Introduction
Salinity, drought, and temperature extremes are among the major environmental constraints to plant productivity worldwide. These constraints will reduce productivity of major agricultural crops by more than 50% by the year 2050 (Wang et al. Reference Wang, Binocur and Altman2003). Both salinity and drought induce osmotic stress, altering cellular homeostasis and ion distribution in plants (Zhu Reference Zhu2001).
The best characterized biochemical response of plants to osmotic stress is the accumulation of compatible solutes, such as glycine-betaine, proline and soluble sugars (McCue & Hanson Reference McCue and Hanson1990). Compatible solutes mediate osmotic adjustment, protect subcellular structures and reduce oxidative damage caused by free radicals produced in response to osmotic stress (Crowe et al. Reference Crowe, Crowe, Leslie and Fisk1993, Yeo Reference Yeo1998, Holmstrom et al. Reference Holmstrom, Somersalo, Mandal, Palva and Welin2000, Chen & Murata Reference Chen and Murata2002).
In plants, a wide range of abiotic stress factors can induce oxidative stress. Salinity, drought, and oxidative stress are accompanied by the formation of reactive oxygen species (ROS) molecules such as superoxide anion O2-, hydrogen peroxide H2O2, and hydroxyl ion OH-, which damage membranes and macromolecules (Noctor & Foyer Reference Noctor and Foyer1998, Asada Reference Asada1999, Mittler Reference Mittler2002).
Different antioxidant defence systems have been developed in aerobic cells to counteract the damaging effects of ROS. Plants possess antioxidant defence systems comprised of enzymatic and non-enzymatic components, which normally maintain ROS balance within the cell. For instance, they may use a diverse array of enzymes such as superoxide dismutase (SOD), catalases (CAT) and peroxidases as well as low molecular mass antioxidants (glutathione, ascorbate phenolic compounds and lignans) to scavenge different types of ROS (Foyer et al. Reference Foyer, Descourvieres and Kunert1994, Pietta Reference Pietta2000).
Deschampsia antarctica Desv. (Poaceae) is the only grass endemic to the Antarctic, a habitat simultaneously affected by various environmental conditions such as extreme high UV-B radiation, low temperatures, high levels of salinity and low water availability (Alberdi et al. Reference Alberdi, Bravo, Gutiérrez, Gidekel and Corcuera2002). It has been reported that D. antarctica presents an efficient photosynthetic system, which results in a large accumulation of sugars (Zúñiga et al. Reference Zúñiga, Alberdi and Corcuera1996). However, the degree of tolerance to abiotic stress under natural conditions has been not described.
The aim of this research was to evaluate some enzymatic and non enzymatic responses of in vitro shoots of D. antarctica subjected to osmotic stress.
Materials and methods
Plant material and in vitro growth conditions
Deschampia antarctica plants were collected from King George Island, South Shetland Islands (62°14′S; 58°48′W), and then moved to the laboratory for in vitro culture at the Universidad de Santiago de Chile. Shoots were propagated vegetatively in soil: peat mixture (3:1) at 16°C ± 2°C in a growth chamber with photon flux density of 40 umol m-2 s-1 and 16/8 hr light/dark period. Crown tissue from axenic plants was used to initiate cultures into culture vessels (250 ml) containing MS Propagation Medium (Murashige & Skoog Reference Murashige and Skoog1962), (3% sucrose, 0.6% agar-agar, pH adjusted at 5.6 and autoclaved at 120°C for 20 min). Cultures were incubated at 14 ± 2°C under 16 hr photoperiod using a cold, white fluorescent light irradiance of 40 μmol m-2 s-1. Explants were sub-cultured to fresh medium at four week intervals.
Osmotic stress simulation was carried out over one year by culturing plants in MS media supplemented with PEG-8000 (-0.3 MPa). Sub-culturing of treated populations was carried out using the same timeframe as used for conventional propagation of shoots.
Physiological parameters of damage
Lipid peroxidation was estimated by measuring the concentration of malondialdehyde (MDA) by thiobarbituric acid (TBA) assay (Ederli et al. Reference Ederli, Pasqualini, Batini and Antonielli1997). 0.1 g of leaves were homogenized with 2 ml of TCA (1%) and centrifuged at 10.000 g for 5 min. 250 μl of the supernatant was mixed with 1 ml of TBA (0.5%) in TCA (20%). Mixtures were incubated in boiling water for 30 min, and then cooled to room temperature. Absorbance was determined at 532 nm and non-specific absorbance at 600 nm (Hodges et al. Reference Hodges, Delong, Forney and Prange1999). MDA content was determined using a molar extinction coefficient of 155 mol-1 cm-1.
