Introduction
Phenology is often crucial in trophic interactions involving spring-feeding lepidopteran larvae. Spring-feeding larvae must hatch synchronously with host plant budburst and feed on young foliage to achieve high fitness (van Asch & Visser, Reference van Asch and Visser2007). Likewise, higher trophic levels (i.e. natural enemies) must time their foraging to a narrow temporal peak of larval abundance in spring and early summer (e.g. Buse et al., Reference Buse, Dury, Woodburn, Perrins and Good1999; Visser et al., Reference Visser, Holleman and Gienapp2006). Thus, differential phenological responses to climatic variation in larvae and key interactants might modify both bottom-up and top-down impacts on spring-feeding lepidopteran demography. So far, however, most studies of climatic impacts on larvae-interactant phenology relationships have focused on the plant-insect interaction (reviewed in van Asch & Visser, Reference van Asch and Visser2007). More studies of how climate affects the phenological basis for larvae-enemy interactions, therefore, would be valuable for improved understanding of how climate can influence demography in lepidopteran spring-feeders.
Larval parasitoids (i.e. parasitoids that oviposit in larvae) are an important group in the context of larvae-enemy phenology relationships, due to their high attack rates on many spring-feeding lepidopterans (e.g. Berryman, Reference Berryman1996) and potentially strong dependency on phenological synchronization (i.e. matching) with their hosts. Larvae can vary greatly in susceptibility to parasitism between instars due to age- or size-specific changes in morphological, behavioural and/or physiological defences against parasitoids (Weseloh, Reference Weseloh1976; Dijkerman, Reference Dijkerman1990; Gross, Reference Gross1993; Benrey & Denno, Reference Benrey and Denno1997; Lu et al., Reference Lu, Hu and Fu2006). The instar of larvae at the time of parasitism can also affect the development rate and final size of parasitoid progeny (e.g. Harvey & Strand, Reference Harvey and Strand2002; Jenner & Kuhlmann, Reference Jenner and Kuhlmann2006). Moreover, the development and activity of parasitoids can be highly sensitive to climate, especially temperature (Hance et al., Reference Hance, van Baaren, Vernon and Boivin2007) and precipitation (Nyrop & Simmons, Reference Nyrop and Simmons1986), but not necessarily to the same degree as in their host larvae (Porter, Reference Porter1983; Van Nouhuys & Lei, Reference Van Nouhuys and Lei2004). Climatic variation in space or time can, thereby, disrupt larvae-parasitoid synchrony relations (e.g. Van Nouhuys & Lei, Reference Van Nouhuys and Lei2004), which in turn may disrupt parasitoid recruitment and promote rapid host population growth if parasitism is otherwise a limiting factor.
The present study examined the role of phenology in interactions between the spring-feeding geometrid moth Operophtera brumata (L.) (Winter moth, Lepidoptera:Geometridae) and its guild of larval parasitoid wasps in the coastal mountain birch (Betula pubescens ssp. czerepanovii Orlova) forests of sub-arctic northern Norway. O. brumata dynamics in this system are characterized by regionally synchronized population cycles, with regular 9–10 year periodicity. However, poorly understood mechanisms cause moth cycles to show wide spatiotemporal variation in amplitude. Larval densities in individual cycle peaks range from levels causing little visible foliage damage to outbreaks that defoliate and kill extensive tracts of birch forest (Tenow, Reference Tenow1972; Jepsen et al., Reference Jepsen, Hagen, Karlsen and Ims2009). Moreover, in the coastal district of northern Norway, outbreaks occur most frequently at or near the altitudinal treeline (Hagen et al., Reference Hagen, Jepsen, Ims and Yoccoz2007).
Climate-induced variation in phenological matching between larvae and birch host plants has been suggested to drive both temporal (Jepsen et al., Reference Jepsen, Hagen, Karlsen and Ims2009) and altitudinal (Hagen et al., Reference Hagen, Jepsen, Ims and Yoccoz2007) variation in moth cycle amplitude. However, several factors suggest that match-mismatch effects between larvae and parasitoids may also be generating spatiotemporal amplitude differences. Spring and early summer in north Fennoscandian coastal regions show wide inter-annual variation in temperature, with start of the growing season differing by up to 3–5 weeks between years (Karlsen et al., Reference Karlsen, Solheim, Beck, Høgda, Wielgolaski and Tømmervik2007). There can also be rapid shifts between cool, precipitation-rich and warmer, dryer conditions, within springs and summers. The potential for between-year variation in the degree of larvae-parasitoid synchrony, therefore, could be high if the development and activity of larvae and parasitoids are differentially climate sensitive. In the spatial dimension, differential climate-sensitivity could cause larvae-parasitoid synchrony to vary along altitudinal temperature gradients. Moreover, larval parasitism of O. brumata frequently reaches 30–60% (Schott et al., Reference Schott, Hagen, Ims and Yoccoz2010) and, thus, may be an important limiting factor for moth abundance, given that larvae-parasitoid synchrony is maintained.
