1. Introduction
Hadrosaurids are among the most diverse and widespread megaherbivore dinosaurs of the Late Cretaceous (Prieto-Márquez, Reference Prieto-Márquez2010). Between 1939 and 1940, crews from the California Institute of Technology (Caltech) collected two partial hadrosaurid skeletons (LACM/CIT 2760 and 2852, Fig. 1) from the upper Maastrichtian Moreno Formation of the Panoche Hills of Fresno County (central California, USA). Morris (Reference Morris1973) referred these skeletons to cf. Saurolophus sp. Subsequently, Bell & Evans (Reference Bell and Evans2010) redescribed the skull of LACM/CIT 2852 and found no unequivocal evidence for assigning this specimen to Saurolophus. They referred this individual to ‘Hadrosaurinae’ (Saurolophinae) indeterminate. More recently, Prieto-Márquez & Wagner (Reference Prieto-Márquez and Wagner2013a ) described the postcranium of LACM/CIT 2852, as well as the skull and postcranium of LACM/CIT 2760, presenting the results of a phylogenetic analysis that integrated the character data available for both specimens. Their comparative osteological observations and analytical results supported the original, tentative referral of the specimens to Saurolophus by Morris (Reference Morris1973), and led to the recognition of a new species, S. morrisi, for the Moreno Formation specimens.

Figure 1. Skeleton of a referred specimen of Augustynolophus morrisi, LACM/CIT 2852. (a) The specimen partially excavated, cropping out in upper Maastrichtian strata of the Moreno Formation in the Tumey Hills of Fresno County, California, western USA. (b) Mounted skull upon preparation of the specimen.
Additional preparation of LACM/CIT 2760 allows for new osteological observations that correct some inaccuracies of a previous contribution on the anatomy and systematics of the Moreno Formation saurolophine (Prieto-Márquez & Wagner, Reference Prieto-Márquez and Wagner2013a ). We identify the nasal bone of LACM/CIT 2760, demonstrate that it formed part of the cranial crest of the animal and show that the skull morphology of this specimen and LACM/CIT 2852, although similar to that of Saurolophus, does not support the assignation of these specimens to Saurolophus. On the contrary, the cranial morphology of these exemplars is sufficiently distinct from that of Saurolophus to justify the erection of a new genus. The recognition of a supraspecific taxon in the latest Cretaceous of California is important because of the limited knowledge of the dinosaurian faunas of the west coast of North America. This study has important implications for understanding the diversity of the North American dinosaur assemblages of the latest Cretaceous.
2. Institutional abbreviations
The repository institutions for the specimens discussed in the text are indicated by the following acronyms: AMNH – American Museum of Natural History, New York, New York, USA; CIT – California Institute of Technology, Pasadena, California, USA; LACM – Natural History Museum of Los Angeles County, Los Angeles, California, USA; PIN – Paleontological Institute, Moscow, Russia; ROM – Royal Ontario Museum, Toronto, Ontario, Canada.
3. Systematic palaeontology
DINOSAURIA Owen, Reference Owen1842
ORNITHISCHIA Seeley, Reference Seeley1887
ORNITHOPODA Marsh, Reference Marsh1881
IGUANODONTIA Dollo, Reference Dollo1888
HADROSAURIDAE Cope, Reference Cope1870
SAUROLOPHINAE Brown, Reference Brown1914 (sensu Prieto-Márquez, Reference Prieto-Márquez2010)
SAUROLOPHINI taxon nov.
Definition. Saurolophine hadrosaurids more closely related to Saurolophus osborni Brown, Reference Brown1912 than to Kritosaurus navajovius Brown, Reference Brown1910, Edmontosaurus regalis Lambe, Reference Lambe1917, Brachylophosaurus canadensis Sternberg, Reference Sternberg1953, or Lambeosaurus lambei Parks, Reference Parks1923.
Diagnosis. Saurolophine hadrosaurids possessing premaxilla with broad arcuate contour of rostrolateral region of thin everted oral margin (convergent in Gryposaurus latidens); medial and lateral processes of premaxilla slightly converging caudally; narrow, slit-like apertura ossis nasi; prefrontal included in circumnarial fossa; quadrate with widely arcuate, asymmetrical quadratojugal notch; narrow dorsal margin of infratemporal fenestra (in large adults); dentary ramus with prominent ventral convexity rostral to coronoid process (convergent in Edmontosaurus); and dentary with flat, steeply inclined occlusal surface of dental battery.
Type genus. Saurolophus Brown, Reference Brown1912.
Content. Saurolophus Brown, Reference Brown1912; Prosaurolophus Brown, Reference Brown1916; Augustynolophus gen. nov.
Comments. This taxon name is established to characterize and differentiate one of the few hadrosaurid subclades consistently recovered in the majority of phylogenetic analyses of Saurolophinae published over the last two decades (e.g. Weishampel & Horner, Reference Weishampel, Horner, Weishampel, Dodson and Osmólska1990; Weishampel, Norman & Grigorescu, Reference Weishampel, Norman and Grigorescu1993; Kirkland, Reference Kirkland1998; Hu et al. Reference Hu, Zhengwu, Qiqing and Xiafosi2001; Prieto-Márquez, Reference Prieto-Márquez2005, Reference Prieto-Márquez2010, Reference Prieto-Márquez2012, Reference Prieto-Márquez2014; Gates & Sampson, Reference Gates and Sampson2007; Godefroit et al. Reference Godefroit, Hai, Yu and Lauters2008; Godefroit, Bolotsky & Lauters, Reference Godefroit, Bolotsky and Lauters2012; McGarrity, Campione & Evans, Reference McGarrity, Campione and Evans2013; Prieto-Márquez & Wagner, Reference Prieto-Márquez and Wagner2013a ), that is, the Prosaurolophus-Saurolophus sister relationship. We have applied a branch-based definition in order to provide room for the future discovery of close relatives outside of that exclusive clade. This taxon name was first used informally by Brett-Surman (unpub. Ph.D. thesis, George Washington University, 1989) and complements Brachylophosaurini (Gates et al. Reference Gates, Horner, Hanna and Nelson2011) and Kritosaurini (Prieto-Márquez, Reference Prieto-Márquez2014). At the rank of tribe, this taxon must be named Saurolophini following article 37.1 of the International Code of Zoological Nomenclature (International Commission of Zoological Nomenclature, 1999), regardless of the unfortunate potential for phonetic confusion with Saurolophinae.
Genus Augustynolophus gen. nov.
