Introduction
The conceptualization of psychosis as an extended phenotype encompasses both psychosis spectrum disorder (any psychotic disorder) and subthreshold psychotic symptoms that are experienced by individuals in the general population (van Os, Reference van Os2016). About 7% of the general population may experience subthreshold psychotic experiences (Linscott and van Os, Reference Linscott and van Os2013). Subthreshold psychotic experiences are transitory in around 80%, persistent in around 20% and evolve into a psychotic disorder in 7%, with an annual transition rate of 0.5–1% (Linscott and van Os, Reference Linscott and van Os2013).
The pathway leading to psychotic disorder remains unknown. It is likely to involve a complex dynamic interaction between familial genetic risk, non-genetic factors such as exposure to environmental risks and interactions within and between symptoms themselves (Smeets et al., Reference Smeets, Lataster, Dominguez, Hommes, Lieb, Wittchen and van Os2012; van Os, Reference van Os2013). There is accumulating evidence that the earliest pathway to psychosis involves an interaction between ‘affective dysregulation’ (Myin-Germeys and van Os, Reference Myin-Germeys and van Os2007; Kramer et al., Reference Kramer, Simons, Wigman, Collip, Jacobs, Derom, Thiery, van Os, Myin-Germeys and Wichers2014) and ‘aberrant salience’ (Reininghaus et al., Reference Reininghaus, Kempton, Valmaggia, Craig, Garety, Onyejiaka, Gayer-Anderson, So, Hubbard, Beards, Dazzan, Pariante, Mondelli, Fisher, Mills, Viechtbauer, McGuire, van Os, Murray, Wykes, Myin-Germeys and Morgan2016). Studies suggest that psychosis in a group of patients begins when, first, affective dysregulation, under the influence of genetic and environmental risk factors, becomes ‘complicated’ by aberrant salience, which in turn, under the influence of the same genetic and environmental risk factors, may progress to full-blown psychotic symptoms (Hafner et al., Reference Hafner, Loffler, Maurer, Hambrecht and an der Heiden1999; Guloksuz et al., Reference Guloksuz, van Nierop, Lieb, van Winkel, Wittchen and van Os2015, Reference Guloksuz, van Nierop, Bak, de Graaf, Ten Have, van Dorsselaer, Gunther, Lieb, van Winkel, Wittchen and van Os2016; van Os and Reininghaus, Reference van Os and Reininghaus2016). Genetic and environmental risk factors thus may operate by impacting the degree of psychosis admixture (none, attenuated psychosis, overt psychotic symptoms) in initially simple states of affective dysregulation.
The role of affective dysregulation in the onset of psychosis derives from experimental and observational studies (Garety et al., Reference Garety, Kuipers, Fowler, Freeman and Bebbington2001; Hanssen et al., Reference Hanssen, Bak, Bijl, Vollebergh and van Os2005; Freeman et al., Reference Freeman, Dunn, Fowler, Bebbington, Kuipers, Emsley, Jolley and Garety2013a, Reference Freeman, Startup, Dunn, Cernis, Wingham, Pugh, Cordwell and Kingdon2013b; Bird et al., Reference Bird, Waite, Rowsell, Fergusson and Freeman2017), as well as experience sampling studies that found that a primary increase in stress-reactivity was associated with subsequent increase in psychotic experiences (Myin-Germeys and van Os, Reference Myin-Germeys and van Os2007; Kramer et al., Reference Kramer, Simons, Wigman, Collip, Jacobs, Derom, Thiery, van Os, Myin-Germeys and Wichers2014; Klippel et al., Reference Klippel, Myin-Germeys, Chavez-Baldini, Preacher, Kempton, Valmaggia, Calem, So, Beards, Hubbard, Gayer-Anderson, Onyejiaka, Wichers, McGuire, Murray, Garety, van Os, Wykes, Morgan and Reininghaus2017). In a recent experience sampling study, increased negative affect was found to result in later paranoia over the subsequent 180 min (Kramer et al., Reference Kramer, Simons, Wigman, Collip, Jacobs, Derom, Thiery, van Os, Myin-Germeys and Wichers2014). A network analysis of psychopathology similarly found that affective symptoms were likely to be on the pathway between environmental exposure (childhood adversity and cannabis use) and clinical psychosis (Isvoranu et al., Reference Isvoranu, Borsboom, van Os and Guloksuz2016a, Reference Isvoranu, van Borkulo, Boyette, Wigman, Vinkers and Borsboom2016b). Affective dysregulation has also been found to mediate the association between childhood adversity and psychosis (van Nierop et al., Reference van Nierop, van Os, Gunther, van Zelst, de Graaf, ten Have, van Dorsselaer, Bak, Myin-Germeys and van Winkel2014) and, conversely, childhood adversity is associated with greater stress-reactivity in individuals at risk of psychosis (Veling et al., Reference Veling, Counotte, Pot-Kolder, van Os and van der Gaag2016; Klippel et al., Reference Klippel, Myin-Germeys, Chavez-Baldini, Preacher, Kempton, Valmaggia, Calem, So, Beards, Hubbard, Gayer-Anderson, Onyejiaka, Wichers, McGuire, Murray, Garety, van Os, Wykes, Morgan and Reininghaus2017).
Aberrant salience, or the attribution of salience to typically non-salient stimuli, is a concept rooted in the dopaminergic hypothesis of psychosis (Kapur et al., Reference Kapur, Mizrahi and Li2005; Winton-Brown et al., Reference Winton-Brown, Fusar-Poli, Ungless and Howes2014). The concept suggests that a primary dopaminergic dysfunction results in attention and action-selection being redirected to irrelevant internal or external stimuli or being directed diffusely, leading to sensory overload. This state of aberrant salience leads to delusion formation, in an attempt by the individual to make sense of the experience (Winton-Brown et al., Reference Winton-Brown, Fusar-Poli, Ungless and Howes2014). Experience of aberrant salience was found to be associated with greater risk of psychotic experiences in at-risk individuals (Reininghaus et al., Reference Reininghaus, Kempton, Valmaggia, Craig, Garety, Onyejiaka, Gayer-Anderson, So, Hubbard, Beards, Dazzan, Pariante, Mondelli, Fisher, Mills, Viechtbauer, McGuire, van Os, Murray, Wykes, Myin-Germeys and Morgan2016). Psychotic experiences or attenuated psychosis has been shown to predict conversion to psychotic disorder (Brucato et al., Reference Brucato, Masucci, Arndt, Ben-David, Colibazzi, Corcoran, Crumbley, Crump, Gill, Kimhy, Lister, Schobel, Yang, Lieberman and Girgis2017; Crump et al., Reference Crump, Arndt, Grivel, Horga, Corcoran, Brucato and Girgis2017), suggesting that they lie on a spectrum of increasing psychopathology.
