INTRODUCTION
Species of the monogenean genus Gyrodactylus von Nordmann, 1832 are ectoparasites of fish with a varying level of host specificity (Bakke et al. 2002). The short generation time and hyperviviparity of gyrodactylids are considered ideal prerequisites for host switching (Cable and Harris, 2002; Boeger et al. 2003). Indeed, host switching is considered common for Gyrodactylus species (Ziętara and Lumme, 2002; Huyse and Volckaert, 2002, 2005) and has been put forward as an explanation for the speciation and radiation within the genus as well as for its large biodiversity (Brooks and McLennan, 1993; Boeger and Kritsky, 1997; Ziętara and Lumme, 2002, 2003; Meinilä et al. 2004).
The identification of gyrodactylids is traditionally based on the morphology of the hard parts of the posterior attachment apparatus (opisthaptor) (Malmberg, 1970), but a high degree of plasticity has been reported (Shinn et al. 2004). Environmental factors such as, for example, temperature may cause substantial variability in size and, to a lesser extent, in shape of the opisthaptoral hard parts and must be considered in taxonomic studies (Mo, 1991a,b,c, 1993; Dávidova et al. 2005). Several molecular markers, such as the internal transcribed spacers 1 and 2 (ITS-1 and ITS-2) and the intergenic spacer (IGS) of the nuclear ribosomal gene cluster as well as the mitochondrial cytochrome oxidase I (COI) have also been applied to study the taxonomy and systematics of gyrodactylids (see e.g. Matějusová et al. 2001; Ziętara and Lumme, 2002; Sterud et al. 2002; Cunningham et al. 2003; Hansen et al. 2003, 2006, Meinilä et al. 2004).
G. salaris Malmberg, 1957 is a pathogen of Norwegian Atlantic salmon stocks (Salmo salar L.) (Johnsen et al. 1999) and farmed rainbow trout (Oncorhynchus mykiss (Walbaum)) (see Mo, 1991c; Meinilä et al. 2004), and will experimentally infect and reproduce on other salmonids including anadromous Arctic charr (Salvelinus alpinus (L.)) (Bakke et al. 1996, 2002). G. salaris is difficult to discriminate morphologically from the closely related G. thymalli Žitnaň, 1960. Also molecular markers such as the nuclear ribosomal ITS (Ziętara and Lumme, 2003) and the mitochondrial COI sequences (Hansen et al. 2003, 2006) fail to unambiguously discriminate both species. IGS has been suggested to discriminate G. salaris and G. thymalli (Sterud et al. 2002). However, Hansen et al. (2006) challenge this interpretation but agree that specific arrangements of 23bp repeats in the IGS may in part be useful to discriminate parasites infecting salmon from those infecting rainbow trout.
The fish genus Salvelinus has a circumpolar distribution and includes several species and subspecies (Brunner et al. 2001), but Arctic charr is the only Salvelinus species with a natural distribution in Norway. Resident populations of Arctic charr occur in freshwater all over the country, but anadromous populations are restricted to northern Norway (Klemetsen et al. 2003). Until now, 9 Gyrodactylus spp. have been recorded on Salvelinus species worldwide (Harris et al. 2004). In northern Norway, with heavy Gyrodactylus-infected anadromous Arctic charr have been reported from the Rivers Skibotnelva and Signaldalselva. Both these rivers drain into the same fjord system and hold Atlantic salmon stocks (Mo, 1988; Johnsen et al. 1999; Knudsen et al. 2004; Kristoffersen et al. 2005). In Skibotnelva, Gyrodactylus on Arctic charr have been identified as G. salaris (see Mo, 1988), while those on Arctic charr in Signaldalselva have not yet been properly characterized.
In Buskerud County (southern Norway) G. birmani Konovalov, 1967 has been reported on resident Arctic charr in one locality (Sterud, 1999), while a pilot study revealed another Gyrodactylus sp. infection on the same host species in Lake Pålsbufjorden.
The main goal of this study was to identify the Gyrodactylus spp. infections found on anadromous Arctic charr in River Signaldalselva and on resident Arctic charr in Lake Pålsbufjorden in Norway. We therefore characterized and compared the Gyrodactylus spp. in both localities using molecular and morphometric methods. Further, we compared parasite specimens from the anadromous Arctic charr with those recovered from Atlantic salmon in the same river.
