Introduction
Leishmaniasis is a neglected disease of high prevalence and distribution directly linked to social, environmental and climatological factors. It is endemic in 98 countries, with 350 million people at risk, and is the cause of 50 000 deaths every year worldwide (Alvar et al., Reference Alvar, Velez, Bern, Herrero, Desjeux, Cano, Jannin and den Boer2012; World Health Organization, 2019). The majority of cutaneous leishmaniasis cases occur in Afghanistan, Algeria, Brazil, Colombia, the Islamic Republic of Iran, Pakistan, Peru, Saudi Arabia and the Syrian Arab Republic (World Health Organization, 2019).
The epidemiology of cutaneous leishmaniasis in the Americas is complex, with multiple circulating Leishmania species in the same geographical area, several reservoirs, hosts and sand fly vectors, as well as variable clinical manifestations and responses to therapy (Burza et al., Reference Burza, Croft and Boelaert2018). The genetic complexity of the causative agents of leishmaniasis is vast, as shown by studies on the taxonomy of these trypanosomatids through molecular analysis of V7V8 SSU rRNA, Hsp70 and gGAPDH genes (Jirku et al., Reference Jirku, Yurchenko, Lukes and Maslov2012; Espinosa et al., Reference Espinosa, Serrano, Camargo, Teixeira and Shaw2018); this may translate to the variability in clinical manifestations of the disease. American cutaneous leishmaniasis (ACL) is associated with at least 15 Leishmania species belonging to the subgenera, Viannia, Leishmania and Mundinia, allocated into the subfamily Leishmaniinae (Espinosa et al., Reference Espinosa, Serrano, Camargo, Teixeira and Shaw2018; Silveira, Reference Silveira2019). Leishmania braziliensis (subgenera Viannia) and Leishmania amazonensis (subgenera Leishmania) are the dominant pathogenic species in the Americas, and both are involved in cutaneous leishmaniasis which usually presents as a self-limited ulcer that heals in 3–18 months. Leishmania braziliensis is also involved in mucosal leishmaniasis, a potentially life-threatening condition that occurs in up to 10% of patients (Burza et al., Reference Burza, Croft and Boelaert2018).
Besides, L. amazonensis is responsible for anergic diffuse cutaneous leishmaniasis (DCL), accounting for nearly 1% of all ACL cases each year in Brazil (de Lima et al., Reference de Lima, Teixeira, Lopes, de Morais, Torres, Braga, Rodrigues, Santiago, Martins and Nagao-Dias2014; Brasil, Ministério da Saúde, 2017; Machado et al., Reference Machado, Prates and Machado2019). The anergic DCL is characterized by massive dermal infiltrates, is chronic with frequent relapses, and presents with clinical, immunological, parasitological, anatomopathological and therapeutic aspects different from other forms of ACL (Costa et al., Reference Costa, Uthant, CostaMl, Bezerril and Barral2009). Leishmania amazonensis infection induces inhibition of the delayed type hypersensitivity of skin, low production of interferon γ (IFN-γ) and high production of interleukin-10 (IL-10) and transforming growth factor-β, leading to inefficient activation of infected macrophages and non-response to conventional treatment (Silveira et al., Reference Silveira, Lainson, Shaw, De Souza, Ishikawa and Braga1991, Reference Silveira, Blackwell, Ishikawa, Braga, Shaw, Quinnell, Soong, Kima, McMahon-Pratt, Black and Shaw1998; Silveira, Reference Silveira2019).
The standard treatment comprises of pentavalent antimonials, liposomal amphotericin B, pentamidines and miltefosine (Pelissari et al., Reference Pelissari, Cechinel, de Sousa-Gomes and Ferreira de Lima Junior2011). These commonly used drugs are toxic, resulting in severe side-effects, such as pancreatitis, leukopenia and more importantly, cardiac arrhythmia (Machado et al., Reference Machado, Pires, Dinis, Santos-Rosa, Alves, Salgueiro, Cavaleiro and Sousa2012). Standard drug therapy is further compounded with high costs and requires administration in a hospital environment, leading patients to discontinue treatment. Parasite resistance to antimonials has been reported, as well as disease recurrence (Sen and Chatterjee, Reference Sen and Chatterjee2011). To achieve control of leishmaniasis, the discovery of new potentially efficient and safer active compounds is necessary.
Diseases have been treated using plants since the dawn of humankind, resulting in the discovery of many bioactive substances, which are the origin for almost half of the existent drugs (Lam, Reference Lam2007; Ganesan, Reference Ganesan2008). Scientific investigations using plants are fundamental, not only for the discovery of new active substances with fewer side-effects, more efficiency and lower cost, but also to amplify knowledge on biodiversity. Much phytochemical research has been conducted employing extracts, essential oils (EOs) and their fractionated compounds. EOs are secondary substances of plants; they have a complex composition, with many compounds, which confer multiple actions to EOs. Studies have demonstrated antileishmanial activity of EOs or isolated compounds from medicinal plants on Leishmania species (de Lima et al., Reference de Lima, Teixeira, Lopes, de Morais, Torres, Braga, Rodrigues, Santiago, Martins and Nagao-Dias2014; Islamuddin et al., Reference Islamuddin, Sahal and Afrin2014). Additionally, it is observed that there is selectivity in relation to the species of Leishmania on which EOs act. Thus, the EO of Nectandra hihua was 121-times more active for L. infantum than for L. amazonensis (Bosquiroli et al., Reference Bosquiroli, Dos Santos Ferreira, Farias, da Costa, Matos, Kadri, Rizk, Alves, Perdomo, Carollo and Pinto de Arruda2017). The EO of Piper demeraranum was 3.8 times more active for L. amazonensis than for L. guyanensis (Moura do Carmo et al., Reference Moura do Carmo, Amaral, Machado, Leon and Silva2012).
Considering the severity of the disease induced by L. amazonensis and the potential of EOs as a source of new bioactive drugs, we systematically investigated previous studies on in vivo and in vitro activity of EOs on this species.
Methods
Search strategy
In-depth search was conducted on PubMed and Web of Science databases to retrieve articles describing the area of interest, published between April 2005 and April 2019. PRISMA statement recommendations were followed in this systematic review (Moher et al., Reference Moher, Shamseer, Clarke, Ghersi, Liberati, Petticrew, Shekelle and Stewart2015). The descriptors or MeSH terms (Medical Subject Headings) were defined independently by five reviewers of group 1 (CELS, JO, FBPF, MPPS, TVAL, RCLS) and Three specialists (MVCL, MSTM and JJVT). The MeSH terms were divided into three blocks – block 1 (‘plant oils’, ‘volatile oils’); block 2 (‘complementary therapies’, ‘anti-infective agents’, ‘antiprotozoal agents’, ‘phytotherapy’, ‘plants medicinal’, ‘biological products’); and block 3 (‘Leishmania’, ‘leishmaniasis’), and combined to retrieve all potential published articles. The descriptors were also researched in the titles of the articles, in individual or combined pairs to increase research sensitivity.
Articles selection
Inclusion and exclusion criteria: All studies that evaluated the therapeutic effects of EOs against L. amazonensis were included. Database filters were used and abstracts were read to select studies. Only original studies published in Portuguese, English, Spanish or French languages with accessible abstracts were included. Through database filtering and abstract readings, experimental studies were selected for the systematic review. We excluded review studies, case reports, editorials, comparisons, editor comments, clinical assays, letters, news and guidelines. Articles not found through databases and those that did not meet the selection criteria were not selected.
Quality evaluation: At this stage, full-text selected articles, which constituted the articles of potential interest, were retrieved and randomly distributed to researchers in group 1. The final validation of the selected publications was conducted by consensus among three judges in group 2 (MVCL, MSTM and JJVT). The references listed in each paper were also explored for potential articles of interest not identified in the initial phase.
