I. INTRODUCTION
Sorbents capable of separating gas mixtures efficiently and cost-effectively are in high demand for a large number of industrial applications, including pre- and post-combustion carbon capture, natural gas sweetening, air purification, and isotopic separations (Li et al., Reference Li, Kuppler and Zhou2009; Sumida et al., Reference Sumida, Rogow, Mason, McDonald, Bloch, Herm, Bae and Long2012; Wang and Zhao, Reference Wang and Zhao2017). One broad category of materials under investigation for such applications are porous solid frameworks, including metal–organic frameworks (MOFs) constructed of metallic ions or clusters linked by organic ligands in a porous crystalline array. Despite the discoveries to date of many MOFs exhibiting promising gas separation behaviour (Millward and Yaghi, Reference Millward and Yaghi2005; Li and Yang, Reference Li and Yang2007; Hamon et al., Reference Hamon, Llewellyn, Devic, Ghoufi, Clet, Guillerm, Pirngruber, Maurin, Serre, Driver, Van Beek, Jolimaître, Vimont, Daturi and Férey2009; Yang et al., Reference Yang, Sun, Ramirez-Cuesta, Callear, David, Anderson, Newby, Blake, Parker, Tang and Schröder2012), a need still exists for the development of novel MOF separators, which combine strong performance under realistic operating conditions with the fulfilment of additional pragmatic criteria, such as high cyclability and low cost. The targeted design of MOFs for separation applications is reliant upon a fundamental understanding of the mechanisms of selective adsorption characteristics. Considerable efforts are therefore being directed toward atomic-scale characterisation of sorbent–guest interactions and the structural responses of the host framework to guest binding, in order to better understand and exploit the origins of guest selectivity in MOFs.
Zn(hba) (hba = the dianion of 4-hydroxybenzoic acid) is a tetragonal square-channel MOF (P4122 symmetry) with an internal square dimension of ~6 Å between opposite van der Waals surfaces (White et al., Reference White, Abrahams, Babarao, Dharma, Hudson, Maynard-Casely and Robson2015). It belongs to a set of isotopological MOFs constructed using Zn nodes with different phenolic carboxylate anion linkers, the larger members being Zn(cma) (cma = the dianion of coumaric acid; internal ~8 Å squares) and Zn(hbpc) (hbpc = the dianion of 4′-hydroxy-4-biphenylcarboxylic acid; ~10 Å squares) (White et al., Reference White, Abrahams, Babarao, Dharma, Hudson, Maynard-Casely and Robson2015). An attractive feature of this series of frameworks is the ease with which the bridging ligands can be functionalised, raising the prospect of tailoring framework sets with a range of pore sizes for specific applications. For example, a recent study of the adsorption of inhalation anaesthetics by members of this series (Abrahams et al., in press) exemplified the utility of pore size tunability by identifying a size-related trade-off between maximum uptake and low-pressure adsorption performance in these materials.
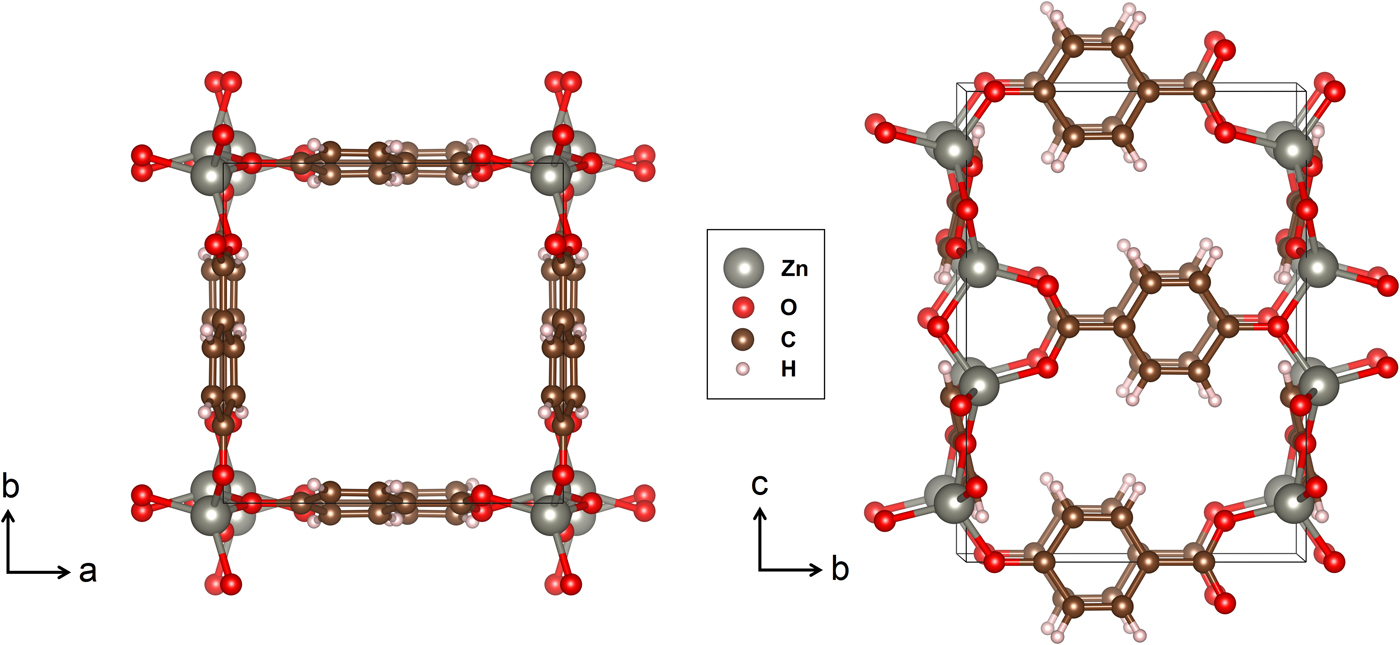
The fully ordered form of Zn(hba) is depicted in Figure 1. The framework is constructed by Zn nodes stacked along the c-axis at the corners of the square channels, with the hba linkers on the a–c and b–c unit-cell faces forming the pore “walls”. Because of the asymmetry of the hba linker, the Zn nodes are displaced from the corners of the square in a 41-type screw arrangement, and the planar linker molecules tilt away from the unit-cell faces at an angle of ~14°.