Among the environmental risk factors, childhood adversity (Varese et al., Reference Varese, Smeets, Drukker, Lieverse, Lataster, Viechtbauer, Read, van Os and Bentall2012), urbanicity (Vassos et al., Reference Vassos, Pedersen, Murray, Collier and Lewis2012; Heinz et al., Reference Heinz, Deserno and Reininghaus2013), and regular cannabis use (D’Souza et al., Reference D'Souza, Radhakrishnan, Sherif, Cortes-Briones, Cahill, Gupta, Skosnik and Ranganathan2016; Marconi et al., Reference Marconi, Di Forti, Lewis, Murray and Vassos2016) have been shown to increase the risk of developing psychotic experiences, persistent psychotic symptoms, and psychotic disorders in epidemiological studies. There is also emerging evidence that some environmental risk factors have stronger effects if there is also evidence of (proxy) genetic risk (van Os et al., Reference van Os, Kenis and Rutten2010). In the current study, we wished to examine the role of environmental risk factors in relation to the earliest ontogenesis of psychosis, defined as the degree of psychosis admixture ‘complicating’ an early state of affective dysregulation.
We wished to test the hypothesis that the association with known environmental risk factors (cannabis use, childhood adversity, and urbanicity) would grow progressively stronger across higher levels of psychosis admixture occurring across more severe affective states, in interaction with evidence of proxy genetic risk. In this analysis, we used evidence of familial affective dysregulation as a proxy for genetic liability, given earlier evidence that (i) molecular genetic risk for schizophrenia can be modeled through affective dysregulation in the relatives (van Os et al., Reference van Os, van der Steen, Islam, Guloksuz, Rutten and Simons2017); (ii) evidence indicating overlap in genetic risk for affective and psychotic disorder (Cardno et al., Reference Cardno, Marshall, Coid, Macdonald, Ribchester, Davies, Venturi, Jones, Lewis, Sham, Gottesman, Farmer, McGuffin, Reveley and Murray1999; Cross-Disorder Group of the Psychiatric Genomics Consortium et al., Reference Lee, Ripke, Neale, Faraone, Purcell, Perlis, Mowry, Thapar, Goddard, Witte, Absher, Agartz, Akil, Amin, Andreassen, Anjorin, Anney, Anttila, Arking, Asherson, Azevedo, Backlund, Badner, Bailey, Banaschewski, Barchas, Barnes, Barrett, Bass, Battaglia, Bauer, Bayes, Bellivier, Bergen, Berrettini, Betancur, Bettecken, Biederman, Binder, Black, Blackwood, Bloss, Boehnke, Boomsma, Breen, Breuer, Bruggeman, Cormican, Buccola, Buitelaar, Bunney, Buxbaum, Byerley, Byrne, Caesar, Cahn, Cantor, Casas, Chakravarti, Chambert, Choudhury, Cichon, Cloninger, Collier, Cook, Coon, Cormand, Corvin, Coryell, Craig, Craig, Crosbie, Cuccaro, Curtis, Czamara, Datta, Dawson, Day, De Geus, Degenhardt, Djurovic, Donohoe, Doyle, Duan, Dudbridge, Duketis, Ebstein, Edenberg, Elia, Ennis, Etain, Fanous, Farmer, Ferrier, Flickinger, Fombonne, Foroud, Frank, Franke, Fraser, Freedman, Freimer, Freitag, Friedl, Frisen, Gallagher, Gejman, Georgieva, Gershon, Geschwind, Giegling, Gill, Gordon, Gordon-Smith, Green, Greenwood, Grice, Gross, Grozeva, Guan, Gurling, De Haan, Haines, Hakonarson, Hallmayer, Hamilton, Hamshere, Hansen, Hartmann, Hautzinger, Heath, Henders, Herms, Hickie, Hipolito, Hoefels, Holmans, Holsboer, Hoogendijk, Hottenga, Hultman, Hus, Ingason, Ising, Jamain, Jones, Jones, Jones, Tzeng, Kahler, Kahn, Kandaswamy, Keller, Kennedy, Kenny, Kent, Kim, Kirov, Klauck, Klei, Knowles, Kohli, Koller, Konte, Korszun, Krabbendam, Krasucki, Kuntsi, Kwan, Landen, Langstrom, Lathrop, Lawrence, Lawson, Leboyer, Ledbetter, Lee, Lencz, Lesch, Levinson, Lewis, Li, Lichtenstein, Lieberman, Lin, Linszen, Liu, Lohoff, Loo, Lord, Lowe, Lucae, MacIntyre, Madden, Maestrini, Magnusson, Mahon, Maier, Malhotra, Mane, Martin, Martin, Mattheisen, Matthews, Mattingsdal, McCarroll, McGhee, McGough, McGrath, McGuffin, McInnis, McIntosh, McKinney, McLean, McMahon, McMahon, McQuillin, Medeiros, Medland, Meier, Melle, Meng, Meyer, Middeldorp, Middleton, Milanova, Miranda, Monaco, Montgomery, Moran, Moreno-De-Luca, Morken, Morris, Morrow, Moskvina, Muglia, Muhleisen, Muir, Muller-Myhsok, Murtha, Myers, Myin-Germeys, Neale, Nelson, Nievergelt, Nikolov, Nimgaonkar, Nolen, Nothen, Nurnberger, Nwulia, Nyholt, O'Dushlaine, Oades, Olincy, Oliveira, Olsen, Ophoff, Osby, Owen, Palotie, Parr, Paterson, Pato, Pato, Penninx, Pergadia, Pericak-Vance, Pickard, Pimm, Piven, Posthuma, Potash, Poustka, Propping, Puri, Quested, Quinn, Ramos-Quiroga, Rasmussen, Raychaudhuri, Rehnstrom, Reif, Ribases, Rice, Rietschel, Roeder, Roeyers, Rossin, Rothenberger, Rouleau, Ruderfer, Rujescu, Sanders, Sanders, Santangelo, Sergeant, Schachar, Schalling, Schatzberg, Scheftner, Schellenberg, Scherer, Schork, Schulze, Schumacher, Schwarz, Scolnick, Scott, Shi, Shilling, Shyn, Silverman, Slager, Smalley, Smit, Smith, Sonuga-Barke, St Clair, State, Steffens, Steinhausen, Strauss, Strohmaier, Stroup, Sutcliffe, Szatmari, Szelinger, Thirumalai, Thompson, Todorov, Tozzi, Treutlein, Uhr, van den Oord, Van Grootheest, Van Os, Vicente, Vieland, Vincent, Visscher, Walsh, Wassink, Watson, Weissman, Werge, Wienker, Wijsman, Willemsen, Williams, Willsey, Witt, Xu, Young, Yu, Zammit, Zandi, Zhang, Zitman, Zollner, Devlin, Kelsoe, Sklar, Daly, O'Donovan, Craddock, Sullivan, Smoller, Kendler and Wray2013); and (iii) evidence that family history of affective dysregulation is an indicator of more severe illness (Milne et al., Reference Milne, Caspi, Harrington, Poulton, Rutter and Moffitt2009). Thus, in this analysis, we sought to examine whether childhood adversity, urbanicity, and cannabis use (i) impacted, in a dose–response fashion, the level of psychosis admixture (from none to attenuated psychosis to overt psychotic symptoms) across different severity states of affective dysregulation, (ii) interacted with proxy genetic risk, showing, for a given level of psychosis admixture, greater effect size in the familial stratum compared with the non-familial stratum.
