Introduction
Metastatic bone cancer is a common and severe complication in advanced diseases.Reference Serafini1, Reference Pandit-Taskar, Batraki and Divgi2 It develops in up to 70% of patients with prostate cancer and breast cancer, and in up to 30% of those with cancers of the lung, bladder and thyroid.3, Reference Lipton4 In these patients who have progressive disease despite treatment, a systemic bone-avid radiopharmaceutical for treatment of widespread bony metastases has obvious potential benefits.Reference Eary, Collins and Stabin5
Nowadays, many radiopharmaceuticals are developed for treatment of painful metastases. An impressive array of radionuclides such as 32P, 89Sr, 186Re, 188Re, 153Sm, 166Ho and 177Lu is used to develop these radiopharmaceuticals. β − particles of low energies are recommended for bone pain palliation, whereas those with higher energies are used for the bone marrow ablation.Reference Bouchet, Bolch and Goddu6 The most important point that should be considered in developing radiopharmaceuticals as the bone pain palliative or the bone marrow ablative agents is the dose delivered to the bone marrow.
Among the therapeutic radionuclides, 153Sm with the favourable radiation characteristics [t 1/2=1·93d, β max=0·81 MeV (20%), 0·71 MeV (49%), 0·64 MeV (30%) and γ=103 keV (30%)],Reference Ouadi, Loussouarn and Morandeau7 is the most widely used radionuclide for radiotherapy of the bone metastases in the form of 153Sm-EDTMP (Lexidronam) in the United States.Reference Pandit-Taskar, Batraki and Divgi8 However, other 153Sm bone-seeking agents have been developed paving the way for better pharmacokinetics with less side effects. 153Sm-triethylene tetramine hexa (methylene phosphonic acid) (153Sm-TTHMP) and 153Sm-propylene di-amino tetra methy1enephosphonicacid (153Sm-PDTMP) are the novel reported agents indicated as potential for clinical applications.Reference Majali, Mathakar and Shimpi9, Reference Naseri, Jalilian and Nemati Kharat10
The main goal in radiotherapy is to deliver the absorbed dose to the target organs in the highest possible amount, while as regards the other organs, especially within the critical organs, the absorbed dose is kept as low as possible. The absorbed dose plays an important role in evaluating the risks associated with the administration of radiopharmaceuticals and thus the maximum amount of activity that should be undertaken.Reference Stabin, Tagesson and Thomas11 Nowadays, in nuclear medicine, the most commonly used procedure for making the internal dose estimates is the radiation dose assessment resource (RADAR) method.Reference Stabin and Siegel12
In this piece of research work, for better evaluation of the therapeutic effects of 153Sm-TTHMP and 153Sm-PDTMP, these complexes were prepared and their biodistribution in the Syrian rats were studied up to 48 hours post injection. In addition, with regard to the importance of the absorbed dose in developing new therapeutic agents, the absorbed dose to human organs for these complexes was evaluated based on biodistribution studies on rats by RADAR method and was compared with 153Sm-EDTMP as the most clinically used bone pain palliative agent.
Materials and methods
Samarium-152 with purity of >98% was obtained from ISOTEC Inc. (Miamisburg, Ohio, USA). 153Sm was produced by 152Sm (n, γ) 153Sm nuclear reaction. All chemicals were purchased from Sigma-Aldrich Chemical Co. Whatman No. 2 paper was obtained from Whatman (UK). Radio-chromatography was performed by using a thin layer chromatography scanner, Bioscan AR2000 (Paris, France). A high purity germanium (HPGe) detector coupled with a Canberra™ (model GC1020-7500SL, Oak Ridge, USA) multichannel analyzer and a dose calibrator ISOMED 1010 (Dresden, Germany) were employed for counting distributed activity in the rat organs. Calculations were based on the 103 keV peak for 153Sm. All values were expressed as mean±standard deviation (mean±SD) and the data were compared using Student’s t-test. Statistical significance was defined as p<0·05. Animal studies were performed in accordance with the UK Biological Council’s Guidelines on the Use of Living Animals in Scientific Investigations, 2nd edition.