Data extraction
For the organization and structuring of the tables, researchers in group 1 extracted the content of interest from the papers with the support of group 2. This strategy improved the quality and precision of content extracted from each article. To reduce the risk of bias regarding the content extracted from the papers, a standardized instrument was used for the researchers. The following relevant information was extracted and inserted in the table: family, plants species, in vitro results (promastigotes, amastigotes), in vivo results and references. Researchers critically validated the information from each selected paper in pairs.
Risk of bias
The quality of the selected publications was carried out through the risk of bias in individual studies independently by two researcher specialists (JJVT and MVCL). For analysis of 23 studies exclusively in vitro, we use a checklist consisting of 15 domains, based on the CONSORT (Consolidated Standards of Reporting Trials) guidelines (Faggion, Reference Faggion2012). For analysis of two studies exclusively in vivo, we use the Rob (Risk of Bias) tool for animal intervention studies (Systematic Review Center for Laboratory Animal Experimentation – SYRCLE RoB tool), consisting of 12 domains (Hooijmans et al., Reference Hooijmans, Rovers, de Vries, Leenaars, Ritskes-Hoitinga and Langendam2014), characterized as an adjusted risk model Cochrane of the bias tool. Six studies presented experiments both in vivo and in vitro. Currently, no checklist allows evaluating in vitro and in vivo studies simultaneously. Thus, we apply the checklists separately for each design model. The analysis of the methodological quality of each publication was carried out according to the scores from zero to ten for in vivo studies and from zero to 15 for in vivo studies, where high scores allowed the high quality of publications.
Results
Published studies were identified in two electronic databases. During the analysis of titles and abstracts of 436 publications by the reviewers, 146 duplicated articles were excluded. Following the application of exclusion criteria, 243 studies were excluded, and 47 were selected since they corresponded to the inclusion criteria, and 14 were excluded for not meeting goals of the study (Fig. 1). The 33 confirmed primary studies were printed in PDF format, analysed thoroughly for data extraction, and were organized and structured (Table 1). This study identified 46 plants species, and all were pre-clinical and experimental. The majority (75.7%) being in vitro studies, 6.1% in vivo and 18.2% in vitro and in vivo. Several of the in vitro studies simultaneously investigated the effect of EO on promastigote (35 plant species) and amastigote (33 plant species) forms of L. amazonensis.
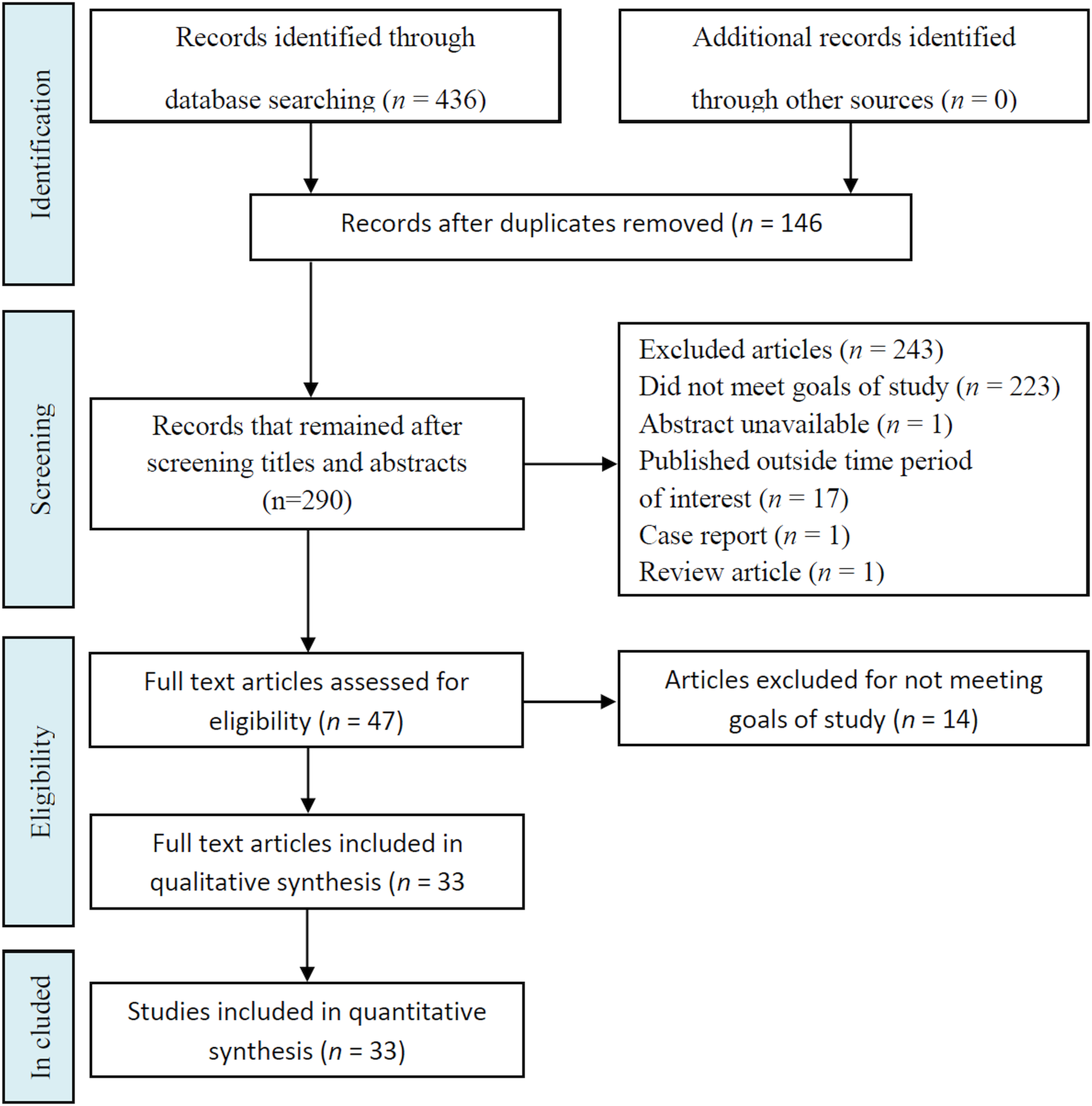
Fig. 1. Flowchart of the different phases of articles included in systematic review.
Table 1. Main characteristics of primary studies in vitro and in vivo with essential oils on Leishmania amazonensis

EO, essential oil; IC50, concentration of the EO that reduced the survival of Leishmania parasites by 50% compared with untreated parasites; CC50, cytotoxicity to cells 50%; 1IC50, obtained with intracellular amastigote on peritoneal macrophages of Balb/c mouse infected with Leishmania; 2IC50, obtained with axenic Leishmania amastigote; NO, nitric oxide; NR, not reported.
Among the EOs of 35 plant species identified in this systematic review study, 45.7% had a 10 < IC50 ⩽ 50 μg mL−1 and 14.3% had a 10 < IC50 μg mL−1 for promastigote forms of L. amazonensis (Supplementary File S1 and S1a). Cymbopogon citratus species had the lowest IC50 (1.7 μg mL−1). However, we identified bioactive agents from ten plant species with IC50 values higher than 100 μg mL−1 for Leishmania promastigotes. However, 33 species of plants investigated for activity against intracellular amastigote forms of L. amazonensis, 39.4% had a 10< IC50 ⩽ 50 μg mL−1 and 33.3% had a 10 < IC50 μg mL−1. The EO of Aloysia gratissima showed the lowest IC50 (0.16 μg mL−1) for intracellular amastigotes (Garcia et al., Reference Garcia, Soares, Santana, Saraiva, Siani, Ramos, Danelli, Souto-Padron and Pinto-da-Silva2018).
