Article contents
- Abstract
- CENTRALIZATION WITHIN THE FRENCH HEALTH-CARE SYSTEM
- HISTORICAL PERSPECTIVE ON CENTRALIZATION/DECENTRALIZATION OF HTA IN FRANCE
- VARIETY OF HTA ACTIVITIES AND CUSTOMERS
- PROVIDERS OF HTA IN FRANCE
- ANAES
- IMPACT OF HTA REPORTS
- RELATIONSHIPS BETWEEN THE PROVIDERS OF HTA
- NEED FOR FURTHER COLLABORATION
- CONCLUSIONS
- References
Overview of health technology assessment in France
Published online by Cambridge University Press: 23 April 2004
- Abstract
- CENTRALIZATION WITHIN THE FRENCH HEALTH-CARE SYSTEM
- HISTORICAL PERSPECTIVE ON CENTRALIZATION/DECENTRALIZATION OF HTA IN FRANCE
- VARIETY OF HTA ACTIVITIES AND CUSTOMERS
- PROVIDERS OF HTA IN FRANCE
- ANAES
- IMPACT OF HTA REPORTS
- RELATIONSHIPS BETWEEN THE PROVIDERS OF HTA
- NEED FOR FURTHER COLLABORATION
- CONCLUSIONS
- References
Abstract
Health technology assessment (HTA) in France covers a variety of activities performed for different customers (e.g., health professionals in the field and policy makers in government) for the benefit of patients. To promote the improvement of quality in health care, France has set up a series of distinct agencies that report to the Ministry of Health but are also accountable to their other customers. We place particular emphasis on ANAES (National Agency for Accreditation and Evaluation in Health) whose main remit is HTA. We show how the diversity of HTA activities and their decentralization suggests tight collaboration among all the different bodies which perform HTA or are closely involved with HTA, and we provide examples of such collaboration.
Keywords
- Type
- Research Article
- Information
- International Journal of Technology Assessment in Health Care , Volume 20 , Issue 1 , January 2004 , pp. 25 - 34
- Copyright
- © 2004 Cambridge University Press
The French health-care system has been described as a “centralized Bismarckian system” offering a high level of coverage to virtually the entire population, and yet it is a system that offers great freedom of choice to the individual patient. Patients can choose their doctor and their hospital/clinic. There is no gatekeeping process for access to specialists. Not only patients but also clinicians have freedom of choice in that they can decide where they practice, how they practice, and what they prescribe. Specialists can choose to work in the hospital and/or ambulatory sector and yet always be part of the state system. In brief, in the name of equity, the state offers coverage; in the name of individual freedom, it leaves the option of choice. The World Health Organization (WHO) has rated the French health-care system as the best in the world (22), although several criticisms have been voiced regarding this rating and how it was derived. The extensive access to health care was an important criterion in the rating. France devotes 9.5 percent of its gross national product to health, one of the highest percentages in Europe. Social Security Funds finance 75.5 percent of care, supplementary and complementary insurance schemes approximately 12 percent, and patients approximately 11 percent.
The present overview of health technology assessment (HTA) in France will illustrate how this duality between centralized/decentralized aspects—centripetal/centrifugal forces—permeates the French health-care system. We shall briefly describe the national approach to health care, list agencies and organizations that have been set up to meet specific needs, and explain how their independence reconciles both public health demands and individual patient needs in the field of HTA. The emphasis will be primarily on the agency ANAES (Agence Nationale d'Accréditation et d'Evaluation en Santé—formerly ANDEM), which was set up specifically to perform HTA. We shall illustrate how the variety of customers and types of HTA reflect a decentralized system. The working methods, priority setting, and impact of ANAES reports will be examined, and we conclude that, while decentralization helps to meet different types of needs, it requires tight collaboration among agencies and other institutions involved in HTA.
CENTRALIZATION WITHIN THE FRENCH HEALTH-CARE SYSTEM
The state's role is to safeguard public interest and improve the health of the general population. It deals mainly with prevention, surveillance, the fight against major diseases, education of health professionals, and quality standards in health-care facilities and pharmaceutical production. It exerts control over the relationships between the institutions that finance health care, health professionals and patients, and defines the rules of health-care coverage. It regulates the inputs of care available (staff, facilities, and equipment). It is also in charge of planning. Each year, parliament defines health-care objectives and the funds to be allocated. The Public Health Committee (Haut Comité de santé publique) helps define these objectives and prepares an annual report that is submitted to the National Health Council (Conférence nationale de Santé) and parliament. It is the Conférence nationale de Santé that makes proposals with regard to priorities and directions in health care. It is composed mainly of health-care professionals and representatives from health-care organizations and from the regional health councils (Conférences régionales de santé). The regional councils include local professionals and also users. Their role is to determine health-care needs and priorities at the local level.