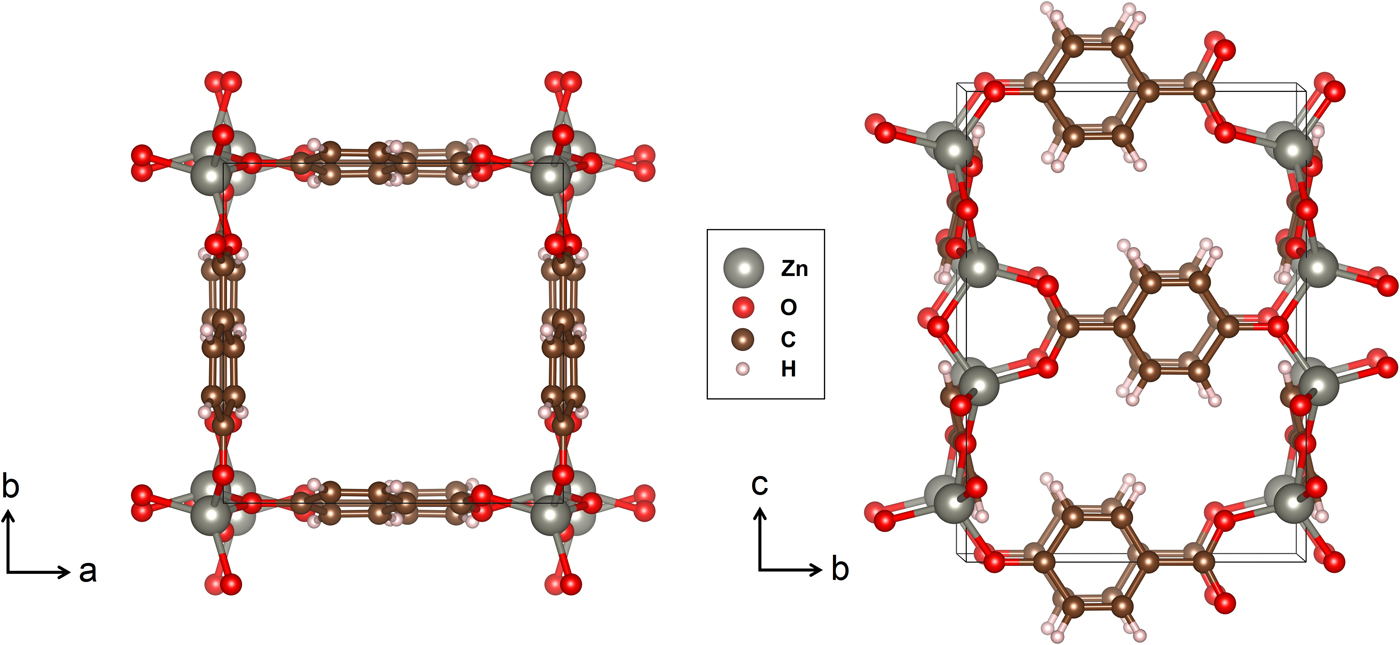
Figure 1. (Colour online) The structure of Zn(hba) depicted along the [001] and approximate [100] projections. Black lines denote the unit cell [Figure 4 drawn using VESTA (Momma and Izuma, Reference Momma and Izuma2008)].
Zn(hba) has been shown experimentally to exhibit selective uptake of CO2 and CH4 relative to H2 and N2 at moderate pressures near room temperature (White et al., Reference White, Abrahams, Babarao, Dharma, Hudson, Maynard-Casely and Robson2015). Interaction energies of almost 30 kJ mol−1 for CO2 and 21.5 kJ mol−1 for CH4 were obtained by fitting virial equations to adsorption isotherms and supported by the results of dispersion-corrected density functional theory (DFT-D2) calculations. However, little information has yet been reported regarding the structural aspects of guest adsorption in this MOF family, including the effects of guest loading on the host framework, and even the guest binding locations in Zn(hba) have so far been determined only theoretically for CO2 by classical simulated annealing (White et al., Reference White, Abrahams, Babarao, Dharma, Hudson, Maynard-Casely and Robson2015). While an indication of guest–host interactions within the material was gained from the DFT-D2 calculations, the structure of the empty framework that formed the basis of these calculations was unconfirmed experimentally under CO2-loaded conditions.
