INTRODUCTION
Endocoelantheae is an interesting but poorly known taxon of sea anemone (Cnidaria: Anthozoa: Actiniaria) whose members primarily inhabit deep or polar waters and for which specimens are rare. Despite its small size (two families and fewer than 20 species), Endocoelantheae is ranked as a suborder because its members have an arrangement of mesenteries unlike that of all other members of the subclass Hexacorallia: after the development of the first 12 pairs of mesenteries, new mesenteries arise in the lateral endocoels (space enclosed by a pair of mesenteries), with the retractor muscles oriented as in the directives. This unique arrangement of the mesenteries has been hypothesized to have arisen once (Carlgren, Reference Carlgren1949) and has been proposed as the rationale for higher-level status for the group (Grebelny, Reference Grebelny1982). Furthermore, members of this suborder lack some morphological characters (basilar and marginal sphincter muscles) whose polarities are controversial and ambiguous for actiniarians in general (see Daly et al., Reference Daly, Chaudhuri, Gusmão and Rodríguez2008). Because specimens are rare, the diversity and morphology of the group is rather poorly understood.
The genus Halcurias is the type genus of the most species-rich family in Endocoelantheae. Halcurias pilatus McMurrich, Reference McMurrich1893—the type species of the genus—was initially described from three specimens collected in Southern Chile (McMurrich, Reference McMurrich1893). Later, McMurrich (Reference McMurrich1898) added a few details to the description of H. pilatus based on six additional specimens collected in the Bahamas, although this lead to a disjunct geographical distribution of H. pilatus (South Pacific and North Atlantic). At the time, differences between specimens were attributed to the state of preservation of the material (McMurrich, Reference McMurrich1898). In his revision of the species of Halcurias from Japan, Uchida (Reference Uchida2004) commented on H. pilatus stating that he considered the material from H. pilatus from the Bahamas to represent a different species, H. macmurrichi [sic] (Uchida, Reference Uchida2004). However, his comments were based on the literature rather than specimens and he did not provide a formal description or add any data for the new species.
We formally redescribe and illustrate Halcurias mcmurrichi Uchida, Reference Uchida2004 from newly collected material from deep-water reefs north off the Straits of Florida. Furthermore, we redescribe and illustrate H. pilatus from newly collected material from Southern Chile. We provide new records and anatomical and cnida data for both species. These species differ in the distribution and cnidom of nematocyst batteries in the column, development of microcnemes, cnidae, and geographical distribution. Furthermore, we find that H. pilatus can have a weak mesogleal marginal sphincter and thus modify the higher-level taxonomic diagnoses accordingly.
MATERIALS AND METHODS
Specimens of Halcurias pilatus were observed, photographed, and collected during SCUBA dives between 1998 and 2012, in shallow water (to 40 m depth) at more than 55 sites along the Chilean Patagonian coast from Punta Chaica/Lenca, Seno de Reloncavi (41°38′18.0″S 72°40′10.0″W) to Canal Pasaje, Central Patagonian Zone (50°27′49.2″S 75°07′41.6″W) (Figure 1). Specimens of H. mcmurrichi were collected in deep water (to 619 m depth) on the ‘Deep Coral’ (on-board RV ‘Edwin Link’) and the ‘Bioluminescence 2009’ (on-board RV ‘Seward Johnson’) cruises—funded by the NOAA Office of Ocean Exploration—to the Straits of Florida during the summers of 2006 and 2009, respectively (Figure 1).

Fig. 1. Geographical distribution of Halcurias pilatus McMurrich, Reference McMurrich1893 (new localities, black dots; type locality, triangle) and H. mcmurrichi Uchida, Reference Uchida2004 (new localities, asterisks; type locality, square).
All specimens were fixed in 10% sea-water formalin; specimens of Halcurias pilatus were relaxed with menthol crystals prior to fixation. Additionally, some specimens of H. pilatus were preserved in 96% ethanol for molecular studies. Preserved specimens (23 of H. pilatus and 2 of H. mcmurrichi) were examined whole, in dissection and as serial sections. Fragments of several specimens (9 of H. pilatus and 2 of H. mcmurrichi) were dehydrated in graded ethanol series and then embedded in paraffin. Histological sections 8–10 μm thick were cut and then stained with Ramon y Cajal's Triple Stain (Gabe, Reference Gabe1968) or Azocarmin Triple Stain (Humason, Reference Humason1967).