The French health-care system falls under the jurisdiction of two ministries—the Ministry of Social Affairs and the Ministry of Health—and of three directorates in particular: Social Security (DSS), Hospitals and Health Care Organisation (DHOS), and Health (DGS). The Ministry of Economy and Finance, of course, intervenes as far as expenditure is involved. These ministries operate a powerful top-down approach. A typical way of dealing with situations is to set up public institutions with competence in specific areas; to promulgate laws, decrees, and orders; and to issue internal circulars. These institutions may be different types of legal entities, such as agencies, committees, or nonprofit organizations. The main ones reporting to the DGS are indicated in Table 1, together with their principal remits as ordained by law or ministerial decree. Many of these organizations were created because of safety concerns; they oversee measures regarding organ transplants, blood supply, food, and medical goods and services. The agency most intimately involved in HTA, besides ANAES, is the French Health Products Safety Agency, AFSSAPS (Agence Française de Sécurité Sanitaire des Produits de Santé). Its main activity is the market authorization process for pharmaceuticals.
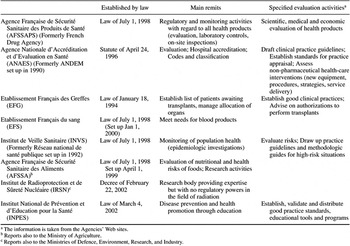
HISTORICAL PERSPECTIVE ON CENTRALIZATION/DECENTRALIZATION OF HTA IN FRANCE
As in other countries, the percentage of gross domestic product spent on health care rose rapidly in France from 1970 onward, leading to speculations about the cause of the increase and attempts to curb it. However, all attempts by government to control costs by restricting access to care (13) had limited impact or were not sanctioned politically. Approximately 85 percent of the population were not only covered by national health insurance but also owned private medical insurance policies, which increased policy holders' choice of services and did not encourage them to limit their consumption. Given the situation, there were two ways forward to bring about change.
The first was to encourage a centrifugal process of inducing cultural and behavioral changes in health-care providers by introducing the concept of evaluation in health care. Independent evaluation initiatives had to be promoted, and health-care professionals needed to acquire sound working methods with which they could formulate their own proposals for improving the quality of care (15). This led to the creation of the National Agency for the Development of Medical Evaluation (ANDEM) in 1990, and which in 1996 became ANAES. ANDEM's role was to set up “consensus conferences” on major health issues and to promote evaluation among health-care professionals by providing them with appropriate methods and tools, as well as genuine examples of their application. In 1993, within the framework of a constructive collaboration with the US Agency for Health Care Policy and Research (AHCPR), ANDEM started to produce clinical practice guidelines (14;20).
The second way forward was to set up a centralized process of controlling supply. Currently, a health map establishes the equipment and hospital beds required in each region of France while regional quotas define the numbers of medical students who will be allowed to register in the university medicine faculties. For instance, the geographical distribution of high-cost imaging equipment such as computed tomography (CT) scanners or magnetic resonance imaging (MRI) machines is strictly controlled by means of the health map. Moreover, in such a controlled system, the usefulness and cost-effectiveness of the equipment has to be assessed. Is a CT scanner a worthwhile financial investment? In the 1970s, French hospitals, unwilling to wait for domestically produced scanners from the Compagnie Générale de Radiologie (CGR), purchased their CT scanners abroad. Health technology was accused of being the “culprit” behind escalating health costs, especially in the public sector (21). It became increasingly obvious that policy makers needed access to systematic factual evidence on equipment rather than just relying on the opinion of experts. Thus, they turned to ANDEM for comprehensive and up to date scientific and technical information on which to base their decisions (8). In 1991, therefore, ANDEM developed a method for producing HTA reports for policy makers based on a critical appraisal of the literature and consultation with experts.
