Introduction
Human alveolar echinococcosis (AE) is a severe zoonotic disease caused by the larval stage of the tapeworm Echinococcus multilocularis, which is endemic in areas of Europe, Asia and North America, and is currently the highest ranked foodborne parasite in Europe (Deplazes et al., Reference Deplazes, Rinaldi, Alvarez, Torgerson, Harandi, Romig, Antolova, Schurer, Lahmar, Cringoli, Magambo, Thompson and Jenkins2017; Bouwknegt et al., Reference Bouwknegt, Devleesschauwer, Graham, Robertson and van der Giessen2018). This disease poses an uncontrolled health problem especially in developing and resource-poor regions (Kern et al., Reference Kern, Menezes da Silva, Akhan, Müllhaupt, Vizcaychipi, Budke and Vuitton2017), being its mortality rate more than 90% within 10–15 years of diagnosis in untreated or inadequately treated patients (Ammann and Eckert, Reference Ammann and Eckert1996). The E. multilocularis life cycle involves canids as definitive hosts, and a variety of mammals as intermediate hosts (mostly voles, but accidentally humans, dogs, captive monkeys and beavers). Intermediate hosts get orally infected through parasite eggs that reach the environment by definitive host feces. Each egg contains an oncosphere which develops into the multivesicular larval stage called metacestode (Romig et al., Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi and de la Rue2017). In humans, metacestodes primarily affect the liver, but they can also form metastases in other organs (Kern et al., Reference Kern, Menezes da Silva, Akhan, Müllhaupt, Vizcaychipi, Budke and Vuitton2017). Morphologically, metacestodes are delimited by an outer protective acellular and carbohydrate-rich laminated layer and an inner cellular germinal layer, which may give rise to brood capsules by asexual budding. The germinal layer is composed of muscle cells, nerve cells, glycogen storage cells, connective tissue and undifferentiated stem cells (Koziol et al., Reference Koziol, Rauschendorfer, Rodríguez, Krohne and Brehm2014).
The preferred therapeutic option for AE is the surgical resection of the parasite mass accompanied by monotherapy with benzimidazole carbamate derivatives (BMZ), such as albendazole (ABZ) and mebendazole, for a minimum of 2 years to reduce the relapse. For inoperable patients, chemotherapy is the only choice and might be prescribed indefinitely to slow the progression of the disease (WHO, 1996; Brunetti et al., Reference Brunetti, Kern and Vuitton2010; Hemphill et al., Reference Hemphill, Stadelmann, Rufener, Spiliotis, Boubaker, Müller, Müller, Gorgas and Gottstein2014). Treatment with these drugs has been proven to inhibit parasite proliferation but is rarely curative, resulting in an elevated risk of adverse effects and low adherence to chronic treatment (Kern et al., Reference Kern, Menezes da Silva, Akhan, Müllhaupt, Vizcaychipi, Budke and Vuitton2017). Considering the shortcomings of current treatments, a large number of compounds have been tested against E. multilocularis in rodent models and in vitro assays (Lundström-Stadelmann et al., Reference Lundström-Stadelmann, Rufener, Ritler, Zurbriggen and Hemphill2019). However, to date, no new drugs have been approved for the treatment of this disease since only the BMZs were shown to be active also against human AE. Therefore, a multi-target treatment approach that reduces the doses and adverse effects related to BMZs could be a reasonable strategy for sustained treatment of AE.
