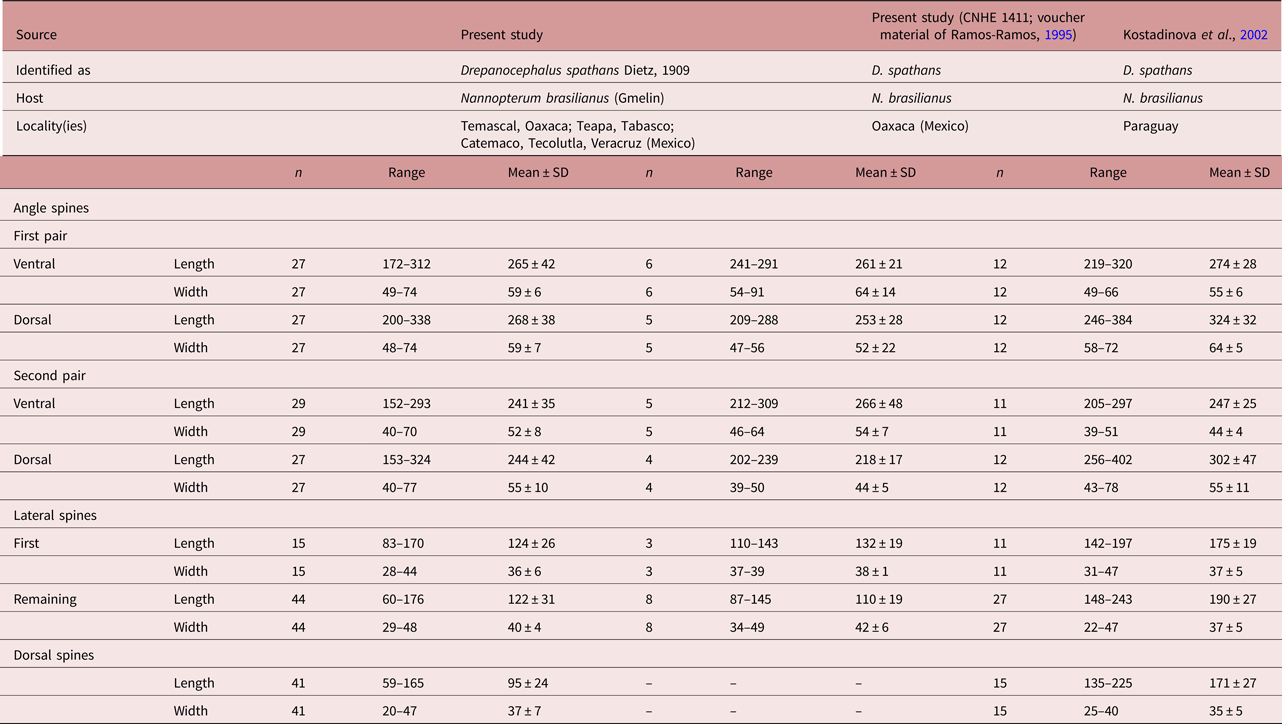
Table 5b. Measurements of head-collar spines of Drepanocephalus spathans Dietz, 1909 and D. mexicanus Lamothe-Argumedo & Pérez-Ponce de León, 1989, continued.