Method
Sample
Data were derived from three waves of the second Netherlands Mental Health Survey and Incidence Study (NEMESIS-2), a longitudinal study of the prevalence, incidence, course, and consequences of psychiatric disorders in the Dutch general population. The study was approved by the standing medical ethics committee. Participants were selected based on a multistage random sampling procedure, resulting in a sample that was representative of the Dutch adult population in terms of age, region, and population density. Participants were included between the ages of 18 and 65; insufficient fluency in Dutch was an exclusion criterion. The participants were interviewed at home by trained interviewers, who were not clinicians, with the Composite International Diagnostic Interview (CIDI) version 3.0 (Alonso et al., Reference Alonso, Angermeyer, Bernert, Bruffaerts, Brugha, Bryson, de Girolamo, Graaf, Demyttenaere, Gasquet, Haro, Katz, Kessler, Kovess, Lepine, Ormel, Polidori, Russo, Vilagut, Almansa, Arbabzadeh-Bouchez, Autonell, Bernal, Buist-Bouwman, Codony, Domingo-Salvany, Ferrer, Joo, Martinez-Alonso, Matschinger, Mazzi, Morgan, Morosini, Palacin, Romera, Taub and Vollebergh2004; de Graaf et al., Reference de Graaf, ten Have, Burger, Buist-Bouwman and Kessler2008) and additional questionnaires. A more detailed description of NEMESIS-2 methodology is presented elsewhere (de Graaf et al., Reference de Graaf, Ten Have and van Dorsselaer2010, Reference de Graaf, ten Have, van Gool and van Dorsselaer2012).
In the first wave (T0), a total of 6646 persons aged 18–64 years were included. Participants were approached for two follow-up surveys, respectively, 3 years (T1) and 6 years (T2) after baseline. At T1, 5303 persons were interviewed again (response rate 80.4%; excluding those who deceased). At T2, 4618 persons were interviewed (response rate 87.8%). Attrition (T0–T1 and T1–T2) was not associated with any of the 12-month mental disorders at T0 (controlled for sociodemographic factors), except for alcohol and drug dependence at T1, which was significantly associated with attrition at T2 (de Graaf et al., Reference de Graaf, van Dorsselaer, Tuithof and ten Have2013, Reference de Graaf, van Dorsselaer, Tuithof and ten Have2015). The mean period between the baseline interview and second follow-up interview was 6 years and 6 days. A more comprehensive description of the design can be found elsewhere (van Nierop et al., Reference van Nierop, Viechtbauer, Gunther, van Zelst, de Graaf, Ten Have, van Dorsselaer, Bak and van Winkel2015).
Assessment of psychopathology
Affective dysregulation
Depressive, manic, and anxiety symptoms were assessed with CIDI 3.0 (de Graaf et al., Reference de Graaf, ten Have, Burger, Buist-Bouwman and Kessler2008). As described elsewhere (van Nierop et al., Reference van Nierop, Viechtbauer, Gunther, van Zelst, de Graaf, Ten Have, van Dorsselaer, Bak and van Winkel2015), affective dysregulation was coded as a binary variable [i.e. considered present if participants experienced at least one of the CIDI 3.0 core symptoms of depressive episode, panic disorder, social phobia, generalized anxiety disorder, and manic episode, assessed at baseline (assessing lifetime occurrence) and each follow-up visit (assessing interval occurrence)].
Psychosis
Presence of psychotic experiences was assessed using a questionnaire based on CIDI 1.1 specifically developed for the purpose of assessing psychotic symptoms, since studies on earlier CIDI versions concluded that the instrument did not adequately measure psychotic symptoms (Andrews and Peters, Reference Andrews and Peters1998). Participants were asked at baseline and each follow-up whether they had experienced any of a list of 20 positive psychotic symptoms (van Nierop et al., Reference van Nierop, Viechtbauer, Gunther, van Zelst, de Graaf, Ten Have, van Dorsselaer, Bak and van Winkel2015). For each symptom category, symptoms were considered present when participants endorsed at least one symptom. All symptoms were assessed using ‘yes’ or ‘no’ questions, and sum scores were obtained by adding reported psychotic symptoms. If symptoms were endorsed, subjects were asked whether they had sought help for these symptoms.
Family history of affective disorders
Family history was assessed as a person-level characteristic in two stages. First, the following psychiatric diagnoses were assessed by self-report in participants who had screened positive for affective dysregulation: depression, mania, and anxiety disorders (panic disorder, social phobia, agoraphobia, generalized anxiety disorder). A total of 44% of the sample thus screened positive at any of the three interview waves. Second, at the first follow-up, self-reported parental history of ‘severe anxiety or phobias’, ‘severe depression’, and ‘delusions or hallucinations’ were assessed in the entire sample: an additional 20% thus screened positive, bringing the total screening positive for family history at 64% (hereafter called ‘FH’). Using these two sources of information, the proportion of the sample in which family history could be assessed was 94%.
Strata of psychopathology
Level of psychopathology was defined based on the degree of admixture of affective and psychotic symptom dimensions and the severity of psychotic experiences: 0 = no symptoms, 1 = any psychotic experience but no affective dysregulation, 2 = affective dysregulation but no psychotic experience, 3 = affective dysregulation and one or two psychotic experiences that did not require help-seeking for psychotic experiences (hereafter: ‘attenuated psychosis’), 4 = affective dysregulation and psychotic experience in more than two domains that did not require help-seeking for psychotic experiences or affective dysregulation and any psychotic experience that required help-seeking for psychotic experiences (hereafter: ‘clinical psychosis’).