The study of Leishmania infection in animal models has been described in eight articles, including the study of antileishmanial activity of EOs of six plant species, Pluchea carolinensis (Garcia et al., Reference Garcia, Scull, Satyal, Setzer and Monzote2017), Bixa orellana (Monzote et al., Reference Monzote, Garcia, Scull, Cuellar and Setzer2014a), C. martii (dos Santos et al., Reference dos Santos, Costa, Ueda-Nakamura, Dias-Filho, da Veiga-Junior, de Souza Lima and Nakamura2011; Dhorm Pimentel de Moraes et al., Reference Dhorm Pimentel de Moraes, Tavares, Soares Rocha, de Paula and Giorgio2018), Tetradenia riparia (Cardoso et al., Reference Cardoso, de Mello, Lopes, Demarchi, Lera, Pedroso, Cortez, Gazim, Aristides, Silveira and Lonardoni2015), C. guianensis (Dhorm Pimentel de Moraes et al., Reference Dhorm Pimentel de Moraes, Tavares, Soares Rocha, de Paula and Giorgio2018) and C. ambrosioides (Monzote et al., Reference Monzote, Montalvo, Almanonni, Scull, Miranda and Abreu2006, Reference Monzote, Montalvo, Scull, Miranda and Abreu2007, Reference Monzote, Garcia, Montalvo, Linares and Scull2009). Of the 33 publications selected, eight used the in vivo model, and the same study used between one and three routes of administration. Among the EOs of these species, EOs of C. ambrosioides (Monzote et al., Reference Monzote, Garcia, Montalvo, Linares and Scull2009), C. martii (dos Santos et al., Reference dos Santos, Costa, Ueda-Nakamura, Dias-Filho, da Veiga-Junior, de Souza Lima and Nakamura2011; Dhorm Pimentel de Moraes et al., Reference Dhorm Pimentel de Moraes, Tavares, Soares Rocha, de Paula and Giorgio2018) and C. guianensis (Dhorm Pimentel de Moraes et al., Reference Dhorm Pimentel de Moraes, Tavares, Soares Rocha, de Paula and Giorgio2018), when administered by the oral route, were effective in reducing parasitic load and lesion volume in L. amazonensis-infected BALB/c mice. EOs of B. orellana (Monzote et al., Reference Monzote, Garcia, Scull, Cuellar and Setzer2014a) and C. ambrosioides (Monzote et al., Reference Monzote, Montalvo, Almanonni, Scull, Miranda and Abreu2006) were effective when administered intraperitoneally. EO of T. riparia, when given topically, did not alter the volume of lesions, but reduced the parasitic load on the spleen and lymph nodes of L. amazonensis-infected BALB/c mice.
Risk of bias assessment
The quality assessments of 33 selected publications were mentioned in Supplementary Tables S2–S5. The score of in vitro studies ranged from 5 to 9 out of a total of 15 points (Supplementary File S2 and S3). All in vitro studies performed the domains for structured abstract, scientific background, and rationale, objectives and/or hypotheses, and intervention of each group. No studies reported allocation sequence generation, allocation concealment mechanism, implementation, outcomes and estimation. Only one publication (Monzote et al., Reference Monzote, Garcia, Scull, Cuellar and Setzer2014a, Reference Monzote, Pastor, Scull and Gille2014b) applied the blinding domain. The articles with the in vivo experimental model had scores ranging from 3 to 7, among ten domains (Supplementary File S4 and S5). All publications developed the domains baseline characteristics, selective outcome reporting, other sources of bias. The allocation sequence generation domain was applied in most publications. The domain blinding of personnel and participants, and random outcome assessment was performed in only one study (dos Santos et al., Reference dos Santos, Costa, Ueda-Nakamura, Dias-Filho, da Veiga-Junior, de Souza Lima and Nakamura2011).
Discussion
Natural plant products are essential sources for drug innovation, and the selection and validation of extracts or molecules with relevant pharmacological action requires strategies for choosing effective bioproducts (Clardy and Walsh, Reference Clardy and Walsh2004; Cos et al., Reference Cos, Vlietinck, Berghe and Maes2006). In this systematic review, we retrieved studies on EOs of plants that specifically act against L. amazonensis, a principal causative agent in cutaneous leishmaniasis. Various screening approaches are available to identify the primary pharmacological actions of natural products on Leishmania. Promastigote forms of Leishmania multiply in defined culture media, and thus, the determination of IC50 of a bioproduct is widely employed among studies. Another interesting bioassay method evaluates the concentration capable of killing 50% of amastigote forms (IC50) in peritoneal macrophages of BALB/c mice infected with Leishmania (Cos et al., Reference Cos, Vlietinck, Berghe and Maes2006). Several articles also address the mechanisms of action of these bioproducts by ultrastructural analysis using transmission electron microscope (TEM), investigation of cell death induction and studies on microbicidal metabolites of macrophages, such as nitric oxide (NO) and various cytokines that modulate the immune response. Measurement of the 50% toxicity concentration of bioproducts on murine cells or cell lines (CC50), and the action on erythrocytes allows an assessment of toxicity the bioproducts may be. These studies usually precede animal model trials in which mice are infected, develop the disease and are subsequently treated using various protocols. Each test should contain at least one reference drug to ascertain test performance and proper interpretation of the screening results. Relevant and selective activity is related to IC50-values below 100 μg mL−1 for extracts and below 25 μ m for pure compounds (Cos et al., Reference Cos, Vlietinck, Berghe and Maes2006). In this review, we studied the EOs of plant species from the following families:
Anacardiaceae family
Myracrodruon urundeuva: M. urundeuva is native to South America and is used in traditional medical practices in Brazil for the treatment of mycoses, candidiasis, bacterial infections and as an anti-inflammatory agent (Pereira et al., Reference Pereira, Barros, Brito, Duarte and Maia2014). The EO of this species (MuEO) contains monoterpene and sesquiterpene hydrocarbons, the main constituent being β-myrcene (42.46%), followed by α-myrcene (37.23%) and caryophyllene (4.28%). β-myrcene showed anti-L. amazonensis activity, as previously reported (Carvalho et al., Reference Carvalho, Sobrinho-Junior, Brito, Nicolau, Carvalho, Moura, Rodrigues, Carneiro, Arcanjo, Cito and Carvalho2017; Machado et al., Reference Machado, Pires, Dinis, Santos-Rosa, Alves, Salgueiro, Cavaleiro and Sousa2012), preferably against the intracellular amastigote forms, which are involved in the development of the clinical manifestations of leishmaniasis. In optical microscopy, it was observed that MuEO-induced morphological changes in promastigotes, showing cells with round or spherical shapes, as well as the presence of cell debris (typical of cell lysis), suggesting leishmanicidal activity (Carvalho et al., Reference Carvalho, Sobrinho-Junior, Brito, Nicolau, Carvalho, Moura, Rodrigues, Carneiro, Arcanjo, Cito and Carvalho2017). MuEO decreased macrophage viability only at high concentrations (>200 μg mL−1). However, cytotoxicity against erythrocytes was low, with no alteration on lysosomal activity or NO production in macrophages, which indicates probable lack of direct involvement in immunomodulatory mechanisms (Carvalho et al., Reference Carvalho, Sobrinho-Junior, Brito, Nicolau, Carvalho, Moura, Rodrigues, Carneiro, Arcanjo, Cito and Carvalho2017).
Asteraceae family
Achillea millefolium: A. millefolium, which is native to Europe, North America and South Australia, is a herbaceous plant with a perennial life cycle and is popularly referred to as ‘thousand leaves’. EO from the leaves and flowers of A. millefolium was active against L. amazonensis with a promising IC50 of 6.5 μg mL−1 and CC50 of 72.0 μg mL−1 (Santos et al., Reference Santos, Santin, Yamaguchi, Cortez, Ueda-Nakamura, Dias-Filho and Nakamura2010). In scanning electron microscopy (SEM), the parasites revealed alterations of shape and size, and in TEM, ultrastructural changes in the flagellar membrane, abnormal membrane structures, rupture of the plasma membrane, atypical vacuoles, myelin-like figures and vesicles that resembled autophagic vacuoles were observed (Santos et al., Reference Santos, Santin, Yamaguchi, Cortez, Ueda-Nakamura, Dias-Filho and Nakamura2010). Achillea millefolium extracts have also been reported to possess anti-inflammatory activity (Tadic et al., Reference Tadic, Arsic, Zvezdanovic, Zugic, Cvetkovic and Pavkov2017), which may be related to its content of phenolic compounds, more specifically, dicaffeoylquinic acids, luteolin, apigenin and its glycosides. Additionally, Villalva et al. (Reference Villalva, Jaime, Villanueva-Bermejo, Lara, Fornari, Reglero and Santoyo2019) reported the inhibitory effect of A. millefolium fractions on IL-1β, IL-6 and tumour necrosis factor-α (TNF-α) secretion.