Production and quality control of 153SmCl3
One milligram of enriched 152Sm2O3 (152Sm, 98·7% from ISOTEC Inc.) was irradiated in a thermal neutron flux of 5×1013 n/cm2/second in a research reactor. The irradiated target was dissolved in 100 µL of 1·0 M HCl, to prepare 153SmCl3. The solution was filtered through a 0·22 µm filter (Millipore, Millex GV, County Cork, Irland). The radionuclidic purity of the solution was checked utilising β spectroscopy as well as HPGe spectroscopy. Also the radiochemical purity of the 152SmCl3 was studied using instant thin layer chromatography (ITLC) method by two solvent systems [A: 10 mM DTPA pH 4 and B: ammonium acetate 10%: methanol (1:1)].
Preparation and quality control of 153Sm-TTHMP and 153Sm-PDTMP
153Sm-TTHMP and 153Sm-PDTMP were prepared according to the previously mentioned procedure.Reference Naseri, Jalilian and Nemati Kharat10 Briefly, a stock solution was prepared by dissolving specified values of TTHMP and PDTMP (250, 200, 150, 100, 50, 10 mg) in 1·5 mL NaOH (2 N) and 3·5 mL distilled H2O. Then 300 µL of the stock solution was added to 200 µL of 153SmCl3 (210 MBq). The effect of pH was investigated by adjustment with phosphate buffer. The reaction mixtures were incubated by stirring at room temperature for 2 hours. The radiolabeling yield of the ligand was determined with paper chromatography employing Whatman No. 2 paper in NH4OH:MeOH:H2O (2:20:40) mixture.
Biodistribution of 153Sm-TTHMP and 153Sm-PDTMP in wild-type rats
Two-hundred microlitre of final 153Sm-TTHMP and 153Sm-PDTMP solutions with 5·55 MBq radioactivity were injected intravenously into rats through their tail vein. The total amount of the injected radioactivity into each animal was measured by counting the syringe before and after injection in a dose calibrator with fixed geometry. The animals were sacrificed at the exact time intervals (2, 4, 24, 48 hours). The activity concentration (A) of each tissue was calculated using an HPGe detector as13
where, ε is the efficiency at photopeak energy, γ is the emission probability of the γ line corresponding to the peak energy, t s is the live time of the sample spectrum collection in seconds, m is the mass (kg) of the measured sample, k 1, k 2, k 3, k 4 and k 5 are the correction factors for the nuclide decay from the time the sample is collected to start the measurement, the nuclide decay during counting period, self-attenuation in the measured sample, pulses loss due to random summing and the coincidence, respectively. N is the corrected net peak area of the corresponding photopeak given as
where N s is the net peak area in the sample spectrum, N b is the corresponding net peak area in the background spectrum and t b is the live time of the background spectrum collection in seconds.
The percentage of the injected dose per gram (%ID/g) for different organs was calculated by dividing the activity concentration of each tissue (A) to the injected activity and the mass of each organ. Five rats were sacrificed for each time interval. All values were expressed as mean±standard deviation and the data were compared using Student’s t-test.
Calculation of accumulated activity in human organs
The accumulated source activity for each organ of animals was calculated according to Equation (3), where A (t) is the activity of each organ at time t:
For this purpose, the data points representing the percentage-injected dose were created. A linear approximation was made between the two experimental points of times. The curves were extrapolated to infinity by fitting the tail of each curve to a monoexponential curve with the exponential coefficient equal to physical decay constant of 153Sm. The accumulated activity was calculated by computing the area under the curves.