Currently, however, better knowledge of larvae-parasitoid phenology relations is required in order to evaluate if larvae-parasitoid match-mismatch effects can explain spatiotemporal variation in O. brumata cycle amplitude. Key parameters defining the potential for mismatch events are the number of larval instars that can be successfully parasitized (i.e. parasitoid instar-specificity) and the duration of parasitoid flight periods. Because instars in O. brumata are typically completed synchronously across larval populations within 1–2 weeks, parasitoids showing high instar-specificity must time their flight accurately in order to achieve successful parasitism. Such timing is especially critical if parasitoid flight periods are short. Accordingly, high instar-specificity and short flight periods put parasitoids at high risk of becoming phenologically mismatched with larvae due to climatic fluctuations. Parasitoids showing low instar-specificity and/or long flight periods, on the other hand, will have smaller risk of loosing the temporal overlap between their flight and susceptible instars. In addition, parasitoid guild diversity matters in this context. Species-rich guilds, with high functional diversity in terms of different flight times and host instar-specificities, ought to be less prone to climate-induced guild level asynchrony with hosts, compared to species-poor and/or phenologically homogenous guilds.
Although the phenology of host-parasitoid interactions can potentially be subject to climate-induced disturbances, with large effects on the dynamics of populations and communities (Stireman et al., Reference Stireman, Dyer, Janzen, Singer, Lill, Marquis, Ricklefs, Gentry, Hallwachs, Coley, Barone, Greeney, Connahs, Barbosa, Morais and Diniz2005; Hance et al., Reference Hance, van Baaren, Vernon and Boivin2007; Klapwijk et al., Reference Klapwijk, Grobler, Ward and Wheeler2010), there are very few studies on this matter (Hance et al., Reference Hance, van Baaren, Vernon and Boivin2007). Even basic descriptions of temporal attack patterns and degrees of instar-specificity among major groups of larval parasitoids of important forest defoliators such as O. brumata are often absent or highly incomplete. This lack of knowledge motivated us to map the phenology of larval parasitoid attacks in an outbreaking population of O. brumata, according to an observational study design. Specifically, we employed sequential within-season sampling of larvae, to reveal the temporal patterning of attacks by different parasitoid species groups throughout the five instars of the moth's larval stage. Moreover, to explore the possibility of a causal relationship between altitudinal variation in O. brumata cycle amplitude and in larvae-parasitoid phenology-matching, we replicated the sampling along an altitudinal temperature gradient harbouring a local O. brumata outbreak at the treeline. To the extent such an observational study allows, we evaluated the probability of mismatching phenologies between parasitoids and larvae, and assessed whether larvae-parasitoid match-mismatch effects are likely to be important drivers of temporal and altitudinal variation in the amplitude of O. brumata population cycles.
Materials and methods
Study species
O. brumata is a geometrid moth with a holarctic distribution and univoltine life cycle. The larvae are highly polyphagous, but their main host plants in northern Fennoscandia are pubescent birch, Betula pubescens (Ehrhart), and mountain birch, Betula pubescens czerepanovii (Orlova) (Tenow, Reference Tenow1972). In our study area in northern Norway, larvae hatch approximately at the time of birch budburst in late May to early June. Larval development spans five instars and lasts about 1–1.5 months, depending on temperature and forage quality. Pupation occurs in the soil during July, and adult moths eclose in mid-September to October (Peterson & Nilssen, Reference Peterson and Nilssen1998). The flightless females oviposit in trees, where the eggs overwinter.