Matricaria chamomilla: The gas chromatography-mass spectrometry (GC-MS) analyses for M. chamomilla EO identified the main constituents as β-farnesene (52.73%), bisabolol oxide (12.09%), α-farnesene (10.34%) and α-bisabolo (9.83%) (Andrade et al., Reference Andrade, Azevedo, Motta, Dos Santos, Silva, de Santana and Bastos2016). This same study compared the IC50/24 h (60.16 μg mL−1) for promastigote forms of L. amazonensis, and cytotoxicity against L6 cells (CC50/24 h 173.04 μg mL−1) using the selectivity index (173.04 μg mL−1), and it was considered moderately active (50 < IC50 ⩽ 150 μg mL−1) (Andrade et al., Reference Andrade, Azevedo, Motta, Dos Santos, Silva, de Santana and Bastos2016).
Matricaria recutita: M. recutita is native to northern Europe and grows wild in Central European countries, Eastern Europe, western Asia, the Mediterranean region of North Africa and the Americas. Chamomile is one of the plants most cited for medicinal purposes in qualitative studies, for adult or paediatric use (Brasil, Ministério da Saúde, 2015). It has uses mentioned in pharmacopoeias, ethnobotanical studies, folk medicine, complementary and alternative medicine. A bio-guided study conducted by Hajaji et al. (Reference Hajaji, Sifaoui, López-Arencibia, Reyes-Batlle, Jiménez, Bazzocchi, Valladares, Akkari, Lorenzo-Morales and Piñero2018) identified the mechanism involved in Tunisian chamomile EO leishmanicidal action. The IC50 values for L. amazonensis promastigote were low and ranging from 10.8 μg mL−1 for the EO to 16.0 μg mL−1 for (−)-α-bisabolol. The CC50 on macrophages J774.A1 was 31.9 μg mL−1, and the selectivity index was 5.5. The (−)-α-bisabolol was able to activate a programmed cell death process. This compound induced phosphatidylserine externalization and membrane damage, decrease the mitochondrial membrane potential and total ATP levels in the promastigote of L. amazonensis (Hajaji et al., Reference Hajaji, Sifaoui, López-Arencibia, Reyes-Batlle, Jiménez, Bazzocchi, Valladares, Akkari, Lorenzo-Morales and Piñero2018). Matricaria recutita and its active compound ( − )-α-bisabolol can be a natural potential alternative to the available drugs.
Mikania micrantha: M. micrantha is native to Central and South America and is considered as an invasive weed, growing in cattle fields. Laurella et al. (Reference Laurella, Cerny, Bivona, Sanchez Alberti, Giberti, Malchiodi, Martino, Catalan, Alonso, Cazorla and Sulsen2017) isolated and identified four sesquiterpene lactones of the germacranolide type from M. micrantha organic extracts: mikanolide, dihydromikanolide and deoxymikanolide scandenolide. They observed that mikanolide and deoxymikanolide showed significant activity against L. braziliensis promastigotes, while dihydromikanolide displayed moderate activity. Houel et al. (Reference Houel, Gonzalez, Bessiere, Odonne, Eparvier, Deharo and Stien2015) used a strategy to discover bioactive natural products based on bioinspiration, which allows the transposition of these desirable properties to a corresponding research field. These authors evaluated the antileishmanial properties of selected anti-dermatophytic plants, looking for a match with antileishmanial activity. Mikania micrantha EO showed weak to non-existent activity against selected dermatophytic filamentous fungi but achieved action against L. amazonensis axenic amastigotes, while presenting low cytotoxicity to VERO cells and BALB/c mice peritoneal macrophages (Houel et al., Reference Houel, Gonzalez, Bessiere, Odonne, Eparvier, Deharo and Stien2015).
Pluchea carolinensis: This plant has a broad native distribution, from Mexico and Central America to South America. Garcia et al. (Reference Garcia, Scull, Satyal, Setzer and Monzote2017) described that the EO from aerial parts of P. carolinensis contained at least 44 compounds, the main component being selin-11-en-4α-ol. This EO inhibited the growth of promastigote and amastigote forms of L. amazonensis, while its cytotoxicity was 5-fold higher for peritoneal macrophages than that for the parasites. In an experimental model of infection, BALB/c mice treated with five doses of the EO (30 mg kg−1) by intralesional route presented reduced lesion size and parasite burden. This potential EO may target specific molecules or pathways in the amastigote form or induce defence mechanisms in the macrophages that contributed to the antileishmanial activity.
Vernonia brasiliana: V. brasiliana is a bush tree observed in the Brazilian savannah, also known as ‘assa-peixe’. The EO from flowers, roots and leaves of V. brasiliana did not display cytotoxicity against Vero (ATCC CCL 81) and RAW 264.7 cell lines, but they showed inhibitory activity against T. cruzi (Martins et al., Reference Martins, de Aquino, de Oliveira, do Nascimento, Chang, Borges, de Melo, da Silva, Machado and de Morais2015). The only study about V. brasiliana, by Martins et al. (Reference Martins, de Aquino, de Oliveira, do Nascimento, Chang, Borges, de Melo, da Silva, Machado and de Morais2015) showed that (a) in the EOs obtained by hydrodistillation, the major components found in the flowers were (E)-hex-2-enal (4.0%), hexan-1-ol (4.2%), (Z)-hex- 2-en-1-ol (6.3%) and palmitic acid (8.3%); (b) in the roots, the major components were α-isocomene (15.4%), α-gurjunene (9.6%), β-isocomene (10.3%), transcaryophyllene (10.4%) and palmitic acid (5.3%); (c) the major components of EOs in the leaves were trans-caryophyllene (8.7%), germacrene-D (10.2%) and caryophyllene oxide (4.5%).
Bixaceae family
Bixa orellana: B. orellana, also known as the ‘anatto’ or ‘achiote’ plant, is a small perennial tree, native of South and Central American forests (Lopes et al., Reference Lopes, Desoti, Caleare Ade, Ueda-Nakamura, Silva and Nakamura2012). Monzote et al. (Reference Monzote, Garcia, Scull, Cuellar and Setzer2014a) analysed the EO of the seed of B. orellana by gas chromatography-mass spectrometry analysis and reported ishwarane (18.6%), geranylgeraniol (9.1%), and bicyclogermacrene (8.4%) as the major components. This EO was active against L. amazonensis and controlled the progression of cutaneous disease in BALB/c mice; at the same time, the CC50 was 7-fold higher for host cells when compared with that for the parasites (Monzote et al., Reference Monzote, Garcia, Scull, Cuellar and Setzer2014a). The EO of B. orellana seeds has a complex chemical composition, as its activity could be attributed to geranylgeraniol, which induced mitochondrial alteration, abnormal chromatin condensation in the nucleus, and an increase in superoxide anion production ultimately leading to apoptosis-like cell death (Lopes et al., Reference Lopes, Desoti, Caleare Ade, Ueda-Nakamura, Silva and Nakamura2012).