Figures 1–7
Type species. Augustynolophus morrisi Prieto-Márquez & Wagner, Reference Prieto-Márquez and Wagner2013a .
Etymology. In recognition of Mrs Gretchen Augustyn and her family, who have provided instrumental support to the scientific and educational programs of the Dinosaur Institute of the Natural History Museum of Los Angeles County. The suffix ‘-lophus’ refers to the phylogenetic affinities of this taxon with members of the Saurolophini tribe.
Diagnosis. As per the only known species.
Augustynolophus morrisi (Prieto-Márquez & Wagner, Reference Prieto-Márquez and Wagner2013a )
Figures 1–7
Synonymy. cf. Saurolophus sp. Morris, Reference Morris1973 (p. 555, fig. 2); Hadrosaurinae gen. et sp. indet. Bell & Evans, Reference Bell and Evans2010 (p. 1419–1424, figs 3–9); Saurolophus morrisi Prieto-Márquez & Wagner, Reference Prieto-Márquez and Wagner2013a (p. 259–263, figs 1–6, 9B).
Holotype. LACM/CIT 2852, including fragmentary premaxillae, both maxillae, right postorbital, right jugal, right quadratojugal, partial right quadrate, distal fragment of caudoventral process of right squamosal, distal fragment of paroccipital process of right exoccipital, predentary, right and partial left dentary, partial surangular, angular and splenial, various cervical, dorsal and caudal vertebrae, right scapula, humerus, both ulnae, radius, femora, tibiae and various manual and pedal elements representing a single individual.
Referred material. LACM/CIT 2760, consisting of skull roof (including distal nasals, frontals, parietal, partial squamosals, partial postorbitals, prootics, supraoccipital and fragmentary exoccipitals), both maxillae, right quadrate, left and posterior half of right dentary, partial surangular and angular, various isolated dentary teeth, right coracoid, partial left scapula, left humerus, distal end of right humerus, proximal regions of both ulnae and radii, fragments of both femora, proximal left and right tibiae, proximal right fibula, left metatarsal III and various fragmentary manual and pedal elements.
Occurrence. The holotype and referred specimen of Augustynolophus morrisi were collected from upper (not lower, as incorrectly reported by Prieto-Márquez & Wagner, Reference Prieto-Márquez and Wagner2013a ) Maastrichtian strata of the Moreno Formation (D. J. McGuire, unpub. Ph.D. thesis, Standford University, 1988) cropping out in western Fresno County, central California, near the Pacific coast of the United States. Specifically, LACM/CIT 2852 came from LACM locality CIT 357 (no coordinates available) of the Tumey Hills, whereas LACM/CIT 2760 was collected in LACM locality CIT 336 (36°40′21″N, 120°42′42″E) of the Panoche Hills.
Diagnosis. Saurolophine hadrosaurid dinosaur characterized by the following autapomorphies: base of frontal caudodorsal process crescentic, both left and right processes forming a U-shape in dorsal view; base of frontal caudodorsal process extending caudolaterally caudal to rostral end of frontal ‘dome’ (at least in juveniles); solid nasal crest projecting caudodorsally above skull roof with elongate triangular distal region ending in knob-like process that is gently but abruptly inflected rostrally; circumnarial depression lightly incised and weakly emarginated, adjacent to caudolateral margin of nasal and occupying two-thirds of width of lateral surface of distal region of crest; caudal surface of distal nasal crest subrectangular.
Comments. LACM/CIT 2760 might represent an immature specimen of Augustynolophus morrisi, as suggested by the relatively small size of the specimen within the context of Saurolophini and the shorter rostral region of the dentary compared to that of LACM/CIT 2852. The relatively longer dentary edentulous margin in the larger specimen is congruent with the general trend in hadrosaurid ontogeny toward elongation of the dentary (Prieto-Márquez, Reference Prieto-Márquez2010). Putative adult specimens of all known saurolophin hadrosaurids reach body lengths in excess of 8m, with 1m long skulls (Brown, Reference Brown1913; Parks, Reference Parks1924; Rozhdestvensky, Reference Rozhdestvensky1957; Bell, Reference Bell2011). The skull roof of LACM/CIT 2760 is of similar size (175mm of interorbital width by 190 mm in length from the caudal corner of the squamosal to the rostral apex of the postorbital) to that of a juvenile specimen of S. angustirostris (PIN 551/359; its equivalent dimensions being 176 mm in width by 194 mm in length), that has a skull length nearly 40% of that of the larger individuals of this species (Bell, Reference Bell2011). Although it cannot be assumed with certainty that A. morrisi followed the same growth trajectory as other saurolophins, the skull of LACM/CIT 2760 is slightly less than half of that of LACM/CIT 2852 as determined by dentary lengths (350 mm v. 710 mm in length, respectively). This supports consideration of LACM/CIT 2760 as a juvenile specimen of A. morrisi, following Evans’ (Reference Evans2010) age classification in which a skull length of less than 50% of the maximum observed skull length corresponds to the juvenile stage.