This paper presents results of in situ neutron powder diffraction (NPD) measurements performed on a deuterated sample of Zn(hba), which yield the first insights into the dependence of the framework lattice on the concentration of CO2 and CD4 guests, and reveals that the framework lattice response is dependent only on the number of guests for these two gas species.
II. EXPERIMENTAL
A. Sample preparation
4-Hydroxybenzoic acid-h (7.0 g, 51 mmol) was placed into a 450 ml Parr vessel, followed by deuterium oxide (D2O, 200 ml), 10 wt% platinum on carbon (1.5 g), and 40 wt% sodium deuteroxide (10.4 ml, two equivalents). The vessel was affixed to the Parr reactor, sealed, and the stirrer turned on. The vessel was purged with N2, then H2, before heating to 453 K for 18 h. The vessel was then cooled, and the reaction mixture filtered through celite. The celite was washed with water (2 × 100 ml). The filtrate was acidified to pH 2 by slow addition of 1 N HCl. Saturated sodium chloride solution (150 ml) was then added, followed by extraction into ethyl acetate (3 × 200 ml). The organic phase was dried over Na2SO4, filtered, and concentrated to provide the crude product mixture as an orange solid (6.1 g). This was adsorbed onto silica and purified by column chromatography (3:7–1:0 ethyl acetate/petroleum ether) to yield 4-hydroxybenzoic acid-d4 as a white-cream solid [1.6 g; overall 78% D by mass spectrometry (MS) and 13C nuclear magnetic resonance (NMR) analysis; 58% D at the 2-position and 98% D at the 3-position by quantitative 13C NMR analysis (Darwish et al., Reference Darwish, Yepuri, Holden and James2016); details of the NMR and MS spectra and deuteration analysis are provided in the online Supplementary Material].
Zn(OAc)2·2H2O (240 mg) and D2hba-d4 (150 mg) were dissolved in hot methanol (20 ml), and pentanol (20 ml) was added to the heated solution. The slow evaporation of methanol at 323 K yielded colourless crystals of Zn(hba-d4)· x pentanol, which were filtered and air dried. Repetition of this procedure yielded a batch of seven individual samples (190–232 mg), which were combined prior to the NPD experiment.
B. Data collection and analysis
The as-prepared sample was sealed and evacuated at 490 K for 18 h. The desolvated sample (1.198 g) was transferred to a 9 mm diameter V can inside a He-filled glove box and attached to an air-isolated cryofurnace centrestick. This centrestick supports gas delivery to the sample as well as temperature sensing and control, and is described elsewhere (Lee et al., Reference Lee, Chevreau, Booth, Duyker, Ogilvie, Imperia and Peterson2016). Using this apparatus, the sample was positioned inside a He cryofurnace mounted on the high-intensity neutron diffractometer WOMBAT (Studer et al., Reference Studer, Hagen and Noakes2006) at the OPAL reactor facility in Australia. WOMBAT features an area detector covering 120° in 2θ, and NPD data were collected continuously during isothermal adsorption and desorption procedures using 2 min acquisition times with an incident wavelength of λ = 2.9589(7) Å (as determined using the NIST La11B6 standard reference material 660b). The simultaneously measured adsorption isotherms were controlled using a Hiden Isochema IMI computerised manometric dosing system, and were performed at 273 K.
For each isotherm experiment, an “equilibration” data set was compiled by extracting the last NPD pattern acquired before application of each dose in the isotherm sequence. These limited data sets were subjected to Pawley fitting, which was performed using the sequential refinement feature available in the GSAS2 software package (Toby and Von Dreele, Reference Toby and Von Dreele2013). The tetragonal unit-cell parameters a and c were refined. The Pawley intensities of 56 reflections were selected for refinement after initial test fits, with the intensities of most unobserved reflections manually fixed to zero to avoid biasing the background model. Peak profiles were modelled using the Gaussian parameters U, V, and W, and the background was modelled using a four-term Chebyschev polynomial. The zero shift and peak asymmetry parameters, along with the centre, area, and width of a spurious peak (at 2θ ≈ 93°) arising from the sample environment, were allowed to refine only for the first pattern in the CO2 isotherm series, after which they were fixed to the same values for all subsequent model refinements against data collected for both guests.
Following the initial sequential fitting procedure, it became apparent that the background was poorly fitted at high loadings of both CO2 and CD4 because of the emergence of a very broad peak centred near 2θ = 40–50°. After refining the position, intensity, and width of this peak against the final NPD pattern in each sequence (in which the broad feature was most intense), the position and width values were fixed and the sequential Pawley fit was repeated with only the peak intensity allowed to refine across the series.