Burseraceae family
Protium heptaphyllum: P. heptaphyllum, commonly called ‘almecegueira’, is known to produce an amorphous resin which is obtained from the stem; its constituents are compounds such as α- and β-amyrin, taraxastan-3-oxo-20-ol, and sitostenonein (Nogueira et al., Reference Nogueira, Oliveira, Adjafre, de Moraes and Aragao2019). Houel et al. (Reference Houel, Gonzalez, Bessiere, Odonne, Eparvier, Deharo and Stien2015) examined whether the antidermatophytic activity of the EO may be an indicator for the discovery of active natural products against L. amazonensis. Since P. heptaphyllum exhibited a good anti-dermatophytic activity against filamentous fungi and axenic amastigotes (IC50 3.7 μg mL−1), it indicates a correspondence between both the activities. P. heptaphyllum is rich in the aromatic monocyclic monoterpene, p-cymene [1-methyl-4-(1-methyl ethyl) benzene], that is the biological precursor of carvacrol (de Cassia da Silveira et al., Reference de Cassia da Silveira, Lima, da Nobrega, de Brito and de Sousa2017). The p-cymene was also shown to diminish NO production in murine macrophages incubated with lipopolysaccharide (de Santana et al., Reference de Santana, Guimarães, Chaves, Silva, Bonjardim, de Lucca Júnior, Ferro, Barreto Ede, dos Santos, Soares, Villarreal, Quintans Jde and Quintans-Júnior2015).
Chenopodiaceae family
Chenopodium ambrosioides: C. ambrosioides is an aromatic and medicinal plant found in the tropics and several regions of America and Africa (Cruz et al., Reference Cruz, Pereira, Patricio, Costa, Sousa, Frazao, Aragao-Filho, Maciel, Silva, Amaral, Barroqueiro, Guerra and Nascimento2007). Monzote et al. (Reference Monzote, Montalvo, Almanonni, Scull, Miranda and Abreu2006) observed an intense inhibitory action of the EO against promastigote and amastigote forms of L. amazonensis, with IC50 values of 3.7 and 4.6 μg mL−1, respectively. Additionally, they noted that BALB/c mice infected and treated with the EO (30 mg kg−1) for 15 days by intraperitoneal route, presented with a reduction in the size of the lesions and suppression of the number of parasites in the infected footpads when compared to the control animals. Monzote et al. (Reference Monzote, Montalvo, Scull, Miranda and Abreu2007) confirmed these results; intraperitoneal treatment reduced parasite burden, oral administration delayed infection, and the intralesional route was not valid. Monzote et al. (Reference Monzote, Garcia, Montalvo, Linares and Scull2009) compared the antileishmanial effect of C. ambrosioides EO in different doses administered by oral route in BALB/c mice and the conventional drugs, glucantime, amphotericin B and pentamidine, all administered for 15 days. The EO in a 150 mg kg−1 dose exhibited better antileishmanial activity and no macroscopic toxic effects. Interestingly, a study demonstrated synergism between C. ambrosioides EO with pentamidine against L. amazonensis promastigotes (Monzote et al., Reference Monzote, Montalvo, Scull, Miranda and Abreu2007). The high-resolution gas chromatography-mass spectrometry (HRGC-MS) analysis of this EO showed that its main components were carvacrol (62.36%) and ascaridole (22.54%). Chenopodium ambrosioides EO, combined with ascaridole compound, exhibited potent antileishmanial activity against promastigote and amastigote forms. Exploration of its mechanism of action suggests a breakdown of mitochondrial membrane potential and a modification of redox indices (Monzote et al., Reference Monzote, Pastor, Scull and Gille2014b).
Fabaceae family
Copaifera martii: Copaifera are trees found in Latin America and West Africa; they live for about 400 years, reaching between 25 and 40 m in height. The origin of the name seems to have come from the native language of the Tupi Indians ‘cupa-yba’ which means ‘depot tree’. Santos et al. (Reference Santos, Ueda-Nakamura, Dias Filho, Veiga Junior, Pinto and Nakamura2008) studied eight different kinds of Brazilian Copaifera oils for antileishmanial activity and observed activity against promastigote forms of L. amazonensis (IC50 5–22 μg mL−1). Oral administration with C. martii oil caused a significant reduction in average lesion size in L. amazonensis infection (dos Santos et al., Reference dos Santos, Costa, Ueda-Nakamura, Dias-Filho, da Veiga-Junior, de Souza Lima and Nakamura2011). Morphological and ultrastructural analyses demonstrated notable changes in parasite cells treated with this oil. The main ultrastructural effect was mitochondrial swelling. Copaifera martii EO led to an increase in plasma membrane permeability and depolarization in the mitochondrial membrane potential in parasite cells. Development of C. martii oil formulation as a nanoemulsion in a delivery system led to a reduction in L. amazonensis infection levels in macrophage cultures and ultrastructural analyses by SEM revealed that exposure to nanoemulsions induced change in parasite cell shape to oval and retracted flagellae. The treatment of L. amazonensis-infected BALB/c mice with nanoemulsions showed significant beneficial effects on lesion size, parasite burden and lesional histopathology (Dhorm Pimentel de Moraes et al., Reference Dhorm Pimentel de Moraes, Tavares, Soares Rocha, de Paula and Giorgio2018).
Vouacapoua americana: V. americana exhibits slow growth and has potential economic value, occurring in small subpopulations in the French Guiana, Guyana, Peru, Suriname and the Brazilian states of Amapá, Pará, Amazonas and Maranhão. Its wood is widely used in construction and shipbuilding. Moreover, the species grows in areas that undergo strong anthropization and the decline of habitat quality is constant (Brasil, Ministério da Saúde, 2017). Vouacapoua americana EO shows activity against Microsporum gypseum, M. canis and Trichophyton mentagrophytes, important dermatophytic fungi. On axenic amastigote forms of L. amazonensis, the IC50 of the EO was 7.2, with no cytotoxicity in BALB/c mice peritoneal macrophages and VERO cells (Houel et al., Reference Houel, Gonzalez, Bessiere, Odonne, Eparvier, Deharo and Stien2015).
Pterodon pubescens: The components extraction from P. pubescens by ‘Supercritical CO2 extraction’ (scCO2), which does not use organic solvents and is environmentally sustainable, detected high geranylgeraniol and 14,15-epoxy-geranylgeraniol content (da Silva Santos et al., Reference da Silva Santos, Garcia, Outuki, Hoscheid, Nunes de Goes, Cardozo-Filho, Nakamura and Carvalho Cardoso2016). This extract had high inhibitory activity against intracellular amastigotes of L. amazonensis (IC50 1.9 μg mL−1), and the effect was likely due to the high geranylgeraniol derivative content of the fluid, which resulted in superoxide anion production, leading to parasite death (Lopes et al., Reference Lopes, Desoti, Caleare Ade, Ueda-Nakamura, Silva and Nakamura2012; da Silva Santos et al., Reference da Silva Santos, Garcia, Outuki, Hoscheid, Nunes de Goes, Cardozo-Filho, Nakamura and Carvalho Cardoso2016).