Measurements of cnidae were made from preserved and living (in Halcurias pilatus) material; small pieces of tissue were smeared on slides and examined using differential interference microscopy (DIC) at 1000× magnification. We scanned through the slides and haphazardly chose 40 capsules of each type (when possible) to measure to generate a range: frequencies given are subjective impressions based on all the cnidae seen on the slides. For each type, a mean and the standard deviation has been provided to give an idea of the distribution and sizes; these are not statistically significant (see Williams (Reference Williams1998, Reference Williams2000) for minimal requirements for statistical significance in cnida sizes) but provide some qualitative information about variability in capsule size for each type of nematocyst. Cnida terminology follows Mariscal (Reference Mariscal, Muscatine and Lenhoff1974). The studied material has been deposited in the American Museum of Natural History (AMNH) in New York City and the Zoologische Staatssammlung München (ZSM) in Germany.
RESULTS
SYSTEMATICS
Order ACTINIARIA Hertwig, Reference Hertwig1882
Suborder ENDOCOELANTHEAE Carlgren, Reference Carlgren1925
Family HALCURIIDAE Carlgren, Reference Carlgren1918
Genus Halcurias McMurrich, Reference McMurrich1893
DIAGNOSIS
Halcuriidae with distinct disc-like aboral end. Body elongate, not lobed distally, almost smooth but often with batteries of nematocysts. Margin tentaculate or with parapet. Marginal sphincter muscle very weak, mesogleal or muscle absent. Tentacles usually 68 but probably to 100, arranged in cycles, typically 18 (10 + 8) + 10 + 16 + 8 + 16 + 32. More mesenteries (microcnenes) distally than proximally; microcnemes more developed distally. To 50 pairs of mesenteries arranged in five cycles (6 + 4 + 8 + 16 + 16). Ten pairs of macrocnemes, all fertile. Single well-developed siphonoglyph. Retractor muscles restricted. Parietal muscles well developed to fairly weak. Longitudinal muscles of tentacles and radial muscles of oral disc ectodermal. Cnidom: spirocysts, basitrichs, holotrichs, and microbasic p-mastigophores (modified after Carlgren (Reference Carlgren1949) and Uchida (Reference Uchida2004), changes in italics).
TYPE SPECIES
Halcurias pilatus McMurrich, Reference McMurrich1893, by original designation.
Halcurias pilatus McMurrich, Reference McMurrich1893
(Figures 1–5; Table 1)
Non Halcurias pilatus: McMurrich, Reference McMurrich1898.
Halcurias pilatus: McMurrich, 1901.
MATERIAL
Two specimens in formalin (coordinates: 41.638333°S 72.669444°W, Punta Chaica/Lenca, Seno de Reloncavi, Chile; water depth: 13 m); [ZSM–20051684]; coll. V. Häussermann/G. Försterra (VH/GF), 13 February 1998.
Two specimens in formalin (coordinates: 41.672550°S 72.656650°W, Lenca, Seno de Reloncavi, Chile; water depth: 6 m); [ZSM–20051689]; coll. VH/GF, 24 January 2000.
Nine specimens in formalin (coordinates: 43.150000°S 73.583333°W, Punta Yenecura, Chile; water depth: 6 m); [ZSM–20051685]; coll. VH/GF, 22 December 1998.
One specimen in ethanol (coordinates: 43.150000°S 73.583333°W, Punta Yenecura, Chile; water depth: 6 m); [ZSM–20051707]; coll. VH/GF, 22 December 1998.
Five specimens in formalin (coordinates: 43.150000°S 73.583333°W, Isla Cailín, off Chiloé Island, Chile; water depth: 12 m); [ZSM–20060559]; coll. VH/GF, 26 December 1999, 31 January 2001.
Four specimens in formalin (coordinates: 42.546278°S 72.616722°W, Caleta Gonzalo, Chile; water depth: 20 m); [ZSM–20120662]; coll. VH/GF, 7 February 2001.
Four specimens in formalin (coordinates: 42.546278°S 72.616722°W, Caleta Gonzalo, Chile; water depth: 25 m); [ZSM–20051682]; coll. VH/GF, 23 February 2001.