Since E. multilocularis consumes high levels of glucose (Ritler et al., Reference Ritler, Rufener, Li, Kämpfer, Müller, Bühr, Schürch and Lundström-Stadelmann2019), agents that affect its energy metabolism by interfering with ATP production, proliferation and cell survival strategies might be useful in treating AE and provide new opportunities for their repurposing. Metformin (Met) is a small molecule of herbal origin which acts as a metabolic modulator with low toxicity and is widely used to treat type II diabetes, non-alcoholic fatty liver disease, premature puberty, polycystic ovarian syndrome and as a successful repurposed drug for cancer therapy given that it also exerts anti-proliferative effects (Quinn et al., Reference Quinn, Kitagawa, Memmott, Gills and Dennis2013; Lv and Guo, Reference Lv and Guo2020). Its general molecular mechanisms of cell growth inhibition involve suppression of mitochondrial complex I, activation of the adenosine monophosphate-activated protein kinase (AMPK) signalling pathway and/or inhibition of the insulin signalling pathway (Morales and Morris, Reference Morales and Morris2015). The efficacy of Met against the larval stage of E. multilocularis has been previously investigated and this drug was found to have an anti-parasitic effect on an early-infection model of AE by targeting the target of rapamycin (TOR) pathway (Loos et al., Reference Loos, Dávila, Brehm and Cumino2020). Therefore, in the current study, the efficacy of Met was assessed in the presence of a sub-optimal dose of ABZ, increasing the parasitic challenge in the experimental murine model of AE.
Materials and methods
Ethics statement
Female CF-1 mice (8 weeks of age) were supplied by the National Health Service and Food Quality (SENASA), Mar del Plata, and housed in specific pathogen-free (SPF) facilities at the bioterium of the National University of Mar del Plata (UNMdP). Animal experiments were performed in strict accordance with the 2011 revised form of The Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Experimental protocols for using mice were evaluated and approved by the Animal Experimental Committee at the Faculty of Exact and Natural Sciences, UNMdP (permit number: RD 493-2017).
Parasite maintenance and isolation
Echinococcus multilocularis (isolates 8065 and J2012) was maintained by serial intraperitoneal passage in CF-1 mice as described by Spiliotis and Brehm (Reference Spiliotis and Brehm2009). Homogenized metacestode material and protoscoleces were isolated from in vivo-cultivated parasite material according to previously established protocols (Spiliotis and Brehm, Reference Spiliotis and Brehm2009).
Chemicals
Met (1,1-dimethylbiguanide hydrochloride) was obtained from Sigma-Aldrich (USA) and ABZ and albendazole sulfoxide (ABZSO) were kindly provided by C. Salomon (National University of Rosario, Argentina). For in vitro assays, Met and ABZSO were kept as a 100 mm and a 100 μ m stock solution in water and in dimethyl sulfoxide, respectively, and added to the medium either separately or in combination. For in vivo experiments, an aqueous solution of Met and an oil solution of ABZ (corn oil, Sigma-Aldrich) were prepared every 2 days from solid drug and maintained under refrigeration (3–5°C).
Drug treatment and viability assays
Protoscoleces were incubated in medium 199 (Gibco, USA) supplemented with antibiotics (penicillin, streptomycin and gentamicin; 100 μg mL−1) at physiological (5 mm) and supra-physiological (25 mm) concentrations of glucose. In vitro treatments were performed with 1 and 10 mm Met, 3 μ m ABZSO and the combination of 1 mm Met plus 3 μ m ABZSO for 8 days. Viability was subsequently assessed using a methylene blue staining assay (Loos and Cumino, Reference Loos and Cumino2015). In all cultures, medium was changed every third day, including fresh addition of Met and/or ABZSO. All experiments were carried out at least three times independently.
Additionally, to investigate the potential parasiticidal activity of Met, the viability of in vitro-treated metacestode suspension was assessed by bioassay in mice as was previously reported by Spicher et al. (Reference Spicher, Roethlisberger, Lany, Stadelmann, Keiser, Ortega-Mora, Gottstein and Hemphill2008). Metacestode suspension (obtained by passing parasite mass isolated from mice through a sterile metal sieve) was distributed into Leighton tubes (100 μl per tube) containing 10 ml culture medium (M199 supplemented with antibiotics and 25 mm glucose). Metacestode suspensions were cultured under control condition or with 10 mm Met for 21 days. Medium was changed with the addition of fresh drug every 7 days. On day 21, the metacestode suspension was concentrated by short centrifugation and mice (10 animals per group) were infected by intraperitoneal injection of 200 μl of inoculum. At 14 weeks post-inoculation, mice were euthanized and the parasite tissue weight was determined.