Lamiaceae family
Tetradenia riparia: T. riparia is native to South Africa, where it is one of the most aromatic and popular medicinal plants (Gazim et al., Reference Gazim, Rodrigues, Amorin, de Rezende, Sokovic, Tesevic, Vuckovic, Krstic, Cortez, Colauto, Linde and Cortez2014). The EO from T. riparia is a complex mixture of terpenoids, including monoterpenes, sesquiterpenes and diterpenes, the most representative class (Gazim et al., Reference Gazim, Amorim, Hovell, Rezende, Nascimento, Ferreira and Cortez2010, Reference Gazim, Rodrigues, Amorin, de Rezende, Sokovic, Tesevic, Vuckovic, Krstic, Cortez, Colauto, Linde and Cortez2014). The most considerable amount of EO in T. riparia was found during winter, and the oil content decreased significantly in spring (Gazim et al., Reference Gazim, Amorim, Hovell, Rezende, Nascimento, Ferreira and Cortez2010). In this context, the EOs of T. riparia obtained in spring, summer, autumn and winter showed similar activity against L. amazonensis. However, BALB/c mice infected with L. amazonensis and treated topically did not present with a reduction in lesion size, but parasite load in the spleen decreased significantly (Cardoso et al., Reference Cardoso, de Mello, Lopes, Demarchi, Lera, Pedroso, Cortez, Gazim, Aristides, Silveira and Lonardoni2015). Demarchi et al. (Reference Demarchi, Thomazella, de Souza Terron, Lopes, Gazim, Cortez, Donatti, Aristides, Silveira and Lonardoni2015) observed that the EO of T. riparia is more effective in promoting the death of promastigote and amastigote forms of L. amazonensis than an isolated diterpene EO, 6,7-dehydroroyleanone. Additionally, increased inducible nitric oxide synthase (iNOS) mRNA expression or nitrite production by macrophages infected with L. amazonensis did not occur after 24 h, suggesting that this EO does not act on parasites through this important elimination pathway. The EO and diterpene derived from T. riparia probably promoted the death of Leishmania parasites through mitochondrial metabolism pathways, a mechanism of cell death that resembles apoptosis. TEM showed that the EO was able to modify the promastigote ultrastructures, suggesting autophagy demonstrated as chromatin condensation, blebbing, membranous profiles and nuclear fragmentation (Demarchi et al., Reference Demarchi, Thomazella, de Souza Terron, Lopes, Gazim, Cortez, Donatti, Aristides, Silveira and Lonardoni2015). However, T. riparia EO can modulate an immune response. Demarchi et al. (Reference Demarchi, Terron Mde, Thomazella, Mota, Gazim, Cortez, Aristides, Silveira and Lonardoni2016) reported that IFN-γ production was inhibited in infected macrophages, and the EO blocked this inhibition. Besides, the EO inhibited some of the most critical cytokines necessary for the establishment of infection, including granulocyte-macrophage colony-stimulating factor, IL-4, IL-10 and TNF-α. The diterpene 6,7-dehydroroyleanone induced a decrease in IL-4 levels and an increase in IL-12 (Terron-Monich et al., Reference Terron-Monich, Demarchi, da Silva, Ramos-Milare, Gazim, Silveira and Lonardoni2019). Thus, the EO of T. riparia is an agent that can stimulate a protective immune response against intracellular pathogens and inhibit or suppress pathological immune reactions.
Ocimum canum: Ocimum species comprise medicinal plants used in traditional medicine for the treatment of microbial diseases, helminthic diseases, inflammation, cardiac diseases, hepatic diseases and metabolic diseases, among many others. Uritu et al. (Reference Uritu, Mihai, Stanciu, Dodi, Alexa-Stratulat, Luca, Leon-Constantin, Stefanescu, Bild, Melnic and Tamba2018) and da Silva et al. (Reference da Silva, Almeida-Souza, Teles, Neto, Mondego-Oliveira, Mendes Filho, Taniwaki, Abreu-Silva, da Silva Calabrese and Mouchrek Filho2018) identified the chemical constituents of O. canum, assessed by gas chromatography-mass spectrometry analyses: thymol (42.15%), p-cymene (21.17%) and γ-terpinene (19.81%) were the major compounds. Antiprotozoal activity of the EO against L. amazonensis promastigotes and intracellular amastigotes were high (IC50 17.4 and 13.1 μg mL−1, respectively), with low cytotoxicity against BALB/c peritoneal macrophages (CC50 315.3 μg mL−1). The EO of O. canum induced ultrastructural alterations promastigotes of L. amazonensis, such as the appearance of autophagosome-like structures, discontinuity of nuclear membrane and exocytic activity by the flagellar pocket, and this exacerbated autophagic response can result in parasite cell death (da Silva et al., Reference da Silva, Almeida-Souza, Teles, Neto, Mondego-Oliveira, Mendes Filho, Taniwaki, Abreu-Silva, da Silva Calabrese and Mouchrek Filho2018).
Ocimum gratissimum: O. gratissimum is widely found in several geographical regions in South America and Africa and is used as a medicinal plant with analgesic activity. It contains several proanthocyanidins, which have shown significant antioxidant activity, and tannins, saponins, steroids, alkaloids, terpenoids, flavonoids, phenols and cardiac glycosides (Igbinosa et al., Reference Igbinosa, Uzunuigbe, Igbinosa, Odjadjare, Igiehon and Emuedo2013). The eugenol-rich EO of O. gratissimum inhibited L. amazonensis growth, in promastigote and amastigote forms, with an IC50 of 135 and 100 μg mL−1, respectively. IC50 of eugenol was 80 μg mL−1 (Ueda-Nakamura et al., Reference Ueda-Nakamura, Mendonca-Filho, Morgado-Diaz, Korehisa Maza, Prado Dias Filho, Aparicio Garcia Cortez, Alviano, Rosa Mdo, Lopes, Alviano and Nakamura2006). According to these authors, the EO showed no cytotoxic effects against mammalian cells. Leishmania amazonensis exposed to the EO underwent considerable ultrastructural alterations, as observed by TEM: the appearance of two or more nuclei or flagella suggesting interference in cell division, internal mitochondrial membrane considerably altered with an increased number of crystals, and the mitochondrial matrix appearing less electron-dense in some amastigotes. NO production by the infected macrophages was increased.
Origanum vulgare: Origanum is a genus of herbaceous perennials and shrubs native to Europe, North Africa and much of temperate Asia. The plants have strong aromatic leaves and abundant tubular flowers with long-lasting coloured bracts (Uritu et al., Reference Uritu, Mihai, Stanciu, Dodi, Alexa-Stratulat, Luca, Leon-Constantin, Stefanescu, Bild, Melnic and Tamba2018). The people from old Egypt used Origanum to disinfect and preserve food (Prerna and Vasudeva, Reference Prerna and Vasudeva2015). Teles et al. (Reference Teles, Rosa, Mouchrek, Abreu-Silva, Calabrese and Almeida-Souza2019) identified 20 compounds in O. vulgare EO and the main compounds were cis-p-menth-2-en-1-ol (33.8%) and linalyl acetate (13.9%). However, antiprotozoal activity on promastigotes was low (IC50 405.5 μg mL−1). According to Sanchez-Suarez et al. (Reference Sanchez-Suarez, Riveros and Delgado2013) O. vulgare EO of the Colombian species exhibited activity against promastigotes of L. panamensis.
Lauraceae family
Nectandra amazonum, N. gardneri, N. hihua and N. megapotamica: EOs extracted by hydrodistillation from stem bark/leaves of N. amazonum, N. gardneri and N. hihua presented sesquiterpene compounds as the major constituents, while phenylpropanoids were predominant in the EO extracted from N. megapotamica (Bosquiroli et al., Reference Bosquiroli, Dos Santos Ferreira, Farias, da Costa, Matos, Kadri, Rizk, Alves, Perdomo, Carollo and Pinto de Arruda2017). EOs of N. gardneri and N. megapotamica induced a significant increase in NO production by infected macrophages, which may mediate the intracellular death of L. amazonensis (Bosquiroli et al., Reference Bosquiroli, Dos Santos Ferreira, Farias, da Costa, Matos, Kadri, Rizk, Alves, Perdomo, Carollo and Pinto de Arruda2017).
Cinnamomum zeylanicum: C. zeylanicum, commonly called cinnamon, is used as a seasoning in cooking and is cultivated mainly in countries like India, Sri Lanka and China. Extracts, EOs and cinnamon isolates have applications in food, cosmetics and pesticides due to antimicrobial, antioxidant and antifungal properties. Teles et al. (Reference Teles, Rosa, Mouchrek, Abreu-Silva, Calabrese and Almeida-Souza2019) identified 15 compounds in C. zeylanicum EO, and the major one was cinnamic aldehyde (46.3%). Its anti-promastigote activity was not considered significant (IC50 >500 μg mL−1).