One specimen in formalin (coordinates: 43.660944°S 72.981361°W, Bahia TicToc, Chile; water depth: 26 m); [ZSM–20051681]; coll. VH/GF, 7, 18 February 2001.
DESCRIPTION
External anatomy
Body cylindrical with well-developed, disc-like, delicate aboral end, to 14 mm in diameter in preserved specimens and 15 mm in living specimens (Figures 2, 3). Column of similar width distally and proximally, to 14 mm diameter and 25 mm height (to 75 mm height in living specimens), smooth but slightly corrugate in preserved specimens. Column with more or less regular longitudinal rows of nematocyst batteries distinctly visible as white spots (Figure 2E); margin tentaculate. Distinct, smooth, delicate parapet proximal to tentacles (Figures 2C, D, E, 3). Retracted column completely covers tentacles (Figure 3D).

Fig. 2. Halcurias pilatus McMurrich, Reference McMurrich1893, in vivo: (A) specimens covering large areas of steep rocky substratum in the shallow subtidal of semi-protected habitats (Lilihuapi Island, Comau Fjord, 5 m); (B) detail of specimen, showing eggs in the tentacles; see also the eggs in small specimen in (C); notice protruded actinopharynx. Photograph Roland Meyer; (C) specimens can strongly expand their column (in aquarium); (D) delicate parapet in distal part of the column (lower arrow); retracted specimens can completely cover the tentacles (upper arrow); (E) detail of the nematocysts batteries (white spots) more or less regularly arranged in longitudinal rows in the column; notice more delicate distal portion of column; (F) white lines on the oral disc and white circles in the base of the tentacles are a common oral disc pattern. Scale bars: A, 100 mm; B–F, 10 mm.

Fig. 3. Halcurias pilatus McMurrich, Reference McMurrich1893, external anatomy of preserved specimen. Scale bar: 10 mm.
Oral disc of slightly contracted and preserved specimens slightly narrower in diameter than proximal end, to 12 mm (to 13 mm in living specimens) (Figure 2C, F). Tentacles 50–98, usually about 70, arranged in four cycles, on outer 1/3 of oral disc, slender, to 8 mm in length in slightly contracted and preserved specimens and to 20 mm in living specimens (Figures 2, 3). Outer tentacles considerably shorter than inner ones.
Internal anatomy
Ten pairs of macrocnemes arranged in one cycle (Figure 4F), all fertile; equally developed proximally and distally; two pairs of directives, only one attached to one well-developed siphonoglyph (Figure 4G). Few pairs of microcnemes more developed distally but present at actinopharynx level (Figure 4H). Large oral stoma, round marginal stoma not present on all mesenteries. Actinopharynx deeply furrowed, often protruding through mouth. Gonochoric, specimens with well-developed oocytes and spermatic vesicles (to 0.33 mm and 0.08 mm in diameter, respectively) collected between December and March; gametes not always equally developed in mesenteries of a pair (Figure 4D). Retractor muscles restricted, reniform, strong (Figure 4F–H); not always equally developed within one pair. Parietal muscles well-developed, differentiated on all mesenteries; muscle fibres on thin, branched mesogleal processes (Figure 4D, G, H). Basilar muscles not differentiated (Figure 4E).

Fig. 4. Halcurias pilatus McMurrich, Reference McMurrich1893, internal anatomy of preserved specimens: (A) longitudinal section of distal part of the column with tentacles; notice thinner distal most area (arrow); (B) detail of muscle fibres embedded in the mesoglea forming a weak marginal sphincter muscle (arrow); (C) cross-section of the tentacle showing the ectodermal longitudinal musculature (arrow); (D) cross-section of the column showing oocytes; (E) detail of proximal end showing no trace of basilar muscles; (F) cross-section of the column at the level of the actinopharynx showing the 10 pairs of macrocnemes; (G) detail of directive mesenteries, siphonoglyph, retractor and parietal muscles (arrows); (H) details of microcnemes appearing in the endocoel at the level of the actinopharynx (arrows). Abbreviations: d, directive mesenteries; ep, epidermis; ga, gastrodermis; me, mesoglea; S, siphonoglyph. Scale bars: A, F, 5 mm; B–E, G, H, 1 mm.