Seven species or species groups (see below) of hymenopteran larval parasitoids were reared from O. brumata in this study (table 1). Most have a poorly known biology, but all are koinobionts (Askew & Shaw, Reference Askew, Shaw, Waage and Greathead1986) and appear to be univoltine in the study area (although Protapanteles species exhibit bivoltinism in other locations (Teder et al., Reference Teder, Tanhuanpää, Henriksson, Kaitaniemi and Ruohomäki2000)). Table 1 summarizes the major biological characteristics and taxonomic affinities of each parasitoid. Larvae of early-eclosing parasitoid species eclose from fourth or fifth instar host larvae, while late-eclosing species eclose from host pupae (see also Klemola et al. (Reference Klemola, Tanhuanpää, Korpimäki and Ruohomäki2002, Reference Klemola, Klemola, Andersson and Ruohomäki2007, Reference Klemola, Heisswolf, Ammunèt, Ruohomäki and Klemola2009) and Vindstad et al. (Reference Vindstad, Hagen, Ims and Schott2010), and references therein). Parasitoids with similar cocoon morphology were treated as single taxonomic units in this study. Because some of these units are uncertain (Vindstad et al., Reference Vindstad, Hagen, Ims and Schott2010), and may contain several morphologically similar parasitoid species, we adopt the term parasitoid ‘group’ (Schott, Reference Schott2007) as a substitute for parasitoid ‘species’ throughout the remainder of this paper.
Table 1. Taxonomic and biological characteristics of the individual parasitoid species/groups reared from O. brumata in this study. Early-eclosing groups emerge from host larvae, while late-eclosing groups emerge from host pupae.

1 Recent results from genetic analysis suggest that this parasitoid group is likely to include at least two different parasitoid species (Vindstad et al., unpublished data).
2 Corresponds to ‘yellow cluster’ in Schott (Reference Schott2007) and Vindstad et al. (Reference Vindstad, Hagen, Ims and Schott2010).
Study area, system characteristics and sampling design
The study was conducted in 2008 at the location Skogsfjord (69°55′ N, 19°18′ E) in Troms County in northern Norway. Five parallel altitudinal transects were established in a southwest-facing slope, with continuous mature birch forest extending from the shore of a large lake at 20 m to about 250 m elevation. Transects were spaced by 200 m intervals and had sampling stations at 50, 100, 170 and 240 m a.s.l. The mean Euclidian distance between stations at neighbouring altitudes was 530 m (SD=118 m, range=411–813 m). Mjaaseth et al. (Reference Mjaaseth, Hagen, Yoccoz and Ims2005) documented that the phenology of O. brumata larvae differed by approximately one week between 30 and 240 m altitudes in a similarly designed altitudinal gradient on the island of Reinøya (located about 20 km east of Skogsfjord). This suggests that we can reasonably assume a roughly one-week difference in larval phenology between 50 and 240 m during the present study.
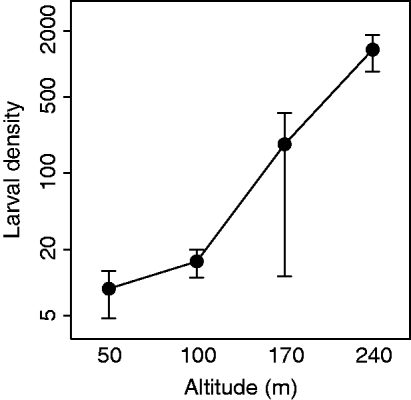
To quantify the altitudinal variation in larval abundance, larval densities were indexed by haphazardly gathering ten 80 cm birch branches within 20 m of each sampling station and counting the number of larvae dislodged after shaking the branches thoroughly over a large plastic box. According to standard methodology, density measurements were conducted when most larvae were in the third to fourth instar (Ims et al., Reference Ims, Yoccoz and Hagen2004). Larval density was high enough to completely defoliate the birch forest at the treeline but dropped gradually with decreasing altitude to levels not causing visible foliage damage at 50 and 100 m (fig. 1). The year of sampling (2008) was the last year before the local O. brumata outbreak at Skogsfjord collapsed.
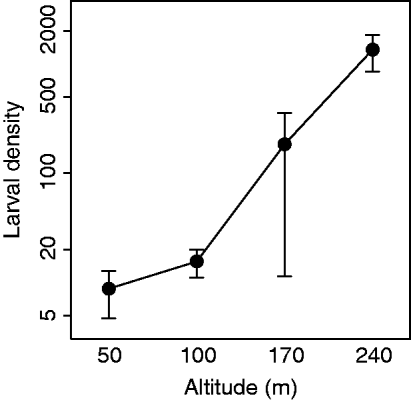
Fig. 1. Estimated larval densities (number of larvae per ten haphazardly collected 80 cm birch branches) for each of the altitudes 50, 100, 170 and 240 m a.s.l. (means across five sampling stations, with 95% confidence intervals).