MATERIALS AND METHODS
Collection of fish and parasites
In southern Norway, Arctic charr were collected with gill nets in Lake Pålsbufjorden (Buskerud County) during the autumn of 2003 and 2004, and in Lake Tinnsjøen (Telemark County), during the autumn of 2003 and 2005. In northern Norway, Arctic charr and Atlantic salmon were collected concurrently by electro-fishing in Signaldalselva, Troms County, in 2001 and 2004 (Table 1).
Table 1. Details on the Norwegian Arctic charr (Salvelinus alpinus) and Atlantic salmon (Salmo salar) specimens used in this study (All fish were screened for Gyrodactylus infection.)

The fish were killed by a blow to the head and the fins of adults were cut off with a pair of scissors and fixed in 96% ethanol (EtOH) immediately on capture. Parr were fixed whole in 96% EtOH. Later, the fish and fins were screened for Gyrodactylus infection under a stereo-microscope. Parasites were removed from as many fish specimens as possible from each locality and transferred into Eppendorf-tubes containing 96% EtOH and stored at −20 °C for later processing.
DNA extraction, amplification and sequencing
The opisthaptors of the Gyrodactylus parasites were excised from the body. The remaining bodies of 1–3 parasites per population were individually used for molecular analyses. DNA was extracted according to Cunningham et al. (2001). The primer pair ITS1A (5′-GTAACAAGGTTTCCGTAGGTG-3′) and ITS2 (5′-TCCTCCGCTTAGTGATA-3′) (Matějusová et al. 2001) was used to amplify a fragment spanning the 3′ end of the 18S gene, the ITS1, the 5.8S gene, the ITS2, and the 5′ end of the 28S gene. The IGS repeat region was amplified using the primers IGSV3 (5′-CTGGCTATAATCACGTAAGACTGC-3′) IGSV4 (5′- AAGATACTCATTTGACTCGGTGTG-3′) designed by Collins and Cunningham (2000). The mitochondrial cytochrome oxidase I gene (CO1) was amplified using the primer pairs of Hansen et al. (2003). PCR reactions were carried out using the amplification protocols published along with the primer sequences. The PCR-products were purified using a QIAquick PCR Purification Kit (Qiagen) according to the manufacturer's recommendations. Both DNA strands were sequenced using BigDye chemistry Version 1.1 (Applied Biosystems) and an ABI3100 automatic sequencer (Applied Biosystems). For sequencing of ITS, the PCR primers and the internal primers ITS4.5 (5′-CATCGGTCTCTCGAACG-3′) and ITSR3A (5′-GAGCCGAGTGATCCACC-3′) (Matějusová et al. 2001) and ITS28F (5′-TAGCTCTAGTGGTTCTTCCT-3′) (Ziętara and Lumme, 2003) were used. Both IGS and CO1 were sequenced using the PCR primers. All sequences were submitted to a BLASTN (Altschul, 1991) search in GenBank to establish possible identity.
Morphometric analyses
After excision, the opisthaptors to be analysed by both light and scanning electron microscopy (SEM) were prepared according to a slightly modified version of Harris et al. (1999). A Leica DC 500 camera mounted on a Leica DM 6000B stereo microscope was used to take digitalized light microscope photographs of the opisthaptoral hard parts (at magnifications of 1000, 1250 or 1600). All distances were measured with the help of the Leica IM1000 software system using a digital calliper and a point-to-point tool.
Preparations of opisthaptoral hard parts for SEM were sputter-coated with a gold-palladium mixture using a Polaron E5000 SEM coating unit for later examination in a JEOL JSM-6400 scanning electron microscope.
Only slides containing all 3 opisthaptoral hard parts: hamuli, ventral bridge, and marginal hooks were used for morphological analyses. Fifteen to 30 specimens from each population were measured. Thirty-three different linear measurements and 1 angular measurement converted to cosine values (most measurements have been described by Shinn et al. (2004)) were applied (see Fig. 1 and Table 2).

Fig. 1. (A–D) Scanning electron micrographs of the opisthaptoral hard parts from Gyrodactylus sp. from Arctic charr (Salvelinus alpinus) in Signaldalselva illustrating the morphometric characters used. The numbers refer to the characters listed in Table 1. (A) Hamuli; (B) ventral bar; (C) marginal hook; (D) marginal hook sickle.