Marginal sphincter muscle mesogleal, present in all specimens examined but very weak (Figure 4A, B). Longitudinal muscles of tentacles and radial muscles of oral disc ectodermal (Figure 4C). Column wall thick, with similar thickness entire length but in parapet: epidermis 0.01–0.02 mm; mesoglea 0.04–0.07 mm thick, and gastrodermis 0.01–0.02 mm thick at level of actinopharynx. Ectodermal muscles in distal column.
Cnidom
Gracile and robust spirocysts, basitrichs, holotrichs, and microbasic p-mastigophores (Figure 5). See Table 1 for size and distribution.

Fig. 5. Halcurias pilatus McMurrich, Reference McMurrich1893, cnidae: basitrichs (A, B, D, G, J, M, N); microbasic p-mastigophores (C, K, O); holotrichs (E, F), spirocysts (H, I, L, P).
Table 1. Size ranges of the cnidae of Halcurias pilatus McMurrich, Reference McMurrich1893 and H. mcmurrichi Uchida, Reference Uchida2004. Mean values marked with an asterisk are based on fewer than 40 capsules. Values of H. mcmurrichi from pooled samples.

M, Microbasic;
![]() ${\bar {\rm X}}$
, mean length by mean width of capsules; SD, standard deviation; S, ratio of number of specimens in which each cnidae was found to number of specimens examined; N, total number of capsules measured; F, frequency; +++, very common; ++, common; +, rather common; —, sporadic.
${\bar {\rm X}}$
, mean length by mean width of capsules; SD, standard deviation; S, ratio of number of specimens in which each cnidae was found to number of specimens examined; N, total number of capsules measured; F, frequency; +++, very common; ++, common; +, rather common; —, sporadic.
Colour
Most living specimens orange to pink; pedal disc, tentacles, and oral disc translucent orange, actinopharynx bright orange; some specimens translucent whitish to beige with white actinopharynx (Figure 2). Often specimens have white rings in bases of tentacles and/or white radial lines on oral disc. Small longitudinal rows of whitish spots on the column corresponding to nematocyst batteries (Figure 2E). Preserved material uniform whitish to beige (Figure 3).
GEOGRAPHICAL AND BATHYMETRIC DISTRIBUTION
Halcurias pilatus was originally described on the Pacific coast off Southern Chile at 821 m (449 fathoms) depth (McMurrich, Reference McMurrich1893). The material of this study has been collected at shallower depths (5–30 m) in the Chilean fjord region between the Seno de Reloncavi (41°38′18.0″S 72°40′10.0″W) and the Central Patagonian Zone (50°27′49.2″S 75°07′41.6″W) (see Figure 1). For additional sites where H. pilatus was found see Table 2.
Table 2. Additional sites where Halcurias pilatus has been found.

REMARKS
McMurrich (Reference McMurrich1898) added details to the original description of Halcurias pilatus based on specimens from the Bahamas. However, these specimens belong to Halcurias mcmurrichi and thus this material is not a synonymy of H. pilatus. He had eliminated any details corresponding to specimens of H. mcmurrichi in the description of H. pilatus.
Halcurias mcmurrichi Uchida, Reference Uchida2004
(Figures 1, 6–8; Table 1)
Halcurias pilatus: McMurrich, Reference McMurrich1898.
Halcurias macmurrichi: Uchida, Reference Uchida2004.
MATERIAL
One specimen, adult, male, partially dissected and sectioned in several slides (‘Bioluminiscence 2009’ cruise, JSLII, Dive–3697; coordinates: 27°03.4519′N 77°19.2865′W, Little Bahamas Bank lithoherms; water depth: 588–619 m) [AMNH–5125]; coll. T. Frank, 29 July 2009.
One specimen, adult, male, partially dissected and sectioned in slides. (‘Deep Coral’ Cruise, JSLII, Dive–3498; coordinates: 26°05.6696′N 79°50.6030′W, Miami Terrace off Fort Lauderdale, Florida; water depth: 300 m) [AMNH–5120]; coll. T. Frank, 1 June 2006.
DESCRIPTION
External anatomy
Body cylindrical with well-developed, disc-like aboral end, to 23 mm in diameter (Figure 6A, B). Remains of thin cuticle in aboral end. Column wider distally than proximally, to 32 mm diameter and 40 mm height, smooth but slightly corrugate in preserved specimens. Column with abundant scattered nematocyst batteries distally (Figures 6C, 7B, C); margin tentaculate. Distinct parapet proximal to tentacles marked by decrease in thickness of mesoglea (Figures 6A, 7A).