VARIETY OF HTA ACTIVITIES AND CUSTOMERS
Thus, as it developed, HTA was offered to both health professionals and policy makers. In fact, the very nature of HTA explains that it can be useful to a broad range of customers. HTA can be viewed not only in terms of its subject matter—“equipment, devices, and drugs and the medical and surgical procedures used in prevention, diagnosis, treatment, and rehabilitation of disease as well as the organisational and supply systems used in the delivery of health care” (5)—but its format and purpose. Is HTA an element of scientific debate, a means of changing practices, a decision-making aid in the public health arena, or even a step toward exerting market control? Different customers require different types of report for different topics and objectives.
Although both AFSSAPS and ANAES report to the Health Directorate (DGS), the DGS is not their only, or even most important, customer. The main activities of AFSSAPS are evaluation, regulation, and monitoring, but its primary customer is the patient, by means of pharmaceutical companies, which seek market approval for their products. AFSSAPS holds the statutory power to authorize market approval for drugs and can withdraw from use any medical devices that are deemed to present a risk. On the other hand, ANAES has an essentially advisory capacity. Its customers are the national health insurance funds (financed by employees and employers), academic societies, health-care institutions, and professionals. For both agencies, these customers provide the lion's share of their funds. In fact, it is the very variety of activities and customers that leads to decentralization.
An HTA topic can be dealt with in several ways, depending on progress in the field concerned. At different moments in time, it can be viewed from different angles and commissioned by different customers. For instance, diagnosis of osteoporosis can be thought of as an issue that primarily concerns researchers (11). If scientific evidence is poor, it may be viewed as a highly controversial topic that needs to be debated publicly among health professionals during a consensus conference (17). On the other hand, for some, it may be a matter of public health that can be addressed in an HTA report for policy makers (7). In time, sufficient information and expertise may become available for a clinical guideline to be drafted for health-care professionals (1). All these types of assessment, excluding research, are addressed in France by the same agency—ANAES.
Some of the differences between these different types of assessment (consensus conferences, HTA reports, and clinical practice guidelines) are highlighted in Table 2.

Priorities for introducing clinical practice guidelines are mostly determined by practitioners' needs (bottom-up demand), although policy makers may also identify issues for which they would like to promote clinical guidelines. In all cases, however, the content of a guideline remains the responsibility of the health professionals who prepared the document (either on behalf of ANAES or as approved by the agency). HTA reports are requested by policy makers who may require a rapid answer to an urgent problem (top-down demand). In 2002, 55 percent of requests to ANAES for HTA reports came from the Ministry of Health (DGS and DHOS) or from the Department of Social Security. The remainder, which represent a substantial proportion (45 percent), were from professional or academic societies (bottom-up demand). These bodies required up to date reports on state-of-the-art technology that highlight the need for a policy decision or investment and that can be used to lobby policy makers. For example, in 2000, ANAES was asked to evaluate virtual colonoscopy, an emerging technology with a possible impact on mass screening for colorectal cancer as, in theory at least, it could replace traditional colonoscopy. The report was not commissioned by the DGS but by the French Society of Radiology and the Society of Digestive Endoscopy.
In drafting guidelines and HTA reports, ANAES supplies the methods, know-how, and infrastructure. Panels of experts provide professional expertise and hands-on knowledge of the field. In some cases, ANAES approves well-prepared reports from consensus conferences and guidelines from professional societies. Its methodologic expertise is recognized and valued by policy makers and health professionals alike. However, the decisional aspects arising from ANAES's work fall upon the client groups who commissioned a report and not ANAES itself. This advisory capacity on the sidelines is reflected in one of the agency's latest mandates. The DGS wishes to introduce practice appraisals in France to reinforce the work already done by ANAES in promoting improvements in quality and clinical audit methods. The system initially will apply only to general practitioners and will operate through self-assessment; participation is voluntary. It is ANAES's role to define the methodology for preparing standards from existing guidelines, to approve those standards, and to train the “helper-doctors” (facilitators) who will “teach” self-assessment. However, the regional medical unions will be responsible for implementing the practice appraisal procedure. Similarly, in the context of the proposed reform of the French classification (nomenclature) system for fee-for-service medical and surgical procedures, ANAES's remit is to provide technical advice on the medical and surgical procedures to be included in the new list (see last column in Table 2). The decision on whether to list a procedure and on whether to reimburse fees remains with the HTA customers—the national health insurance funds, the DSS, and the DGS (16).
The relationship between providers and purchasers of HTA in France, in relation to activity, is shown in Table 3 for several bodies funded, directly or indirectly, by the state. This table is by no means exhaustive. The scope of HTA is vast, and many other organizations carry out some aspect of HTA.