Total chlorophyll content in leaves was analysed according to Lichtenthaler methodology (Lichtenthaler & Wellburn Reference Lichtenthaler and Wellburn1990). 0.1 g of leaves was homogenized with 2 ml of acetone 80%, maintaining the mortar on ice. Samples were centrifuged at 4°C for 10 min at 10.000 g. 50 μl of the supernatant was mixed with 1 ml of acetone 80% and absorbance was determined at 663.6 and 646.6 nm. Total chlorophyll content was calculated using the formula: Chl a + b = 17.76A646.6 + 7.34A663.6.
Hydrogen peroxide content was determined using a reflectometric method by used Rqflex (Merck), and applying a sensitivity range between of 0.2–20 mg l-1. Fresh tissue (0.1 g) was macerated with 2 ml of 50 mM sodium phosphate (pH 7.0), and immediately used for analysis.
Non-enzymatic antioxidant response
The extracts were prepared by taking 100 mg of tissue and extracting with 3 ml of 100% methanol. Extracts were sonicated at 50/60 Hz (Cole-Parmer, Model 8851) for 4 hr, and kept a 4°C for 96 hr in darkness prior to analysis.
The total phenolic content was determined using a modified Folin-Ciocalteu colorimetric method (Asami et al. Reference Asami, Hong, Barrett and Mitchell2003). Results were expressed as milligrams of gallic acid equivalents. The flavonoid content was determined using a modified colorimetric method as described by Liu et al. (Reference Liu, Li, Weber, Lee, Brown and Liu2002). Results were expressed as milligrams of quercetine equivalents. Data are reported as means ± SD for at least three replicates.
Antioxidant activity of methanolic extracts was measured by the bleaching of 1,1-Difenil-2-Picril-Hidrazil (DPPH) cation radical. The DPPH solution is purple (λ max = 517 nm), but loses its colour when the radical molecules are stabilized by antioxidants (Blois 1958). The measurement was carried out by determining the curve consumption DPPH in 180 sec, evaluating 50 μl extract with 950 μl of DPPH solution (Brand-Williams et al. Reference Brand-Williams, Cuvelier and Berset1995).
Ascorbate content was determined using a reflectometric method, Rqflex (Merck), and applying a sensitivity range of 25 to 450 mg l-1. Fresh tissue (0.1 g) was macerated with 2 ml of 50 mM sodium phosphate (pH 7.0) and immediately used for analysis.
Effects of PEG-8000 on the enzymatic antioxidant system
To determine the activities of the antioxidant enzymes, 0.1 g of leaves were homogenized with 2 ml of potassium phosphate buffer 50 mM adjusted at pH 7.0 (extraction buffer). Samples were centrifuged at 4°C for 15 min at 11 000 g and the supernatant used for activity determinations. Protein content of samples was determined using a modified Bradford method (1 ml of Bradford reactive, 80 μl of 0.15 M NaCl and 20 μl of supernatants (Bradford Reference Bradford1976). Protein concentration was calculated using a calibration curve made with BSA (1 mg ml-1).
APX (EC 1.11.1.11) activity was tested measuring the decomposition of ascorbate at 290 nm for 45 sec. The reaction mixture contained 1 ml of extraction buffer, 5 μl of 30% H2O2, 40 μl of 10 mM ascorbic acid and 20 μl of the supernatant. Enzyme activity was calculated using a molar extinction coefficient of 2.8 mM-1 cm-1 (Zhao & Blumwald Reference Zhao and Blumwald1998).
CAT (EC 1.11.1.6) activity was tested by measuring the decomposition of hydrogen peroxide at 240 nm for 45 sec. The reaction mixture contained 1 ml of extraction buffer, 3 μl of 30% H2O2 and 20 μl of the supernatant. Enzyme activity was calculated using a molar extinction coefficient of 39.4 mM-1 cm-1 (Pinhero et al. Reference Pinhero, Rao, Paliyath, Murr and Fletcher1997).