Fig. 6. Halcurias mcmurrichi Uchida, Reference Uchida2004, external anatomy of preserved specimens: (A) lateral view (AMNH–5125); (B) lateral view (AMNH–5120); (C) detail of scattered nematocyst batteries in the distal column (black arrows); (D, E) detail of distal-most column showing a pair of microcnemes (arrows) appearing in the endocoels of the pairs of macrocnemes. Abbreviation: te, tentacle. Scale bars: A, B, 20 mm; C–E, 5 mm.
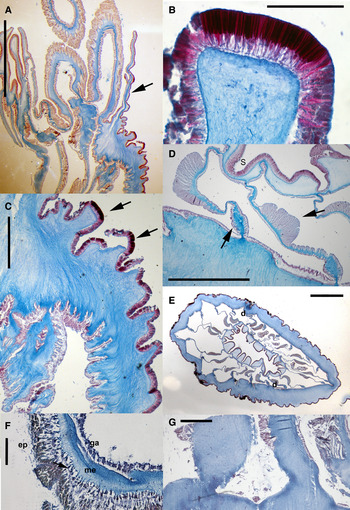
Fig. 7. Halcurias mcmurrichi Uchida, Reference Uchida2004, internal anatomy of preserved specimens: (A) longitudinal section of distal part of the column; notice the parapet marked by a considerable decrease of the thickness of the mesoglea (arrow); (B) detail of a nematocyst battery in distal column; notice the densely packed nematocysts; (C) detail of proximal column showing no trace of marginal sphincter muscle; notice nematocyst batteries (black arrows); (D) detail of directive mesenteries, retractor and parietal muscles (arrows); (E) cross-section of the column at the level of the actinopharynx showing the pairs of mesenteries; (F) cross-section of the tentacle showing the ectodermal longitudinal musculature (arrow); (G) detail of proximal end showing no trace of basilar muscles. Abbreviations: d, directive mesenteries; ep, epidermis; ga, gastrodermis; me, mesoglea; S, siphonoglyph. Scale bars: A, 5 mm; B, 0.2 mm; C, 1 mm; D, 1.5 mm; E, 3 mm; F, 0.1 mm; G, 0.5 mm.
Oral disc of slightly contracted and preserved specimens wider in diameter than proximal end, to 37 mm (Figure 5A, B). Tentacles 64–68, arranged in three cycles, to 15 mm in length in slightly contracted and preserved specimens (Figure 6A, B).
Internal anatomy
Ten pairs of macrocnemes arranged in one cycle, all fertile; two pairs of directives, only one attached to one well-developed siphonoglyph (Figure 7D, E). Few pairs of microcnemes in distal-most column, only immediately beneath tentacles (Figure 6D, E). No stomata (?). Actinopharynx deeply furrowed. Gonochoric; both specimens male, with well-developed spermatic vesicles (to 0.1 mm in diameter) collected in June and July. Retractor muscles restricted, relatively strong. Parietal muscles well-developed, differentiated on all mesenteries (Figure 7D); muscle fibres on-broad, branched mesogleal base. Basilar muscles not differentiated (Figure 7G).
No marginal sphincter muscle (Figure 7A, B). Longitudinal muscles of tentacles and radial muscles of oral disc ectodermal (Figure 7F). Column wall thick, with similar thickness entire length but in parapet: epidermis 0.02–0.04 mm; mesoglea 0.11–0.13 mm thick, and gastrodermis 0.02–0.03 mm thick at level of actinopharynx. Ectodermal muscles in distal column.
Cnidom
Gracile and robust spirocysts, basitrichs, and microbasic p-mastigophores (Figure 8). See Table 1 for size and distribution.

Fig. 8. Halcurias mcmurrichi Uchida, Reference Uchida2004, cnidae: basitrichs (A–E, H, K); spirocysts (F, G, J, M); microbasic p-mastigophores (I, L).
Colour
Preserved material uniform yellowish to whitish; tentacles fading to white toward the tips (Figure 6).