4. Osteological remarks on diagnostic cranial elements
4.a. The postorbital of Augustynolophus morrisi
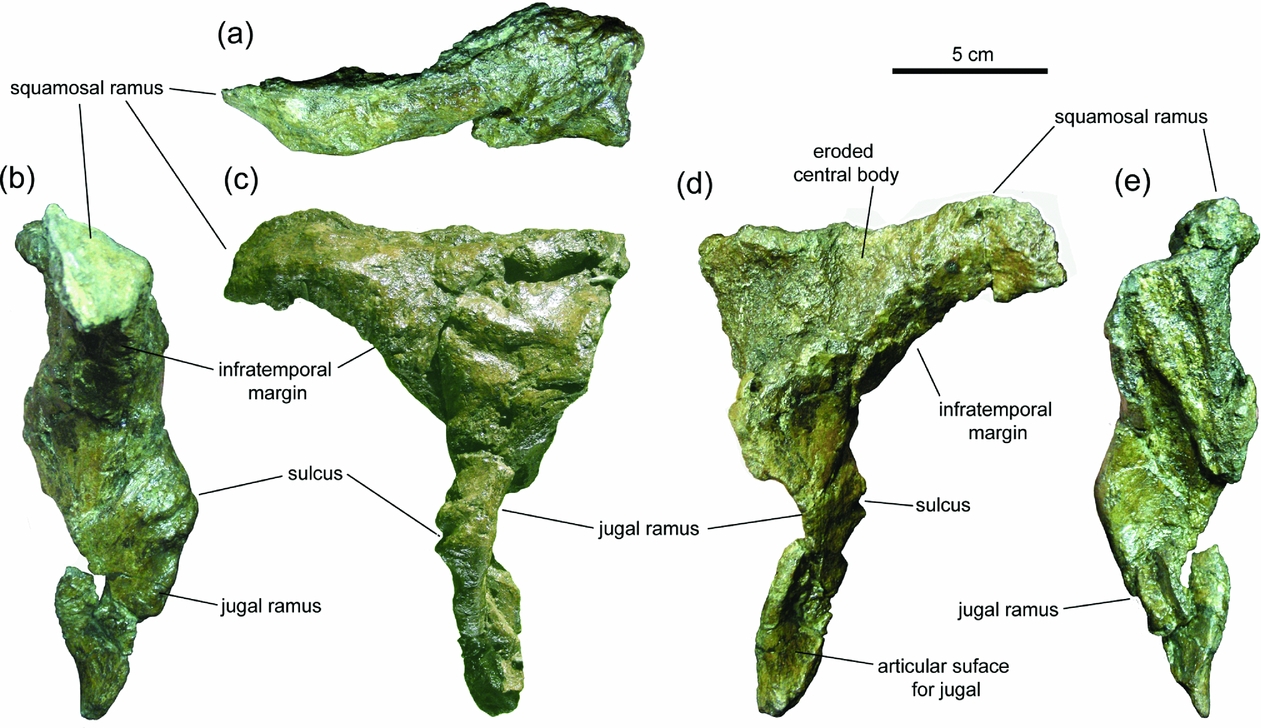
The postorbital of LACM/CIT 2852 apparently departs morphologically from that typically present in hadrosaurids, and this might cast doubt on our identification of the element. Specifically, the bone exhibits an undulating ventral ramus and lacks the overall triradiate shape characteristic of the hadrosaurid postorbital, the medial laterosphenoid cotylus present in archosaurs ancestrally and a V-shaped facet on the caudal process for the reception of the squamosal medially (Fig. 2). The apparent lack in LACM/CIT 2852 of a triradiate morphology stems from the fact that the prefrontal ramus is missing. This is indicated by truncation of the rostral extent of the bone; the resulting rostral surface shows a rugose and irregular texture bounded by sharp and uneven edges. The orientation of the rostroventral and dorsal margins of the rostral broken border converge rostrodorsally. These observations are congruent with breakage and subsequent detachment of the prefrontal ramus from the central region of the postorbital.

Figure 2. Postorbital of Augustynolophus morrisi gen. nov. (LACM/CIT 2852) in (a) dorsal, (b) caudal, (c) lateral, (d) medial and (e) rostral views.
The medial surfaces of both the central body and the proximal region of the squamosal ramus are so severely damaged that most of the bone surface of these areas is eroded away (Fig. 2d). Consequently, any trace of the laterosphenoid cotylus and the facet for reception of the postorbital ramus of the squamosal has been erased. The jugal ramus tapers ventrally; its unusual undulating morphology is attributable to the pathology hypothesized below, probably in conjunction with diagenetic plastic deformation of the bone, a distortion that is pervasive throughout the skull of LACM/CIT 2852 (Bell & Evans, Reference Bell and Evans2010; Prieto-Márquez & Wagner, Reference Prieto-Márquez and Wagner2013a ). Part of the articular surface for the postorbital ramus of the jugal is preserved along the ventral segment of the ventral ramus as a concave caudal surface (Fig. 2d). The dorsal half of the process is flat to gently convex and shows no trace of a facet. A similar configuration is found in well-preserved postorbitals of other hadrosaurids (e.g. Brachylophosaurus canadensis MOR 1071-7-13-99-87-L) in which the facet also appears as a concave surface restricted to the ventral segment of the jugal ramus.
An L-shaped cleft is present on the lateral surface of the central body of the bone, which would seem to separate two different elements (Fig. 2c). However, medially and caudally the elongate ventrally directed jugal ramus is seamlessly continuous with the ventral tapering region of the central body of the element. This argues against this bone being composed of two different elements. The cleft probably represents a fissure and partial detachment of the proximal region of the jugal ramus from the lateral surface of the postorbital. In summary, the element of LACM/CIT 2852 is quite reasonably interpreted as the postorbital, and doubts cast upon that referral by authors Bell & Evans (Reference Bell and Evans2010) are rejected here.
Prieto-Márquez & Wagner (Reference Prieto-Márquez and Wagner2013a ) regarded as the sole autapomorphy of Augustynolophus morrisi a short sulcus that cuts transversely the caudolateral surface of the jugal ramus of the postorbital of LACM/CIT 2852 (Fig. 2a–d). It is not possible to know whether this sulcus was also present in LACM/CIT 2760 because the jugal ramus is not preserved in the postorbitals of this exemplar. However, after additional preparation and re-examination of the LACM/CIT 2852 postorbital, we now doubt that the sulcus is an actual ornamental feature of this bone. The jugal ramus is slightly bent rostrally, and the inflexion point of this curvature coincides with the location of the sulcus. The breadth of the jugal ramus increases in the area containing the sulcus; this increase is particularly important mediolaterally, so that the surface carved by the sulcus protrudes laterally forming a prominent ‘swelling’. These observations indicate that the sulcus may be pathological in origin, an area where the jugal ramus of the postorbital experienced some sort of trauma. The presence of this sulcus is therefore not a diagnostic feature of A. morrisi.
4.b. The diagnostic Y-shaped postorbital of Saurolophus
Bell (Reference Bell2011) proposed that a Y-shaped postorbital is a synapomorphy uniting larger individuals of Saurolophus angustirostris and S. osborni. This condition, caused by dorsal inflection of the prefrontal and squamosal rami of the postorbital, is certainly absent in most other saurolophines (Prieto-Márquez & Wagner, Reference Prieto-Márquez and Wagner2013a ), although a shallowly inflected version of this character is present in Kundurosaurus (Godefroit, Bolotsky & Lauters, Reference Godefroit, Bolotsky and Lauters2012) and at least some Prosaurolophus (McGarrity, Campione & Evans, Reference McGarrity, Campione and Evans2013). Subsequently, Prieto-Márquez & Wagner (Reference Prieto-Márquez and Wagner2013a ) opposed diagnosing Saurolophus on the basis of a Y-shaped postorbital because of the presence of a T-shaped postorbital in both LACM/CIT 2760 (Figs 3, 4) and 2852 (Fig. 2) and the attribution of the Moreno Formation species to Saurolophus. However, removal of the Moreno Formation hadrosaurid to its own new monotypic genus here leaves Saurolophus as the only taxon with a strongly inflected Y-shaped postorbital. We therefore concur with Bell (Reference Bell2011) in that this condition is apomorphic for larger specimens of Saurolophus.