Each of these five levels of psychopathology was combined with FH to construct 10 vulnerability strata.
Exposure to environmental risks
Childhood adversity
Childhood adversity was assessed using a questionnaire based on the NEMESIS-1 trauma questionnaire (de Graaf et al., Reference de Graaf, Ten Have and van Dorsselaer2010). Whenever a subject reported having experienced one of five types of childhood adversity [two times or more emotional neglect (not listened to, ignored, or unsupported), physical abuse (kicked, hit, bitten, or hurt with object or hot water), psychological abuse (yelled at, insulted, unjustly punished/treated, threatened, belittled, or blackmailed), peer victimization (bullying), and one time or more sexual abuse (any unwanted sexual experience) before the age of 16], they were asked to state how often it had occurred. The item ‘sexual abuse’ was rated on a scale of 1 (once) to 5 (very often), while all other items (namely, emotional neglect, physical abuse, psychological abuse, and peer victimization or bullying) were rated and on a scale of 1 (sometimes) to 5 (very often). Conforming with previous work in this area, the childhood adversity score was dichotomized at the 80th percentile (van Dam et al., Reference van Dam, van Nierop, Viechtbauer, Velthorst, van Winkel, Bruggeman, Cahn, de Haan, Kahn, Meijer, Myin-Germeys, van Os and Wiersma2015).
Cannabis use
Cannabis use was assessed in the section Illegal Substance Use of the CIDI 3.0. Conforming with previous work, the cut-off of use of once per week or more in the period of most frequent use was used to define a binary variable for regular cannabis use (van Winkel et al., Reference van Winkel, van Beveren and Simons2011).
Urbanicity
The extent of the exposure to urban environment until age 16 years was constructed at five levels based on the Dutch classification or population density: (1) countryside (distances to amenities is larger), (2) village (<25 000 inhabitants), (3) small city (25 000–50 000 inhabitants), (4) medium city (50 000–100 000 inhabitants), (5) large city (>100 000 inhabitants). Consistent with previous work, the cut-off of >50 000 inhabitants was used to define the binary variable of urban area (Guloksuz et al., Reference Guloksuz, van Nierop, Lieb, van Winkel, Wittchen and van Os2015).
Statistical analysis
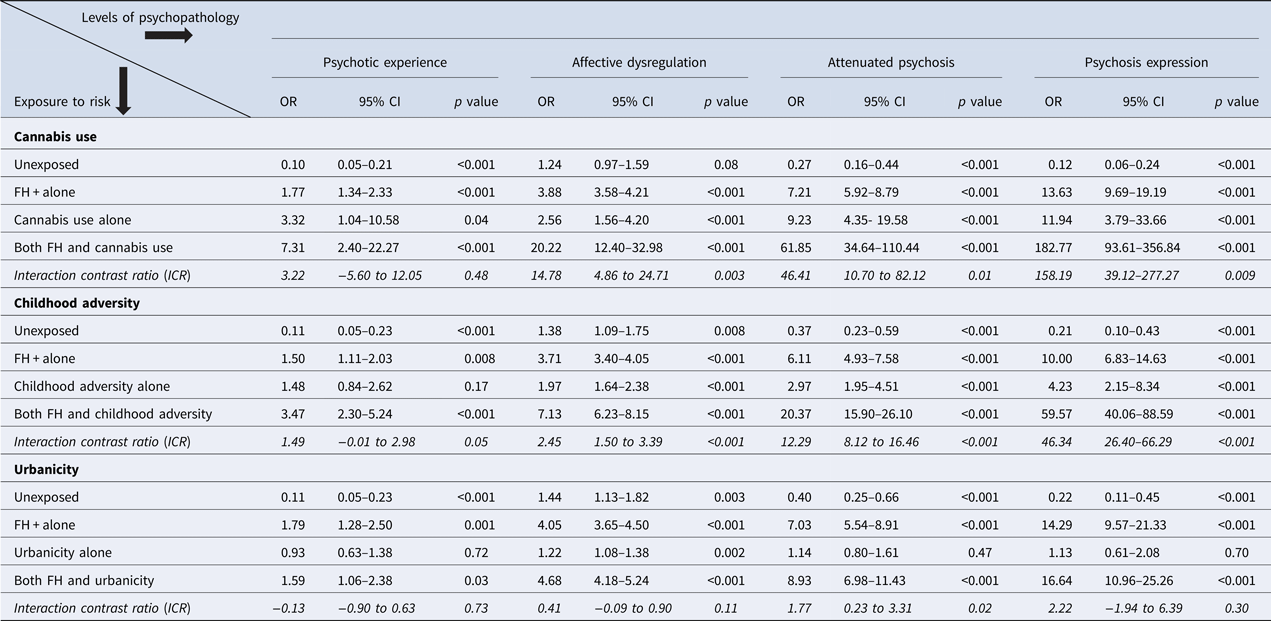
All analyses were performed using STATA, version 14. The level of significance (α) was set at 0.05. In line with previous analyses in this sample (Guloksuz et al., Reference Guloksuz, van Nierop, Lieb, van Winkel, Wittchen and van Os2015), cross-sectional data analysis was performed using the ‘long format’ [each individual contributing three observations (T0, T1, and T2)], adding precision to the estimates. Consistent with previous work in this area (Morgan et al., Reference Morgan, Reininghaus, Reichenberg, Frissa, Hotopf and Hatch2014; Guloksuz et al., Reference Guloksuz, van Nierop, Lieb, van Winkel, Wittchen and van Os2015), the five psychopathology outcomes were modeled as a function of the joint effects of FH and each environmental risk factor (regular cannabis use, childhood adversity, urbanicity) under a model of additive interaction, expressed as the interaction contrast ratio (ICR), calculated with the NLCOM option in Stata. The ICR is the excess risk due to interaction relative to the risk without exposure. The ICR method as suggested by Knol et al. (Reference Knol, van der Tweel, Grobbee, Numans and Geerlings2007) allows use of odds ratios (ORs) derived from logistic models to estimate the relative excess risk as a result of synergy for combinations of dichotomous, ordinal, and continuous exposures (i.e. ICR = OR (exposure A and exposure B) – OR (exposure A only) – OR (exposure B only) + 1. An ICR greater than zero is defined as a positive deviation from additivity.