Sampling of larvae for parasitoid rearing was conducted at 7–10 day intervals three times during summer at all sampling stations. Due to logistic constraints, altitudes were sampled pairwise over a two-day period as follows: sample 1: 27 June at 50 and 100 m, 28 June at 170 and 240 m; sample 2: 7 July at 50 and 100 m, 8 July at 170 and 240 m; sample 3: 15 July at 50 and 100 m, 16 July at 170 and 240 m. The larvae along the altitudinal gradient were mostly in the third instar at the time of sample 1 (second instar at 240 m), fourth instar at sample 2 and fifth instar at sample 3. Larvae were collected by shaking branches of haphazardly chosen birch trees in a large plastic box or by picking rolled up leaves with larvae inside.
Larvae were separated according to instar within 12 h of sampling and, subsequently, reared to record parasitism, following standard laboratory procedures (Hagen et al., Reference Hagen, Sørlibråten, Ims and Yoccoz2006). With experience, instars can be reliably distinguished by the size of sclerotized larval head capsules by unaided visual examination. Note that in the context of statistical analysis and rearing of parasitoid groups (see below) ‘instar’ refers to the instar of the larvae when they were sampled. Due to difficulties in removing very small larvae from rolled up leaves without causing mortality, the youngest larvae from sample 1 at 240 m could not be closely examined and may have included some first instars, although second instars clearly predominated. The smallest of these larvae mostly perished during rearing, and it was consequently assumed for the analysis that the survivors had been second instars when sampled. We, thereby, obtained samples that, with certainty, covered the second through fifth instar. Larvae dying from unidentified cause or with unknown fate were excluded from the samples before estimation of parasitism rates. Instar-specific sample sizes and parasitism rates are given in Appendices 1 and 2, respectively.
Data analysis
Parasitism (i.e. prevalence) rates were taken as binomial proportions given by P ijkm=N p, ijkm Ntot, ijkm −1, where N tot, ijkm and N p, ijkm are the number of larvae cultured and parasitized, respectively, from sampling station i, instar j, sampling date k and altitude m.
To investigate the temporal and altitudinal patterning of parasitism (i.e. P ijkm), we conducted logistic regressions using sampling date, altitude and their interaction as predictors. Because nonlinear effects of altitude and date were apparent for some parasitoid groups (fig. 2), both predictor variables were treated as categorical. The levels of date were taken as 1 (27 and 28 June), 2 (7 and 8 July) and 3 (15 and 16 July). Models for individual parasitoid groups were fitted using data only from dates when they occurred in larval samples.

Fig. 2. Observed parasitism rates, with 95% confidence intervals, within each of the altitudes 50, 100, 170 and 240 m a.s.l., on each of the three larval sampling dates, for individual larval parasitoid groups (a–f) and the larval parasitoid guild as a whole (g). The presented values are maximum likelihood estimates of the mean parasitism rate and its associated confidence interval across the five sampling stations within each altitude on each date. The estimates were obtained by fitting a separate logistic regression model, containing only an intercept, to the parasitism data for each date-altitude combination and, subsequently, back transforming the intercept and associated confidence interval from each model from logit to proportion scale. Note different scaling of the y-axis in (g).
Subsequently, parasitism (i.e. P ijkm) was related to larval phenology within dates and altitudes. This was done by adding larval instar and its two-way interactions with altitude and date as additional predictors to the selected models from the analysis of date and altitude effects (above). Thus, we first did the analysis with only date and altitude as terms in the models, to interpret the effects of these two factors; and, then, as a second step, we added instar in order estimate the effect of this variable. This procedure was necessary to obtain unbiased estimates of the effects of date and altitude since there were missing values for instars (i.e. no larvae above or below a certain size) across altitudes and over time. For the same reason, it was not possible to analyze the full data set with instar as categorical predictor variable. However, plots of partial residuals from models controlling for date and altitude showed no evidence for nonlinear effects of instar for any parasitoid group or for total parasitism rates (fig. 3). Instar, therefore, was treated as a continuous variable. Statistical analysis of instar effects was only meaningful for early-eclosing parasitoid groups and total parasitism rates, due to the almost exclusive occurrence of late-eclosing groups in fifth instar larvae. In addition, the early-eclosing Eulophus larvarum was too rare to justify statistical modelling of instar-specific parasitism patterns. Note, also, that second instar larvae were omitted from analysis in Braconidae indet. and P. anchisiades/P. immunis/Cotesia salebrosa because these groups, respectively, showed absence and only a single occurrence in this instar. This resulted in slightly different datasets between the two steps of the analysis for these two parasitoid groups.