Figure 3. Skull roof of a referred specimen of Augustynolophus morrisi gen. nov. (LACM/CIT 2760). (a) Dorsal view. (b) Interpretive line drawing of the dorsal view. (c) Ventral view. (d) Interpretive line drawing of the ventral view. Diagonal line patterns indicate broken or eroded bone surfaces.

Figure 4. Skull roof of a referred specimen of Augustynolophus morrisi gen. nov. (LACM/CIT 2760). (a) Rostral view. (b) Interpretive line drawing of the rostral view. (c) Caudal view. (d) Interpretive line drawing of the caudal view. (e) Right lateral view. (f) Interpretive line drawing of the right lateral view. (g) Left lateral view. (h) Interpretive line drawing of the left lateral view Diagonal line patterns indicate broken or eroded bone surfaces.
4.c. Breadth of the apertura ossis nasi in LACM/CIT 2852
According to Prieto-Márquez & Wagner (Reference Prieto-Márquez and Wagner2013a ), Augustynolophus morrisi might differ from Prosaurolophus and Saurolophus in possessing a broader apertura ossis nasi, unlike the elongate and slit-like opening in the latter two genera. However, further examination of the preserved rostroventral margin of the apertura ossis nasi in LACM/CIT 2852 reveals signs of breakage, including a rough texture and discontinuous outline. The apparent breadth of the apertura ossis nasi of LACM/CIT 2852 may have been caused by post-depositional distortion and/or fracture of the premaxilla and may not be as distinctive as previously considered (Prieto-Márquez & Wagner, Reference Prieto-Márquez and Wagner2013a ). We have therefore amended the diagnosis of A. morrisi to remove the shape of the apertura ossis nasi.
4.d. The temporal region of the skull of Augustynolophus morrisi
In dorsal aspect, the lateral margins of the skull roof (composed of the postorbital and squamosal) are angled caudomedially with the result that the caudal portion of the skull tapers caudally, and the skull table is broader across the postorbitals than across the quadrates (Fig. 3). Among saurolophins, the caudal region of the skull tapers in Prosaurolophus (McGarrity, Campione & Evans, Reference McGarrity, Campione and Evans2013, fig. 4; also exemplified by ROM 787 and 1928, TMP 84.1.1). However, in Saurolophus (Bell, Reference Bell2011) and Augustynolophus morrisi (Fig. 3a, b) the intertemporal bars are roughly parallel and the skull is approximately as broad across the postorbitals as it is across the quadrates. In LACM/CIT 2760 the lateral surface of the left postorbital is heavily eroded (Fig. 4g), forming the impression that the skull is slightly broader across the squamosals (Fig. 3a). However, the lateral surface of the right postorbital is relatively complete (Fig. 4e). This indicates that no caudal tapering occurs in the skull roof in A. morrisi.
The skull roof of Saurolophus is rostrocaudally ‘compressed’, apparently in concert with retraction of the crest to a position over the cranium. In the larger Saurolophus specimens, the dorsal margin of the infratemporal fenestra is as wide as (e.g. S. osborni AMNH 5220) or narrower than (e.g. S. angustirostris PIN 551/358) the quadrate cotylus of the squamosal. In juveniles (e.g. S. angustirostris PIN 551/359) the dorsal margin of the infratemporal fenestra is still relatively narrow, being only slightly wider than the quadrate cotylus. One manifestation of this condition is that the upper portion of the jugal process of the postorbital is drawn close to the dorsal terminus of the quadrate, causing a narrowing of the infratemporal fenestra at its dorsal extreme. Morphogenetically, this is likely related to bowing of the quadrate in S. angustirostris (Rozhdestvensky, Reference Rozhdestvensky1957; Bell, Reference Bell2011) as part of the extreme compression of the skull roof associated with crest retraction in that species. Narrowing of the infratemporal fenestra is also present in large Prosaurolophus (e.g. AMNH 5386; although the extent of narrowing is variable), but absent in smaller specimens of that taxon (e.g. ROM 1928). As mentioned before, juveniles of Saurolophus (Bell & Evans, Reference Bell and Evans2010; Bell, Reference Bell2011) show narrowing of the infratemporal fenestra while the presumably immature LACM/CIT 2760 displays relatively rostrocaudally broad upper infratemporal fenestrae. This condition is only preserved on the left side of the skull in LACM/CIT 2760; although only the proximal extent of the jugal ramus and the prequadratic process are preserved, the distance between these two structures is nearly double the width of the quadrate cotylus of the squamosal, which is indicative of an unabbreviated infratemporal fenestra (Fig. 4g, h). The right side however is distorted due to post-depositional rostral compression of the squamosal against the postorbital and parietal, with concomitant rostrocaudal shortening of the supratemporal fenestra (Fig. 3a, b). The unabbreviated infratemporal fenestra of LACM/CIT 2760 is therefore similar to immature Prosaurolophus but it remains uncertain whether this condition would have been present in adult A. morrisi.
As preserved, LACM/CIT 2760 shows a slight sagittal elevation of the squamosals at the midline (Fig. 4c). However, the dorsoventral crushing experienced by the specimen indicates that the elevation of the squamosals was probably greater than observed. This deformation is particularly evident in the right squamosal, which has been collapsed and pushed rostrally (Fig. 3a), resulting in rostrocaudal compression of the right supratemporal fenestra and the upper margin of the right infratemporal fenestra described above. In Prosaurolophus there is no appreciable elevation of the squamosals (see McGarrity, Campione & Evans, Reference McGarrity, Campione and Evans2013, fig. 20). One large skull, TMP 1981.001.0001 (McGarrity, Campione & Evans, Reference McGarrity, Campione and Evans2013, fig. 12) appears to show substantially elevated squamosals; however, the mediolateral post-depositional compression experienced by this skull (evidenced by the fact that the left dorsal supratemporal region is visible in right lateral view) probably accounts for this conformation. Although not developed to the extent present in Saurolophus (e.g. AMNH 5220), a rostrocaudally abbreviated, caudally deepening and down-warped sagittal crest is present in LACM/CIT 2760. We therefore consider the condition in Augustynolophus morrisi to be sufficiently developed to be comparable to that in Saurolophus, the adult specimens of which have a prominent sagittal crest of the parietal that deepens caudally and is strongly down-warped. It is therefore conceivable to hypothesize that adult A. morrisi would probably exhibit a sagittal crest comparable to that of Saurolophus.