Table 2. The morphometric results of 34 characters measured on the opisthaptoral hard parts: hamuli, ventral bar and marginal hook of Gyrodactylus salaris from Arctic charr (Salvelinus alpinus) (N=30) from Pålsbufjorden, and Arctic charr (N=23) and Atlantic salmon (Salmo salar) (N=23) from Signaldalselva (Each measure is given as micrometer (μm) ±standard deviation (S.D.), range in parentheses. The results of a Mann-Whitney U-test based on the different individual measurements are also presented (statistically significance, P<0·05).)

Principal component analysis (PCA) was employed to analyse the multivariate morphometric datasets. Having identified the axes of maximal variance, the principal components scores (PCA-scores) were compared by Mann-Whitney U tests. By negating PC1, the component that often best expresses size variation, the effects of having a between-group bias in size was assumed minimal. In addition the PCA-scores from all components, both including and excluding PC1, were subjected to Two-group permutation tests. Kruskal-Wallis and Mann-Whitney U tests were employed to explore differences in single measures between populations. All calculations and graphical illustrations were performed with the programme PAST vs 1·29 (Hammer et al. 2001).
RESULTS
Infection data
Both Arctic charr from Lake Pålsbufjorden and River Signaldalselva, and Atlantic salmon from Signaldalselva were infected with Gyrodactylus (Table 1). The abundance of Gyrodactylus sp. on Arctic charr was 0·9 in Pålsbufjorden in 2003 and 8·0 in Signaldalselva in 2004. All specimens of Atlantic salmon in Signaldalselva in 2004 were infected. No parasites were observed on Arctic charr from Lake Tinnsjøen.
Identification of Gyrodactylus sp. from Arctic charr in Signaldalselva and comparison with G. salaris on sympatric Atlantic salmon
The ITS sequences obtained from the Gyrodactylus specimens from both Arctic charr and Atlantic salmon matched GenBank Accession number AF328871 and were thus identified as G. salaris. The mitochondrial COI sequences from Arctic charr were identical to the haplotype reported earlier from G. salaris on Atlantic salmon from Signaldalselva (GenBank Accession number AY486497) and Skibotnelva (GenBank Accession number AY486525) (Hansen et al. 2003).
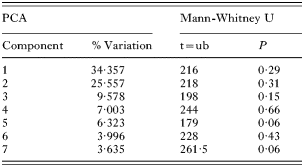
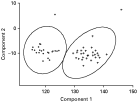
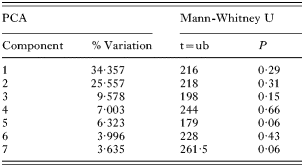
The individual measurements taken of G. salaris from both Arctic charr (n=23) and Atlantic salmon (n=23) showed that only 6 out of 34 measures of the opisthaptoral hard parts differed significantly (Mann-Whitney U tests, P<0·05; see Table 2). A PCA-plot demonstrates a high degree of overlap in the first and the second principal components (PC1 and PC2; see Fig. 2). The loadings of PC1 were mostly negative, and PC1 were therefore interpreted as a component representing variance related mainly to size. The variances of the PC2-7 were interpreted as reflecting shape because of both negative and positive loadings. There were no significant differences between G. salaris from Arctic charr and Atlantic salmon along PC1-7 (Mann-Whitney U test, P>0·05, Table 3) which collectively accounted for 70% of the variation in the dataset. Neither were there significant differences between G. salaris on Arctic charr and salmon when all PCA components were considered collectively, nor when PC1 was excluded from the analyses (Two-group permutation tests, P>0·05).

Fig. 2. PCA plot of the morphometric data of all measurements (see Table 1) of Gyrodactylus sp. from Arctic charr (Salvelinus alpinus) (circle) and G. salaris from Atlantic salmon (Salmo salar) (triangle) from Signaldalselva in the two first planes (Component 1 vs Component 2) of the PCA plot (ellipses represent 95% confidence intervals about the mean).

Fig. 3. (A–D) Scanning electron micrographs of the opisthaptoral hard parts of Gyrodactylus salaris from Arctic charr (Salvelinus alpinus) in Pålsbufjorden. (A) Hamuli; (B) ventral bar; (C) marginal hook; (D) marginal hook sickle.
Table 3. The percentage variation described by the 7 first components of the PCA analyses of Gyrodactylus salaris from Arctic charr (Salvelinus alpinus) and Atlantic salmon (Salmo salar) in Signaldalselva (The results of a Mann-Whitney U-test based on the PCA-scores of the different components are also presented.)