PROVIDERS OF HTA IN FRANCE
A brief description of the HTA activities of the organizations listed in Table 3 is provided below.
AFSSAPS and the Commissions It Chairs
AFSSAPS is the French Health Products Safety Agency and, as mentioned above, has regulatory powers. Its main mission is to evaluate drug dossiers before granting marketing approval. An economic evaluation is not part of this approval process. Once approval is granted, the drugs are reviewed by the “Commission de transparence,” which is chaired by AFSSAPS but is not an integral part of the agency. The drugs are ranked according to a multilevel grid from “a major contribution” down to “no contribution” to health care (ASMR, Amélioration du Service Médical Rendu). Economic arguments, provided to the commission, may be taken into account in establishing this ranking, especially for clinically equivalent drugs, but no formal rules have been established. Decisions are on a case-by-case basis. The commission's decisions may then contribute to price determination procedures.
AFSSAPS is also in charge of market monitoring, both for drugs and medical devices. It decides whether certain marketed devices need to be evaluated to either restrict use or redefine indications and/or conditions of patient follow-up. Several bodies in France have the authority to give EC approval to medical devices; the largest is G-MED. Evaluation by these organizations focuses essentially on the product's characteristics, particularly on biocompatibility issues, and not a great deal on its clinical relevance. However, quality of manufacture and production processes cannot guarantee safety of use. In fact, no organization systematically evaluates medical devices. Public hospitals are free to use any EC-approved device, regardless of price, and the cost of the device is included in the hospital's overall budget. However, manufacturers have to apply for an official reimbursement price for a device to be used in private institutions. The Product and Services Evaluation Commission, CEPP (Commission d'Evaluation des Produits et des Prestations), chaired by AFSSAPS, assesses medical devices to be used in the private sector before decisions are taken on reimbursement. Manufacturers now have to show that medical devices, like drugs, improve medical provision on the basis of disease severity, safety and effectiveness, usefulness compared with other therapies, and usefulness from a public health standpoint. The CEPP sends its assessment to the commission in charge of price determination. Price negotiations are based on the CEPP report and information provided by the manufacturers. The DGS can override a CEPP decision on financial grounds.
ANAES
As indicated in Table 3, ANAES meets the needs of a variety of customers. It is under DGS supervision and satisfies its specific demands (centralization). Yet the preparation of clinical practice guidelines, assessment criteria, and standards for clinical practice in hospitals and ambulatory care, and HTA reports for academic societies are notably orientated toward medical and allied professions, and toward patients (decentralization).
We shall focus just on HTA reports in this discussion. The annual program for these reports is defined after customer consultation and ends with validation by ANAES's administrative board (Table 4).

Table 5 provides examples of reports published since 1999 and currently in preparation. Important topics are imaging techniques, emerging techniques, and public health issues. Imaging techniques make up a substantial part of the program because of complaints from both health-care professionals and patients about lack of access to equipment. Medical devices are not evaluated per se but in terms of their usefulness within a treatment strategy and overall patient management, or as part and parcel of a comprehensive public health approach to general medical and surgical care that includes prevention and mass screening.

Currently, ANAES's HTA program comprises two major types of assessment: (i) evidence-based assessment of widely used technologies and of technologies on the verge of being disseminated. ANAES defines the relevance and conditions of use of these technologies. The procedure for the preparation of a standard HTA report is given in Table 6. Although most reports are based on an evidence-based appraisal of the literature, ANAES is introducing other methods such as expert panels when data are limited, and modeling methods for comparing public health strategies in clinical and economic terms. (ii) Rapid assessment of emerging technologies with a short half-life, fast-developing technologies, and emerging public health issues. ANAES advises on the correct positioning of emerging technologies and, as of last year, produces experimental “milestone HTA reports” (23). When the scope of the question relating to the technology under study is highly focused and when good use can be made of available high-quality reviews or reports from other sources, the time taken to produce an HTA report can be reduced from 12 to less than 6 months.

CEDIT
Hospitals need HTA reports on the equipment they wish to purchase. The CEDIT (Committee for the Evaluation and Diffusion of Innovative Technologies), in operation since 1982, is a hospital-based committee that advises AP-HP hospitals (Assistance Publique–Hôpitaux de Paris; AP-HP is a group of fifty university hospitals in the Paris area) on the diffusion of diagnostic and therapeutic technical innovations (3;4). CEDIT has a pragmatic rather than systematic approach to technology assessment. Any medical or administrative staff member of AP-HP can seek CEDIT's advice. The heads of medical departments will want the benefits of a new technology to be recognized, whereas administrative staff will seek advice on a new investment at hospital or institutional level. As CEDIT is the only hospital-based unit involved in HTA, its reports may also be passed on to other hospitals. They may be used by the Ministry of Health to promote national awareness on specific issues.