Fig. 3. Partial residuals (on logit scale) from logistic models controlling for the effects of sampling date and altitude on parasitism plotted against larval instar. Solid lines represent the estimated effects of instar on parasitism, when controlling for date and altitude, while dashed lines represent 95% confidence intervals for the estimated instar effects. (a) Protapanteles anchisiades/P. immunis/Cotesia salebrosa. (b) Phobocampe sp./Sinophorus crassifemur. (c) Braconidae indet. (d) total larval parasitism. Note that the second instar was omitted from analysis in P. anchisiades/P. immunis/C. salebrosa and Braconidae indet. due to lack of parasitism by these parasitoid groups in second instar larvae. Observed instar-specific parasitism rates for individual parasitoid groups and the larval parasitoid guild, as a whole, are given in Appendix 2.
For both steps of the analysis, we conducted goodness-of-fit (GOF) tests based on sums of squared Pearson residuals from the most complex models considered. There was evidence of overdispersion in the model for date and altitude effects in the case of total parasitism rates (χ2=133.19; df=98; P=0.01) and the Phobocampe sp./Sinophorus crassifemur group (χ2=133.46; df=98; P=0.01). We corrected for this by implementing quasi-likelihood methodology. Minimization of the Akaike information criterion (Burnham & Anderson, Reference Burnham and Anderson2002), adjusted for small sample size (AICc) and additionally for overdispersion (QAICc) when necessary, was the basis for all model selection. The minimal models always retained the main effects of all predictor variables. This ensured that the biological effects could be estimated and that date and altitude were controlled for when analyzing instar effects.
All statistical analysis and plotting of data were performed with the statistical package R (R Development Core Team, 2008).
Results
The parasitism rates of individual parasitoid groups were mostly lower than 10% at all altitudes and dates (fig. 2a–f) but were still adequate to justify statistical analysis for all groups except Cryptus titubator and Agrypon flaveolatum. In the first step of the analysis (i.e. not including instar as a predictor), the models supported by AICc or QAICc for all individual groups, as well as for total parasitism rates, included only the main effects of date and altitude without their interactions (Appendix 3; all P>0.28 for likelihood ratio tests for interaction terms). The adequacy of additive models is also evident from graphical representations of date and altitude-specific parasitism rates (fig. 2), showing that the temporal patterning of parasitism was largely similar among altitudes (or alternatively that the altitudinal patterning was similar over time) for each parasitoid group and for the larval parasitoid guild as a whole.
Gradually declining parasitism rates with increasing altitude characterized most parasitoid groups (table 2a–e. fig. 2a–f). Notable exceptions were a lack of significant altitudinal variation in parasitism by Phobocampe sp./S. crassifemur on any date (table 2a, fig. 2a), and a less systematic altitudinal pattern for P. anchisiades/P. immunis/C. salebrosa at 240 m on date 2 (fig. 2b). The altitudinal clines displayed by most parasitoid groups gave a predominantly negative relationship between total parasitism rates and altitude throughout the study. However, because total parasitism increased slightly between 170 and 240 m on date 1 and 2, but decreased between these altitudes on date 3, the estimated main effects of these two altitudes were similar (table 2f, fig. 2 g).
Table 2. Coefficients from the selected logistic regression models (Appendix 3) relating sampling date and altitude to group-specific (a–e) and total (f) parasitism rates, and the coefficient for larval instar at sampling when it was added as an additional predictor to these models, all with 95% confidence intervals in brackets. The intercept in all models is the predicted logit value for 50 m altitude on date 1.

Statistically significant effects are shown in bold and the number of stars indicates significance levels: *, P<0.05;
** P<0.01; *** P<0.001.
A successional attack pattern within the guild of parasitoid groups was apparent from the temporal patterning of group-specific parasitism rates (fig. 2a–f), the main effects of date in the group-specific logistic models (table 2a–e), and the instars from which different parasitoid groups were reared (Appendix 2). Phobocampe sp./S. crassifemur was common already in samples from date 1 and showed no further increase in prevalence on later dates (table 2a, fig. 2a). It was reared from the second through fifth instar. Braconidae indet. and P. anchisiades/P. immunis/C. salebrosa were rare on date 1; but their prevalence increased significantly to date 2, followed by stagnation and a slight decrease to date 3, respectively (table 2b, c, fig. 2b, c). The groups were reared from the third through fifth instar (except for occurrence of the latter group in a single second instar larva). Eulophus larvarum was absent on date 1 and rare on date 2, but increased significantly to date 3 (table 2d, fig. 2d). It was reared from the fourth and fifth instar, mainly from the latter. Cryptus titubator and Ichneumonidae indet. occurred only on date 3 (fig. 2e, f). The former group was reared from the fourth and fifth instar, predominantly from the fifth, while the latter, except for occurrence in a single fourth instar larva, was reared exclusively from the fifth instar. Finally, A. flaveolatum was reared from a single fifth instar larva on date 3. The sequential attacks of different parasitoid groups produced a steady increase in total larval parasitism rates between all dates (table 2f, fig. 2 g).