III. RESULTS AND DISCUSSION
A. Adsorption isotherms
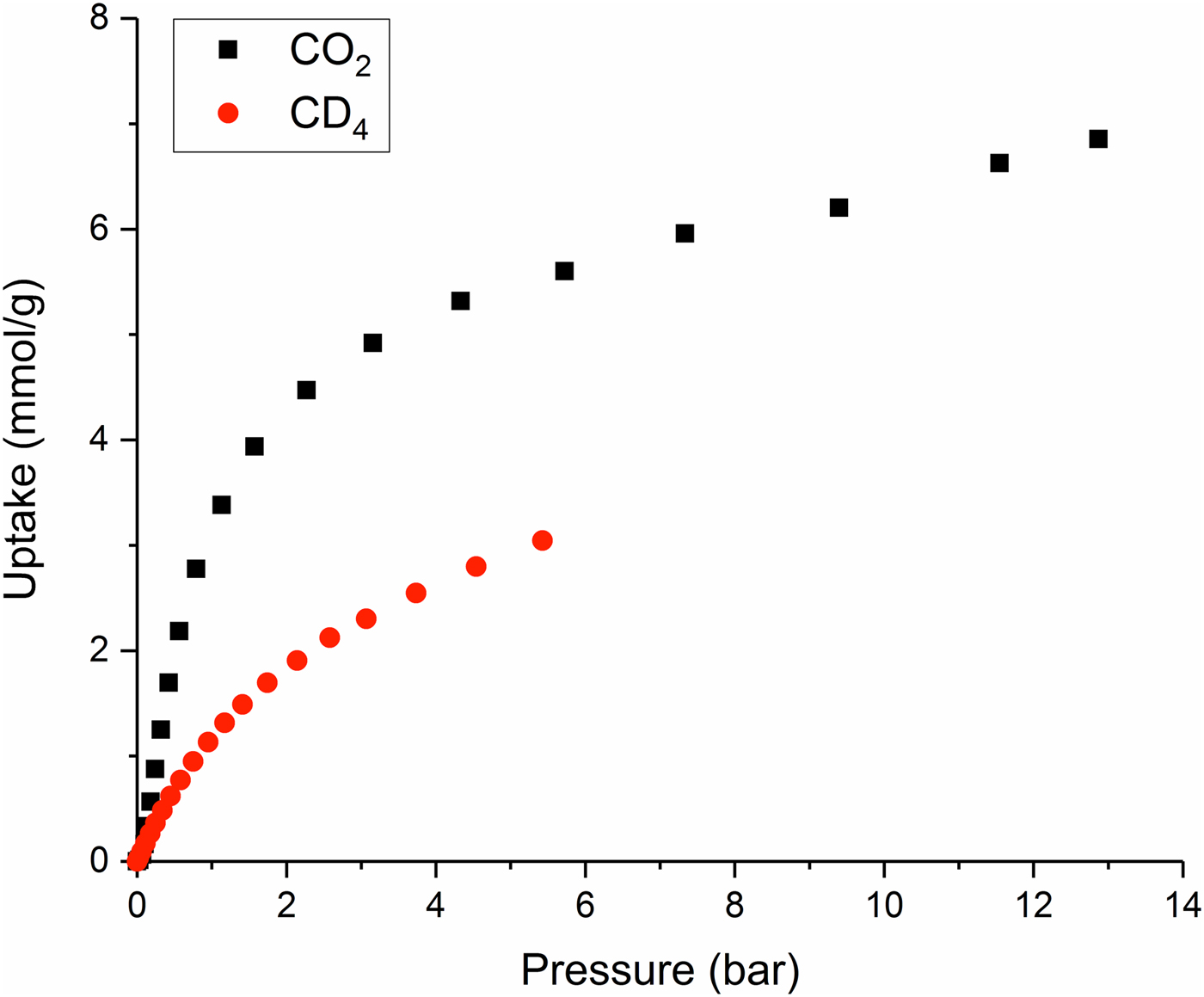
Adsorption isotherms measured for the Zn(hba) sample during the collection of in situ NPD data (Figure 2) are in good agreement with those reported previously by White et al. (Reference White, Abrahams, Babarao, Dharma, Hudson, Maynard-Casely and Robson2015). Because of a technical issue, no data were obtained for Zn(hba):CD4 at equilibrium pressures higher than 5.43 bar. Nevertheless, the form of the isotherm curve and the previously published data both indicate that the maximum CD4 uptake achieved during this experiment was ~80% of the expected saturated uptake. As observed by White et al. (Reference White, Abrahams, Babarao, Dharma, Hudson, Maynard-Casely and Robson2015), Zn(hba) demonstrates a modest selectivity of around 2:1 for CO2 over CD4 at 273 K.