GEOGRAPHICAL AND BATHYMETRIC DISTRIBUTION
Halcurias mcmurrichi was initially described south of the Bahamas Islands between 201 and 212 m (110–116 fathoms) depth (McMurrich, Reference McMurrich1898). Our material was collected in bathyal depths (300–619 m) off the Miami Terrace and at the Little Bahamas Bank lithoherms (see Figure 1).
REMARKS
Uchida (Reference Uchida2004) established Halcurias macmurrichi [sic] as a nomen novum for the material of H. pilatus from the Bahamas based on the literature but he did not provide a formal description. However, according to the International Code of Zoological Nomenclature (ICZN), Art. 13.1.1 (International Commission on Zoological Nomenclature, 1999), because he provided diagnostic characters to differentiate this new taxon in his key to species of Halcurias from Japan, his new name is legitimate (ICZN, 1999). However, the correct spelling for the species name is H. mcmurrichi (Art. 29.3.1; ICZN, 1999).
DISCUSSION
On the type species Halcurias pilatus
The relatively large amount of material available for this study provided new data on the type species of Halcurias. Although some of our findings do not correspond completely with the original description of H. pilatus, we consider most of these discrepancies due to low number of studied specimens (three) in the original description and thus evidence of intraspecific variability.
One of the cited diagnostic characters of the suborder Endocoelantheae is the lack of a marginal sphincter muscle (Carlgren, Reference Carlgren1949). However, in all studied specimens of Halcurias pilatus we found muscles fibres embedded in the mesoglea of the distal column, forming a weak mesogleal marginal sphincter (Figure 4A, B). This finding is not correlated to preservation, degree of contraction, or size. Furthermore, although McMurrich (Reference McMurrich1898) confirmed the absence of this muscle in H. pilatus providing an image of a distal longitudinal section of the column, his observations were based on specimens corresponding to H. mcmurrichi. Thus, the diagnoses of the suborder Endocoelantheae and the family Halcuriidae need to be modified to include the possibility of a weak mesogleal marginal sphincter in at least some members. Similarly, although H. pilatus (and other species of the genus) is described as having only up to 70 tentacles and four cycles of mesenteries (to 34 pairs), we found that most specimens had between 70 and 98 tentacles, and thus up to five cycles of mesenteries (to 50 pairs and probably up to 100 tentacles); the possibility of H. pilatus developing a fifth cycle was reflected by Carlgren (Reference Carlgren1914). We modified the diagnosis of Halcurias to reflect these new findings (marked in italics).
Halcurias pilatus was originally reported at 821 m depth (McMurrich, Reference McMurrich1893). We found H. pilatus living in the shallow subtidal (to 40 m) in small holes, rocky chinks, below stones, in dead barnacles, between polychaete tubes, or on rocky walls in the inner fjords as well as channels and semi-exposed areas of the Chilean fjord region. However, deep-water species are commonly found in shallow waters (e.g. Försterra, Reference Försterra, Häussermann and Försterra2009), a phenomenon called deep-water emergence. Thus, we here extend the bathymetric distribution of H. pilatus from bathyal to shallow depths.
We report for the first time holotrichs in the nematocyst batteries of a species within Halcurias (Uchida, Reference Uchida2004). Halcurias pilatus can be locally very abundant to dominant, densely covering the substrate at semi-exposed sites (Figure 2A). It settles densely on recruitment plates at Lilihuapi Island but is relatively rare at ‘Cross Huinay’, a site in the inner fjord (unpublished results). When specimens co-occur, they are likely sexually produced because we found developed eggs in all studied specimens (see Figures 2B, 3A, D). However, it is possible that these are not meiotically produced eggs, being instead asexual buds, or parthenogenic eggs rather than participants in mictic reproduction (e.g. Thorpe & Carter, Reference Thorpe and Carter1982; Ayre, Reference Ayre1988). The morphology and size ranges of the holotrichs in the columnar nematocyst batteries of H. pilatus are very similar to those holotrichs associated to structures in the margin of the column (acrorhagi) in members of some endomyarian families (e.g. Actiniidae Rafinesque, Reference Rafinesque1815). Acrorhagi are used in agonistic behaviour in some clonal sea anemones putatively due to space limitation in the intertidal (Francis, Reference Francis1973; Ayre & Grosberg, Reference Ayre and Grosberg2005). Holotrichs have also been reported in the column of non-clonal endomyarian species (e.g. Epiactis Verrill, Reference Verrill1869) and in catch (or fighting) tentacles of some acontiate species (e.g. Metridium de Blainville, Reference Blainville1824). Although some species have been shown to have holotrichs, they are not present in all individuals analysed (Fautin & Chia, Reference Fautin and Chia1986; Edmands & Fautin, Reference Edmands and Fautin1991). This observation, in combination with the fact that fighting-tentacles can be induced (Watson & Mariscal, Reference Watson and Mariscal1983), has led to the hypothesis that holotrichs might be inducible (Fautin & Chia, Reference Fautin and Chia1986). The apparent success of H. pilatus competing for substrate might be related to the presence of holotrichs in the nematocyst batteries of the column. This could also explain their absence in H. mcmurrichi. However, further studies are necessary to address asexual reproduction in H. pilatus and the suggested relationship between holotrichs and competition for substrate in this species.