For the three parasitoid groups where instar effects could be modelled and for the larval parasitoid guild as a whole, the best supported models in the second step of the analysis included only the main effects of instar, date and altitude (Appendix 4; all P>0.11 for likelihood ratio tests for interaction terms). Furthermore, in all models, the effect of instar was highly significant and negative (table 2a, b, c and f), indicating that parasitism decreased consistently with increasing larval instar within dates and altitudes (fig. 3).
Discussion
Phenology of larvae-parasitoid interactions
The temporal parasitism patterns in our study indicate that individual parasitoid groups had attacked mainly one or two larval instars. Parasitism by Phobocampe sp./S. crassifemur did not vary significantly between altitudes and dates, indicating that the group had completed parasitism along the entire altitudinal gradient before sampling date 1. Because most larvae at 240 m (the phenologically most delayed altitude) were in the second instar on this date, this suggests attack mainly on first and/or second instar larvae. Parasitism by Braconidae indet. and P. anchisiades/P. immunis/C. salebrosa occurred mainly between date 1 and 2, suggesting attack mostly on third and/or fourth instar larvae, which predominated along the altitudinal gradient during this period (although rearing of the latter group from a single second instar larva showed that it was also able to parasitize this instar). Parasitism by E. larvarum happened mainly between date 2 and 3 (seemingly somewhat earlier at 50 m), when most larvae were in the fourth to fifth instar. Rearing of the group from both instars, but mainly from the fifth, suggests that both had been attacked. Finally, late-eclosing parasitoid groups (Ichneumonidae indet., C. titubator and A. flaveolatum) occurred only in samples from date 3 and were reared almost exclusively from fifth instar larvae, suggesting attack mostly on larvae that were close to pupation.
In addition, parasitism by the three major early-eclosing groups decreased significantly with increasing instar within dates and altitudes (fig. 3). This pattern would indicate that the groups were targeting, and/or having higher success with, the smallest larvae available during their flights. However, because the instar of larvae, when they were parasitized, is unknown, we cannot determine if parasitoid attack and/or success rates were higher in given instars or simply in the relatively smallest larvae encountered per se. Predisposition to parasitism in relatively smaller larvae would not be surprising since the efficiency of anti-parasitoid defences based on morphology (e.g. cuticle thickness (Beckage & Riddiford, Reference Beckage and Riddiford1978)), behaviour (e.g. thrashing of the larval body (Gross, Reference Gross1993)) or physiology (e.g. encapsulation (Lu et al., Reference Lu, Hu and Fu2006)) is often greater in larger hosts. A negative relationship between larval size and parasitism could conceivably also result if parasitized larvae suffered reduced development rate immediately following parasitism (as observed by, e.g. Shi et al., Reference Shi, Liu and Li2002). However, in this case, parasitized larvae should show a progressively larger developmental delay over time, resulting in negative date-instar interactions. Model selection did not support such interactions. Also, personal experience from larval rearing suggests that these parasitoids do not affect larval development appreciably until very late in the larval stage (O.P.L. Vindstad, personal observation), a pattern which has also been documented for other parasitoid wasps (e.g. Smith & Smilowitz, Reference Smith and Smilowitz1976).
Accurate assessment of parasitoid capacity for host stage utilization requires experimental data, but the above results may nevertheless suggest that individual larval parasitoid groups of O. brumata show considerable host size or instar-specificity. Consequently, these parasitoids may run a high risk of host asynchrony in the climatically unstable birch forests of coastal northern Fennoscandia. Asynchronies, for instance, could result from moth eggs overwintering in birch canopies, while parasitoid cocoons lie on the ground under the snow (Bylund, Reference Bylund1999). Developmental responses to inter-annual variation in spring temperature may diverge in these different microhabitats, thereby causing the relative timing of larval hatching and parasitoid eclosion to shift between years. As another example, prolonged periods of rain could prevent parasitoids from searching while larvae pass through instars susceptible to parasitism. Fink & Völkl (Reference Fink and Völkl1995) and Weisser et al. (Reference Weisser, Völkl and Hassell1997) showed that rain can be highly detrimental to parasitoid searching efficiency and oviposition rate. If the time window for larvae-parasitoid interactions is limited, parasitoids may miss the chance for parasitism due to even small climatic effects.