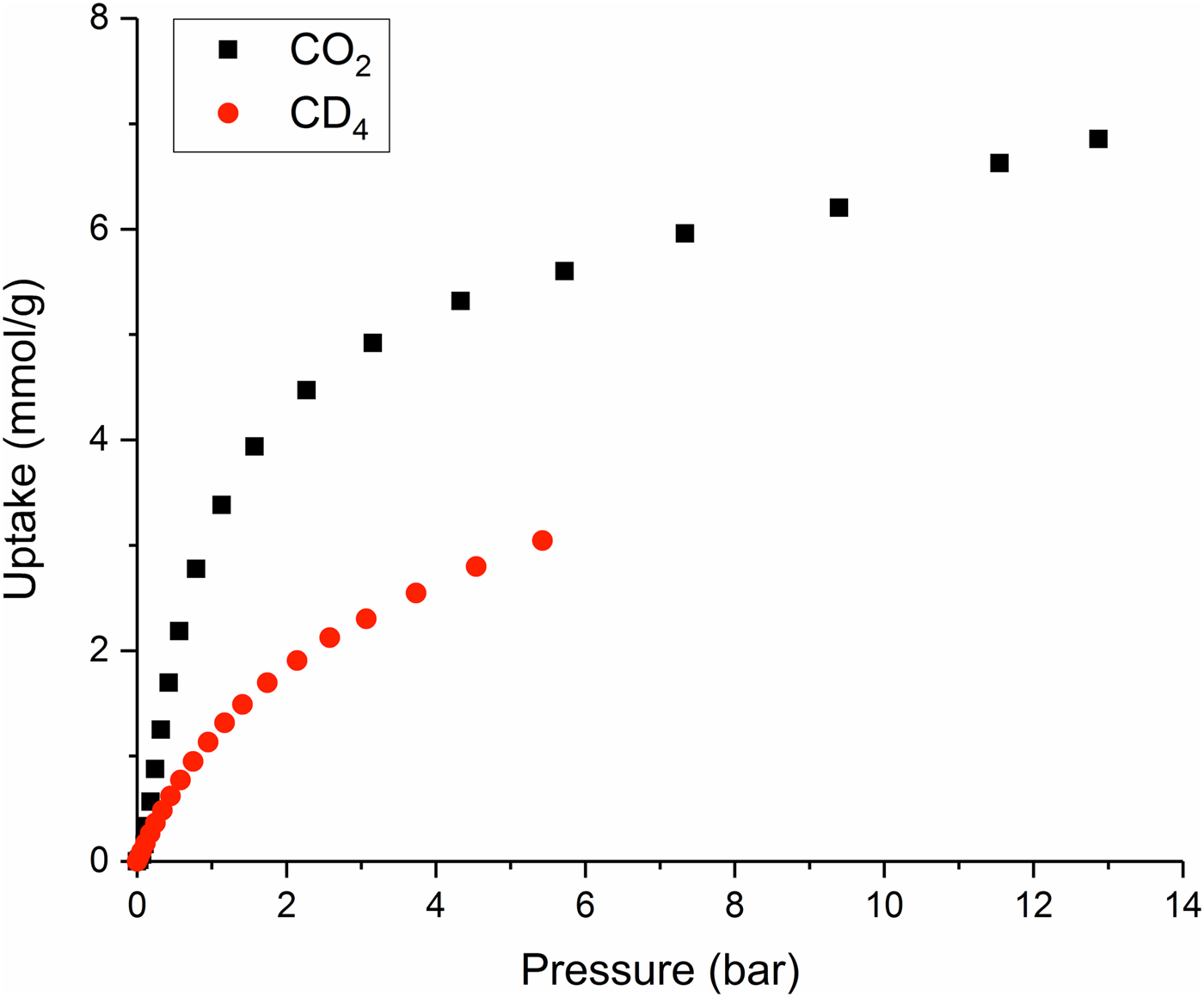
Figure 2. (Colour online) Adsorption isotherms recorded for Zn(hba) during simultaneous collection of NPD data.
B. Sequential Pawley fits
The in situ NPD data collected for Zn(hba) were well indexed using a tetragonal unit cell with P4122 symmetry [a = 9.1178(3), c = 12.6306(19) Å for the desolvated framework]. This model represents the structure reported by White et al. (Reference White, Abrahams, Babarao, Dharma, Hudson, Maynard-Casely and Robson2015) having fully ordered hba ligand orientations (Figure 1). However, single-crystal X-ray diffraction studies previously revealed evidence for considerable orientational disorder among the hba linkers and Zn nodes (White et al., Reference White, Abrahams, Babarao, Dharma, Hudson, Maynard-Casely and Robson2015). For this reason, an alternative model was constructed incorporating fourfold disorder of the framework by reflection along [100] and [010], resulting in P42/mmc symmetry and a c-axis half that of the starting model. This cell failed to index several very weak reflections corresponding to l-odd reflections of the data, despite Pawley refinement yielding good agreement values, so the larger unit cell of the ordered model was adopted for all sequential Pawley refinements. The low intensity of the l-odd reflections is noted as likely evidence of poor orientational order. No evidence for a phase transition requiring modification of the unit cell at elevated guest loadings was observed in any of the NPD data series.