The accumulated activity in the animals was extrapolated to the accumulated activity in humans by the proposed method of Sparks et al. [Equation (3)]:Reference Sparks and Aydogan14
 $$\tilde{\!\!A}_{{{\rm human\,organ}}} =\tilde{\!\!A}_{{{\rm animal \,organ}}} {\times}{{{{Organ mass_{{human}} } \over {Body mass_{{human}} }}} \over {{{Organ mass_{{animal}} } \over {Body mass_{{animal}} }}}}$$
$$\tilde{\!\!A}_{{{\rm human\,organ}}} =\tilde{\!\!A}_{{{\rm animal \,organ}}} {\times}{{{{Organ mass_{{human}} } \over {Body mass_{{human}} }}} \over {{{Organ mass_{{animal}} } \over {Body mass_{{animal}} }}}}$$
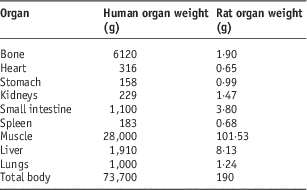
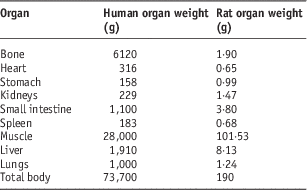
In order to extrapolate this accumulated activity to human, the standard mean weights of each organ for human and rat were used (Table 1).Reference Stabin and Siegel12, Reference Yousefnia, Zolghadri and Jalilian15
Table 1 The mean weights of organs for human and rat
Absorbed dose calculation
The absorbed dose in human organs was calculated by RADAR formalism based on biodistribution data in the rats:Reference Stabin and Siegel12
where à is the accumulated activity for each human organ, and DF is
where n i is the number of radiations with energy E emitted per nuclear transition, E i is the energy per radiation (MeV), ϕ i is the fraction of energy emitted that is absorbed in the target, m is the mass of the target region (kg) and k is some proportionality constant ![]() $$\left( {{{mGy \cdot {\rm k}g} \over {MBq \cdot s \cdot MeV}}} \right)$$
. DF represents the physical decay characteristics of the radionuclide, the range of the emitted radiations, and the organ size and configurationReference Bevelacqua16 expressed in mGy/MBq·s. DFs have been taken from the OLINDA/EXM software.Reference Stabin and Siegel12 It should be notified that D in Equation (5) is the absorbed dose in the target organ from a source organ and as a result, the total absorbed dose for each target organ was computed by the summation of the absorbed dose received from each source organ.
$$\left( {{{mGy \cdot {\rm k}g} \over {MBq \cdot s \cdot MeV}}} \right)$$
. DF represents the physical decay characteristics of the radionuclide, the range of the emitted radiations, and the organ size and configurationReference Bevelacqua16 expressed in mGy/MBq·s. DFs have been taken from the OLINDA/EXM software.Reference Stabin and Siegel12 It should be notified that D in Equation (5) is the absorbed dose in the target organ from a source organ and as a result, the total absorbed dose for each target organ was computed by the summation of the absorbed dose received from each source organ.
Results and discussion
Production and quality control of 153SmCl3
The radionuclide was prepared in a research reactor with a specific activity of 12·8 GBq/mg. After counting the samples on an HPGe detector, radionuclidic purity was higher than 99·99% [154Eu<4·7×10−5% of 153Sm and 155Eu<2·4×10−5% of 153Sm]. Radiochemical impurities in the 153Sm sample used in the radiolabeling step were checked by the two solvent systems. Whatman No. 2 was used as a stationary phase for paper chromatography system. In %10 ammonium acetate:methanol, the free samarium cation in 153Sm3+ form remained at the origin (R f=0·0), while other 153Sm species migrated to higher R fs (0·8). Another eluent for 153Sm3+ detection was 10 mM DTPA aqueous solution at pH 3 (R f=0·8).
Quality control of 153Sm-TTHMP and 153Sm-PDTMP
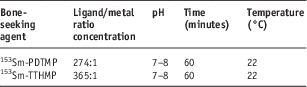
In order to obtain maximum complexation yield, several experiments were carried out by varying different reaction parameters such as ligand concentration, pH and reaction time. The optimised condition for radiolabeling of 153Sm-PDTMP and 153Sm-TTHMP are given in Table 2. The radiolabeled complexes were prepared by radiochemical purity of higher than 99% in the optimised conditions. ITLC chromatograms of 153SmCl3 and radiolabeled solutions in NH4OH: MeOH: H2O (2:20:40) are shown in Figure 1.