However, only evaluating the risk of phenological asynchrony for individual parasitoid groups may lead to biased conclusions regarding the likely impacts of larvae-parasitoid match-mismatch effects on host demography. Our results suggest that there is high potential for phenological diversity among parasitoid groups to buffer overall larval parasitism rates against mismatch events. The attacks of different groups seemed to follow each other phenologically in a successional manner throughout the study period and collectively expose larvae to parasitism during most or all of their development. With the activity of different parasitoid groups distributed across the entire larval season, there is little chance that climatic events will cause the entire parasitoid guild to become asynchronous within a single year. Moreover, it is conceivable that asynchrony in some groups can be compensated for, with respect to total parasitism rates, via reduced interspecific competitive effects (e.g. multiparasitism) on groups flying at other times. We recently showed that total larval parasitism rates of O. brumata in north Fennoscandian coastal regions can be largely independent of the identity of the parasitoid group dominating the parasitoid guild (Vindstad et al., Reference Vindstad, Hagen, Ims and Schott2010). This suggests that there is a high degree of functional equivalence among guild members and, accordingly, potential for potent compensatory dynamics if one or a few parasitoid groups become mismatched with larvae. Generally, phenological diversity within enemy guilds could represent one important dimension of the biocomplexity that may significantly contribute to counteract the effect of abiotic disturbances (Hooper et al., Reference Hooper, Chapin, Ewel, Hector, Inchausti, Lavorel, Lawton, Lodge, Loreau, Naeem, Schmid, Setala, Symstad, Vandermeer and Wardle2005; Duffy, Reference Duffy2009). Thus, for the present study system, we find it unlikely that climate-induced larvae-parasitoid asynchronies have a major impact on O. brumata demography or contribute importantly to the profound temporal variation characterizing the amplitude of the moth's population cycles. These conclusions complement the ones derived from the multi-year studies of Hagen et al. (Reference Hagen, Jepsen, Schott and Ims2010) and Schott et al. (Reference Schott, Hagen, Ims and Yoccoz2010), namely that larval parasitoids are unlikely to drive (i.e. generate) the population cycles of O. brumata (and the closely related Epirrita autumnata (Bkh.) (Autumnal moth, Lepidoptera:Geometridae)) in coastal northern Fennoscandia.
Parasitoid prevalence in relation to altitude: a result of mismatch with host phenology?
Except for Phobocampe sp./S. crassifemur, all of the common parasitoid groups in our study showed declining prevalence with increasing altitude on all sampling dates when they occurred. Thus, we cannot rule out that the increase in moth density towards higher altitudes (fig. 1), to some extent, can have been due to reduction in parasitism pressure (although this will have to be further assessed by additional research). Concerning the cause of the altitudinal clines in parasitism, their presence could reflect altitudinal variation in numerous biotic and abiotic factors unrelated to our present focus of larvae-parasitoid phenology-matching (Hodkinson, Reference Hodkinson2005). First, parasitoid performance may have declined along the altitudinal gradient due to harsh abiotic conditions at higher altitudes, e.g. high wind speed and low temperature, which are both known to reduce parasitoid functional efficiency (Gu & Dorn, Reference Gu and Dorn2001; Menon et al., Reference Menon, Flinn and Dover2002; Zamani et al., Reference Zamani, Talebi, Fathipour and Baniameri2006). Second, if parasitoids showed weak reproductive and/or aggregative responses to spatial variation in host density, the sharp increase in larval density with increasing altitude may have been the cause (i.e. rather than the consequence) of the altitudinal clines in parasitism. Such a ‘parasitoid swamping’ effect due to the extreme larval densities at the highest altitudes seems highly probable. Finally, the phenologically latest parasitoid groups may have been active after our last sampling date, implying that their final performance along the gradient is unknown.