Identification of Gyrodactylus sp. from Arctic charr in Pålsbufjorden and comparison with Gyrodactylus sp. from Arctic charr in Signaldalselva
The ~1250 bp ITS sequences (GenBank Accession number DQ898302) obtained from 5 Gyrodactylus specimens from Arctic charr from Pålsbufjorden (3 collected in 2003 and 2 collected in 2004) were identified as G. salaris. Only one G→A transition at position 288 of the ITS 2 region was detected consistently in all individuals when compared to GenBank Accession number AF328871. This particular G→A transition has never been observed before. The 720 bp mitochondrial COI sequence (GenBank Accession number DQ923578) was identical to the haplotype reported from G. salaris on hatchery reared rainbow trout and on Atlantic salmon from the Norwegian rivers Drammenselva, Lierelva and Lærdalselva (Hansen et al. 2003; Meinilä et al. 2004). The 685 bp of the IGS repeat region (GenBank Accession number DQ898303) contained a particular arrangement of 23 bp repeats (ABBABBBBBB – PPPSURVQ) that is identical to that of some clones obtained for G. salaris from hatchery reared rainbow trout (GenBank Accession numbers AY490400-AY490402) (Hansen et al. 2006). Such a repeat arrangement has never been reported in Norwegian G. salaris from salmon (Hansen et al. 2006).
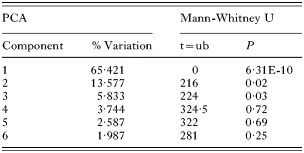
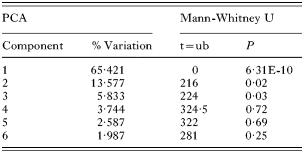
A comparison of the morphology of G. salaris from Arctic charr from Pålsbufjorden (n=30) (see Fig. 3) and Signaldalselva (n=23) (see Fig. 1) showed that 24 out of 34 measures of the opisthaptoral hard parts differed significantly (Mann-Whitney U tests, P<0·05; see Table 2). In a PCA-plot there is almost no overlap of the morphological measurements of G. salaris from Arctic charr in Pålsbufjorden and Signaldalselva along PC1 (Fig. 4). The loadings of PC1 were mostly positive so the variance in this axis seems mainly to originate from size-differences. The 2 G. salaris populations were significantly different in PC1-3 (Mann-Whitney U tests, P<0·05; Table 4), which collectively account for 89% of the variance. In PC4-6, which collectively account for 8% of the variance, the morphometric differences between these populations are small and not statistically significant (Mann-Whitney U tests, P>0·05; Table 4). The morphometry of the 2 G. salaris populations was significantly different when all components were considered collectively (Two-group permutation test, P<0·05). When PC1 were excluded from the analysis, there were no significant differences (Two-group permutation test, P>0·05).

Fig. 4. PCA plots of the two first planes of the morphometric data of all measurements (see Table 1) of Gyrodactylus salaris on Arctic charr (Salvelinus alpinus) from Pålsbufjorden (cross) and Signaldalselva (circle) (ellipses are 95% confidence intervals about the mean).
Table 4. The percentage variation described by the first 6 components of the PCA analyses of Gyrodactylus salaris from Arctic charr (Salvelinus alpinus) in Pålsbufjorden and Signaldalselva (The results of a Mann-Whitney U-test based on the PCA-scores of the different components are also shown.)
DISCUSSION
Gyrodactylus specimens from Arctic charr from both the north Norwegian Signaldalselva and the south Norwegian Pålsbufjorden were identified as G. salaris by means of molecular and morphometric methods. The parasites from the 2 localities belong to 2 different mitochondrial lineages. In addition, the morphometric measurements of the parasites from both Signaldalselva and Pålsbufjorden fall within the relatively wide range of measurements for the up to 15 characters for G. salaris published earlier by Mo (1991a,b,c) and Shinn et al. (2001, 2004) (except for 1 individual from Pålsbufjorden that had an atypically large ventral bar).