To test our hypotheses on synergism, the combination of each environmental factor (childhood trauma, urbanicity, cannabis use) and family history of affective dysregulation were included as independent variables (three dummy variables with non-exposed state as the reference category), and strata of psychopathology was included as the dependent variable in logistic models (Knol et al., Reference Knol, van der Tweel, Grobbee, Numans and Geerlings2007). Using the ORs derived from these models, ICRs (e.g. ICR = OR (childhood trauma and psychosis expression) – OR (childhood trauma) – OR (psychosis expression) + 1) for each model were calculated using the Stata NLCOM command.
All analyses were corrected for sex, age, education [(1) primary school, (2) lower secondary education, (3) higher secondary education, (4) higher professional education), and first-generation minority status (dichotomized as born in the Netherlands v. other).
Results
The sample size for the analysis was 16 140 observations from surveys of 6646 participants at three time points (T0, T1, and T2) [n = 6646 in the first wave (T0), n = 5303 in second wave (T1), and n = 4618 in third wave (T3)]. The demographics of the NEMESIS-2 sample has been published elsewhere (de Graaf et al., Reference de Graaf, Ten Have and van Dorsselaer2010, Reference de Graaf, ten Have, van Gool and van Dorsselaer2012). Pertinent to this paper, the descriptive data in the different strata with respect to age, sex, minority status, and education are detailed in Table 1. Figure 1a–c shows the rates of regular cannabis use, childhood adversity, and urbanicity across strata of five increasing levels of psychopathology complicating affective dysregulation and two levels of family history. The figures show that (i) for each environmental risk and for each of the five outcome levels, the exposure rate is higher in the familial history stratum as compared with the non-familial history stratum; and (ii) for childhood adversity and cannabis use, but not urbanicity, exposure rate is progressively higher with higher levels of psychosis admixture complicating affective dysregulation.
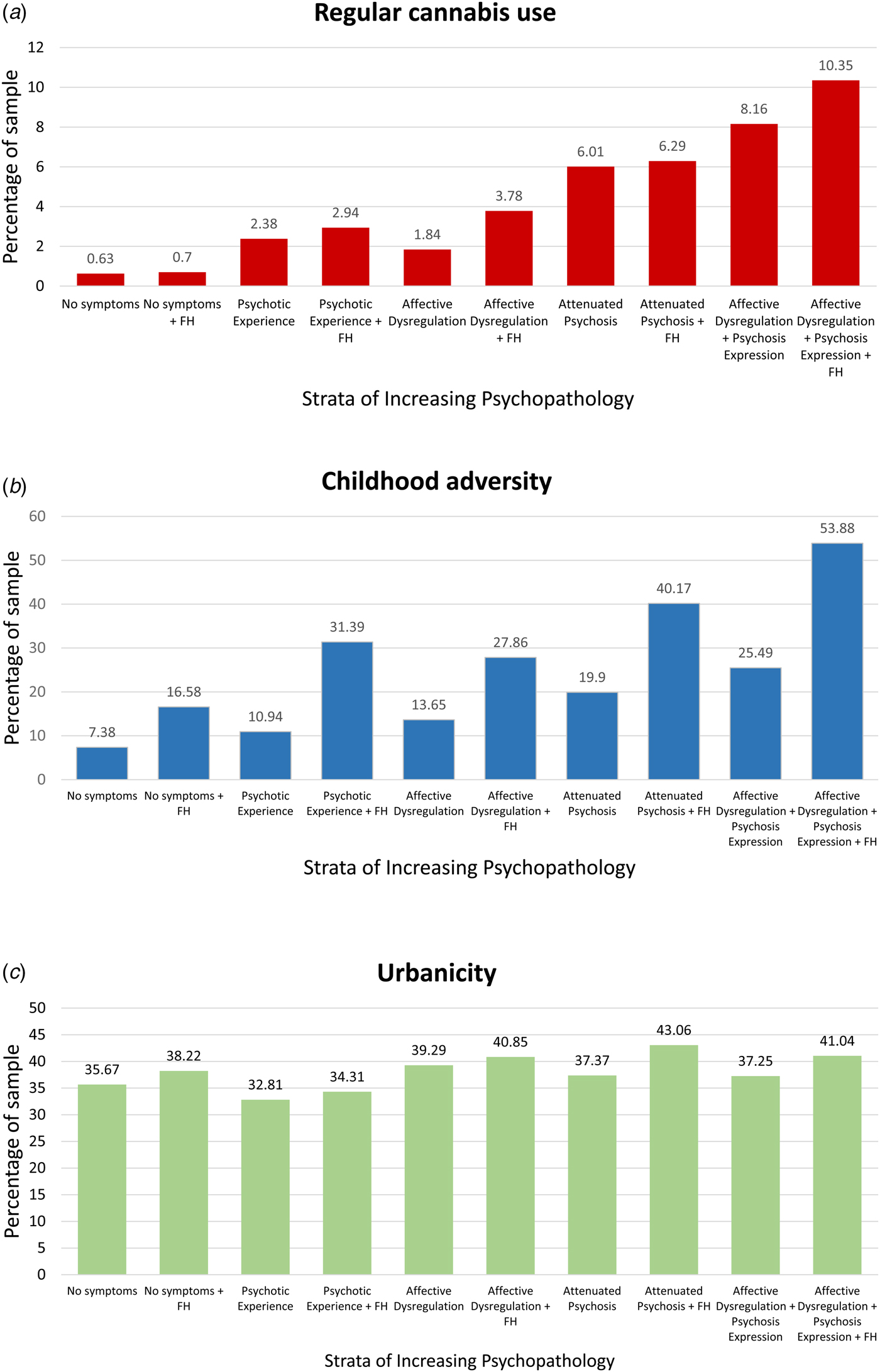
Fig. 1. (a) Depicts rates of regular cannabis use across strata of increasing levels of psychopathology complicating affective dysregulation. Rates of regular cannabis use is greater with increasing levels of psychopathology. (b) Depicts rates of childhood adversity across strata of increasing levels of psychopathology complicating affective dysregulation. Rates of childhood adversity is greater with increasing levels of psychopathology. (c) Depicts rates of urbanicity across strata of increasing levels of psychopathology complicating affective dysregulation. Rates of urbanicity did not increase with increasing levels of psychopathology.
Table 1. Descriptive data per strata

Data from surveys of 6646 participants at three time points (T0, T1, and T2), yielding a total of 16 140 observations for analysis in the ‘long’ format.
a Four-level education: (1) primary school, (2) lower secondary education, (3) higher secondary education, (4) higher professional education, university; FH, family history of affective disorders.