4.e. The caudodorsal process of the frontal of Augustynolophus
Additional preparation of LACM/CIT 2760 clarifies the morphological and osteological relationships of the caudodorsal process that rises from the ectocranial surface of each frontal (Fig. 5a, c, e). The frontal caudodorsal process of Augustynolophus morrisi differs from that of Saurolophus in various aspects. First, the base of the process of A. morrisi constitutes a thick crescentic ridge that is obliquely oriented relative to the sagittal plane of the skull, so that both caudodorsal processes converge (but do not contact one another) rostromedially forming a wide U-shaped profile in dorsal view (Fig. 5c). Each process becomes gradually thicker laterally. In contrast, the base of the caudodorsal process of Saurolophus angustirostris is columnar (e.g. PIN 551/356; Fig. 5b) and its caudal surface is buttressed by a ridge that extends caudolaterally (Bell, Reference Bell2011, p. 712; Fig. 5b). A similar buttress is present in S. osborni (Bell, Reference Bell2010) although the thinner medial part of the ridge, if present, cannot be observed.

Figure 5. Variation in the frontal caudodorsal process of saurolophin hadrosaurids. (a) Caudodorsal process of Augustynolophus morrisi gen. nov (LACM/CIT 2760) in caudodorsal view. (b) Caudodorsal process of Saurolophus angustirostris (PIN 551/356) in caudodorsal view. (c) Caudodorsal process of D. morrisi gen. nov. (LACM/CIT 2760) in dorsal view. (d) Caudodorsal process of a juvenile S. angustirostris (PIN 551/359) in dorsal view. (e) Skull roof of A. morrisi (LACM/CIT 2760) in dorsal view. (f) Skull roof of S. angustirostris (PIN 551/359) in dorsal view. Arrows in (a) and (c) indicate broken surfaces that are continuous with the caudodorsal processes of the frontals and likely indicate their true caudolateral extent.
The caudodorsal process of Augustynolophus morrisi also differs from that of Saurolophus in that it extends farther caudolaterally past the level of the rostral end of the frontal ‘dome’ (Fig. 5c). In fact, the process might have extended even farther caudally than preserved, surrounding the rostral one-half of the frontal ‘dome’. This possibility is supported by the presence of an eroded, elongate surface (arrows in Fig. 5a, c) that is continuous caudolaterally with each caudodorsal process and the incomplete preservation of the caudal ends of both processes. Mediolateral thickening of the caudodorsal process also continues and substantially increases along the eroded, elongate surface that may represent what remains of the complete base of each process. In contrast, in juvenile Saurolophus specimens (e.g. PIN 551/359, with a size comparable to that of LACM/CIT 2760) the caudodorsal process ends just rostral to the frontal ‘dome’ (Fig. 5d). This condition is also present in larger Saurolophus (e.g. PIN 551/356; Fig. 5b), in which the caudodorsal process does not extend further caudolaterally in relation to that of the juveniles of this taxon.
Because only the bases of the two caudodorsal processes are preserved, their dorsal extent cannot be evaluated. Specifically, whether they became strap-like as they stretch caudodorsally underlying a substantial extent of the nasal crest as in larger Saurolophus (e.g. PIN 551/356; see Bell, Reference Bell2011, fig. 3) or remained a stub at the base of the crest as in juvenile Saurolophus (e.g. PIN 551/359; see Bell, Reference Bell2011, fig. 6) cannot be determined. Poor preservation prevents determination in Augustynolophus of the presence of a caudodorsal process of the prefrontal, a process that has been documented in Saurolophus (Bell, Reference Bell2011).
It is now apparent that rather than fusing sagittally to form a single frontal buttress (Prieto-Márquez & Wagner, Reference Prieto-Márquez and Wagner2013a ), both caudodorsal processes are separated by a narrow gap in Augustynolophus morrisi (Fig. 5a). The two caudodorsal processes are also divided medially in species of Saurolophus (e.g. PIN 551/356; Fig. 5b), in this case by a descending process of each nasal. This division extends to the base of the crest in Saurolophus osborni (Bell, Reference Bell2010), a condition also present in A. morrisi (Fig. 5a). It is not clear if this condition, or if the condition in S. angustirostris (wherein the midline nasofrontal suture is on the caudal surface of the crest; Bell, Reference Bell2011, fig. 2), is ancestral.
4.f. The nasal crest of Augustynolophus morrisi
Two paired bone fragments are preserved in LACM/CIT 2760 diagenetically fused onto the rostral margin of the skull roof (Figs 3a, 4a, 6). These elements, previously misidentified as part of the frontal (Prieto-Márquez & Wagner, Reference Prieto-Márquez and Wagner2013a ), occupy the anatomical position in the skull where the nasal is expected to reside, and resemble the distal ascending processes (crest) of the nasals in Saurolophus. We identify these elements in LACM/CIT 2760 as the left and right nasals partly conjoined and displaced to the right side of the skull, crushed ventrally but otherwise nearly in articulation with each other. Evidence of this displacement and crushing is provided by the fact that the lateral margin of the left nasal lies overlapping the rostral surface of the caudodorsal processes of the frontals; the thin bony lamina of that margin is deformed to accommodate the surface relief of the frontal processes (Fig. 6d). Incomplete conjoining of the nasals shows their flat medial faces (Fig. 6h). If the nasals were crushed from approximate life position, most of the preserved portion of these bones would be elevated above the skull roof (Fig. 6b), comparable to that found on juvenile Saurolophus (e.g. PIN 551/359, Fig. 6c; see also Bell, Reference Bell2011).

Figure 6. Distal region of the nasal supracranial crest of Augustynolophus morrisi (LACM/CIT 2760). (a) Skull roof of A. morrisi (LACM/CIT 2760) in dorsal view. (b) Concept model of the skull of A. morrisi based on LACM/CIT 2760 and 2852, in left lateral (and slightly rostral) view. (c) Left lateral view of the skull of a juvenile Saurolophus angustirostris (PIN 551/359). (d) Left and right distal nasal crest of A. morrisi (LACM/CIT 2760) in left lateral view. (e) Interpretive line drawing of (d). (f) Left distal nasal crest of S. angustirostris (PIN 551/359) in lateral view. (g) Rostral view of left and medial view of right distal nasal crest of A. morrisi (LACM/CIT 2760). (h) Rostral view of the left and right distal nasal crest of A. morrisi (LACM/CIT 2760). (i) Rostromedial view of right and medial view of left nasal crest of A. morrisi (LACM/CIT 2760). (j) Medial view of the right and caudomedial view of the left nasal crest of A. morrisi (LACM/CIT 2760). Arrows throughout indicate the inflection of the knob-like process.