Experimental animals and determination of the efficacy of in vivo treatment
Healthy CF-1 mice (30 ± 35 g) were acclimatized for 1 week before initiation of the experiment. Mice were infected by intraperitoneal infection with 200 and 400 μL of homogenized metacestode material (8065 strain) to produce experimental secondary AE (Loos et al., Reference Loos, Dávila, Brehm and Cumino2020). Every 3–4 days per week, animals were placed into a clean cage with fresh wood shavings. All pharmacological treatments were performed by intragastric administration of a drug suspension (0.1–0.25 mL per animal). At the end of experiments, mice were weighted, anaesthetized with ketamine-xylazine (50–5 mg kg−1 per mouse) and euthanized by cervical dislocation. At necropsy, the peritoneal cavity was opened, and the parasite tissues were carefully recovered and weighted. The efficacy of treatment was calculated using the following formula: 100 × {(mean parasite weight of control group)–(mean parasite weight of treated group)}/(mean parasite weight of control group). In addition, samples were processed for scanning electron microscopy (SEM) with a JEOL JSM-6460LV electron microscope as previously described (Loos et al., Reference Loos, Caparros, Nicolao, Denegri and Cumino2014).
Therapeutic effectiveness of metformin and its combination with albendazole
In order to evaluate the effect of Met on the murine experimental model of AE, two different experimental designs were performed:
Using a 400 μL parasite inoculum and starting the treatment at the time point of infection. Forty infected mice were randomly assigned into 4 groups of 10 animals each. Drugs were daily administered per oral gavage for 60 days as follows: control group (receiving corn oil as a placebo), ABZ at 5 mg kg−1 day−1, Met at 100 mg kg−1 day−1 and a combination of ABZ (5 mg kg−1 day−1) plus Met (100 mg kg−1 day−1). At the end of the treatment period, animals were euthanized and necropsied.
Using a standard parasite inoculum of 200 μL and starting the treatment 6 weeks after infection. At 6 weeks post-infection, 40 mice were allocated into 4 groups of 10 animals each. Drugs were daily administered per oral gavage for 60 days as follows: control group (receiving corn oil as a placebo), ABZ at 5 mg kg−1 day−1, Met at 100 mg kg−1 day−1 and a combination of ABZ (5 mg kg−1 day−1) plus Met (100 mg kg−1 day−1). At the end of treatment period, animals were euthanized, necropsy was carried out immediately thereafter, and intracystic glucose concentration was determined from parasite fluid as described below.
Serum and intracystic glucose determination
Serum glucose levels were measured using tail vein blood after puncturing the tail with a 25-gauge needle to produce one drop of blood for each test. Glucose was measured using a Bayer Contour Glucometer and glucose test strips.
To determine the intracystic glucose concentration, the fluid was aseptically removed from the metacestode masses. For that, after extracting and weighing the parasite tissue from each mouse, it was rapidly rinsed with sterile 1 × PBS, and placed into a dry and sterile Petri dish. The fluid from the larger vesicles (with a diameter ≥5 mm in which the presence of fluid is evident) was extracted using a 1 mL syringe and a hypodermic needle (0.5 × 16 mm 25 G 5/8), and collected in sterile Eppendorf tubes previously cooled in ice. Glucose concentration was immediately determined by the glucose oxidase method on a glucose analyser (Beckman Instruments, Inc., Fullerton, CA, USA).
Statistics
Data within experiments were compared using the Student's t-test or the analysis of variance, and differences among groups were considered as statistically significant with P values below 0.05. Statistical analyses were performed using R software (https://www.R-project.org).