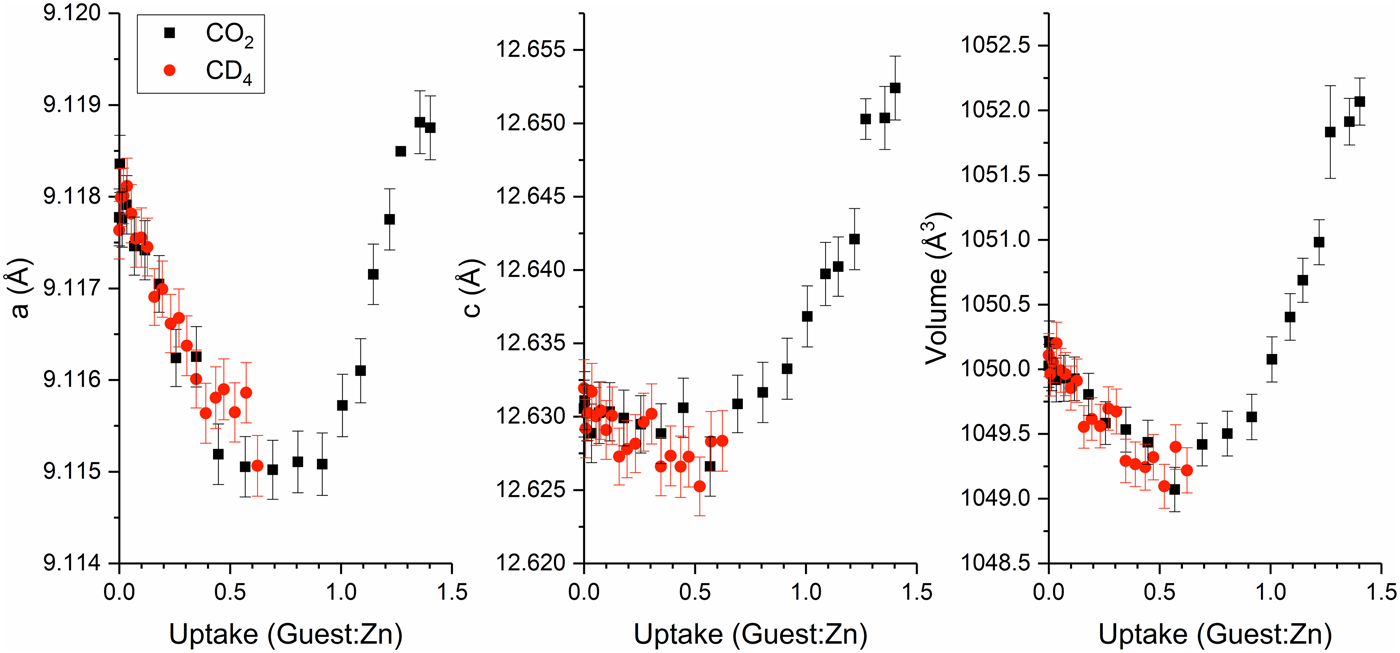
The Zn(hba) lattice responds anisotropically to CO2 loading and exhibits a two-stage behaviour (Figure 3). At low guest loadings, the a-axis contracts with increased guest concentration, while the c-axis remains unchanged within error, resulting in an overall decrease of the unit-cell volume. Guest-induced contraction arising from the attractive force between host and guests is commonly observed in flexible framework systems (Coudert et al., Reference Coudert, Fuchs and Neimark2014). Although the location of the CO2 binding sites in Zn(hba) has not been determined experimentally, simulated annealing methods previously identified a binding site interacting with the hba phenolate and carboxylate oxygen atoms near the channel corner, with a large calculated binding energy of −33 kJ mol−1 (White et al., Reference White, Abrahams, Babarao, Dharma, Hudson, Maynard-Casely and Robson2015). Strong binding at this location may result in contraction of the a-axis as the ends of perpendicular hba linkers are drawn closer together, reducing the square diameter without greatly affecting the c axis.
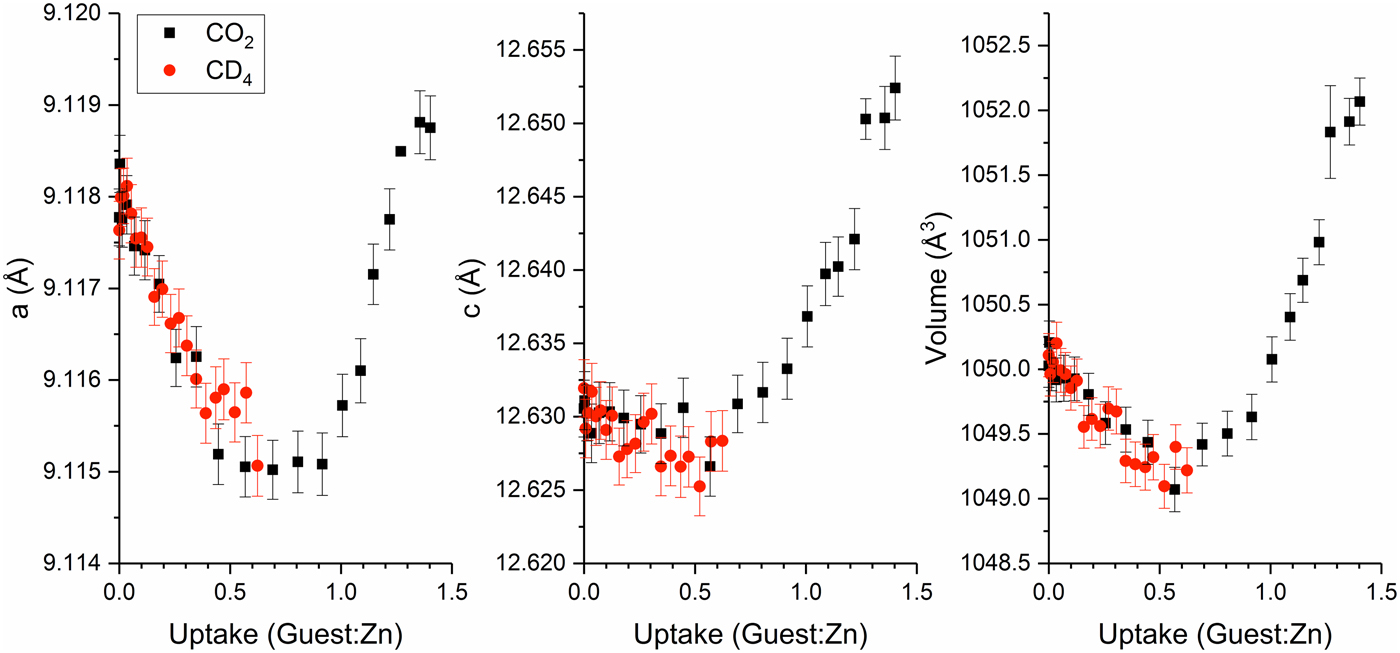
Figure 3. (Colour online) Lattice parameters of Zn(hba) as a function of guest loading, determined by Pawley refinement against in situ NPD data.