POX (EC 1.11.1.7) activity was tested measuring the appearance of tetraguaiacol at 470 nm for 45 sec. The reaction mixture contained 1 ml of extraction buffer, 5 μl of 30% H2O2, 5 μl of guaiacol and 10 μl of the supernatant. Enzyme activity was calculated using a molar extinction coefficient of 26.6 mM-1 cm-1 (Pinhero et al. Reference Pinhero, Rao, Paliyath, Murr and Fletcher1997).
The activity of GR (EC 1.6.4.2) was determined by measuring the oxidation of NADPH at 340 nm for 3 min. The reaction mixture contained 1 ml of extraction buffer, 2 mM Na2EDTA, 0.15 mM NADPH, 0.5 mM GSSG and 100 μl extract. Enzyme activity was calculated using a molar extinction coefficient of 6.2 mM-1cm-1 (Schaedle & Bassham Reference Schaedle and Bassham1977).
Statistical analysis
All studies were performed in triplicate and results were analysed using the t-test with a confidence interval of 95%. Analyses were made using the software Statgraphics Plus 5.1 software (Manugistics Inc, Rockville, MD, USA).
Results
Deschampsia antarctica shoots were successfully introduced into in vitro cultivation by use of PM and a proper sub-culturing rate was set up every 21–30 days. Within five months, shoot populations reached approximately 200 individuals in a general healthy looking state. The population was then split into control shoots kept under PM, and treated shoots, kept and sub-cultured in treatment medium (TM). During one year of cultivation in TM, shoots showed no visual sign of damage by browning, chlorosis, leaf deformation or stunting, and their sub-culturing time was similar to control shoots (Fig. 1).