Results
Coadministration of metformin and a low dose of albendazole reduces the E. multilocularis development in mice infected with a high parasite inoculum
It has been previously reported that after intraperitoneal infection of mice with 200 μl of metacestode material (8065 strain), the oral administration of Met (50 mg kg−1 day−1) for 8 weeks from the day of infection achieved a significant reduction of parasite weight (Loos et al., Reference Loos, Dávila, Brehm and Cumino2020). Here, the study was extended to evaluate the in vivo efficacy of Met using different schemes of infection and treatment, either by increasing the parasitic inoculum or starting the treatment later, but doubling the dose of Met (100 mg kg−1 day−1) in both cases. In the first model, mice were intraperitoneally infected with 400 μL of metacestode tissue and treated from the day of infection by oral administration of vehicle, Met (100 mg kg−1 day−1), ABZ (5 mg kg−1 day−1) or the combination of Met (100 mg kg−1 day−1) plus ABZ (5 mg kg−1 day−1) over a period of 8 weeks. All mice survived until the end of the experiment and showed no signs of stress. As shown in Fig. 1A, only the combined treatment led to a significant reduction in parasite weight (2.1 ± 1.9 g) compared to the untreated group (6.3 ± 2.4 g) or those treated with either a low dose of ABZ (4.7 ± 2.6 g) or Met (5.2 ± 2.2 g) (P < 0.05). Additionally, SEM analysis revealed that, although germinal layers of metacestodes obtained from all treated mice exhibited some kind of damage, this was more pronounced in metacestodes obtained from mice treated with the drug combination (Fig. 1B). Germinal layers of metacestodes collected from mice receiving monotherapy or the combined treatment displayed partial and overall loss of cells, respectively (Fig. 1Bb–d). In contrast, metacestodes from control mice exhibited an intact germinal layer composed of a multitude of different, morphologically intact, cell types (Fig. 1Ba).

Fig. 1. In vivo efficacy of metformin and its combination with a suboptimal dose of albendazole in mice infected with a high parasite inoculum. (A) Box plot showing the comparative distribution of the weight (in grams) of metacestodes recovered from untreated mice (Ct) and treated with albendazole (ABZ, 5 mg kg−1 day−1), metformin (Met, 100 mg kg−1 day−1) and the combination of both drugs (ABZ + Met) for 60 days from the day of infection. A significant metacestode weight reduction (*P < 0.05) was achieved with Met in combination with ABZ. (B) Representative SEM images of metacestodes recovered from untreated mice (a) or treated with Met (b), ABZ (c) and ABZ + Met (d). Bars indicate 20 μm (a, b) and 50 μm (c, d).
Combined treatment of metformin with a low dose of albendazole shows therapeutic effectiveness against fully developed metacestodes
In the second model, mice were infected with a standard parasite inoculum of 200 μL, and treatments were started 6 weeks after infection. The combination of Met (100 mg kg−1 day−1) together with a suboptimal dose of ABZ (5 mg kg−1 day−1) was, again, highly effective in reducing the parasite weight (4.3 ± 1.4 g) compared with the control (11 ± 3.2 g) (Fig. 2A, P < 0.01). Parasite masses developed in mice treated with each drug alone (8.2 ± 2.9 g and 7.4 ± 2.8 g for ABZ and Met treatments, respectively) showed no significant weight reduction in comparison with those recovered from untreated mice. Likewise, although all treatments induced morphological and ultrastructural alterations in the metacestodes, the combination of Met and ABZ showed the highest level of damage (Fig. 2B).

Fig. 2. In vivo efficacy of metformin and its combination with a suboptimal dose of albendazole in mice with an established AE infection. (A) Box plot showing the comparative distribution of the weight (in grams) of metacestodes recovered from untreated mice (C t) and treated with albendazole (ABZ, 5 mg kg−1 day−1), metformin (Met, 100 mg kg−1 day−1) and the combination of both drugs (ABZ + Met) for 60 days from 6 weeks after infection. A significant metacestode weight reduction (*P < 0.05) was achieved with Met in combination with ABZ. (B) Representative SEM images of metacestodes recovered from untreated mice (a) or treated with Met (b), ABZ (c) and ABZ + Met (d). Bars indicate 20 μm (a, b, c) and 10 μm (d). (C) Intracystic glucose concentration from metacestodes recovered from untreated (C t) and drug-treated mice from the experiment indicated in (A).