Only the distalmost segment 90 mm of the nasal crest that projected above the skull roof is preserved. As in Saurolophus (Bell, Reference Bell2010, Reference Bell2011; Fig. 6f), the nasal here takes the form of a bluntly elongate triangular plate (Fig. 6d, e), concave laterally where it is excavated by the distalmost extent of the circumnarial fossa. However, the distal segment of the crest is narrower in Augustynolophus morrisi than in Saurolophus. For example, the width at mid-length of the preserved portion of the nasal crest of LACM/CIT 2760 (Fig. 6d) is c. 70% of the width of the corresponding segment of the crest in S. angustirostris PIN 551/359 (Fig. 6f) (both having skull roofs of similar size).
Distally, the circumnarial fossa of Augustynolophus is weakly emarginated and relatively shallow (Fig. 6d, e), unlike the deeply pocketed emargination of Saurolophus (e.g. PIN 551/359, Fig. 6f). Furthermore, in Saurolophus the circumnarial fossa is wide, spanning from the medial to the lateral margins of the nasal in the distal part of the crest (e.g. PIN 551/359, Fig. 6f). By contrast, in Augustynolophus the circumnarial fossa occupies the lateral two-thirds of the lateral surface of the crest (Fig. 6d, e).
The distal end of the crest of Augustynolophus morrisi is composed of a knob-like process that is gently inflected rostrally (arrows in Fig. 6e, g, i, j). This process is not excavated by the circumnarial fossa and stands in contrast with the excavated ‘cap’ that forms the distal terminus of the nasal crest in Saurolophus (Fig. 6f).
The proximal (ventral) terminus of the preserved nasals has been sectioned transversely, and clearly occurs above the level of the apertura ossis nasi. The cross-section of the nasal is vaguely sigmoid, reflecting its articulation caudally with the arcuate caudodorsal process of the frontal. Given the arcuate shape of the frontal caudodorsal processes, it is reasonable to assume that the nasals wrapped around them with a U-shaped cross-section (convex rostrally). The caudoventral surface of the nasal is flat (Fig. 7a), as in Saurolophus (e.g. PIN 551/359, Fig. 7b). However, whereas in the latter the caudal surface of the nasal narrows to a blunt apex distally (Fig. 7b), in Augustynolophus the width of the caudal surface is only slightly diminished distally (Fig. 7a).

Figure 7. Caudoventral surfaces of saurolophin distal nasal crests. (a) Augustynolophus morrisi (LACM/CIT 2760). (b) Saurolophus angustirostris (PIN 551/359).
Notably, the frontal caudodorsal processes of Augustynolophus morrisi are not significantly inclined caudally, suggesting a more erect crest such as that of Saurolophus osborni (e.g. AMNH 5220) and juvenile S. angustirostris (e.g. PIN 551/359) rather than the strongly backswept crest of adult S. angustirostris (Bell, Reference Bell2011).
5. Discussion
5.a. Generic status and relationships of the Moreno Formation saurolophin
Previously, Prieto-Márquez & Wagner (Reference Prieto-Márquez and Wagner2013a ) concluded that Augustynolophus morrisi is the sister taxon to Saurolophus. These authors ventured that the crest of A. morrisi would have been similar to that in Saurolophus, and referred the former to the latter genus as S. morrisi. Our new observations identify important differences in the skull table and crest of A. morrisi. Saurolophine genera are mainly distinguished on the basis of overall crest morphology and incorporation of the Moreno Formation hadrosaurid into Saurolophus would imply that Augustynolophus sported a rod-like nasal crest like that of the former, an implication that can no longer be supported. Furthermore, such referral would result in substantial changes to the diagnosis of Saurolophus. We therefore here recognize morrisi as the type species of a new genus, Augustynolophus.
We conducted a revised phylogenetic analysis of Saurolophinae to infer the relationships of Augustynolophus with other hadrosaurids of this clade. The morphological character dataset of Prieto-Márquez (Reference Prieto-Márquez2014) constitutes one of the most current for saurolophine hadrosaurids and, as such, we revised these data in the light of the new anatomical observations presented here for use in our phylogenetic analysis. The resulting character-taxon matrix consisted of 272 morphological characters (186 cranial and 86 postcranial) and, in addition to Augustynolophus, included 21 saurolophine species and 12 outgroup taxa (3 lambeosaurines and 9 non-saurolophid hadrosauroids) (see also online Supplementary Material 1, available at http://journals.cambridge.org/geo). In order to evaluate whether there is any phylogenetic support for referring LACM/CIT 2852 to A. morrisi, we coded this specimen and LACM/CIT 2760 as separate operational taxonomic units (OTUs) (see also online Supplementary Material 1, available at http://journals.cambridge.org/geo). A heuristic search of 10000 replicates, using random addition sequences followed by branch swapping using tree-bisection reconnection and holding 10 trees per replicate, was performed in TNT version 1.1 (Goloboff, Farris & Nixon, Reference Goloboff, Farris and Nixon2008). Bootstrap proportions were calculated with PAUP version 4.0b10 (Swofford, Reference Swofford2002), setting the analysis to 5000 replicates using heuristic searches, where each search was conducted using random addition sequences with branch-swapping by subtree pruning and regrafting and 25 replicates. Bremer support was assessed by computing decay indices using the TNT software.
The analysis resulted in a single most parsimonious tree of 629 steps (consistency index CI = 0.61; retention index RI = 0.75). It supported the previous phylogenetic hypothesis of Prieto-Márquez & Wagner (Reference Prieto-Márquez and Wagner2013a ), in which Augustynolophus is sister to Saurolophus (Fig. 8). Synapomorphies supporting this relationship are given in Figure 9. We acknowledge that this placement of Augustynolophus in the saurolophine phylogeny neither refutes nor necessitates the creation of a new genus for the Moreno Formation hadrosaurid, as the genus Saurolophus could simply be expanded to include it.

Figure 8. The single-most parsimonious tree resulting from maximum parsimony analysis of saurolophine relationships. Numbers above branches indicate bootstrap proportions (left) and decay indices (right).