Our finding of G. salaris on wild anadromous Arctic charr in Signaldalselva confirms the suggestion of the presence of this species made by Knudsen et al. (2004) and is in line with observations of G. salaris on Arctic charr in the nearby River Skibotnelva (Mo, 1988; Kristoffersen et al. 2005). Our data also corroborate earlier experimental data which showed that Arctic charr from some anadromous populations can serve as a suitable host for G. salaris (strain from Atlantic salmon in the river Lierelva) (see Bakke et al. 1996). The infections of both Atlantic salmon and Arctic charr in the same locality with G. salaris bearing the same mitochondrial haplotype strongly indicate that both parasite metapopulations belong to the same suprapopulation. It is therefore a reasonable assumption that anadromous Arctic charr can acquire G. salaris from co-occurring infected Atlantic salmon (see Knudsen et al. 2006). Transmission of the parasite may occur through direct contact between infected fish, indirectly from the substrate, or via drift in the water column (Bakke et al. 1992; Soleng et al. 1999). Atlantic salmon parr are frequently found in deeper parts of rivers with strong water currents, whereas parr of Arctic charr are usually found in shallow waters near the shore (Heggberget, 1984). Hence, indirect transmission of G. salaris from salmon to Arctic charr is probably the most important transmission route. Further support for this assumption comes from the observation of Olstad et al. (2006) that G. salaris can survive for up to 2·5 days off its host. It is of course also possible that heavily infected and moribund salmon parr may display abnormal behaviour and move into more shallow waters, thereby facilitating transmission between the host species (see Bakke et al. 1992; Knudsen et al. 2006). However, the relatively high average infection and prevalence of G. salaris on Arctic charr in Signaldalselva also indicates in situ reproduction on Arctic charr (Knudsen et al. 2006).
G. salaris from Arctic charr and Atlantic salmon in Signaldalselva are morphologically almost indistinguishable, which may be taken as further support for both populations belonging to the same gene pool. The minor morphometric differences may represent host-induced differences since the macroenvironment is identical. Previously, host-dependent morphometric differences have not been observed for G. salaris (see Mo, 1991c) or other Gyrodactylus species (e.g. Mo, 1993; Geets et al. 1999). This was, however, suggested by Huyse and Volckaert (2002) who found significant morphometric differences within G. rugiensoides infecting Pomatoschistus pictus and P. minutus. The epidermis of different fish species provide different host-specific microenvironments (Buchmann and Lindenstrøm, 2002) which may affect the phenotype of the opisthaptoral hard parts.
The morphometric differences between G. salaris infecting Arctic charr in Signaldalselva and in Pålsbufjorden exceed the small morphometric differences observed between the parasites infecting Arctic charr and Atlantic salmon in Signaldalselva. The opisthaptoral hard parts of G. salaris from Arctic charr in Pålsbufjorden are larger than those from Arctic charr in Signaldalselva and there are also some differences in shape. Earlier investigations have shown that the size of the opisthaptoral hard parts of G. salaris is negatively correlated with water temperature (Mo, 1991a,b,c). This has also been observed for several other Gyrodactylus species (e.g. Ergens, 1976, 1981; Ergens and Gelnar, 1985; Mo, 1993; Appleby, 1996). Since G. salaris in Signaldalselva was collected at a lower temperature than G. salaris in Pålsbufjorden its opisthaptoral hard parts are expected to be larger. However, the opposite has been observed. Although more comprehensive sampling is required to satisfactorily answer this question, our observation may be taken as an indication that the size differences of G. salaris infecting Arctic charr in Signaldalselva and in Pålsbufjorden may depend on other factors than water temperature.
G. salaris from Arctic charr in Pålsbufjorden and in Signaldalselva belong to different mitochondrial haplogroups. The parasites from Pålsbufjorden carry the same mitochondrial haplotype as parasites from Atlantic salmon in the River Drammenselva (Hansen et al. 2003). Mo (1991c) hypothesized that G. salaris on Atlantic salmon in Drammenselva has larger opisthaptoral hooks than usually observed from parasites on other Atlantic salmon stocks. The morphology of G. salaris from Pålsbufjorden is also slightly different from other G. salaris populations with the same mitochondrial haplotype, i.e. from G. salaris populations on Atlantic salmon in Drammenselva and farmed rainbow trout in western Sweden (Robertsen, 2005). Although these differences may be linked to the macroenvironment, it can currently not be ruled out that G. salaris from Pålsbufjorden already shows adaptation to the new host species, the Arctic charr. However, other explanations for the observed morphological differences between parasites infecting the two Arctic charr populations may be possible.