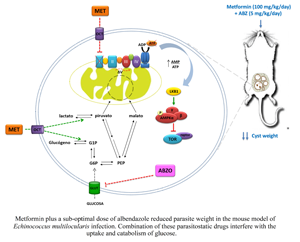
Since Met has been shown to modify the carbohydrate metabolism in Echinococcus granulosus (Loos and Cumino, Reference Loos and Cumino2015), the effect of the drug on the availability of glucose for E. multilocularis was analysed in the murine model. For this purpose, intracystic glucose concentration was measured in metacestodes obtained from pharmacologically treated mice, using fluid from metacestodes of untreated mice as control. Intracystic glucose concentration was significantly lower both in samples from animals treated with Met alone and in those from animals receiving both drugs (Fig. 2C). All mice survived until the end of the experiment and treatments did not affect their body weights and serum glucose levels (Supplementary Fig. S1).
Metformin attenuates parasite growth but lacks parasiticidal effect
It has been previously reported that both primary stem cells and protoscoleces of E. multilocularis are sensitive to Met and that this drug is not only capable of partially arresting the in vitro de-differentiation of protoscoleces into metacestode vesicles, but also reduces the parasite mass in a murine secondary AE model (Loos et al., Reference Loos, Dávila, Brehm and Cumino2020). However, here it was shown that when the parasitic inoculum used to infect the mice is higher than 200 μL, or the in vivo treatments are started 6 weeks after infection, Met alone is not capable of achieving a significant reduction of parasite weight, even at higher doses (Figs 1A and 2A). Thus, to determine whether the effects of Met are parasitostatic or parasiticidal, the regrowth of in vitro-treated parasite was tested in mice. Metacestode suspensions were cultured in vitro for 21 days with or without addition of 10 mm Met. Then, this tissue was injected into mice (10 animals per condition, each infected with 200 μL of suspension), and after 14 weeks, metacestodes were recovered and weighted. The mean weight of the parasite masses obtained from mice infected with treated tissue (0.21 ± 0.3 g) was significantly lower than that from mice infected with untreated tissue (1.54 ± 0.8 g) (P < 0.05) (Fig. 3A). Additionally, the in vitro effect of Met and its combination with the minimum effective concentration of ABZSO on the viability of protoscoleces was investigated. For this purpose, protoscoleces were cultured at either high (25 mm) or physiological (5 mm) glucose concentrations for 8 days. As shown in Fig. 3B, Met exerted a dose-dependent effect on the viability of protoscoleces, and this was slightly more pronounced under physiological glucose concentrations. At 3 μ m ABZO, protoscoleces revealed no changes in viability at high glucose concentration, and 30% of protoscoleces were dead at physiological glucose concentration. Interestingly, an increased anti-echinococcal effect was found when Met was used in combination with ABZSO. In this case, 1 mm Met plus 3 μ m ABZSO increased the protoscolex mortality up to 30% at 25 mm glucose and 60% at 5 mm glucose. These assays indicated that, despite inducing considerable damage in vitro, Met was not capable of exerting a true parasiticidal effect.

Fig. 3. Viability-assessment of in vitro drug-treated metacestode tissue and protoscoleces. (A) Scatter plot showing the weight of parasite masses obtained from mice at 14 weeks after infection with 200 μL of metacestode tissue cultured with 10 mm metformin or without drug for 21 days. Each dot represents 1 animal and the horizontal bar represents the mean. A significant metacestode weight reduction (*P < 0.05) was achieved with Met. (B) Viability of protoscoleces incubated for 8 days with 1 and 10 mm of metformin alone (Met), 3 μ m albendazole sulfoxide (ABZSO) alone and 1 mm Met plus 3 μ m ABZSO in combination, either at physiological (5 mm, black bars) or supra-physiological (25 mm, grey bars) glucose concentrations. Data are the mean ± s.d. of three independent experiments. *Statistically significant difference (P < 0.05) compared with control (C t).