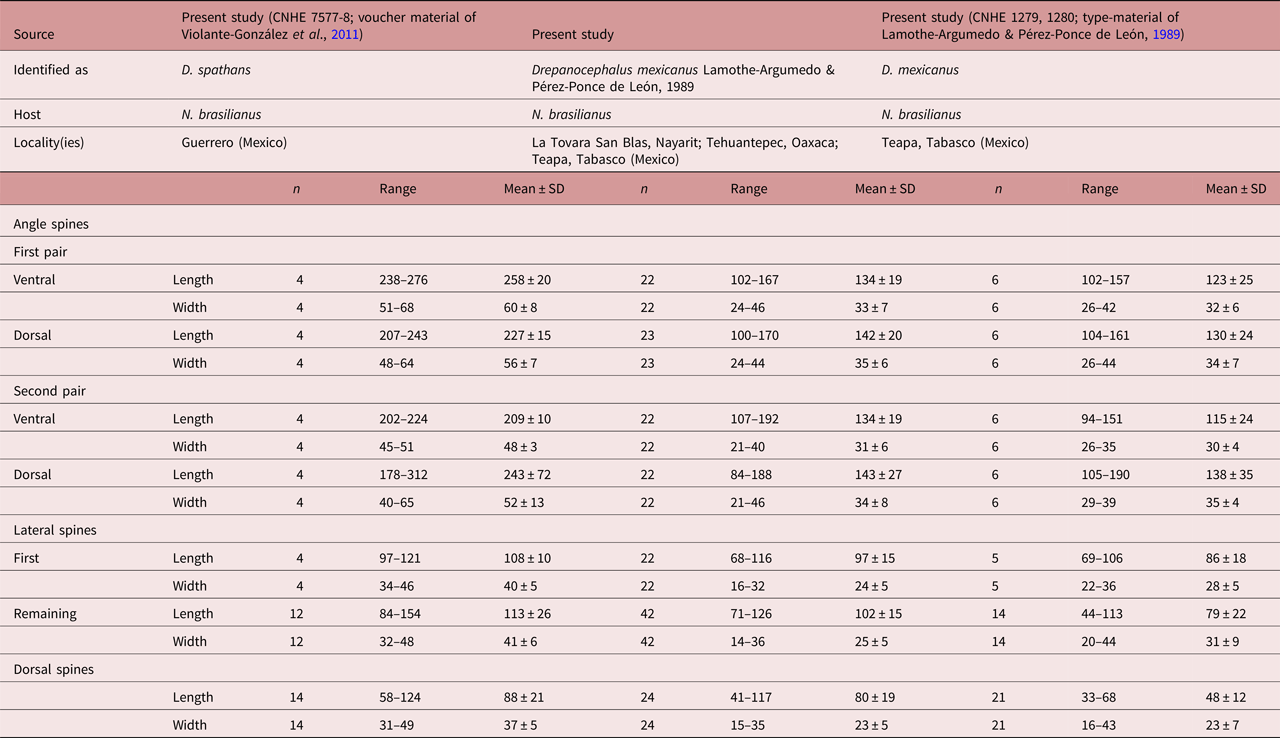
With the re-allocation of D. mexicanus and the synonymy of D. auritus with D. spathans proposed in this study, the genus Drepanocephalus now contains three nominal species: (1) D. spathans, associated with the Neotropical cormorant N. brasilianus, the double-crested cormorant N. auritus and the brown booby S. leucogaster, distributed widely across the Americas, in Argentina, Brazil, Paraguay, Venezuela, Colombia, Mexico, USA and Canada (Ramos-Ramos, Reference Ramos-Ramos1995; Kostadinova et al., Reference Kostadinova, Voucher and Gibson2002; Wagner et al., Reference Wagner, Hoberg, Somer, Soos, Fenton and Jenkins2012; Griffin et al., Reference Griffin, Khoo, Steadman, Ware, Quinou, Mischke, Greenway and Wise2014; O'Hear et al., Reference O'Hear, Pote, Yost, Doffitt, King and Panuska2014; Pinto et al., Reference Pinto, Griffin, Quiniou, Ware and Melo2016; Sheehan et al., Reference Sheehan, Hanson-Dorr, Dorr, Yarrow and Johnson2016); (2) D. mexicanus, only found parasitizing the Neotropical cormorant N. brasilianus distributed in Mexico and Brazil; and, finally, (3) D. olivaceus from N. brasilianus in Venezuela and Brazil.