Cryptocarya aschersoniana: The EO of C. aschersoniana obtained by hydrodistillation presented monoterpene hydrocarbons (48.8%), limonene (42.3%), linalool (9.7%) and nerolidol (8.6%) as predominant constituents. Its EO had high activity against L. amazonensis promastigote forms (IC50 4.46 μg mL−1); however, it also demonstrated relatively high cytotoxicity against mouse peritoneal macrophages (CC50 7.71 μg mL−1) (Andrade et al., Reference Andrade, Melo, Alcoba, Ferreira Junior, Pagotti, Magalhaes, Santos, Crotti, Alves and Miranda2018).
Meliaceae family
Carapa guianensis: C. guianensis is the Amazon's traditional phytotherapeutic, and its EO is used for its anti-microbial and anti-inflammatory properties for skin diseases. The C. guianensis seed EO did not exhibit antileishmanial activity; however, three limonoid-rich EO fractions demonstrated activity against intracellular amastigotes of L. amazonensis, which was attributed to the compounds 11β-hydroxygedunin and 6α and 11β-diacetoxygedunin (Oliveira et al., Reference Oliveira, Moragas Tellis, Chagas, Behrens, Calabrese, Abreu-Silva and Almeida-Souza2018). Dhorm Pimentel de Moraes et al. (Reference Dhorm Pimentel de Moraes, Tavares, Soares Rocha, de Paula and Giorgio2018) reported the development of nanoemulsions as a delivery system for C. guianensis EO (nanoandi), with toxic activity against promastigotes of Leishmania species. Ultrastructural analyses by SEM revealed that exposure to nanoemulsion induced changes in the oval cell shape and flagellae retraction. Interestingly, the C. guianensis EO nanoemulsions were effective orally in BALB/c mice infected with L. amazonensis (Dhorm Pimentel de Moraes et al., Reference Dhorm Pimentel de Moraes, Tavares, Soares Rocha, de Paula and Giorgio2018).
Myrtaceae family
Syzygium cumini: S. cumini is a large tree, popularly known as ‘black plum’, ‘jambolan’, ‘jamum’ or ‘java plum’. The chemical composition and biological potential of the EO extracted from S. cumini leaves revealed a high abundance of monoterpenes (87.12%), and the major components were α-pinene (31.85%), (Z)-β-ocimene (28.98%) and (E)-β-ocimene (11.7%) (Dias et al., Reference Dias, Rodrigues, Carvalho, Carneiro, Maia, Andrade and Moraes2013). This EO showed significant activity against promastigote forms of L. amazonensis (IC50 60 μg mL−1). The α-pinene has activity against L. amazonensis promastigote forms (IC50 19.7 μg mL−1), axenic forms (IC50 15.6 μg mL−1) and intracellular amastigotes (IC50 16.1 μg mL−1) and was more effective than the EO from S. cumini against axenic and intracellular amastigotes (Rodrigues et al., Reference Rodrigues, Amorim, Dias, Moraes, Carneiro and Carvalho2015). The EO and α-pinene antileishmanial effects were mediated by immunomodulatory activity, as evidenced by an increase in both phagocytic and lysosomal activities and elevated NO levels induced by these components of S. cumini (Rodrigues et al., Reference Rodrigues, Azevedo, Chaves, Bizzo, Corte-Real, Alviano, Alviano, Rosa and Vermelho2013).
Piperaceae family
Piper rivinoides, P. mosenii , P. cernuum, P. arboretum, P. aduncum, P. gaudichaudianum, P. xylosteoides, P. mikanianum and P. diospyrifolium: Bernuci et al. (Reference Bernuci, Iwanaga, Fernadez-Andrade, Lorenzetti, Torres-Santos, Faioes, Goncalves, do Amaral, Deschamps, Scodro, Cardoso, Baldin and Cortez2016) evaluated the activity of EOs of nine Piper species on L. amazonensis promastigotes and found IC50 <30 μg mL−1 for EOs of P. rivinoides, P. mosenii , P. cernuum, P. arboretum and P. aduncum. However, the EOs of P. gaudichaudianum, P. xylosteoides and P. mikanianum showed IC50 >100 μg mL−1. Piper diospyrifolium and P. aduncum alone showed activity against intracellular amastigotes. Analyses of EOs obtained from inflorescences and leaves fresh or dried of P. claussenianum identified sesquiterpenes as the main constituents in the leaves, with a predominance of (E)-nerolidol (up to 83%), while in the EO of inflorescences, monoterpenes were the majority, with predominantly linalool (>50%) (Marques et al., Reference Marques, Barreto, Batista, Curvelo, Velozo, Moreira Dde, Guimaraes, Soares and Kaplan2010). Interestingly, only the EO from fresh leaves of P. claussenianum, which was rich in (E)-nerolidol, inhibited the L. amazonensis growth.
Piper demeraranum, P. duckei and P. hispidum: P. demeraranum and P. duckei EOs exhibited biological activity against promastigote and amastigote forms of L. amazonensis, P. duckei EO being the most active (Moura do Carmo et al., Reference Moura do Carmo, Amaral, Machado, Leon and Silva2012). The main constituents found in P. demeraranum EO were limonene (19.3%) and β-elemene (33.1%), and in P. duckei EO, the major components were germacrene D (14.7%) and trans-caryophyllene (27.1%) (Moura do Carmo et al., Reference Moura do Carmo, Amaral, Machado, Leon and Silva2012). The species P. hispidum was identified by Houel et al. (Reference Houel, Gonzalez, Bessiere, Odonne, Eparvier, Deharo and Stien2015) as the most promising Piper EO (IC50 4.7 μg mL−1), and the most abundant compounds found in this EO were sesquiterpenes, notably curzerene and furanodiene.
The EOs are complex mixtures of compounds, which might possess several activities, that act on multiple targets, and cause death of the parasite by various mechanisms (Salehi et al., Reference Salehi, Zakaria, Gyawali, Ibrahim, Rajkovic, Shinwari, Khan, Sharifi-Rad, Ozleyen, Turkdonmez, Valussi, Tumer, Monzote Fidalgo, Martorell and Setzer2019). The ultrastructural alterations induced by EOs of Piper species on L. amazonensis were mitochondrial swelling, intense exocytic activity in the flagellar pocket, and vacuoles in the cytoplasm, suggesting the depletion of ergosterol and alteration of the physical properties of the membranes of the parasites (Vendrametto et al., Reference Vendrametto, Santos, Nakamura, Dias Filho, Cortez and Ueda-Nakamura2010). Additionally, nerolidol from P. Aduncum may lead to parasite death by reduction in cell size, loss of mitochondrial membrane potential, phosphatidylserine exposure and DNA degradation (Ceole et al., Reference Ceole, Cardoso and Soares2017).
Plantaginaceae family
Otacanthus azureus: Houel et al. (Reference Houel, Gonzalez, Bessiere, Odonne, Eparvier, Deharo and Stien2015) described a high in vitro activity (IC50 0.7 μg mL−1) for O. Azureus EO, and the value was similar to that of the reference compound, amphotericin B (0.3 μg mL−1). This EO was composed of sesquiterpenes, with the main component being β-copaen-4-α-ol (23%), alongside α-humulene (10.6%), α-copaene (8.8%), myrtenal (5.6%), viridiflorol (5.1%) and trans-pinocarveol (4.3%). Interestingly, none of the main components of O. azureus EO have been identified as antileishmanial agents.
Poaceae family
Cymbopogon citratus: C. citratus is originally from India, possesses aromatic leaves, and is known as a source of ethnomedicines (Puatanachokchai et al., Reference Puatanachokchai, Kishida, Denda, Murata, Konishi, Vinitketkumnuen and Nakae2002). Santin et al. (Reference Santin, dos Santos, Nakamura, Dias Filho, Ferreira and Ueda-Nakamura2009), through the analysis of a chromatogram obtained by gas chromatography coupled with mass spectrometry, identified the monoterpene, citral, a mixture of the stereoisomers, geranial (42.2%), neral (36.3%) and β-myrcene (13.2%) as the main compound in C. citratus EO. In the same study, these authors suggested the activity of the EO on promastigotes, axenic amastigote and intracellular amastigote forms of L. amazonensis may be explained by the synergistic effects of various compounds of the EO. The promastigote forms of L. amazonensis treated with C. citratus EO presented notable morphological and ultrastructural alterations by light microscopy, SEM and TEM (Santin et al., Reference Santin, dos Santos, Nakamura, Dias Filho, Ferreira and Ueda-Nakamura2009). Houel et al. (Reference Houel, Gonzalez, Bessiere, Odonne, Eparvier, Deharo and Stien2015) identified C. citratus EO as a potent antidermatophytic agent, and with high antileishmanial activity. However, its EO was considered quite toxic and therefore, is not useful as a medicine.