Family history–environment interaction analysis
The FH × environment interaction analyses revealed that, consistent with the results displayed in Fig. 1, the risk associated with environmental exposure was significantly greater if there was also evidence of familial affective liability (Fig. 2). In addition, the greater-than-additive effect of environmental risk in combination with familial affective liability grew progressively greater as psychopathology outcomes were more severe in the sense of more psychosis admixture complicating affective dysregulation (Table 2).

Fig. 2. (a) Depicts individual and joint effects of FH and cannabis use on risk across levels of psychopathology. The risk with both FH + cannabis use is greater than the individual risks with FH alone and cannabis use alone. (b) Depicts individual and joint effects of FH and childhood adversity use on risk across levels of psychopathology. The risk with both FH + childhood adversity is greater than the individual risks with FH alone and childhood adversity alone. (c) Depicts individual and joint effects of FH and urbanicity on risk across levels of psychopathology. The risk with both FH + urbanicity use is greater than the individual risks with FH alone and urbanicity alone.
Table 2. Greater-than-addtive risk of family history of affective dysregulation and environmental exposure across strata of increasing psychopathology
Discussion
Findings
This study investigated the association between the level of psychosis admixture in affective dysregulation and environmental risks, and to what degree these risks were conditional on affective familial liability. The main findings were: (i) for a given stratum of psychosis admixture, the association with environmental risk factors was greater-than-additive if there was also evidence of familial affective liability; (ii) the ICR grew progressively greater over the five psychopathology levels indicative of greater severity due to greater psychosis admixture complicating affective dysregulation.
Affective pathway to psychosis
There is growing evidence that affective dysregulation is an early sign of psychosis, representing the mildest form along the severity dimension of psychosis (Kelleher et al., Reference Kelleher, Keeley, Corcoran, Lynch, Fitzpatrick, Devlin, Molloy, Roddy, Clarke, Harley, Arseneault, Wasserman, Carli, Sarchiapone, Hoven, Wasserman and Cannon2012; Wigman et al., Reference Wigman, van Nierop, Vollebergh, Lieb, Beesdo-Baum, Wittchen and van Os2012). Among the so-called clinical high-risk populations, affective dysregulation is more prevalent in the group with high risk of ‘conversion’ to psychotic disorder (Addington et al., Reference Addington, Cadenhead, Cannon, Cornblatt, McGlashan, Perkins, Seidman, Tsuang, Walker, Woods and Heinssen2007), i.e. the most severe psychopathological state. The association between affective dysregulation and psychosis has long been demonstrated in studies across the psychosis spectrum including among patients with psychotic disorders (McMillan et al., Reference McMillan, Enns, Cox and Sareen2009), those with subthreshold psychotic experiences (van Rossum et al., Reference van Rossum, Dominguez, Lieb, Wittchen and van Os2011; Wigman et al., Reference Wigman, van Nierop, Vollebergh, Lieb, Beesdo-Baum, Wittchen and van Os2012; Stochl et al., Reference Stochl, Khandaker, Lewis, Perez, Goodyer, Zammit, Sullivan, Croudace and Jones2015), clinical high-risk populations (Fusar-Poli et al., Reference Fusar-Poli, Nelson, Valmaggia, Yung and McGuire2014), and prodromal samples (Hafner et al., Reference Hafner, Loffler, Maurer, Hambrecht and an der Heiden1999). In agreement with the current analyses, previous work suggests that early states of affective dysregulation may progress to more severe states characterized by psychotic symptoms following exposure to environmental risk factors (van Os and Reininghaus, Reference van Os and Reininghaus2016). Additionally, recent studies suggest that the relationship between affective dysregulation and psychosis is not dependent on any particular mood state, i.e. anxiety, depression, or mania (Krabbendam et al., Reference Krabbendam, Myin-Germeys, Hanssen, de Graaf, Vollebergh, Bak and van Os2005; van Rossum et al., Reference van Rossum, Dominguez, Lieb, Wittchen and van Os2011; Armando et al., Reference Armando, Lin, Girardi, Righetti, Dario, Saba, Decrescenzo, Mazzone, Vicari, Birchwood and Fiori Nastro2013). In one study, Armando et al. (Reference Armando, Lin, Girardi, Righetti, Dario, Saba, Decrescenzo, Mazzone, Vicari, Birchwood and Fiori Nastro2013) examined anxiety and depressive symptoms and found that they both correlated with psychosis risk (anxiety correlated with increased CAPE positive symptom score, and depressive symptoms correlated with CAPE negative symptom score). van Rossum et al. (Reference van Rossum, Dominguez, Lieb, Wittchen and van Os2011) measured depressive symptoms and hypomanic symptoms and found that they were both associated with greater risk of psychotic experiences, i.e. there was no differential effect of a particular affective state on risk of psychotic experiences. Krabbendam et al. (Reference Krabbendam, Myin-Germeys, Hanssen, de Graaf, Vollebergh, Bak and van Os2005) showed that in people with hallucinatory experience, depressive symptoms increase the risk of development of a psychotic disorder, in agreement with the idea that affective dysregulation increases risk along the dimension of increasing psychopathology.
Environmental risk factors such as cannabis use, childhood adversity, and urbanicity have been shown to increase the risk of psychosis admixture among those with affective disorders (Guloksuz et al., Reference Guloksuz, van Nierop, Lieb, van Winkel, Wittchen and van Os2015, Reference Guloksuz, van Nierop, Bak, de Graaf, Ten Have, van Dorsselaer, Gunther, Lieb, van Winkel, Wittchen and van Os2016); childhood adversity has been associated with an increased risk of admixture of psychotic and non-psychotic psychopathology (van Nierop et al., Reference van Nierop, Viechtbauer, Gunther, van Zelst, de Graaf, Ten Have, van Dorsselaer, Bak and van Winkel2015); and environmental risk factors were shown to act additively in increasing the risk of psychosis (Cougnard et al., Reference Cougnard, Marcelis, Myin-Germeys, De Graaf, Vollebergh, Krabbendam, Lieb, Wittchen, Henquet, Spauwen and Van Os2007).
Although the current analysis does not inform on temporal order, the findings are compatible with the view that progression from an early state of affective dysregulation to more severe states characterized by admixture with, first, attenuated psychosis and, subsequently, clinical psychosis is associated with progressively greater level of exposure to some environmental risks. The current study suggests a complex relationship between affective dysregulation, cannabis use and childhood adversity, and clinical psychosis.