Discussion
Combination therapies represent a promising approach in indications of unmet medical needs. In fact, their use has become a standard strategy to enhance the efficacy of anti-viral and anti-parasitic treatments (Kobylinski et al., Reference Kobylinski, Alout, Foy, Clements, Adisakwattana, Swierczewski and Richardson2014; Zeuzem et al., Reference Zeuzem, Jacobson, Baykal, Marinho, Poordad, Bourlière, Sulkowski, Wedemeyer, Tam, Desmond, Jensen, Di Bisceglie, Varunok, Hassanein, Xiong, Pilot-Matias, DaSilva-Tillmann, Larsen, Podsadecki and Bernstein2014). While there is no doubt about the efficacy of ABZ for the treatment of many helminth diseases, as a single agent it results clearly insufficient for long-term anti-echinococcal therapy given the poor patient adherence and the incomplete response in certain cases. Here, the rationale for proposing a combination therapy in the treatment of AE was to improve the efficacy and reduce the toxicity of high-dose ABZ monotherapy using Met, an inhibitor of respiratory chain complex I as a second drug. It is noteworthy that both the in vitro and in vivo models used here were previously established (Hemphill and Gottstein, Reference Hemphill and Gottstein1995; Siles-Lucas and Hemphill, Reference Siles-Lucas and Hemphill2002).
Met is considered a valuable potential anti-parasitic drug against arthropod-borne protozoa such as Plasmodium sp., Trypanosoma sp. and Leishmania sp. (Vera et al., Reference Vera, Ruivo, Rocha, Marques, Bhatia, Mota and Mancio-Silva2019; Lima et al., Reference Lima, Ferreira, Malta, Bonyek-Silva, Santos, Tavares, de Carvalho Filho and Arruda2020) as well as against helminths, because these parasites are highly dependent on glucose metabolism for energy production (McManus and Smyth, Reference McManus and Smyth1982; Spiliotis and Brehm, Reference Spiliotis and Brehm2009; Loos and Cumino, Reference Loos and Cumino2015; Othman et al., Reference Othman, Abou Rayia, Ashour, Saied, Zineldeen and El-Ebiary2016; Loos et al., Reference Loos, Dávila, Rodrígues, Petrigh, Zoppi, Crocenzi and Cumino2017, Reference Loos, Dávila, Brehm and Cumino2020; Martínez-Flórez et al., Reference Martínez-Flórez, Galizzi, Izquierdo, Bustamante, Rodriguez and Rodriguez2020). Based on preclinical studies, a substantial anti-parasitic effect of Met on E. granulosus and the improvement of the therapy in mice with fully-developed cysts when the drug is used in combination with the lowest recommended dose of ABZ were demonstrated (Loos et al., Reference Loos, Dávila, Rodrígues, Petrigh, Zoppi, Crocenzi and Cumino2017). Likewise, the pharmacological effectiveness of Met against E. multilocularis was also confirmed, demonstrating its anti-parasitic efficacy on stem cell-containing primary cell cultures as well as in secondary murine infection employing the lowest oral dose of this drug used in mice (50 mg kg−1 day−1; Loos et al., Reference Loos, Dávila, Brehm and Cumino2020). Here, the challenge was to evaluate the in vivo efficacy of Met by increasing the parasitic inoculum and delaying the beginning of the treatments. Considering that Met acts in a dose-dependent manner in cancer cells (Chaiteerakij et al., Reference Chaiteerakij, Petersen, Bamlet, Chaffee, Zhen, Burch, Leof, Roberts and Oberg2016; Wu et al., Reference Wu, Hu, Chen, Shi, Fetahu, Wu, Rabidou, Fang, Tan, Xu, Liu, Argueta, Zhang, Mao, Yan, Chen, Dong, Lv, Xu, Wang, Ye, Zhang, Duquette, Geng, Yin, Lian, Murphy, Adler, Garg, Lynch, Yang, Li, Lan, Fan, Shi