At high CO2 loadings, both the a- and c-lattice parameters increase rapidly with guest concentration, resulting in positive volume expansion. The changeover point between the two regimes lies in the range 0.5–0.7 CO2:Zn, but is difficult to determine with precision because of the errors on the refined parameters. Pseudo-isotropic expansion is characteristic of weaker binding interactions between the framework and incoming guests, and is usually associated with the more complete filling of pores and consequential reduction in empty space leading to sterically driven expansion. The observed change in lattice expansion behaviour therefore implies that a second, less energetically favourable binding site is being filled in this regime. The location of this possible binding site has not yet been identified.
The response of the Zn(hba) framework to CD4 loading follows the same trends as for CO2 in the low-concentration regime. The maximum 0.62 CD4:Zn loading achieved during the experiment was insufficient to determine whether a comparable change in expansion behaviour occurs at high loadings of CD4. However, given the appearance of the data in Figure 3, it is doubtful that any such change would be observable before the expected loading of <0.8 CD4:Zn was reached at the high-pressure limit of our experiment.
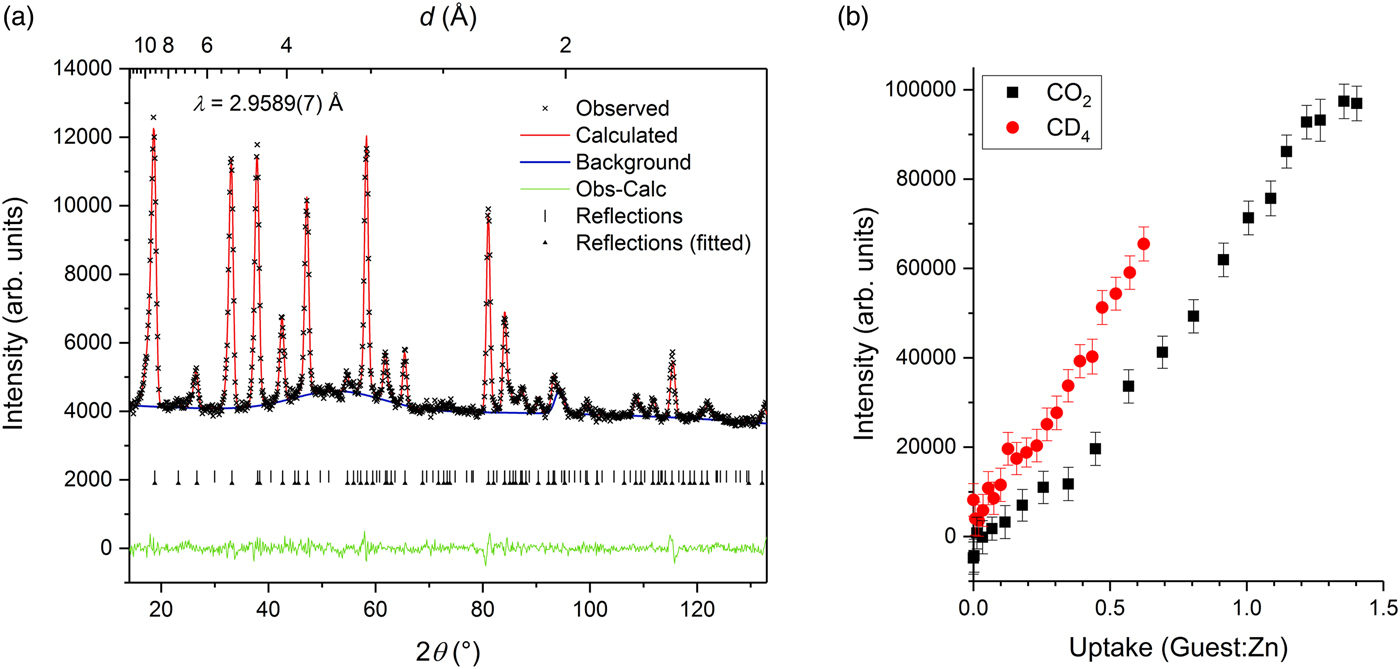
A broad diffuse feature is also clearly evident in the NPD data at higher guest concentration (Figure 4). This feature was modelled using a peak centred around d ≈ 3.3 Å for CO2 and d ≈ 4.0 Å for CD4, and in both cases its intensity increased with guest concentration. The onset of this feature is indicative of a form of poor structural order emerging among the guest molecules or framework components. For the CO2 data series, the intensity of the diffuse feature increases more rapidly above ~0.5 CO2:Zn loading (the approximate commencement point of the change in lattice expansion behaviour), suggesting that the feature probably corresponds to the progressive occupation of a secondary guest binding site that is poorly ordered. The fact that the refined peak centre is guest-dependent also supports the view that the feature is likely to arise directly from partial guest disorder rather than partial framework disorder.