Figure 1 ITLC chromatograms of 153SmCl3 (left) and radiolabeled compounds (right) on Whatman No. 2 paper using NH4OH:MeOH:H2O (0·2:2:4). Abbreviations: ITLC, instant thin layer chromatography.
Table 2 The optimised conditions for radiolabeling of 153Sm-TTHMP and 153Sm-PDTMP
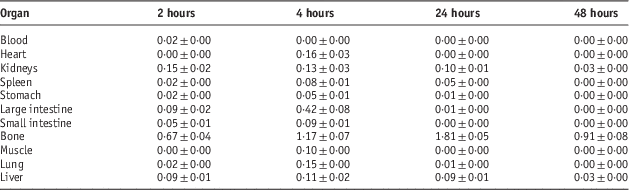
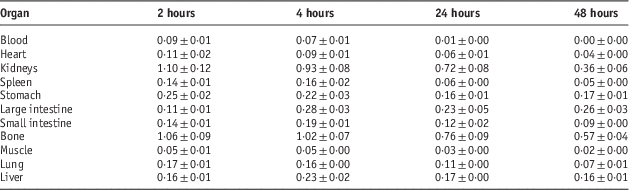
Biodistribution of radiolabelled compounds in Syrian rats
The animals were sacrificed by CO2 asphyxiation at the selected times after injection (2, 4, 24 and 48 hours). Dissection began by drawing blood from the aorta followed by removing the heart, spleen, muscle, bone, kidney, liver, intestine, stomach, lung and skin samples. The tissue uptakes were calculated as the percentage of the area under the curve of the related photopeak per gram of the tissue (%ID/g; Tables 3 and 4). The non-decay-corrected clearance curves from the main organ sources of the rats for 153Sm-TTHMP and 153Sm-PDTMP are shown in Figures 2 and 3, respectively.

Figure 2 Non-decay corrected clearance curves for each organ of Syrian rats after injection of 5·55 MBq 153Sm-TTHMP.

Figure 3 Non-decay corrected clearance curves for each organ of Syrian rats after injection of 5·55 MBq 153Sm-PDTMP.
Table 3 Percentage of injected dose per gram (ID/g %) after intravenous administration of 5·55 MBq 153Sm-PDTMP in Syrian rat tissues at 2, 4, 24 and 48 hours post injection
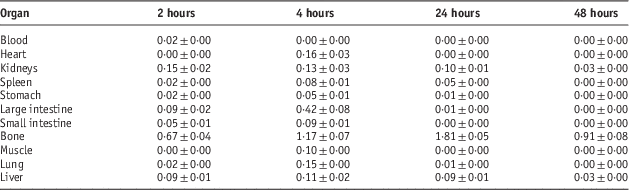
Table 4 Percentage of injected dose per gram (ID/g %) after intravenous administration of 5·55 MBq 153Sm-TTHMP in Syrian rat tissues at 2, 4, 24 and 48 hours post injection
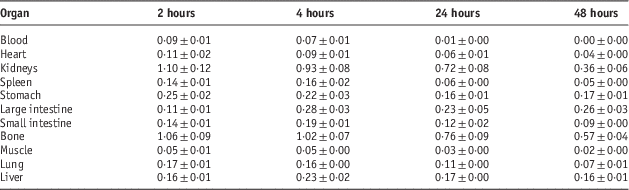
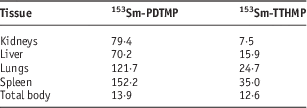
Dosimetric studies
The importance of an ideal radiopharmaceutical lies in the accumulation of the complex in the target organs compared with the critical organs. Due to the high radio-sensitivity of hematopoietic cells within the marrow of the trabecular bone cavities, the bone marrow is an absolutely main critical organ for metastatic bone pain palliation therapy.Reference Turner, Martindale and Sorby17 Therefore, for bone-seeking radiopharmaceuticals, the dose delivered to the bone marrow is one of the most important parameters that should be considered.