INSERM and CNRS
INSERM (the National Health Research Institute) and CNRS (National Centre for Scientific Research) are two of the main state-funded bodies that perform scientific and technologic research in France. Both report to the Ministry of Research, but INSERM also reports to the Ministry of Health.
INSERM produces systematic reviews of biomedical research data that rely on the “collective expertise” of several of their research teams working in the fields of biology, medicine, and health. The aim is to translate scientific data into relevant and pragmatic information on an issue that is defined by the customer and INSERM. Reports summarize the latest scientific innovations, the points raised by experts working for or consulted by INSERM, and end with comments and recommendations designed to enlighten the customer on research questions to be addressed. Reports are often made public but can remain confidential at the customer's request. Examples of reports are to be found on INSERM's Web site (www.inserm.fr). Some are published as scientific articles (6).
CNRS comprises over 1,000 research units, of which 85 percent are in partnership with other research organizations or with institutions of higher education. Research units evaluate public policies—for instance, health economy—and CNRS staff as well as staff from INSERM and universities collaborate within these units. They address topics such as the relevance of economic evaluation in defining health policy, the medical and economic evaluation of health networks, and the diffusion of technologic innovations.
The above bodies impact on decision making at the “macro” (national) or “meso” (regional/local) levels and on practices at the “micro” (individual) level. For example, AFSSAPS, ANAES and INSERM have important roles at the micro level (impact on professional practice) and macro level (public health decisions), whereas CEDIT plays a more significant role at the meso level. However, these distinctions are far from absolute.
IMPACT OF HTA REPORTS
Assessments that are part of a regulatory process have, by their very nature, an impact on decisions and outcomes. Drug files produced by the pharmaceutical industry to gain market approval and their assessment by drug agencies impact directly on the availability of new drugs. The situation is fairly similar for medical devices. Self-assessments of quality of practice produced by French hospitals and their evaluation by ANAES determines whether ANAES will accredit these hospitals. However, ANAES's HTA reports are different in nature. They are either informative overviews on state-of-the-art technologies to be used by stakeholders (such as academic societies) to influence decisions or they are advisory reports to be used by the authorities (e.g., government bodies, national insurance funds) as decision aids. They advise on research to be done (clinical and economic) and on resource requirements (type of health-care organization, equipment, resources, and staff training), but they have no formal status. Those who commission the reports have no obligation to accept, discuss, or even take into account the advice given. For the authorities, many considerations, other than the HTA report, influence decisions; these include budgets, social acceptability, and political priorities. Thus, the impact of ANAES's consensus conferences (10;19), guidelines (9) and HTA reports depends entirely on implementation by the customer. Of course, the report will be all the more persuasive the higher the quality of the assessment method and the more appropriate and attractive the format.
The following examples illustrate some of the different types of impact an HTA report can have:
- In 1998, ANAES advised against mass screening for hemochromatosis. (This advice was later supported by international consensus). This is a typical example of a decision for not doing something because of lack of evidence. Because nothing changed (the screening was not set up), it is difficult to argue that the report had any impact. Had the conclusion of the report been different, would something have been done? The report will be updated when new scientific evidence becomes available. In the meantime, ANAES is setting up a work program for assessing the expectations and preferences of patients with hemochromatosis. The conclusion of the 1998 report may need to be changed in light of the new information that arises from this exercise.
- In 1999, ANAES considered that the two linear accelerators available for stereotactic intracranial surgery were not adequately compared and recommended clinical research in centres where the two processes were available. Such a clinical research protocol is about to be implemented, but it is difficult to judge how far the report influenced this decision.