On the other hand, the negative relationship between altitude and parasitism could also imply that larvae-parasitoid synchrony declined with increasing altitude. Although our observational study was particularly designed to map the phenology of host-parasitoid interactions along altitudinal gradients, the observed spatiotemporal parasitism patterns give limited possibilities of distinguishing altitudinal match-mismatch effects from alternative underlying mechanisms. In particular, there was no statistical support for date-altitude or instar-altitude interactions in the parasitoid groups where such relationships could be modelled (all of the early-eclosing groups, except for E. larvarum in the case of instar related effects). Such interactions could have been indicative of different degrees of synchrony between altitudes. Their absence, on the other hand, does not prove that larvae and parasitoids were well synchronized along the altitudinal gradient. Since sampling dates were separated by 7–10 days, the lack of date-altitude interactions indicates that altitudinal delays in parasitism did not exceed the expected delay in larval phenology, i.e. about one week between 50 and 240 m. However, more extensive altitudinal delays in parasitoid phenology may still have been present; if parasitoids were only able to attack a narrow range of larval sizes or instars, only a phenologically early minority of parasitoids would achieve efficient parasitism at altitudes where parasitoid phenology generally lagged behind the phenology of larvae. Parasitism rates at such altitudes, thereby, would become persistently low, but show little or no indication of being delayed relative to larval phenology. This would produce strong main effects of altitude without interactions, i.e. similar patterns to what we have observed in our study. The lack of instar-altitude interactions, but presence of strong negative main effects of instar, indicates that relatively smaller larvae were most parasitized within all altitudes. This may suggest that the relative timing of parasitoid flight and larval development was similar along the altitudinal gradient. However, this interpretation is only valid if the negative relationship between instar and parasitism resulted from parasitoids attacking specific larval instars. If parasitoids were simply attacking relatively smaller larvae per se, parasitoid flight periods and larval development could shift relative to each other along the altitudinal gradient without causing instar-altitude interactions.
The fact that many different mechanisms could underlie the same altitudinal patterns suggests that further research, applying other approaches than used in the present study, is required to determine if and how larvae-parasitoid phenology relations affect altitudinal parasitism patterns. For instance, approaches yielding more direct information on parasitoid flight periods at different altitudes would be valuable, but depend on adequate field techniques and better taxonomic information about key members of the parasitoid guild, to allow species identification of adult specimens. What can be concluded for now is that nothing in our present results points towards larvae-parasitoid asynchrony as a more plausible explanation for the observed altitudinal parasitism patterns than alternative candidate mechanisms.
Evolutionary implications of larvae-parasitoid phenology relations
The negative relationship between larval size and parasitism risk suggested by our results could impose strong selection pressures on O. brumata larval phenology. However, such selection may largely coincide with that caused by the host plant. Larvae hatching synchronously with budburst gain optimal foliage quality and grow efficiently (van Asch & Visser, Reference van Asch and Visser2007, and references herein). This results in high fecundity and survival, and will reduce parasitism risk if small larvae are predisposed to parasitism. Rapid growth also allows early pupation, which could allow larvae to avoid the late flight periods of late-eclosing parasitoid groups (see also Kaitaniemi & Ruohomäki, Reference Kaitaniemi and Ruohomäki1999). Hatching after budburst subjects larvae to lower foliage quality, which is likely to increase parasitism risk due to poor growth and longer development time. Hatching before budburst will not allow larvae to improve protection from parasitoids (i.e. relative to hatching synchronously with budburst) by growing large and pupating earlier, since larval growth in O. brumata cannot proceed before buds open. Rather, very early hatching may increase parasitism risk, by lengthening the larvae's exposure to phenologically early parasitoids groups. Hatching at budburst, thereby, would seem to both optimize food quality and minimize parasitism risk for the larvae.
Conclusion
Although our study did not enable us to make clear inferences on how climatic variation affects the degree of phenological matching between an outbreaking birch forest geometrid and its different larval parasitoids, it yielded new information on the functioning of the larval parasitoid guild in terms of phenological organization. In particular, our study suggests the possibility that taxonomically diverse parasitoid guilds may harbour sufficient phenological diversity to buffer overall guild functionality against climate-induced asynchrony between hosts and individual parasitoid species. Our results, thus, provide some further weight to previous studies on the interactions between O. brumata and its larval parasitoids, indicating the potential for compensatory mechanisms within the parasitoid guild (Vindstad et al., Reference Vindstad, Hagen, Ims and Schott2010) and a lack of significant effects of larval parasitism on moth outbreak dynamics in time and space (Hagen et al., Reference Hagen, Jepsen, Schott and Ims2010; Schott et al., Reference Schott, Hagen, Ims and Yoccoz2010). Taken together, the present knowledge suggests that larvae-parasitoid interactions are unlikely to explain the wide temporal and altitudinal variation in amplitude characterizing O. brumata population cycles in sub-arctic coastal birch forests.
Acknowledgements
This study was funded by the Department of Arctic and Marine Biology, University of Tromsø and the Research Council of Norway. We thank Einar Stikbakke and Saga Svavarsdóttir for assistance with field and lab work, and Tero Klemola and Ian Hodkinson for constructive comments that helped improve the quality of the manuscript.
Supplementary material
The online Appendices can be viewed at http://journals.cambridge.org/ber.