Differential diagnosis of Halcurias mcmurrichi
The study of the newly collected specimens of Halcurias mcmurrichi showed that the specimens from the Bahamas initially identified by McMurrich (Reference McMurrich1898) as H. pilatus warrant establishing a new species, as proposed by Uchida (Reference Uchida2004).
Halcurias mcmurrichi and H. pilatus differ in external and internal anatomy, cnidae, and geographical distribution. In H. pilatus, the nematocyst batteries in the column are arranged in more or less regular longitudinal rows along the entire column and contain holotrichs and basitrichs whereas in H. mcmurrichi the nematocyst batteries are irregularly arranged, are only present in the distal column, and contain only basitrichs. Halcurias pilatus has up to 98 tentacles and, although the number of available specimens is low, H. mcmurrichi has only up to 70 tentacles. With respect to internal anatomy, the species differ in the mesenterial stomata (oral and marginal in H. pilatus but absent in H. mcmurrichi), retractor and parietal muscles (retractors restricted to reniform and parietal muscles weak in H. pilatus whereas the retractors are strong but not reniform and the parietal muscles are well-developed with fibres on a broad, branched mesogleal base in H. mcmurrichi), and in the microcnemes (more developed distally but present at the actinopharynx level (see Figure 4H) in H. pilatus whereas microcnemes are present only in the most distal part of the column, just immediately beneath the tentacles (Figure 6D, E), in H. mcmurrichi). Based on McMurrich's original description of H. pilatus, Uchida (Reference Uchida2004) used the reduction of four pairs of macrocnemes proximally as a difference to H. mcmurrichi (in which macronecmes are not reduced proximally). However, we did not find reduction of macronecmes proximally in the studied material of H. pilatus; this observation was most probably due to the stage of development of the single specimen McMurrich (Reference McMurrich1893) examined histologically. The distribution and sizes ranges of cnidae are clearly different in both species, particularly in the scapus, nematocyst batteries, and mesenterial filaments (Table 1). Although the number of studied specimens of H. mcmurrichi is rather low (only two), the material is well preserved, and thus, the observed differences in the cnidae between the two species are not likely to be an artefact of preservation. Finally, H. pilatus might have a weak mesogleal marginal sphincter muscle whereas a marginal sphincter muscle has not been detected in H. mcmurrichi.
The geographical distribution of both species is also different: Halcurias mcmurrichi has been collected in deep-water reefs (defined as coral banks, bioherms or lithotherms) off the south-eastern Atlantic coast of the United States, in the Miami Terrace (north of the Straits of Florida) and north of the Miami Terrace, off the Little Bahamas Bank. Halcurias pilatus has been collected in deep and shallow waters (on hard substrates) of the south-eastern Pacific coast of Chile.
ACKNOWLEDGEMENTS
Special thanks to Charles Messing (Nova Southeastern University (NSU) Oceanographic Center, Florida) and Meg Daly (The Ohio State University, Ohio) for making access to the material of Halcurias mcmurrichi possible. Tamara Frank (NSU Oceanographic Center) and John Reed (Florida Atlantic University) are thanked for collecting these specimens and providing collection data. We thank Günter Försterra for collecting material of H. pilatus and his help during fieldwork. Comments from M. Daly significantly improved this work. This research received no specific grant from any funding agency, commercial or not-for-profit sectors. Support for M.M. was partly provided by Benemérita Universidad Autónoma de Puebla (Mexico). This is publication no. 81 from Huinay Scientific Field Station.