Fig. 1 Phenotype of in vitro shoots of D. antarctica in control media or in PEG-8000 (-0.3 MPa) induced osmotic stress media. Shoots were kept for 12 months under those conditions.
Effect of PEG-8000 - physiological parameters of damage
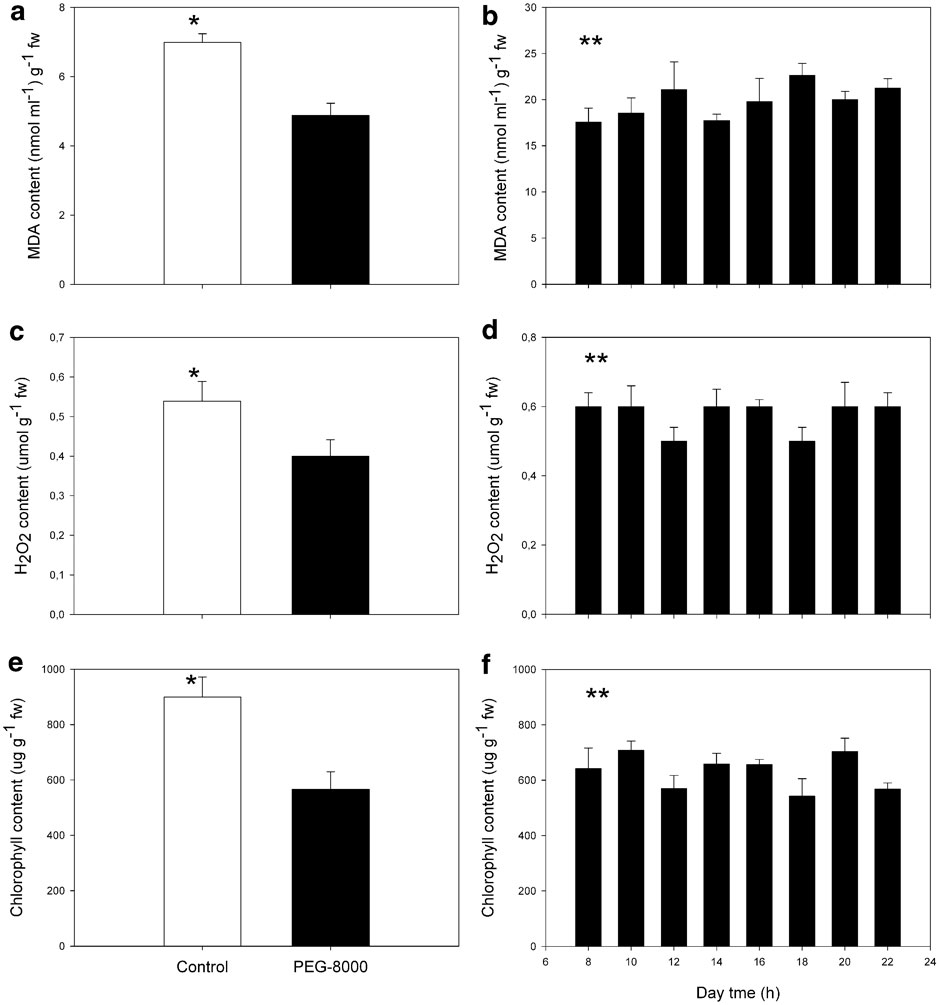
Despite the fact that PEG-8000 did not affect any visual phenotypic characteristic in the shoots, its effect on levels of MDA, chlorophyll and hydrogen peroxide was compared with those in samples of plants growing under field conditions in the Antarctic. Figure 2 shows that MDA content was lower in treated than in control plants. It is important to notice that the levels of MDA in shoots growing in medium without PEG-8000, are significantly lower than levels found in the samples obtained from the Antarctic zone (P < 0.05) (Fig. 2a). In these plants, TBARS levels were about four-fold higher than in vitro, but remained unchanged during the day. Antarctic conditions did not induce signs of oxidative stress in plants of D. antarctica. In addition, the levels of hydrogen peroxide and total chlorophyll in plants treated with PEG-8000 decreased significantly compared to control plants (P < 0.05) (Fig. 2b & c). These results suggest that in vitro shoots of D. antarctica are capable of controlling the oxidative damage induced by PEG-8000.
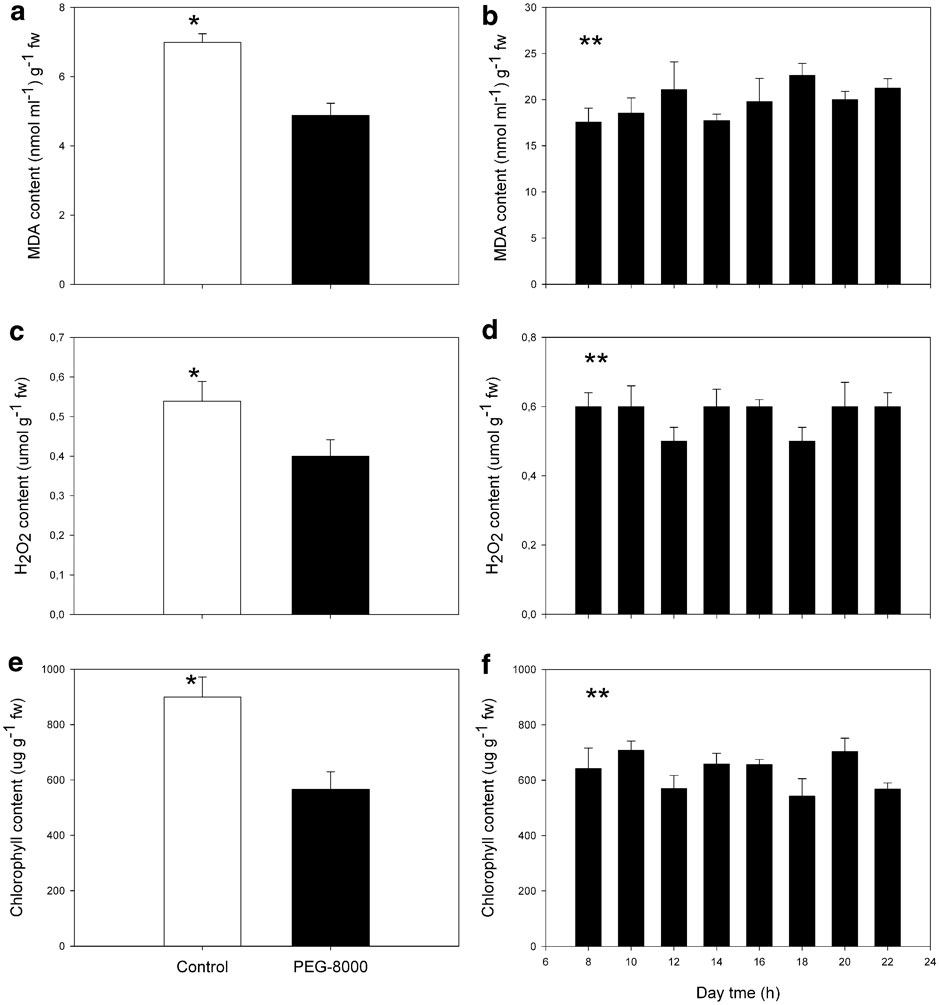
Fig. 2 Content of a, b. Malondialdehyde, c, d. hydrogen peroxide, and e, f. total chlorophyll on in vitro and in situ shoots of D. antarctica. a., c. & e. Control plants and PEG-8000 treated plants, and b., d. & f.in situ growing plants. Each value corresponds to the mean of six replicates ± standard error (g-1 f. wt). Bars with * are significantly different (P < 0.05), ** = no significant difference.