- In 1999, ANAES recommended breast cancer screening for all women between 50 and 74 years of age. A mass screening program was officially introduced in 2002. However, the usefulness of such screening has been queried recently in a widely publicized paper (18), prompting the DGS to ask ANAES whether it still upheld its conclusions. After a critical analysis of the published paper and other data that highlighted several contradictory conclusions, ANAES confirmed its opinion that screening should be implemented. In this case, the request for confirmation of the original recommendation links the DGS's final decision to the HTA assessment. A second example of a request for confirmation concerns a negative decision. Two years after ANAES advised against implementing mass screening for prostate cancer, a committee set up by the DGS to make proposals on cancer policy requested confirmation of this recommendation. The 2001 report on the comparative efficacy of treatments for local prostate cancer reaffirmed the original conclusions advising against mass screening. These two examples indicate that HTA reports may impact on major public health decisions. However, how these reports were taken into account by the authorities and what would have happened if they had not confirmed an earlier decision is not known.
Decisions are, of course, also taken without the support of HTA reports—as was the decision to perform neonatal screening for cystic fibrosis. This raises the question of whether specific criteria determine the need for an HTA report for decision making. Does a general perception of the volume and quality of available scientific information determine whether or not a request is made? Do highly conflicting opinions on a topic prompt requests? The answers to these questions are not known.
RELATIONSHIPS BETWEEN THE PROVIDERS OF HTA
A rather intricate and complex pattern of provider/purchaser relationships has emerged over time. The following are examples of the interfaces between spheres of competence.
- In theory, but this is not always confirmed, the closest interfaces are those within an agency because of geographical proximity. HTA activities in one area can benefit from the expertise of colleagues in other areas. For instance, at ANAES, staff who draft clinical practice guidelines can consult their colleagues who prepare in-house HTA reports; staff who produce standards for appraisals can consult colleagues who produce guidelines upon which the standards are based; those who develop methods and tools for quality assessment and improvement in the ambulatory sector can consult with colleagues working for the hospital sector (12). Coordination ensures greater relevance, exhaustiveness, and consistency.
- AFSSAPS may use ANAES's HTA reports, which often highlight problems of medical follow-up, to decide whether marketed devices need re-evaluation to either restrict their use, or redefine indications and/or conditions of patient follow-up (e.g., the report on stent-grafts for endovascular repair).
- For a particular medical device, CEDIT provides purchasers within the Paris network of public hospitals (AP-HP) with information on comparative efficacy and safety, and with published medico-economic data and a financial analysis, and evaluates the impact of device use on work organization in AP-HP hospitals. ANAES also provides information on the comparative efficacy and safety of medical devices and on published medico-economic data. It analyzes care pathways and suggests operator training requirements and further clinical studies. It also addresses alternatives to hospital treatment (ambulatory care) and economic impact outside the hospital sector. Ethical issues may be similar in and out of hospitals, but because the social perspective is different, the conclusions may differ. AFSSAPS defines instructions for use in relation to proven efficacy and safety (as established by CEDIT and/or ANAES), and carries out monitoring activities.
- ANAES regularly invites one or more members of AFSSAPS's staff to their working group meetings on care pathways involving a medical device or drug that already has been reviewed by AFSSAPS.
Clearly, these manifold interfaces raise the question of whether the existence of several discrete bodies performing HTA is beneficial or detrimental. Is there too much duplication or are there too many gaps? In answering these questions, at least two fundamental points must be considered. First, although each body may have its own sphere of competence, none can ignore the overall complexity of an issue (e.g., device safety, efficacy, distribution, availability; operator training and opinion; legal, economic, ethical, and social issues) and, therefore, must interface with other bodies. Second, the most useful solutions often arise from a confrontation of opinions rather than from a “we know” attitude. Expert panels convened by the agencies cannot agree on all points. This is to be expected—their data sources (published literature, manufacturers' data, etc.) and working methods are not the same, nor are their objectives. Although this situation may lead to some duplication, it is clear that viewing any object (e.g., a medical device) from more than one angle (confrontation of expert opinions) often best reveals the multidimensional structure of the object.