and Shi2018; Zhang et al., Reference Zhang, Zhou, Xie, Nice, Zhang, Cui and Huang2020), the dose of Met was doubled to 100 mg kg−1 day−1 to guarantee its pharmacological action against an aggressive pathogen as E. multilocularis and in a demanding experimental design as the one used here. It was shown that daily oral application of a sub-optimal ABZ dose plus less than a half the Met maximum dose used for tumour cell treatments during 2 months resulted in significantly reduced parasite weight in experimentally infected mice. Moreover, the most severe ultrastructural alterations in metacestode germinal layers were exhibited with this combined therapy, in accordance with the impaired development of the parasite in mice. Successful combination of these drugs represents a case of coalism, given that the weight of the parasite masses recovered from mice treated with either Met or ABZ was not statistically significant compared with that of the control group (Foucquier and Guedj, Reference Foucquier and Guedj2015). In addition, in vitro treatment with Met plus ABZSO exhibited an enhanced effect on protoscolex viability, in comparison with each drug alone, especially at physiological glucose concentrations (5 mm). The in vitro data also showed that the anti-echinococcal effect of Met depends on the availability of glucose in the medium, in agreement with that reported for cancer cells (Zhuang et al., Reference Zhuang, Chan, Haugrud and Miskimins2014). This is to be expected since Met inhibits the complex I of Echinococcus and cancer cells, so they must convert glucose into energy without involving mitochondria, leading then to a slow-down of cell proliferation in abundance of glucose and cell death upon its deprivation (Wheaton et al., Reference Wheaton, Weinberg, Hamanaka, Soberanes, Sullivan, Anso, Glasauer, Dufour, Mutlu, Budigner and Chandel2014; Loos and Cumino, Reference Loos and Cumino2015; Loos et al., Reference Loos, Dávila, Brehm and Cumino2020).
Depending on oxygen availability, E. multilocularis larval stage uses oxidative phosphorylation (particularly when it has access to the portal vein or hepatic vein), which involves the mitochondrial complexes I to V, or fumarate respiration (when it grows within organs), which depends on complexes I and II, for its survival in the host (Matsumoto et al., Reference Matsumoto, Sakamoto, Shinjyo, Kido, Yamamoto, Yagi, Miyoshi, Nonaka, Katakura, Kita and Oku2008; Enkai et al., Reference Enkai, Kouguchi, Inaoka, Irie, Yagi and Kita2021). In both cases, complex I is involved in the generation of a proton gradient that drives ATP synthesis. Here, a decrease in intracystic glucose concentration was shown in the metacestode fluids of mice treated with Met alone or combined with ABZ. Conversely, serum glucose level, which is carefully regulated by hormones, remained constant between the mice of different groups. This suggests a pharmacological interference in parasite glucose uptake and/or an increase in the inefficient consumption of glucose by glycolysis, the only feasible pathway to produce ATP under anaerobic conditions after inhibition of fumarate respiration. In this line of evidence, it has been previously demonstrated that Met-induced inhibition of complex I is accompanied by an increase in glycolysis to compensate for reduced ATP production (Loos and Cumino, Reference Loos and Cumino2015).