Effect of PEG-8000 on antioxidant enzyme activities
ROS can be controlled by efficient enzymes (Jiang & Zhang Reference Jiang and Zhang2002). For this reason we evaluated the effect of PEG-8000 on the activity of antioxidant enzymes such as CAT, APX, GR and POX on in vitro shoots of D. antarctica (Fig. 3). The activity of CAT in plants grown in the presence of PEG increases significantly as compared to control plants (seven-fold) (P < 0.05) (Fig. 3a).

Fig. 3 Effect of PEG-8000 induced osmotic stress on the activities of the antioxidant enzymes a. catalase (CAT), b. guaiacol peroxidase (POX), c. ascorbate peroxidase (APX), and d. glutation reductase (GR) in D. antarctica shoots. Each value represents the mean of six replicates ± standard error. Bars with * are significantly different (P < 0.05), ** = no significant difference.
PEG-induced activities of POX in D. antarctica were four-fold higher than controls (P < 0.05) (Fig. 3b). APX activity did not show significant changes (P < 0.05) (Fig. 3c) in PEG treated plants. Additionally, the activity of GR found in shoots of plants treated with PEG-8000 showed a three-fold increase compared to control shoots (P < 0.05). These results suggest that the null effect observed in plants growing in PEG-8000 could be due, at least in part, to the induction of the activity of these antioxidant enzymes (CAT, GR and POX).
Effect of PEG-8000 on non-enzymatic antioxidant system
In order to determine whether the non-enzymatic antioxidant system is affected by PEG-8000, concentrations of phenolic compounds and flavonoids were measured (Fig. 4). The content of phenolic compounds was significantly increased in plants growing in media with PEG-8000 (five-fold) with respect control shoots (P < 0.05) (Fig. 4a). In addition, the content of flavonoids, did not change significantly between shoots from both media (P > 0.05) (Fig. 4b).

Fig. 4 Effect of PEG-8000 induced osmotic stress in the content of a. total phenolic compounds, and b. flavonoids in D. antarctica shoots. Each value represents the mean of six replicates ± standard error. (QE = quercetine equivalent). Bars with * are significantly different (P < 0.05), ** = no significant difference.
Changes in the content of total phenolic compounds in shoots of control and stressed D. antarctica plants are presented in Fig. 4. The results show that the total content of phenolic compounds in methanolic extracts of PEG-8000 treated plants are higher than in control ones (P > 0.05), suggesting that the increased levels of phenolic compounds could have a function related to ROS detoxification. Methanolic extracts showed antiradical activity against the DPPH radical (Fig. 5).

Fig. 5 Effect of PEG-8000 induced osmotic stress in the antioxidant capacity of leaf extracts of in vitro shoots of D. antarctica. Antioxidant capacity was expressed as percentage of the free radical DPPH+ consumed in 180 sec of reaction. Each value represents the mean of six replicates ± standard error. * is significantly different (P < 0.05).
Other molecules that showed changed levels under osmotic stress were ascorbate and glutathione. Figure 6 shows that the level of ascorbate content in plants subjected to PEG-8000, were significantly lower than levels determined in control shoots (P < 0.05). These differences could be attributed to severe changes in steady-state of this molecule, which could be caused by its over consumption in addition to a relatively low rate of regeneration.

Fig. 6 Effect of PEG-8000 induced osmotic stress in the ASC content in shoots of D. antarctica. Each value represents the mean of six replicates ± standard error. (μmol g-1 f. wt). * is significantly different (P < 0.05).