NEED FOR FURTHER COLLABORATION
Fields of competence may overlap but also leave gaps in assessment. The numbers of technologies to be assessed and aspects to be covered are such that gaps are inevitable. Tighter collaboration is needed among HTA providers in at least four sectors: (i) public hospitals. At present, no agency systematically evaluates the pros and cons of new classes of medical devices for public hospitals. (ii) Early links should be established with clinical research. In France, publicly funded medical research is carried out mostly by INSERM but also by other bodies (e.g., CNRS). In March 2002, the Ministry of Health asked ANAES to review scientific and technologic advances in order to include major, emerging topics and needs for updates in their annual work program. A method to identify new technologies that could have a major impact on public health is currently in preparation. Links with bodies carrying out clinical and technologic research are essential at the “horizon-scanning” stage. Links with clinical research are also required at the feedback stage when HTA reports have found that crucial clinical trials that would guarantee reasonable levels of safety and quality of care are missing and need to be performed. At present, such clinical research is sometimes carried out or prompted by experts taking part in the ANAES working groups. (iii) Links with patients and patient groups need to be reinforced, because the ultimate customer of HTA is the patient. At ANAES, patients' representatives chosen by the Ministry of Health sit on the administrative board and scientific council and take part in some working groups. However, no single individual can enlighten any board or working group on the complexities of patients' rights, information needs, preferences, and quality of life. We need to develop ad hoc scientific methods to obtain overviews of patients' expectations and preferences and also to provide health-care professionals with information (e.g., guidelines) of such clarity that their substance can be easily relayed to patients. Patient education is a vital step toward improving public health. Patients have access to an increasing amount of information of variable quality and, in view of the strong pressure exerted by patients on policy makers by means of the media (i.e., popular press, television, etc.), it is crucial that up to date information be provided to patients, that science and expert opinion be distinguished from hype, and that patients' opinions be consulted. There are currently much stronger links between patient groups and public research bodies (for instance in the funding of genetic research on orphan diseases) than between patients groups and agencies doing HTA. (iv) Links might also be desirable with those bodies that are empowered by policy makers to implement the recommendations of an HTA report. This means tighter informal links not only with bodies performing clinical research, but also with national, regional, or local bodies involved in the implementation of recommendations on conditions of technology use and training requirements. The ARH (Angences Régionales d'Hospitalisation), responsible for reorganizing the regional hospitals network (public and private), have recently been empowered to approve certain heavy equipment purchases (e.g., CT-scanners, MRI equipment) and will require HTA reports.
CONCLUSIONS
An assessment of how to make the best use of high-tech innovations, whether established, new, or emerging, is part of a more general concern to offer customers—that is patients, health professionals, manufacturers, and policy makers—the best information and advice. The challenge for policy makers is to find the right balance between providing access to innovative medical technologies and, at the same time, guaranteeing quality of care, without at any point forgetting cost-effectiveness.
There are several points to be addressed. It is clear that clinical evaluation often lags behind technologic progress (2). To reduce the gap, continuous evaluation is necessary but is also difficult to achieve. In our view, a better option is the early evaluation of new technologies to identify the lines along which they are likely to develop. Moreover, mere technical expertise is not enough. A broader view is necessary. HTA cannot be separated from the settings in which the technology is or will be used (where it is used, by whom, and for which conditions). Institutions performing HTA have to have access to a wide variety of viewpoints (developers, manufacturers, users, and the public). The variety of HTA activities and multiplicity of customers also necessitate strong collaboration within and between agencies and different bodies dealing with HTA. Furthermore, providing health professionals with validated methods and tools to evaluate and improve quality is likely to enhance their acceptance of HTA and the conclusions that are derived. Finally, in HTA, technical expertise and consensus among health professionals go hand in hand; one cannot replace the other.
We thank David Banta (Netherlands Organization for Applied Scientific Research and Swedish Council on Technology Assessment in Health Care), Elisabeth Fery Lemonnier (CEDIT) and Jean-Claude Ghislain (AFSSAPS) for their apt comments on the first version of this paper. We also thank our colleagues at ANAES: Patrice Dosquet (Head of the Guidelines Department), James Goldberg (Head of International Relations), Marie-José Moquet (Head of the Nomenclature Department), Tiiu Ojasoo (Medical writer) and Catherine Rumeau-Pichon (Head of the Economic Evaluation Department) for their contributions and comments.
References

Main Agencies Reporting to the Health Directorate (DGS)

Distinctions Between Different Types of Assessment: Consensus Conferences, Health Technology Assessment (HTA) Reports, and Clinical Practice Guidelines

Main State-Funded Health Technology Assessment (HTA) Activities: Who Assesses What for Whom in France

Defining Priorities and the Annual Health Technology Assessment Program at Agence Nationale d'Accréditation et d'Evaluation en Santé (ANAES)

Technology Assessment Reports at Agence Nationale d'Accréditation et d'Evaluation en Santé (ANAES)

Procedure for Preparing a Standard Health Technology Assessment Report at Agence Nationale d'Accréditation et d'Evaluation en Santé (ANAES)
- 13
- Cited by