The finding that cannabis use and childhood adversity, and to a lesser degree urbanicity, are associated with increased risk of clinical psychosis in those with affective dysregulation, in interaction with the concomitant presence of familial affective liability, suggests some testable hypotheses. First, it is noteworthy that childhood adversity, cannabis use, and urban environment affect individuals at distinct neurodevelopmental stages, namely during childhood (adversity) and adolescence (cannabis use), or both (urban environment) (Pedersen and Mortensen, Reference Pedersen and Mortensen2001; van Os et al., Reference van Os, Kenis and Rutten2010). Yet, they all appear to be associated with increased risk across all strata of psychosis admixture, particularly childhood adversity and cannabis use. This raises the question whether cannabis use and childhood adversity mediate risk via a final common neurobiological pathway, or if they are both environmental risk factors that are mediated by a common latent risk variable. Second, while childhood adversity has been shown to increase stress-reactivity and cannabis use has been shown to be associated with greater affective dysregulation (Dorard et al., Reference Dorard, Berthoz, Phan, Corcos and Bungener2008), the direction of causality remains an open question. Novel analytic techniques such as machine learning, deep phenotyping, and ecological momentary assessment strategies may help answer these questions. Third, the dimensional relationship between affective dysregulation and psychosis risk warrants further study. While psychosis and affective dysregulation are traditionally considered orthogonal, our results show that affective dysregulation is able to increase psychosis risk irrespective of their dimensional relationship, as also noted in other studies (Krabbendam et al., Reference Krabbendam, Myin-Germeys, Hanssen, de Graaf, Vollebergh, Bak and van Os2005).
Gene–environment interplay
While earlier studies have shown that affective dysregulation is associated with expression of psychosis in a bidirectional, dose–response fashion (van Rossum et al., Reference van Rossum, Dominguez, Lieb, Wittchen and van Os2011), the present study extends this association to familial affective liability as well. Research suggests that risk for psychosis is pleiotropically distributed across currently defined diagnostic boundaries (Cardno et al., Reference Cardno, Marshall, Coid, Macdonald, Ribchester, Davies, Venturi, Jones, Lewis, Sham, Gottesman, Farmer, McGuffin, Reveley and Murray1999; Cross-Disorder Group of the Psychiatric Genomics Consortium et al., Reference Lee, Ripke, Neale, Faraone, Purcell, Perlis, Mowry, Thapar, Goddard, Witte, Absher, Agartz, Akil, Amin, Andreassen, Anjorin, Anney, Anttila, Arking, Asherson, Azevedo, Backlund, Badner, Bailey, Banaschewski, Barchas, Barnes, Barrett, Bass, Battaglia, Bauer, Bayes, Bellivier, Bergen, Berrettini, Betancur, Bettecken, Biederman, Binder, Black, Blackwood, Bloss, Boehnke, Boomsma, Breen, Breuer, Bruggeman, Cormican, Buccola, Buitelaar, Bunney, Buxbaum, Byerley, Byrne, Caesar, Cahn, Cantor, Casas, Chakravarti, Chambert, Choudhury, Cichon, Cloninger, Collier, Cook, Coon, Cormand, Corvin, Coryell, Craig, Craig, Crosbie, Cuccaro, Curtis, Czamara, Datta, Dawson, Day, De Geus, Degenhardt, Djurovic, Donohoe, Doyle, Duan, Dudbridge, Duketis, Ebstein, Edenberg, Elia, Ennis, Etain, Fanous, Farmer, Ferrier, Flickinger, Fombonne, Foroud, Frank, Franke, Fraser, Freedman, Freimer, Freitag, Friedl, Frisen, Gallagher, Gejman, Georgieva, Gershon, Geschwind, Giegling, Gill, Gordon, Gordon-Smith, Green, Greenwood, Grice, Gross, Grozeva, Guan, Gurling, De Haan, Haines, Hakonarson, Hallmayer, Hamilton, Hamshere, Hansen, Hartmann, Hautzinger, Heath, Henders, Herms, Hickie, Hipolito, Hoefels, Holmans, Holsboer, Hoogendijk, Hottenga, Hultman, Hus, Ingason, Ising, Jamain, Jones, Jones, Jones, Tzeng, Kahler, Kahn, Kandaswamy, Keller, Kennedy, Kenny, Kent, Kim, Kirov, Klauck, Klei, Knowles, Kohli, Koller, Konte, Korszun, Krabbendam, Krasucki, Kuntsi, Kwan, Landen, Langstrom, Lathrop, Lawrence, Lawson, Leboyer, Ledbetter, Lee, Lencz, Lesch, Levinson, Lewis, Li, Lichtenstein, Lieberman, Lin, Linszen, Liu, Lohoff, Loo, Lord, Lowe, Lucae, MacIntyre, Madden, Maestrini, Magnusson, Mahon, Maier, Malhotra, Mane, Martin, Martin, Mattheisen, Matthews, Mattingsdal, McCarroll, McGhee, McGough, McGrath, McGuffin, McInnis, McIntosh, McKinney, McLean, McMahon, McMahon, McQuillin, Medeiros, Medland, Meier, Melle, Meng, Meyer, Middeldorp, Middleton, Milanova, Miranda, Monaco, Montgomery, Moran, Moreno-De-Luca, Morken, Morris, Morrow, Moskvina, Muglia, Muhleisen, Muir, Muller-Myhsok, Murtha, Myers, Myin-Germeys, Neale, Nelson, Nievergelt, Nikolov, Nimgaonkar, Nolen, Nothen, Nurnberger, Nwulia, Nyholt, O'Dushlaine, Oades, Olincy, Oliveira, Olsen, Ophoff, Osby, Owen, Palotie, Parr, Paterson, Pato, Pato, Penninx, Pergadia, Pericak-Vance, Pickard, Pimm, Piven, Posthuma, Potash, Poustka, Propping, Puri, Quested, Quinn, Ramos-Quiroga, Rasmussen, Raychaudhuri, Rehnstrom, Reif, Ribases, Rice, Rietschel, Roeder, Roeyers, Rossin, Rothenberger, Rouleau, Ruderfer, Rujescu, Sanders, Sanders, Santangelo, Sergeant, Schachar, Schalling, Schatzberg, Scheftner, Schellenberg, Scherer, Schork, Schulze, Schumacher, Schwarz, Scolnick, Scott, Shi, Shilling, Shyn, Silverman, Slager, Smalley, Smit, Smith, Sonuga-Barke, St Clair, State, Steffens, Steinhausen, Strauss, Strohmaier, Stroup, Sutcliffe, Szatmari, Szelinger, Thirumalai, Thompson, Todorov, Tozzi, Treutlein, Uhr, van den Oord, Van Grootheest, Van Os, Vicente, Vieland, Vincent, Visscher, Walsh, Wassink, Watson, Weissman, Werge, Wienker, Wijsman, Willemsen, Williams, Willsey, Witt, Xu, Young, Yu, Zammit, Zandi, Zhang, Zitman, Zollner, Devlin, Kelsoe, Sklar, Daly, O'Donovan, Craddock, Sullivan, Smoller, Kendler and Wray2013). A recent study from the GROUP cohort shows that the polygenic risk score for psychosis was associated with affective dysregulation in both controls and the relatives of patients with a psychotic disorder, supporting this premise (van Os et al., Reference van Os, van der Steen, Islam, Guloksuz, Rutten and Simons2017). Another recent study showed that non-psychotic mental health complaints, particularly depression, in a general population twin sample were associated with polygenic risk for schizophrenia (Nivard et al., Reference Nivard, Gage, Hottenga, van Beijsterveldt, Abdellaoui, Bartels, Baselmans, Ligthart, Pourcain, Boomsma, Munafo and Middeldorp2017).