Discussion
In this study, D. antarctica was shown to be tolerant to PEG-induced osmotic stress. It is already known that free radicals generated under stress conditions induce peroxidation of lipid membranes (Jain et al. Reference Jain, Mathur, Koul and Sarin2001). In the present work, MDA levels and H2O2 did not increase as a result of PEG-8000 treatment. In addition, MDA and H2O2 remained constant during a daily cycle in plants growing under Antarctic conditions. These results suggest that D. antarctica has efficient mechanisms to control the generation of ROS.
To resist oxidative damage, plants possess antioxidant defence systems that include metabolites such as ascorbate and glutathione (GSH), present in tissues at mM concentrations. Another part of the ROS scavenging system depends on enzymes, such as SODs, peroxidases and catalases (Noctor & Foyer Reference Noctor and Foyer1998, Asada Reference Asada1999). These enzymes catalyze redox reactions, many of which rely on electrons supplied by reductants of low molecular weight, (Noctor & Foyer Reference Noctor and Foyer1998). In this work we observed enhanced activities of CAT, POX and GR and high antioxidant activity of methanolic extract in plants treated with PEG-8000. It has been suggested that constitutive levels of CAT and POX were always higher in species tolerant to osmotic stress (Sairam et al. Reference Sairam, Shukla and Saxena1998).
Catalases convert H2O2 to water and O2 (Smirnoff Reference Smirnoff1993). These enzymes have extremely high catalytic rates, but low substrate affinities, since the reaction requires the simultaneous access of two H2O2 molecules at the active site. An alternative mode of H2O2 detoxification is via peroxidases, which are present in most organelles and show higher affinity for H2O2 than CAT. However, peroxidases require a reductant, since they reduce H2O2 to H2O. In plant cells, the most important reducing substrate for H2O2 detoxification is ascorbate. This may explain the decreased content of this molecule in plants treated with PEG-8000. Ascorbate is a powerful antioxidant, which reacts directly with O2- and HO- radicals. It has an important role in preserving the activities of enzymes that contain prosthetic groups with transition metal ions. Apoplastic ascorbate is also considered crucial in scavenging ROS, particularly those arising from exposure to atmospheric pollutants such as ozone. Ascorbate may also be involved in the regulation of the cell cycle (Kerk & Feldman Reference Kerk and Feldman1995). Arrest of cell cycle during oxidative stress prevents replication of damaged DNA, and appears to correlate with a decreased ratio of ascorbate to DHA (Sánchez-Fernández et al. Reference Sánchez-Fernández, Fricker, Corben, White, Sheard, Leaver, van Montagu, Inzé and May1997).
GR also plays a key role in oxidative stress by converting oxidized glutathione, GSSG to GSH (Fadzilla et al. Reference Fadzilla, Finch and Burdon1997). Increased GR activity in corn plants has been reported to be closely related with drought tolerance (Pastori & Trippi Reference Pastori and Trippi1993). In our experiments GR activity increased in D. antarctica under osmotic stress. It has been shown that O2- and H2O2 generated during water stress might be responsible for the induction of GR (Baisak et al. Reference Baisak, Rana, Acharya and Kar1994). The increase in GR in D. antarctica plants treated with PEG-8000, might have resulted in a higher pool of GSH, which could be used in ascorbate generation. GR could play a key role in protection against oxidative stress (Gamble & Burke Reference Gamble and Burke1984).
By analysing the antioxidant activity of shoot extracts, it was demonstrated that methanolic extracts had the capacity to scavenge DPPH free radicals (Fig. 4). A positive correlation was demonstrated between the overall content of phenolic compounds in the extracts and their antioxidant capacity (Fig. 5). The methanolic extract is being characterized to identify the molecules with antioxidant activity.
In conclusion, based on the data obtained from MDA, H2O2 and chlorophyll content, it is clear that D. antarctica is tolerant to osmotic stress. It is possible that this osmotic stress tolerance is associated with its ability to induced activity of CAT, POX and GR resulting in lower H2O2 production and lipid peroxidation.
Acknowledgements
This work has been supported by fellowship for a Doctoral thesis on Antarctic Studies (P. Zamora) at the Chilean Antarctic Institute (INACh) and DICYT-USACH. We thank INACh for logistical support and Cristina Vargas for technical assistance in this study.