Figure 9. Phylogram showing the relationships, geographical provenance and distribution of synapomorphies of saurolophin taxa. Asterisks indicate ambiguous synapomorphies. When only subages (e.g. late Maastrichtian) are available instead of absolute dating estimates, the absolute dating of the taxon is approximated as the mid-point of the available range following the geologic time scale of Walker et al. (Reference Walker, Geissman, Bowring and Babcock2012). Literature sources for the geochronological range of each terminal taxon (in alphabetical order) are as follows: Augustynolophus morrisi, late Maastrichtian (D. J. McGuire 1988, unpub. Ph.D. thesis, Standford University, 1988; Bell & Evans, Reference Bell and Evans2010); Prosaurolophus maximus, 75.7–74.1 Ma range (McGarrity et al. Reference McGarrity, Campione and Evans2013); Saurolophus angustirostris, ?late Campanian – early Maastrichtian range (Bell, Reference Bell2011); S. osborni, early Maastrichtian, 68.4–70.4 Ma range (Eberth et al. Reference Eberth, Evans, Brinkman, Therrien, Tanke and Russell2013).
The phylogeny of Saurolophini presented here agrees well with stratigraphy (Fig. 9). The occurrence of Prosaurolophus in the upper Campanian (McGarrity, Campione & Evans, Reference McGarrity, Campione and Evans2013) suggests a short ghost lineage leading to the Augustynolophus + Saurolophus clade. Saurolophus has been reported from the upper Campanian of North America (Gates & Farke, Reference Gates and Farke2009), but the fragmentary nature of the specimen alleged to belong to this taxon prevents generic and specific determination with certainty. The split between Saurolophus and Augustynolophus predates the lower Maastrichtian appearance of Saurolophus osborni (Bell, Reference Bell2010), which also constrains the dispersal event that resulted in the presence of S. angustirostris in central Asia. This leaves a ghost lineage for Augustynolophus through the lower and part of the upper Maastrichtian. The lower Maastrichtian is a relatively poorly known interval overall, and so the presence of unrecovered diversity is not at all surprising.
5.b. Referral of LACM/CIT 2760 to Augustynolophus morrisi
Doubts concerning the diagnostic nature of the postorbital of LACM/CIT 2852 present an unfortunate situation, in that all of the autapomorphies of Augustynolophus morrisi are preserved on the referred specimen and not the holotype. Yet, while LACM/CIT 2852 does not exhibit any unambiguous autapomorphies, it displays the following saurolophin synapomorphies: broad arcuate, everted premaxillary oral margin, medial and lateral processes of the premaxilla converging caudally, quadrate with wide arcuate, asymmetrical quadratojugal notch, ventral convexity of dentary ramus rostral to coronoid process, and flat, steeply inclined dentary occlusal plane. These characters allow referral of LACM/CIT 2852 to Saurolophini.
Although the material available for LACM/CIT 2852 does not preserve the diagnostic skeletal regions bearing the autapomorphies of LACM/CIT 2760, we choose to refer the two specimens to the same species for two reasons. First, there are various overlapping elements between the referred specimen of Augustynolophus morrisi LACM/CIT 2760, and the type LACM 2852. Those elements include overlapping regions of the maxilla, postorbital, quadrate, dentary and dentary tooth crowns, scapula, ulna, various manual phalanges and metatarsal III. The morphology of these bones in LACM/2852 accords with that of the same elements in LACM/CIT 2760, including saurolophin synapomorphies of the quadrate, dentary and dentition.
Secondly, scoring LACM/CIT 2760 and 2852 as separate OTUs in the aforementioned maximum parsimony analysis of Saurolophinae resulted in both specimens joined together as sister OTUs and nested within Saurolophini as sister taxa to Saurolophus (Fig 8). The LACM/CIT 2760 and 2852 relationship was supported unambiguously by (1) dentaries with a ratio between the distance from the caudal margin of the coronoid process to the inflexion point of the ventral margin and the distance from the caudal margin of the coronoid process to the rostralmost alveolus between 0.66 and 0.78, and (2) uncompressed, relatively broad dorsal margin of the infratemporal fenestra. This result is congruent with referral of LACM 2852 to Augustynolophus morrisi.
Rejecting referral of LACM/CIT 2760 to Augustynolophus morrisi would necessitate the recognition of not one but two saurolophins in the Moreno Formation. In that situation, we could adopt a shorthand such as ‘the Moreno Formation hadrosaurid’, but withholding taxonomic recognition usually has the effect of stifling discussion and not promoting it, and we feel that this is a biogeographically important occurrence that should be considered. Furthermore, in other rock formations there is evidence of only a single saurolophin present at any one time; indeed, it is exceptional for closely related hadrosaurids of any group to be present in the same deposits. This would be an especially interesting result given claims of reduced diversity in the late Maastrichtian.
Regardless of the taxonomic status of these specimens, evaluation of the significance of Augustynolophus (see discussion below) is valid even if the material represents more than one species; two saurolophins are no more expected than one in the latest Maastrichtian.
5.c. Crest evolution in Saurolophini
Placement of Augustynolophus within the phylogeny of Saurolophini permits a clearer understanding of the evolution of the crest in this clade in a phylogenetic context. In Prosaurolophus, the crest is a prominence on the nasals that barely rises above the level of the skull table; we interpret this as being close to the ancestral condition for the group (Fig. 9). In the evolution of the Augustynolophus + Saurolophus clade, caudal elongation of the nasals produced a crest that rises significantly over the skull table (Fig. 9). As part of this transition, the frontal (and probably the prefrontal) were incorporated into the crest. In the evolution of Saurolophus, the base of the crest became appressed against the skull table as exemplified by the steep angle between the parietal and frontals (as seen in lateral view) and development of the Y-shaped postorbital (Bell, Reference Bell2011). The orbital margin also became everted in S. osborni, possibly as a lateral extension of the circumnarial fossa.
Phylogenetically, the development of the crests in Saurolophini agrees with Hopson's (Reference Hopson1975) prediction that such structures should become more prominent during the evolution of a lineage, supporting his hypothesis that the crest is an inter-/intraspecific signaling structure. The circumnarial structure (Hopson, Reference Hopson1975; Wagner, Reference Wagner2004), implicated in display, is first elevated and lengthened then moved above the skull roof. Nowhere is this clearer than in Saurolophus angustirostris, in which the crest is dramatically appressed to the skull roof as it is swept back over the skull (Fig. 9).