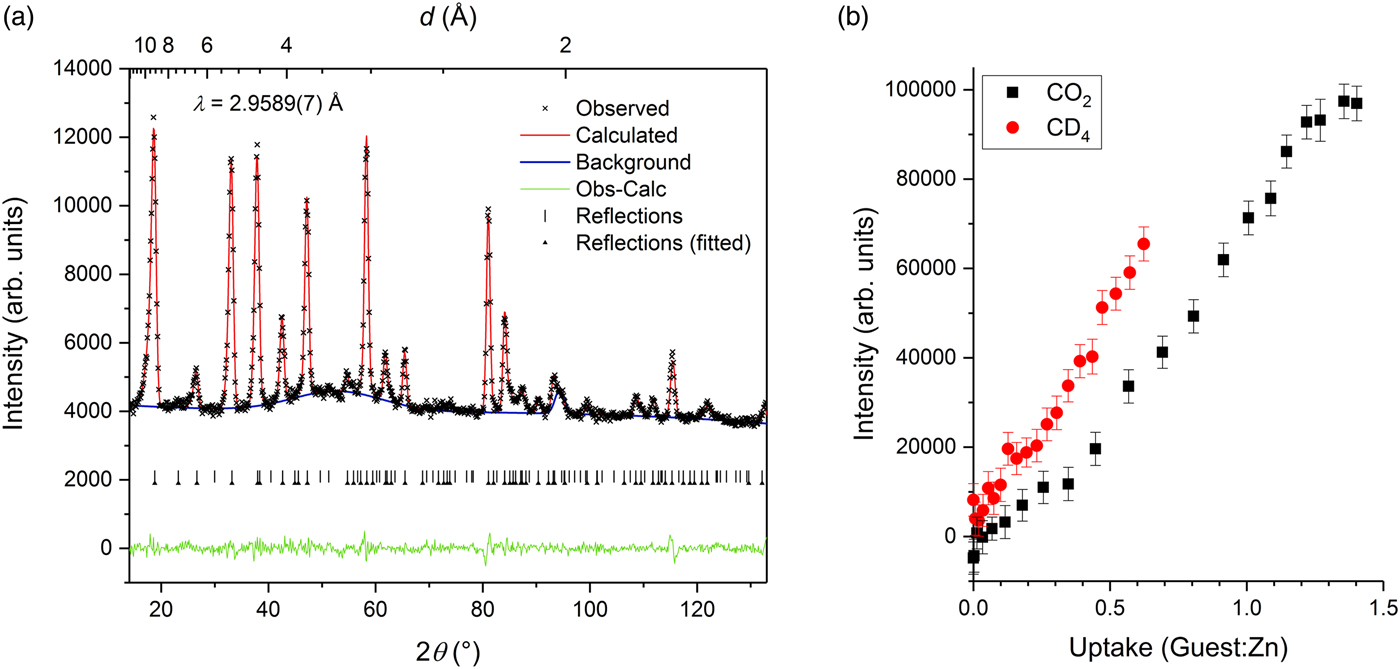
Figure 4. (Colour online) (a) Pawley refinement against NPD data collected at a guest concentration of 1.36 CO2:Zn, exhibiting a diffuse feature centred around d ~ 3.3 Å. Arrowheads indicate reflections for which the Pawley intensity was refined; intensities of the unmarked reflections were fixed to zero. (b) Refined intensity of the diffuse feature as a function of concentration of CO2 and CD4 guests.
It is particularly interesting to note that in the low-concentration regime, the Zn(hba) unit-cell behaviour is essentially indistinguishable for both CO2 and CD4 at equal loadings. Although the binding enthalpy determined for CO2 in Zn(hba) was 8 kJ mol−1 higher than that for CH4 (White et al., Reference White, Abrahams, Babarao, Dharma, Hudson, Maynard-Casely and Robson2015), as is typical in MOFs because of the quadrupole moment of CO2 (D'Alessandro et al., Reference D'Alessandro, Smit and Long2010; Chevreau et al., Reference Chevreau, Liang, Kearley, Duyker, D'Alessandro and Peterson2015), the Zn(hba) lattice expansion appears to be independent of guest interaction strength and dependent only on the total concentration of guest molecules. It can be speculated that the lower observed uptake of CH4 by Zn(hba) results from a steric inability of the bulkier CH4 molecule to access the proposed secondary binding site occupied by CO2 at higher loadings. Further investigation of the structural changes associated with guest loading, along with the determination of preferred binding site locations for both guests, is required in order to fully understand the origins of CO2/CD4 selectivity in this material.
IV. CONCLUSION
In situ NPD data collected during CO2 adsorption in Zn(hba) reveal a two-stage lattice response characterised by framework contraction at low guest concentrations followed by expansion at high concentrations, with no evidence for any accompanying change in symmetry. The experimental results point to the probable occupation of two binding sites, the first strongly interacting and the second weakly interacting and poorly ordered. The lattice behaviour up to 0.62 Guest:Zn loading is indistinguishable for CO2 and CD4 guests, indicating that the mechanism of lattice contraction is not strongly dependent on the interaction strength of the guest, and that the higher observed uptake of CO2 may be related to steric considerations.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0885715617000720.
ACKNOWLEDGEMENTS
This research was supported by the Science and Industry Endowment Fund (RP02-035). The authors thank the sample environment team at the Australian Centre for Neutron Scattering for assistance and support with respect to the gas-delivery equipment used for the NPD experiment. The National Deuteration Facility is partly funded by the National Collaborative Research Infrastructure Strategy (NCRIS), an initiative of the Australian government. RB acknowledges the Australian Research Council for a DECRA fellowship (DE160100987), and the National Computational Infrastructure (NCI) and Pawsey Supercomputing facility (Magnus) for providing computing support.