The finding of G. salaris on resident Arctic charr in Pålsbufjorden in southern Norway is the first record of a viable and sustained infection of G. salaris on another wild salmonid in the absence of salmon and is therefore of particular interest. The mitochondrial haplotype of this G. salaris is the same as that of G. salaris on Atlantic salmon from the Norwegian rivers Drammenselva, Lierelva and Lærdalselva, and of G. salaris on farmed rainbow trout throughout Fennoscandia (Hansen et al. 2003; Meinilä et al. 2004). However, G. salaris from salmon in Lierelva was unable to reproduce on resident Arctic charr from Lake Korssjøen, Southern Norway (Bakke et al. 1996). This may indicate host or parasite specific differences that are not reflected by the currently used genetic markers. The IGS sequences of G. salaris from Pålsbufjorden were most similar to sequences found in specimens from farmed rainbow trout (see Sterud et al. 2002; Cunningham et al. 2003, Hansen et al. 2006). Thus, both CO1 and IGS sequences are congruent with a hypothesis of rainbow trout being the source of the infection of Arctic charr in Pålsbufjorden. Originally, Arctic charr were introduced into Pålsbufjorden from Lake Tinnsjøen between 1910 and 1919 (Aass, 1970). G. salaris may have been introduced simultaneously, however, no gyrodactylids on Arctic charr from Tinnsjøen have been observed yet. The Arctic charr could also have been infected when arriving in Pålsbufjorden, but G. salaris has not been found on brown trout, which is the only native salmonid in the lake (unpublished observations). It is thus more likely that G. salaris was introduced into Pålsbufjorden more recently. Rainbow trout were imported from Jutland (Denmark) and kept in various fish farms in southern Norway before they were introduced to Pålsbufjorden on several occasions during 1962–1964, and to the nearby and connected lake Tunhovdfjorden between 1962 and 1967 (Per Aass, personal communications). According to this line of argument, rainbow trout must have acquired the G. salaris infection in Danish or Norwegian fish farms. A ‘rainbow trout strain’ of G. salaris may subsequently have switched to the resident Arctic charr population in Pålsbufjorden. This hypothetical scenario implies at least 2 host-switches: the first from Atlantic salmon to rainbow trout in Danish or Norwegian fish farms and the second from rainbow trout to Arctic charr in Pålsbufjorden. After the second host switch, the parasite must have adapted rapidly to the new host species, as the rainbow trout vanished from fish catches within only 4 years after its last introduction (Per Aass, personal communications). This hypothesis implies a remarkably rapid host switch, as rainbow trout and Arctic charr probably co-occurred only for about 6 years. The reproductive mode of Gyrodactylus facilitates a rapid speciation by isolation and subsequent genetic diversification after a successful host-switch (Cable and Harris, 2002; Ziętara and Lumme, 2002; Meinilä et al. 2004) since a single worm can give rise to a viable population. In addition, recurrent host-switching has been put forward to promote rapid host-specific adaptation (Cribb et al. 2002; Poulin, 2002; Ziętara and Lumme, 2002).
The finding that G. salaris can infect resident Arctic charr in the absence of alternative hosts indicates the potential for radiation of this species complex. Further speculation about the importance of Arctic charr as a reservoir for G. salaris awaits detailed cross-infection experiments with different G. salaris strains. However, preliminary results suggest that a strain of G. salaris from salmon was more pathogenic to salmon than the Arctic charr strain from Pålsbufiorden (Olstad et al. manuscript in preparation).
The present findings have implications for the Norwegian salmon management and surveillance programmes as Arctic charr may sustain a G. salaris infection and hence be a potential focus for spreading of the parasite to uninfected Atlantic salmon stocks. Thus, the presence of Arctic charr must be taken into consideration along with Atlantic salmon when trying to eliminate G. salaris from an infected water course.
We thank Cathrine Vollelv, Henning Pavels, Finn Smestad, Kjersti Kvalsvik and Bjørn A. Bjerke, NHM, Department for Zoology, University of Oslo (UiO), and Rune Knudsen, NFH, University of Tromsø, for help with the fieldwork, Torill M. Rolfsen for support at the Scanning Electron Microscope Unit, University of Oslo, and Kjetil Olstad, NHM, University of Oslo and Jo Cable, Cardiff University for laboratory assistance and advice. This work was supported by the NRC ‘Wild Salmon Program’ (Project no. 145861/720) and the National Centre for Biosystematics (Project no. 146515/420, co-founded by the NRC and the NHM, UiO).