This sequence of crest evolution closely parallels that seen in lambeosaurines (Prieto-Márquez & Wagner, Reference Prieto-Márquez and Wagner2013b ), in which elevation of the crest preceded relocation of the crest above the frontals. The ancestral saurolophid (i.e. Saurolophinae + Lambeosaurinae, sensu Prieto-Márquez, Reference Prieto-Márquez2010) almost certainly possessed a crest (Wagner, Reference Wagner2004), and the most parsimonious hypothesis is that the ancestral crest was in front of the cranium proper and likely composed only, or at least primarily, of the nasals (Prieto-Márquez & Wagner, Reference Prieto-Márquez and Wagner2013b ). The parallel shift of this structure from the rostrum to the skull roof in Saurolophini and Lambeosaurinae suggests a common selection pressure, possibly for increasing the display area and the visibility of the displayed structure. A similar pattern may be evident in Brachylophosaurini (with the exception of the apomorphically crestless Acristavus Gates et al. Reference Gates, Horner, Hanna and Nelson2011) where the crest is drawn out across the skull roof in Brachylophosaurus, and in Kritosaurini wherein the crest of Kritosaurus is similarly drawn up against the skull roof relative to that in Gryposaurus (Prieto-Márquez, Reference Prieto-Márquez2014).
5.d. Biostratigraphic and palaeozoogeographic implications
The Moreno Formation includes the Maastrichtian–Danian boundary and is interpreted as upper Maastrichtian (D. J. McGuire, unpub. Ph.D. thesis, Standford University, 1988; Ford, Reference Ford2006; Bell & Evans, Reference Bell and Evans2010; contra Prieto-Márquez & Wagner, Reference Prieto-Márquez and Wagner2013a ). Prosaurolophus is Campanian (McGarrity, Campione & Evans, Reference McGarrity, Campione and Evans2013) and Saurolophus osborni is early Maastrichtian (Bell, Reference Bell2010), making A. morrisi the youngest known saurolophin in North America and one of the last surviving hadrosaurids on the continent. Survival of Saurolophini to the terminal Cretaceous contradicts the previous tacit assumption that this clade either went extinct or was extirpated from North America after the early Maastrichtian occurrence of Saurolophus osborni; the clade may have persisted up to the terminal Cretaceous extinction event.
Augustynolophus represents a significant addition to the known diversity of upper Maastrichtian hadrosaurids of North America: all published specimens with material sufficient for diagnosis to the genus level have been referred to Edmontosaurus (Bell & Evans, Reference Bell and Evans2010; Campione & Evans, Reference Campione and Evans2011; Brusatte et al. Reference Brusatte, Butler, Prieto-Márquez and Norell2012). Since this is an interval in which dinosaur diversity has been hypothesized to be decreasing (e.g. Bakker, Reference Bakker1986; Archibald, Reference Archibald1996; Lehman, Reference Lehman, Tanke and Carpenter2001; Campione & Evans, Reference Campione and Evans2011; Brusatte et al. Reference Brusatte, Butler, Prieto-Márquez and Norell2012), the addition of a new hadrosaurid genus to the late Maastrichtian faunal list of North America is particularly significant. This is congruent with recent work suggesting a greater diversity than previously suspected among ceratopsians and basal ornithopods in the late Maastrichtian as well (e.g. Brown, Boyd & Russell Reference Brown, Boyd and Russell2011; Farke, Reference Farke2011). However, in this case the newly discovered diversity lies not in a close offshoot of a surviving lineage (as in Nedoceratops; Farke, Reference Farke2011), but in the survival of a long-diverged lineage. Regardless, this additional diversity argues for caution in the interpretation of the faunal dynamics of the latest Cretaceous.
Lehman (Reference Lehman1987) outlined a hypothesis of faunal provinciality in late Maastrichtian time in which North America was divided into a northern ‘Triceratops fauna’ and a southern ‘Alamosaurus fauna’. It is not clear how Augustynolophus fits into this scenario. No evidence of Augustynolophus has been recovered from the well-sampled upper Maastrichtian units of the northern Great Plains of North America, suggesting faunal differentiation between the Moreno Formation and these classic ‘Triceratops Fauna’ localities, which is minimally consistent with provincialism (Lehman, Reference Lehman1987, Reference Lehman, Tanke and Carpenter2001). Geographically, the Moreno Formation might be expected to record the southern ‘Alamosaurus fauna’. Hadrosaurids from that assemblage are too poorly known to exclude Augustynolophus (e.g. Hunt & Lucas, Reference Hunt and Lucas1991; J. R. Wagner, unpub. Masters thesis, Texas Technical University, Austin, 2001); until better data are available, we are reluctant to refer the Moreno Formation to this fauna although we cannot reject it. The Moreno Formation may otherwise represent a separate biogeographic unit, either another provincial fauna or possibly as a peripheral refugium preserving a relict, endemic fauna. Regardless of the interpretation, the presence of a non-Edmontosaurus hadrosaurid in California reinforces the case for faunal heterogeneity during late Maastrichtian time.
6. Conclusions
The Moreno Formation saurolophine specimens represent a new genus of hadrosaurid dinosaur characterized by unique apomorphies of the frontal and facial skeleton. This taxon, Augustynolophus, is the sister to Saurolophus; together with Prosaurolophus these genera constitute the tribe Saurolophini. Although fragmentary, the morphology of the nasal and surrounding bones of the skull roof of LACM/CIT 2760 lead us to hypothesize the presence in A. morrisi of a solid nasal crest that would extend above the skull roof ending distally in a blunt elongate triangular plate. Among saurolophins, supracranial crests evolved first towards greater height and then towards greater caudodorsal elongation above the skull table, with concomitant lengthening of the circumnarial fossa and involvement of adjacent elements of the facial skeleton. The presence of Augustynolophus in late Maastrichtian North America represents a substantial increase in our knowledge of late Maastrichtian dinosaur diversity, and lends further support to the hypothesis of faunal heterogeneity among large-bodied herbivorous dinosaurs at the time.
Acknowledgements
We thank José Soler and Stephanie Abramowicz (Natural History Museum of Los Angeles County, Los Angeles) for preparing and photographing, respectively, the skull roof of LACM/CIT 2760. Maureen Walsh provided archive images of the Moreno Formation outcrops where LACM/CIT 2852 was found. David C. Evans (Royal Ontario Museum, Toronto) shared insightful comments on the anatomy of LACM/CIT 2760. We are especially grateful to Mrs Gretchen Augustyn and her family for providing support to the Dinosaur Institute and to this investigation. Research funds were provided by the Dinosaur Institute of the Natural History Museum of Los Angeles County.
Declaration of interest
There are no conflicts of interest.
Supplementary materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0016756814000284