Rubiaceae family
Mitracarpus frigidus: Chemical analysis of the M. frigidus EO obtained by hydrodistillation of the aerial parts of the plant resulted in the identification of 12 known compounds, linalool (29.29%) and eugenol acetate (15.85%) were the major constituents, followed by 5-hydroxy-isobornyl isobutyrate (8.41%), 5-methyl-1-undecene (7.69%) and methyl salicylate (6.55%) (Fabri et al., Reference Fabri, Coimbra, Almeida, Siqueira, Alves, Zani and Scio2012). This EO exhibited a potent antifungal effect against Cryptoccocus neoformans, Candida albicans and an expressive activity against L. amazonensis promastigote forms. The antioxidant activity of the EO investigated through 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging was significant (IC50 of 38 μg mL−1) (Fabri et al., Reference Fabri, Coimbra, Almeida, Siqueira, Alves, Zani and Scio2012).
Scrophulariaceae family
Achetaria guianensis: Houel et al. (Reference Houel, Gonzalez, Bessiere, Odonne, Eparvier, Deharo and Stien2015) examined whether the antidermatophytic activity of EOs can be used as an indicator for the discovery of active natural products against L. amazonensis. They examined seven EOs that exhibited potential antimicrobial obtained from fragrant plant species from French Guiana. Achetaria guianensis (EO of leaves and stems) was one of the species studied, but the EO did not show activity against dermatophytic filamentous fungi. The activity of the EO on axenic amastigote forms of L. amazonensis was high (6.3 μg mL−1), and cytotoxicity expressed as median toxic dose (TD50) on BALB/c mice peritoneal macrophages and VERO cells were 32.5 and 30.7 μg mL−1, respectively (Houel et al., Reference Houel, Gonzalez, Bessiere, Odonne, Eparvier, Deharo and Stien2015).
Verbenaceae family
Lippia sidoides: L. sidoides, popularly known as ‘alecrim pimento’, is grown mainly in Northeast Brazil. de Medeiros et al. (Reference de Medeiros, da Silva, Cito, Borges, de Lima, Lopes and Figueiredo2011) analysed the chemical composition of L. sidoides EO through GC/MS and found the oxygenated monoterpene thymol to be its principal constituent (78.4%). Monoterpene hydrocarbons such as ρ-cymene (6.3%) and other compounds were detected in smaller amounts. Study of the EO from L. sidoides and its major compound thymol on L. amazonensis showed significant activity against promastigote forms. However, thymol showed toxicity against peritoneal macrophages and low selectivity against the promastigotes, when compared with the crude EO. However, no cytotoxic effect was observed in macrophages treated with the crude EO. Incubation of L. amazonensis-infected macrophages with L. sidoides EO showed a remarkable reduction in amastigote survival within the macrophages. Significant morphological alterations, such as the accumulation of large lipid droplets in the cytoplasm, disrupted membrane and wrinkled cells, were usually seen in parasites treated with the EO (de Medeiros et al., Reference de Medeiros, da Silva, Cito, Borges, de Lima, Lopes and Figueiredo2011).
Aloysia gratissima: A. gratissima is known as ‘Brazil lavender’ and is commonly used in Brazilian folk medicine for the treatment of digestive and respiratory diseases. The only study on A. gratissima identified in this review was conducted by Garcia et al. (Reference Garcia, Soares, Santana, Saraiva, Siani, Ramos, Danelli, Souto-Padron and Pinto-da-Silva2018). These authors detected in A. gratissima the monoterpene, 1,8-cineole (17.6%) and the sesquiterpene alcohol, guaiol (10.5%), along with other guaiol isomers (azulene-types structures, 7.3%), hydrocarbons of the germacrene-type sesquiterpenes (>17%), trans-caryophyllene and its oxide (7%) in the EO of leaves. The results showed that the EO killed promastigotes and intracellular amastigotes of L. amazonensis at an IC50 of 25 and 0.16 μg mL−1, respectively. The EO of A. gratissima was safe for macrophages with up to 100 μg mL−1, as evaluated by dehydrogenase activity, membrane integrity and phagocytic capacity of macrophages, and did not induce NO in resting macrophages and inhibited the production of NO in lipopolysaccharide-stimulated macrophages. Ultrastructural analysis suggested that the EO and guaiol act directly on parasites, affecting the kinetoplast, mitochondrial matrix and plasma membrane of promastigotes (Garcia et al., Reference Garcia, Soares, Santana, Saraiva, Siani, Ramos, Danelli, Souto-Padron and Pinto-da-Silva2018).
Zingiberaceae family
Curcuma longa: Study on C. longa EO revealed 17 compounds, turmerone (55.43%), β-turmerone (12.02%) and γ-curcumene (6.96%) being the major compounds. Curcuma longa EO has potent antipromastigote activity, which showed a 4.87-fold lower IC50 value for intracellular amastigotes, when compared to the IC50 for promastigotes (Teles et al., Reference Teles, Rosa, Mouchrek, Abreu-Silva, Calabrese and Almeida-Souza2019). According to these authors, there was a reduction of infection in BALB/c mice. Curcuma longa EO inhibited NO production in peritoneal macrophages, suggesting that there may be other possible mechanisms involved in the intracellular anti-amastigote activity of C. longa EO (Teles et al., Reference Teles, Rosa, Mouchrek, Abreu-Silva, Calabrese and Almeida-Souza2019).
Strengths and limitations of the study
We conducted an extensive search in two databases, which was checked and validated by the researchers in this study, to ensure greater accuracy of the findings. The MeSH terms have been tirelessly revised to provide higher sensitivity to research. Our limitations were the search in only two databases, the setting of English, Portuguese, Spanish and French and a limited search period. Despite the vast heterogeneity of the substances identified in the selected studies, we detected a limited number of publications for each EO, which made it difficult to expand the discussion of the findings. It is important to comment that many of the compounds identified in this review study are potential therapeutic targets for the treatment of L. amazonensis infection, as they have low toxicity against host cells and potent antileishmanial action. The risk of bias in the selected in vivo and in vitro studies was moderate. It is essential to highlight that no study reported allocation sequence generation, allocation concealment mechanism, implementation, outcomes and estimation. Another critical point was that only one study applied the domain blinding of personnel and participants, and the random outcome assessment was performed. These flaws tend to increase the risk of bias in publications of an experimental nature.
Conclusion and future directions
The systematic review of 33 experimental assays showed that EOs and other investigated products possess significantly variable antileishmanial activity against promastigote and amastigote forms of L. amazonensis. The in vivo studies identified that the route of administration of the EO was important, and the intraperitoneal route is more effective. The discovery of new leishmanicidal bioactive agents is eminent in the face of increased incidence of leishmaniasis and current treatment difficulties. Plants and their secondary compounds, such as EOs, are a promising source for the discovery of new active agents and formulation of prototypes for potential drugs, as revealed by the selected 23 primary studies, which also reported the accurate safety margins of the bioactive agents. Many researchers have been investigating and tracking bioactive agents of new plants, being of paramount relevance for the advancement of these studies to the phase of controlled clinical assays.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182020001304.
Acknowledgements
We thank Editage for the quality and clarity in the English language review.
Financial support
This study was supported by the CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil), and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brasil).
Conflict interest
The authors declare that there is no conflict of interest.
Ethical standards
Not applicable.