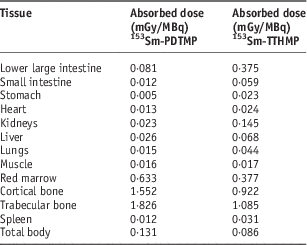
Dosimetric evaluation in human organs was carried out by the RADAR method based on biodistribution data in the rat organs. The absorbed dose in human organs after the injection of these complexes is presented in Table 5. The highest absorbed dose for 153Sm-TTHMP and 153Sm-PDTMP is observed in the trabecular bone with 1·085 and 1·826 mGy/MBq, respectively.
Table 5 The absorbed dose in each human organ after injection of 153Sm-PDTMP and 153Sm-TTHMP
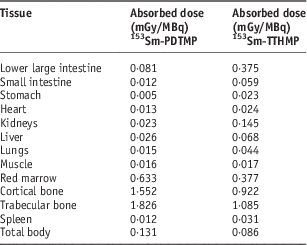
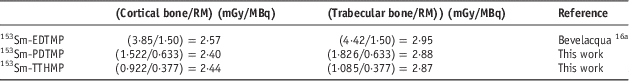
Since 153Sm-EDTMP is the most clinically used Sm-153 bone pain palliative radiopharmaceutical, the average bone/red marrow dose ratio after injection of these bone-seeking agents is compared with this value for 153Sm-EDTMP (Table 6). This ratio, as the target/critical organ dose ratio for these two complexes is approximately the same as 153Sm-EDTMP.
Table 6 The average bone/red marrow dose ratio after injection of 153Sm-EDTMP, 153Sm-TTHMP and 153Sm-PDTMP
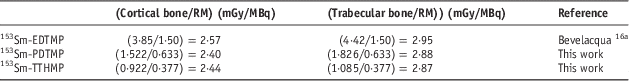
a The data are the average of the absorbed dose for 27 independent measurements after 153Sm-EDTMP injection to patients with skeletal metastases.
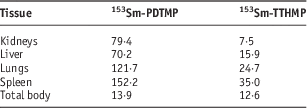
The dose ratio of the trabecular bone to the other critical tissue for 153Sm-TTHMP and 153Sm-PDTMP is compared in Table 7. The dose ratio of the trabecular bone to the other critical tissue for 153Sm-PDTMP is truly greater than 153Sm-TTHMP. This suggests that 153Sm-PDTMP has negligible undesirable uptake and therefore, it is a more appropriate agent for the bone pain palliation.
Table 7 Trabecular bone to other critical tissue dose ratio for 153Sm-TTHMP and 153Sm-PDTMP
Conclusion
153Sm-TTHMP and 153Sm-PDTMP were prepared in high radiochemical purity (>99%, ITLC). The end products of complexes were administered to the Syrian rats and the biodistribution of the complexes was checked 2–48 hours post injection, showing a basic process of accumulation in the bone tissue. Contrary to the bone tissues, all of the rest received almost an insignificant absorbed dose. The bone/red marrow dose ratio for these two complexes is approximately the same as 153Sm-EDTMP. 153Sm-PDTMP indicated lesser undesirable uptake compared with 153Sm-TTHMP. The results showed that these bone-seeking agents, especially 153Sm-PDTMP, have outstanding characteristics in comparison with 153Sm-EDTMP, the most clinically used bone pain palliative radiopharmaceutical and therefore can be good candidates for the bone pain palliation in the patients with the bone metastasis. According to the threshold amount of the absorbed dose for the critical organs, the obtained results can be useful for the determination of the maximum permissible injected activity of these radiopharmaceuticals for radiotherapy of the bone metastases in the treatment planning programs.
Acknowledgement
The authors wish to thank the Nuclear Sience & Technology Research Institute (NSTRI) for the financial support.