The species Petasiger parvicephalum was originally described as a member of Drepanocephalus and transferred to Paryphosomum by Kostadinova et al. (Reference Kostadinova, Voucher and Gibson2002), and more recently to the genus Petasiger (Tkach et al., Reference Tkach, Kudlai and Kostadinova2016). Considering the morphology of the species, it may belong in Drepanocephalus but further molecular data from the type locality in Colombia are necessary to confirm its taxonomic status. As we show in this study, the records of D. olivaceus in Mexico (Pineda-López et al., Reference Pineda-López, Páramo, Trejo, Almeyda, Osorio and Pérez Ponce de León1985; Ramos-Ramos, Reference Ramos-Ramos1995; Violante-González et al., Reference Violante-González, Monks, Gil-Guerrero, Rojas-Herrera, Flores-Garza and Larumbe-Morán2011) are incorrect, and in fact correspond with D. spathans. Our observations of the morphological characters of museum specimens from the studies of Ramos-Ramos (Reference Ramos-Ramos1995) (CNHE 1141) and Violante-González et al. (Reference Violante-González, Monks, Gil-Guerrero, Rojas-Herrera, Flores-Garza and Larumbe-Morán2011) (CNHE 7577-7578) corroborate the conclusion that these specimens belong to D. spathans.
In addition, sequence data were generated from specimens of Drepanocephalus collected in Temascal, Oaxaca – the same host and locality as in the study by Ramos-Ramos (Reference Ramos-Ramos1995), demonstrating that these worms belong to D. spathans. Our results also show that both species of Drepanocephalus (D. spathans and D. mexicanus) occur in sympatry in the Neotropical cormorant in at least five of the sampled localities (see localities 7, 10, 11, 17, 20 in fig. 1), corresponding to the Neotropical biogeographical region. Still, the study of the first and second intermediate hosts in the life cycle of these parasites is necessary to establish a link among life-cycle stages, especially in species such as D. spathans, whose extensive distributional range across the Americas (from Canada to Argentina) is determined primarily by the vagility of their bird hosts, although it is possible that the first and second intermediate hosts are different across the distributional range of the species. We have demonstrated here that, contrary to what could be expected in finding a complex of cryptic species, considering the wide distributional range of D. spathans (Blouin, Reference Blouin2002), they actually represent a single species, genetically very similar but showing some level of intraspecific morphological variability. This seems to represent a case of a widely distributed species across the Americas, whose distributional range is mainly determined by the bird definitive host, although, surely, first and second intermediate hosts must also play an important role in its geographical distribution. Other trematode parasites of both species of cormorants (N. brasilianus and N. auritus), i.e. Austrodiplostomum and Hysteromorpha, may follow the same biogeographical pattern in cormorants across the Americas, from Argentina to Canada (see Drago et al., Reference Drago, Lunaschi and Schenone2011; Locke et al., Reference Locke, McLaughlin, Lapierre, Johnson and Marcogliese2011; García-Varela et al., Reference García-Varela, Sereno-Uribe, Pinacho-Pinacho, Domínguez-Domínguez and Pérez-Ponce de León2016); however, more detailed taxonomic work is required. The case of the status of Austrodiplostomum ostrowskiae Dronen, 2009 in relation to the species from South America, A. mordax Szidat & Nani, 1951 and A. compactum (Lutz, 1928) Dubois, 1936, may result in a single and widely distributed species in cormorants across the Americas (pending molecular analysis including samples from the southern part of the continent), as is the case for Hysteromorpha triloba Rudolphi, 1919.
Acknowledgements
We are grateful to Alejandra López, Carlos Pinacho and Leopoldo Andrade for their help during field work. We also thank Berenit Mendoza for her help with the use of the SEM unit, and Luis García Prieto for providing material from the CNHE.
Financial support
This research was supported by grants from the Programa de Apoyo a Proyectos de Investigación e Inovación Tecnológica (PAPIIT-UNAM) IN206716 and IN202617 to M.G.V. and G.P.P.L., respectively.
Conflict of interest
None.
Ethical standards
Specimens were collected under the Cartilla Nacional de Colector Científico (FAUT 0202 and 0057) issued by the Secretaría del Medio Ambiente y Recursos Naturales (SEMARNAT), to M.G.V. and G.P.P.L., respectively.