The results indicate that the effect sizes of cannabis use and childhood adversity, and to a lesser extent urban environment, on the level of psychosis admixture were strongly moderated by the presence of familial affective liability. These findings are in agreement with earlier work showing moderation of cannabis use and urban environment by variables indexing familial loading of psychosis (van Os et al., Reference van Os, Kenis and Rutten2010; Genetic Risk and Outcome in Psychosis Investigators, 2011). As the terms making up the interaction (FH × cannabis use, FH × childhood adversity, and FH × urbanicity) were all significantly associated with each other, two explanations can be invoked to explain this finding. First, familial affective liability may reflect pleiotropic genetic risk which may render individuals more sensitive to environmental risks under a model of gene–environment interaction. Second, familial affective liability may reflect a high-risk environment associated with greater probability of cannabis use and childhood adversity under a model of gene–environment correlation. It is not possible to distinguish between the two in the current data set but both would be clinically relevant and both may apply to a degree (Van Os and Sham, Reference van Os, Sham, Murray, Jones, Susser, Van Os and Cannon2003).
Gene–environment interplay in the affective pathway to psychosis
Another important finding of this study is that among those with affective dysregulation, gene–environment interplay is more prominent at the more severe level of psychosis admixture, i.e. clinical psychosis, but less at lower levels of severity such as attenuated psychosis. In the formal gene × environment ICR analysis, cannabis use and childhood adversity, and to a lesser extent urbanicity, was associated with a greater-than-additive risk at the level of clinical psychosis. This pattern of simple additivity at lower levels of psychopathology, and greater-than-additive risk at higher level of psychopathology (namely, clinical psychosis) is interesting, as it suggests that clinical relevance occurs when there is more-than-additivity. This points to the relevance of the quality and intensity of the factors associated with environmental and genetic risk, and not merely their presence.
Strengths and limitations
These findings should be interpreted in the light of several strengths and limitations. In terms of strengths, the present study has the advantage of a large number of participants, representative of the Dutch population, with direct questioning of reported psychotic experiences by trained interviewers, which is especially important in general population studies (Linscott and van Os, Reference Linscott and van Os2010; van Nierop et al., Reference van Nierop, van Os, Gunther, Myin-Germeys, de Graaf, ten Have, van Dorsselaer, Bak and van Winkel2012). The study incorporates symptom assessments across diagnostic boundaries, providing an opportunity to examine multiple strata of increasing psychopathology and familial risk as is present in the general population.
The most obvious limitation of the current analysis is its cross-sectional nature, precluding conclusions of causality. Another limitation, although difficult to avoid, is the retrospective nature of the information on childhood adversity, which may have resulted in increased random error. Previous work has found that recall of childhood adversity is reliable, including in individuals with psychotic disorders (Fisher et al., Reference Fisher, Craig, Fearon, Morgan, Dazzan, Lappin, Hutchinson, Doody, Jones, McGuffin, Murray, Leff and Morgan2011). Similarly, the assessment of cannabis use and urbanicity was based on CIDI questions and might be subject to decreased precision in reporting of use. Additionally, assessment of familial risk was by different short instruments where the respondent reported whether parents had mental health problems, and is hence limited by the respondent's knowledge of the same. The NEMESIS study did not include family history of all possible mental disorders since they are of relatively low prevalence. This however does not limit the interpretation of our analysis since the primary question in this paper was focused mainly on the group with affective psychopathology, i.e. whether cannabis use and other risks were associated with increasing psychopathology in the psychosis dimension in this group.
Conclusions
In conclusion, the current study provides evidence that exposure to regular cannabis use and childhood adversity, and to a lesser extent urban environment, results in increasing levels of psychosis admixture in early states of affective dysregulation, supporting the model of an affective pathway to psychosis. In addition, the results suggest that there is interplay between familial affective liability and environmental risks, possibly in the direction of synergism. The findings imply that a multidimensional staging of severity cutting across traditional diagnostic clusters extended by means of genetic and environmental risk tiers may provide a suitable framework to gain insight into early psychopathology that often emerges as a mixed bag of subtle symptoms and may further progress to a more distinct and severe clinical syndrome, such as psychosis spectrum disorder (Guloksuz and van Os, Reference Guloksuz and van Os2017).
Funding
This work was supported by the Ministry of Health, Welfare and Sport (Grant Number 310253), with supplement support from the Netherlands Organization for Health Research and Development (ZonMw) and the Genetic Risk and Outcome of Psychosis (GROUP) investigators. Supported by the European Community's Seventh Framework Program under Grant agreement No. HEALTH-F2-2009-241909 (Project EU-GEI).
Conflict of interest
Rajiv Radhakrishnan, Sinan Guloksuz, Lotta-Katrin Pries, Saskia van Dorsselaer, Margreet ten Have, Ron de Graaf, Nicole Gunter, Christian Rauschenberg, Uli Reininghaus have received no funding or compensation from companies. Maarten Bak has received financial compensation as a speaker from AstraZeneca and Eli Lilly and as a course organizer from Janssen Nederland. In the past 5 years, university research funding managed by Professor Jim van Os has received unrestricted investigator-led research grants or recompense for presenting research from Janssen-Cilag and Lundbeck, companies that have an interest in the treatment of psychosis. Rajiv Radhakrishnan is supported by Dana Foundation David Mahoney program and CTSA Grant Number UL1 TR001863 from the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of NIH.