Mechanistically, Met promotes anti-tumour effects by affecting molecular targets involved in the reprogramming of energy metabolism such as AMPK, phosphatidylinositol 3-kinase (PI3K)-mTOR pathway and oxidative phosphorylation, contributing to induce cytostatic or cytotoxic effects depending on the cancer type (Wu et al., Reference Wu, Hu, Chen, Shi, Fetahu, Wu, Rabidou, Fang, Tan, Xu, Liu, Argueta, Zhang, Mao, Yan, Chen, Dong, Lv, Xu, Wang, Ye, Zhang, Duquette, Geng, Yin, Lian, Murphy, Adler, Garg, Lynch, Yang, Li, Lan, Fan, Shi and Shi2018; Zhang et al., Reference Zhang, Zhou, Xie, Nice, Zhang, Cui and Huang2020). In this way, bioassay results lead to the conclusion that Met induces a cytostatic effect on E. multilocularis development, in the same manner as those compounds that provoke loss of proliferative capacity by interfering with mitochondrial function and regulation of TOR signalling (Short et al., Reference Short, Fielder, Miwa and von Zglinicki2019; Loos et al., Reference Loos, Dávila, Brehm and Cumino2020). Since Met is a reversible non-competitive inhibitor of complex I, the degree of the inhibition induced by this drug is dependent on its concentration (Bridges et al., Reference Bridges, Jones, Pollak and Hirst2014; Vial et al., Reference Vial, Detaille and Guigas2019). In fact, studies on the effect of Met on hepatocarcinoma cells showed that low doses of the drug promoted cell senescence, while higher doses provoked their apoptosis (Yi et al., Reference Yi, He, Zhou, Xian, Yuan, Jia, Hong, He and Liu2013; Short et al., Reference Short, Fielder, Miwa and von Zglinicki2019). Henceforth, the interest will be focused on in vivo studies using higher Met concentrations (up to a maximum dose of 250 mg kg−1 day−1 in mice) to assess whether a better anthelmintic efficacy could be achieved.
Regarding ABZ, its active metabolite binds to β-tubulin, inhibits microtubule polymerization and reduces glucose uptake, with the consequent decrease in glycogen content (Lacey, Reference Lacey1990; Cumino et al., Reference Cumino, Elissondo and Denegri2009). This indicates that Met is more effective against AE if used alongside other treatments that reduce the availability of glucose inside cells, as has previously been reported for cancer treatment (Wheaton et al., Reference Wheaton, Weinberg, Hamanaka, Soberanes, Sullivan, Anso, Glasauer, Dufour, Mutlu, Budigner and Chandel2014). Finally, the recommended ABZ dosage for humans (10–15 mg kg−1 day−1, in two doses of 400 mg) is considered an appropriate initial and maintenance dose and is equivalent to that commonly used in murine models of AE (200 mg kg−1 day−1) (Rufener et al., Reference Rufener, Dick, D'Ascoli, Ritler, Hizem, Wells, Hemphill and Lundström-Stadelmann2018). Here, a combination of Met, a low-toxicity drug for normal mammalian cells which interferes with the main catabolic pathways of the parasite, together with a suboptimal dose of ABZ, 40 times lower than the one commonly used and equivalent to 0.45 mg kg−1 day−1 for humans, was successfully employed for the first time in the experimental AE model. These findings highlight the importance of carrying out further studies to determine the significance of the use of this drug combination in relation to the treatment of AE, with even the potentiality of testing higher and still safe drug doses.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021001633
Acknowledgements
We gratefully acknowledge Dr Patricia Gentili, Dr Santiago Fares Taie and Dr Agustina Fares Taie for their assistance in the biochemical determinations in metacestode fluids. We also thank Med. Vet. Alejandra Goya (SENASA, Mar del Plata, Argentina), Lic. Carolina Kelly (SENASA, Mar del Plata, Argentina) and Lic. Hugo Núnez García (CONICET, Universidad Nacional de Mar del Plata, Argentina) for their collaboration in the welfare assessment of mice.
Financial support
This work was supported by CONICET (PIP 2016 No.11220150100406), ANPCyT (PICT 2017 No. 0950); and Universidad Nacional de Mar del Plata (Grant EXA 963/20 and EXA964/20), Argentina.
Conflict of interest
None.
Ethical standards
Animal experiments were performed in strict accordance with the 2011 revised form of The Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Experimental protocols for using mice were evaluated and approved by the Animal Experimental Committee at the Faculty of Exact and Natural Sciences, UNMdP (permit number: RD 493-2017).